Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6
Abstract
1. Introduction
2. Results and Discussion
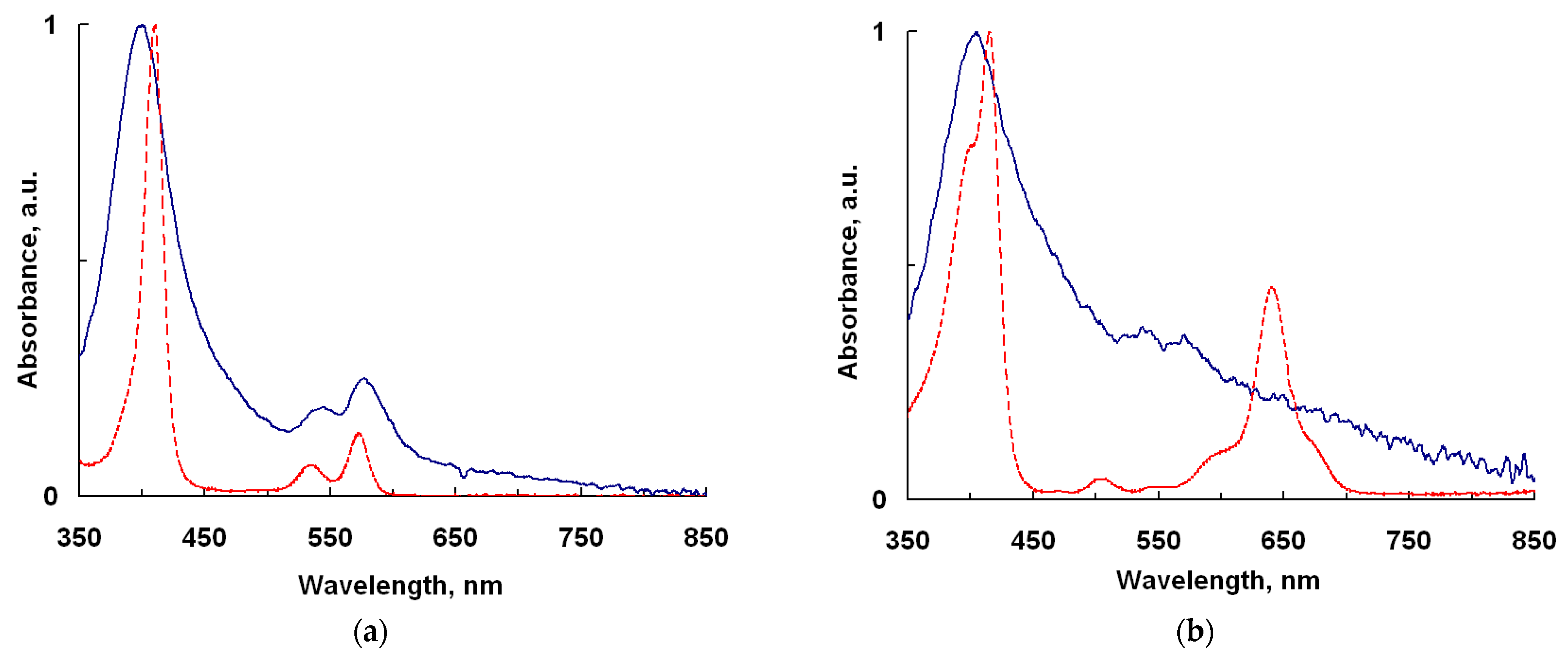
2.1. UV-Vis and IR Spectroscopy
2.2. Stability of the Dye Molecules during Thermal Evaporation
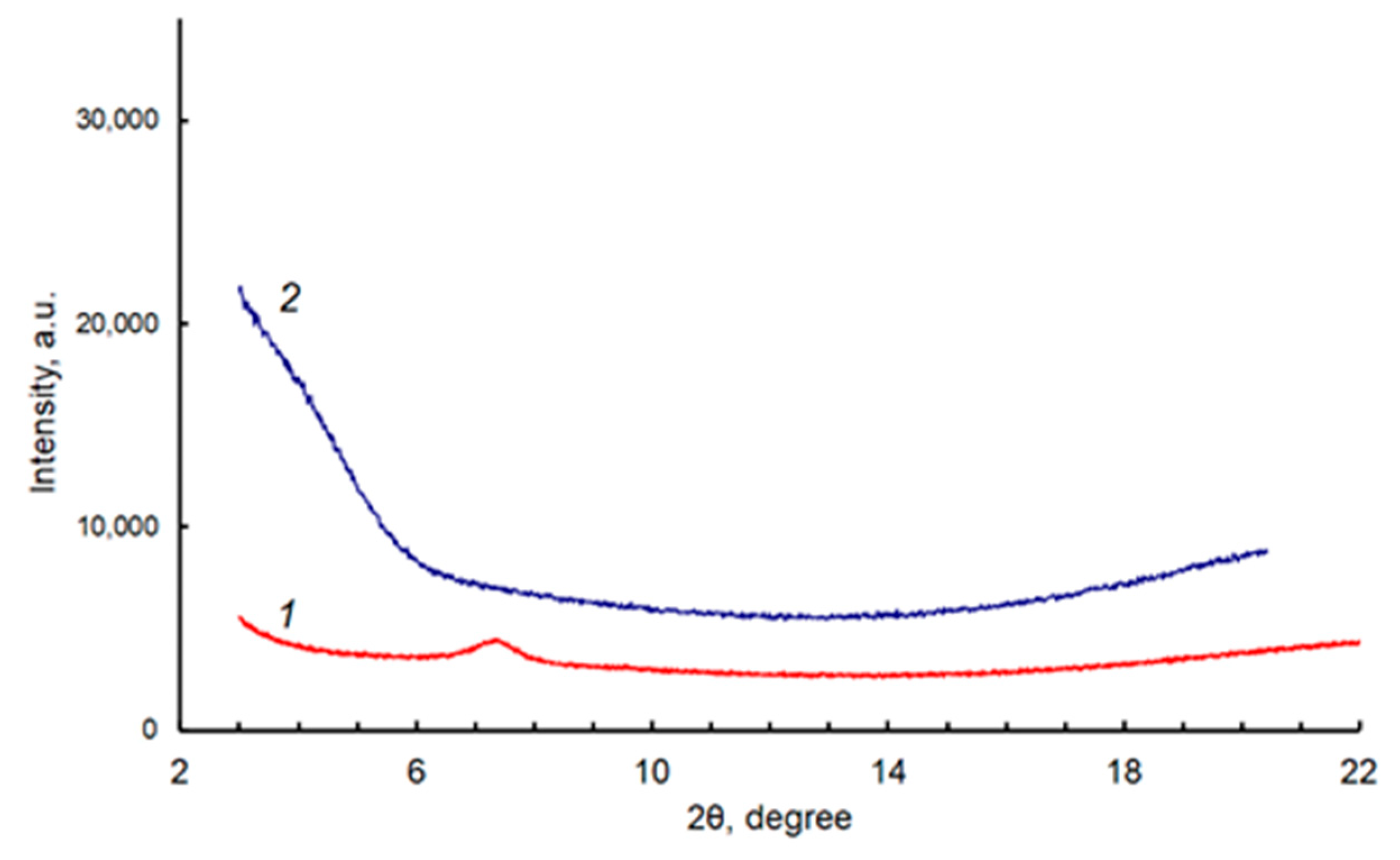
2.3. Dye Solid Layers
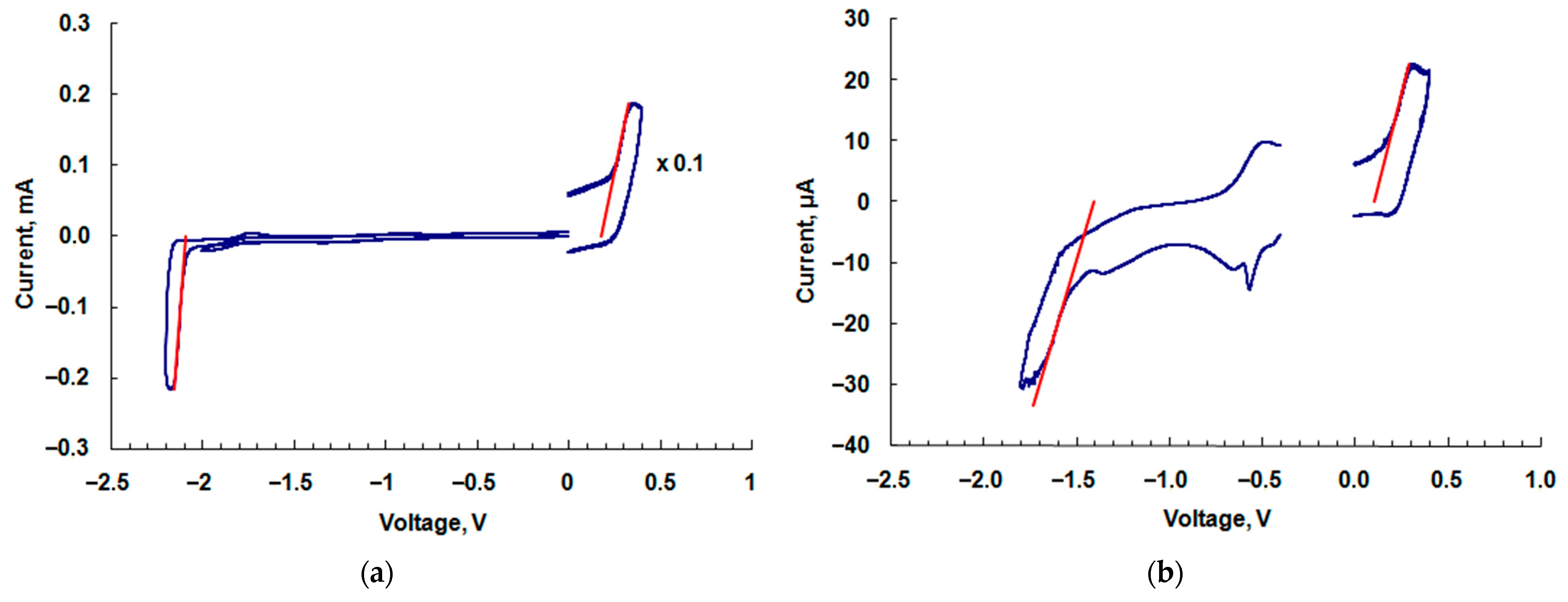
2.4. HOMO and LUMO Levels
2.5. Charge Carrier Mobility
2.6. Photoconduction
2.7. Electroluminescence
3. Materials and Methods
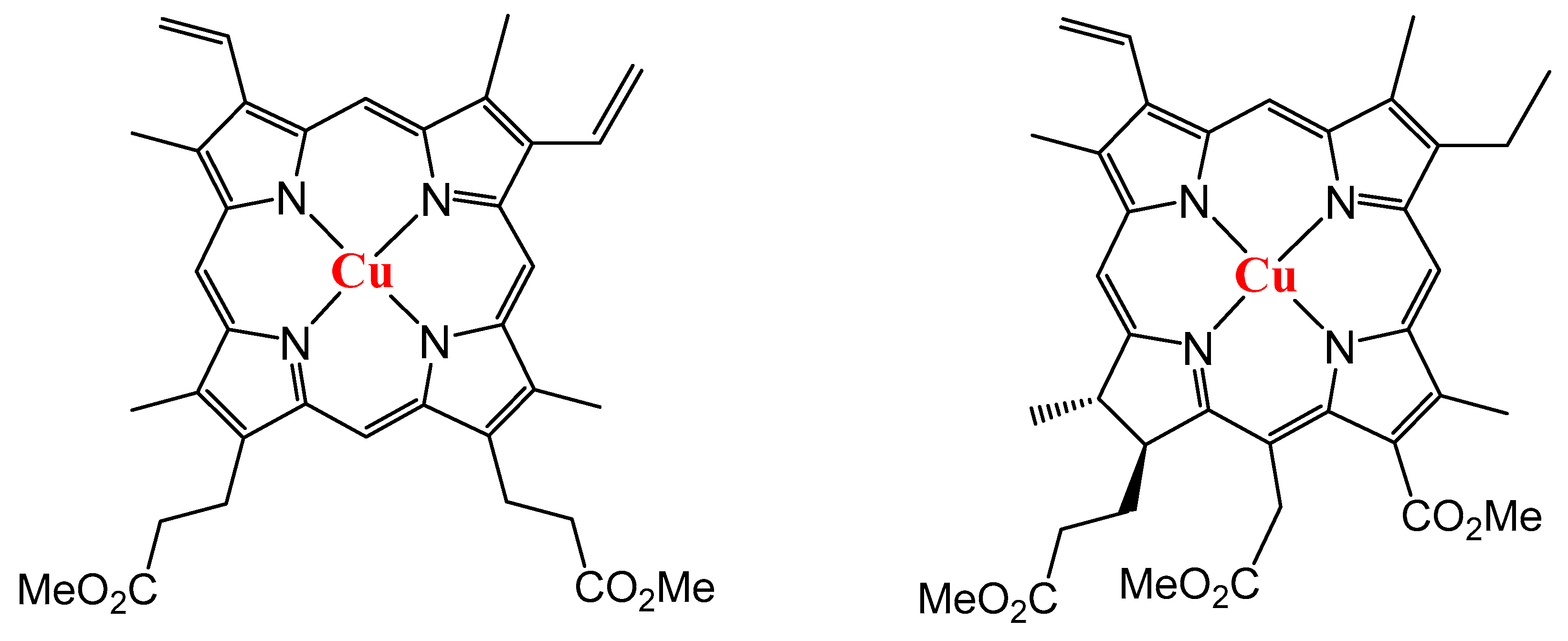
3.1. Synthesis of Compounds
3.2. Photoluminescence Spectra
3.3. Cyclic Voltammetry
3.4. Resistive Thermal Evaporation (RTE)
3.5. Atomic Force Microscopy (AFM)
3.6. Infrared (IR) Spectroscopy
3.7. X-ray Photoelectron Spectroscopy (XPS)
3.8. X-ray Powder Diffraction (XRD)
3.9. Scanning Electron Microscopy (SEM)
3.10. Charge Mobility Measurements
3.11. OLED Fabrication and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook; Academic Press: New York, NY, USA, 2000; Volume 2. [Google Scholar]
- Chernyadyev, A.Y.; Aleksandrov, A.E.; Lypenko, D.A.; Tyurin, V.S.; Tameev, A.R.; Tsivadze, A.Y. Copper (II) Meso-Tetraphenyl- and Meso-TetafluorenylPorphyrinates as Charge Carrier Transporting and Electroluminescent Compounds. ACS Omega 2022, 7, 8613–8622. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Chernyad’ev, A.Y. Metal crown-porphyrin complexes: Preparation, optical properties, and applications (Review). Russ. J. Inorg. Chem. 2020, 65, 1662–1686. [Google Scholar] [CrossRef]
- Belykh, D.V.; Kozlov, A.S.; Pylina, Y.I.; Khudyaeva, I.S.; Benditkis, A.S.; Krasnovsky, A.A. Copper Complexes of Chlorin derivatives of chlorophyll a as potential photosensitizers for medical purposes. Macroheterocycles 2019, 12, 68–74. [Google Scholar] [CrossRef]
- Mal’tsev, E.I.; Brusentseva, M.A.; Lypenko, D.A.; Novikov, S.V.; Vannikov, A.V.; Rumyantseva, V.D.; Mironov, A.F.; Berendyaev, V.I. Electrophosphorescence of aromatic polyimides doped with platinum porphyrins. Polym. Sci. Ser. A 2006, 48, 146–152. [Google Scholar] [CrossRef]
- Drouet, S.; Paul-Roth, C.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Platinum and palladium complexes of fluorenyl porphyrins as red phosphors for light-emitting devices. New J. Chem. 2011, 35, 438–444. [Google Scholar] [CrossRef]
- Gradyushko, A.T.; Tsvirko, M.P. The probability of intercombination transitions in the molecules of porphyrins and metalloporphyrins. Opt. Spektrosk. 1971, 21, 548–556. [Google Scholar]
- Richeter, S.; Thion, J.; van der Lee, A.; Leclercq, D. Synthesis, Structural Characterization, and Properties of Aluminum (III) meso-Tetraphenylporphyrin Complexes Axially Bonded to Phosphinate Anions. Inorg. Chem. 2006, 45, 10049–10051. [Google Scholar] [CrossRef]
- Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C. Thermally Activated Delayed Fluorescence from Sn4+-Porphyrin Complexes and Their Application to Organic Light Emitting Diodes—A Novel Mechanism for Electroluminescence. Adv. Mater. 2009, 21, 4802–4806. [Google Scholar] [CrossRef]
- Chernyad’ev, A.Y.; Tsivadze, A.Y. Synthesis, structure, and luminescence properties of transition metal 12-crown-4-porphyrinates in a polystyrene matrix. Prot. Met. Phys. Chem. Surf. 2015, 51, 533–539. [Google Scholar] [CrossRef]
- Jeoung, S.C.; Kim, D.; Hahn, S.J.; Ryu, S.Y.; Yoon, M. Lowest Excited State of Oxovanadyl (IV) Tetraphenylporphyrin. J. Phys. Chem. A 1998, 102, 315–322. [Google Scholar] [CrossRef]
- Gladkov, L.L.; Starukhin, A.S.; Shulga, A.M. Vibrational states of chlorophyll and related compound molecules. Metal complexes of octaethylchlorin. Spectrochim. Acta Part A Mol. Spectrosc. 1987, 43, 1125–1134. [Google Scholar] [CrossRef]
- Gupta, R.K.; Das, D.; Gupta, M.; Pal, S.K.; Iyer, P.K.; Achalkumar, A.S. Electroluminescent room temperature columnar liquid crystals based on bay-annulated perylene tetraesters. J. Mater. Chem. C 2017, 5, 1767–1781. [Google Scholar] [CrossRef]
- Juska, G.; Arlauskas, K.; Viliunas, M. Extraction Current Transients: New Method of Study of Charge Transport in Microcrystalline Silicon. Phys. Rev. Lett. 2000, 84, 4946–4949. [Google Scholar] [CrossRef]
- Tameev, A.R.; He, Z.; Milburn, G.H.W.; Kozlov, A.A.; Vannikov, A.V.; Puchala, A.; Rasala, D. Electron drift mobility in polystyrene doped with bispyrazolopyridine derivatives. Appl. Phys. Lett. 2002, 81, 969–971. [Google Scholar] [CrossRef]
- Kim, W.; Nishikawa, Y.; Watanabe, H.; Kanazawa, A.; Aoshima, S.; Fujii, A.; Ozaki, M. Stereoregularity effect on hole mobility in poly(N-vinylcarbazole) thin film evaluated by MIS-CELIV method. Jpn. J. Appl. Phys. 2019, 59, SDDA01. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Nyman, M.; Dahlström, S.; Sandén, S.; Törngren, B.; Smått, J.-H.; Österbacka, R. On the validity of MIS-CELIV for mobility determination in organic thin-film devices. Appl. Phys. Lett. 2017, 110, 153504. [Google Scholar] [CrossRef]
- Kawabe, Y.; Abe, J. Electron mobility measurement using exciplex-type organic light-emitting diodes. Appl. Phys. Lett. 2002, 81, 493–495. [Google Scholar] [CrossRef]
- Das, D.; Gopikrishna, P.; Narasimhan, R.; Singh, A.; Dey, A.; Iyer, P.K. White polymer light emitting diodes based on PVK: The effect of the electron injection barrier on transport properties, electroluminescence and controlling the electroplex formation. Phys. Chem. Chem. Phys. 2016, 48, 33077–33084. [Google Scholar] [CrossRef]
- Shin, S.B.; Gong, S.C.; Lee, H.M.; Jang, J.G.; Gong, M.S.; Ryu, S.O.; Lee, J.Y.; Chang, Y.C.; Chang, H.J. Improving light efficiency of white polymer light emitting diodes by introducing the TPBi exciton protection layer. Thin Solid Films 2009, 517, 4143–4146. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Loginov, D.A.; Lypenko, D.A.; Dmitriev, A.V.; Pozin, S.I.; Aleksandrov, A.E.; Tameev, A.R.; Martynov, I.L.; Chernyadyev, A.Y.; Osipov, S.N. New unsymmetrically substituted benzothiadiazole-based luminophores: Synthesis, optical, electrochemical studies, charge transport, and electroluminescent characteristics. Molecules 2021, 26, 7596. [Google Scholar] [CrossRef] [PubMed]
- Malov, V.V.; Tanwistha, G.; Nair, V.C.; Maslov, M.M.; Katin, K.P.; Unni, K.N.N.; Tameev, A.R. Hole mobility in thieno [3,2-bthiophene oligomers]. Mendeleev Commun. 2019, 29, 218–219. [Google Scholar] [CrossRef]
- Mal’tsev, E.I.; Lypenko, D.A.; Bobinkin, V.V.; Tameev, A.R.; Kirillov, S.V.; Shapiro, B.I.; Schoo, H.F.; Vannikov, A.V. Near-infrared electroluminescence in polymer composites based on organic nanocrystals. Appl. Phys. Lett. 2002, 81, 3088–3090. [Google Scholar] [CrossRef]
- Renikuntla, B.R.; Rose, H.C.; Eldo, J.; Waggoner, A.S.; Armitage, B.A. Improved photostability and fluorescence properties through polyfluorination of a cyanine dye. Org. Lett. 2004, 6, 909–912. [Google Scholar] [CrossRef] [PubMed]


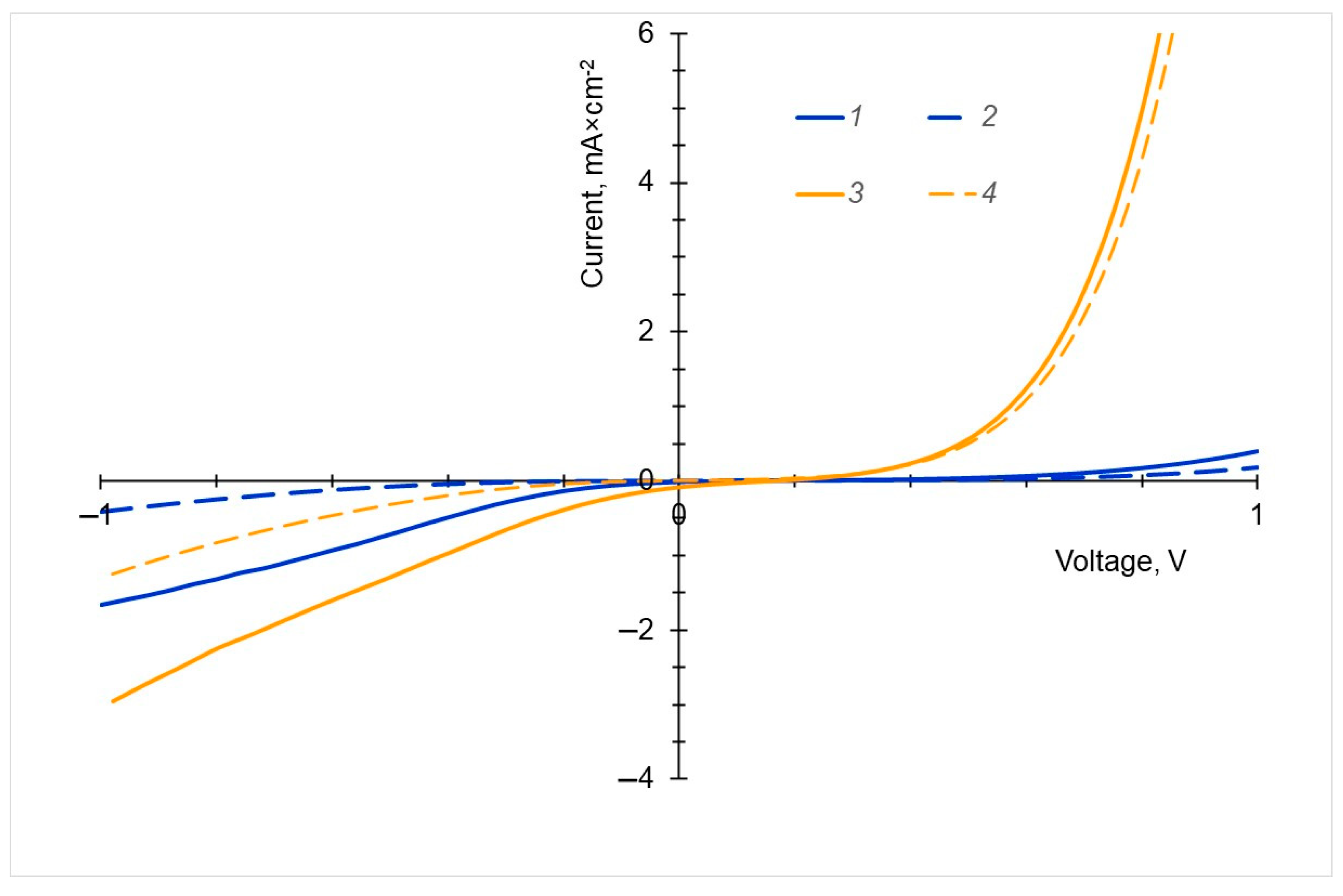
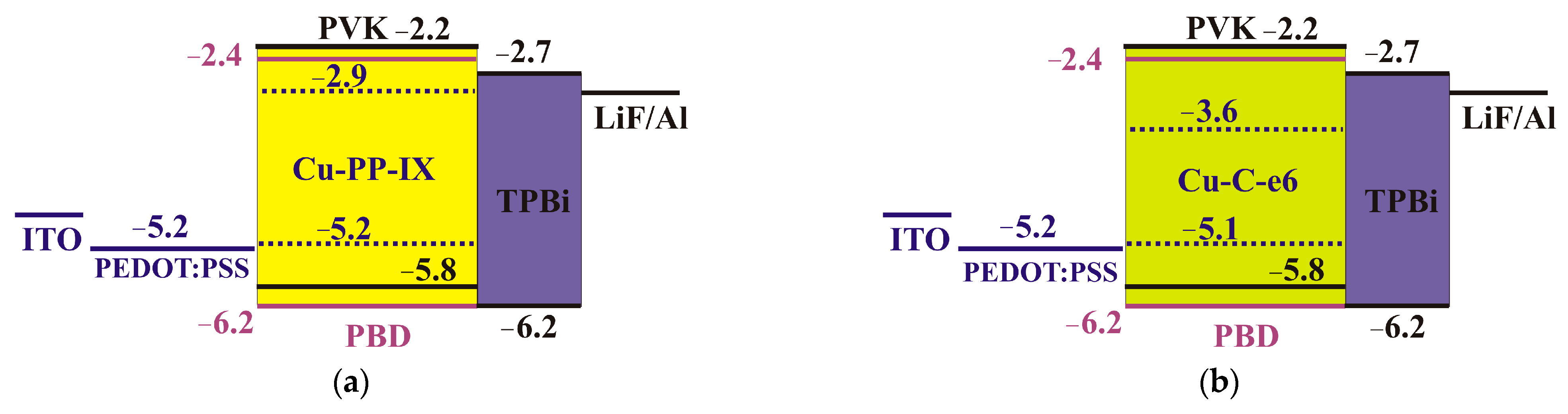
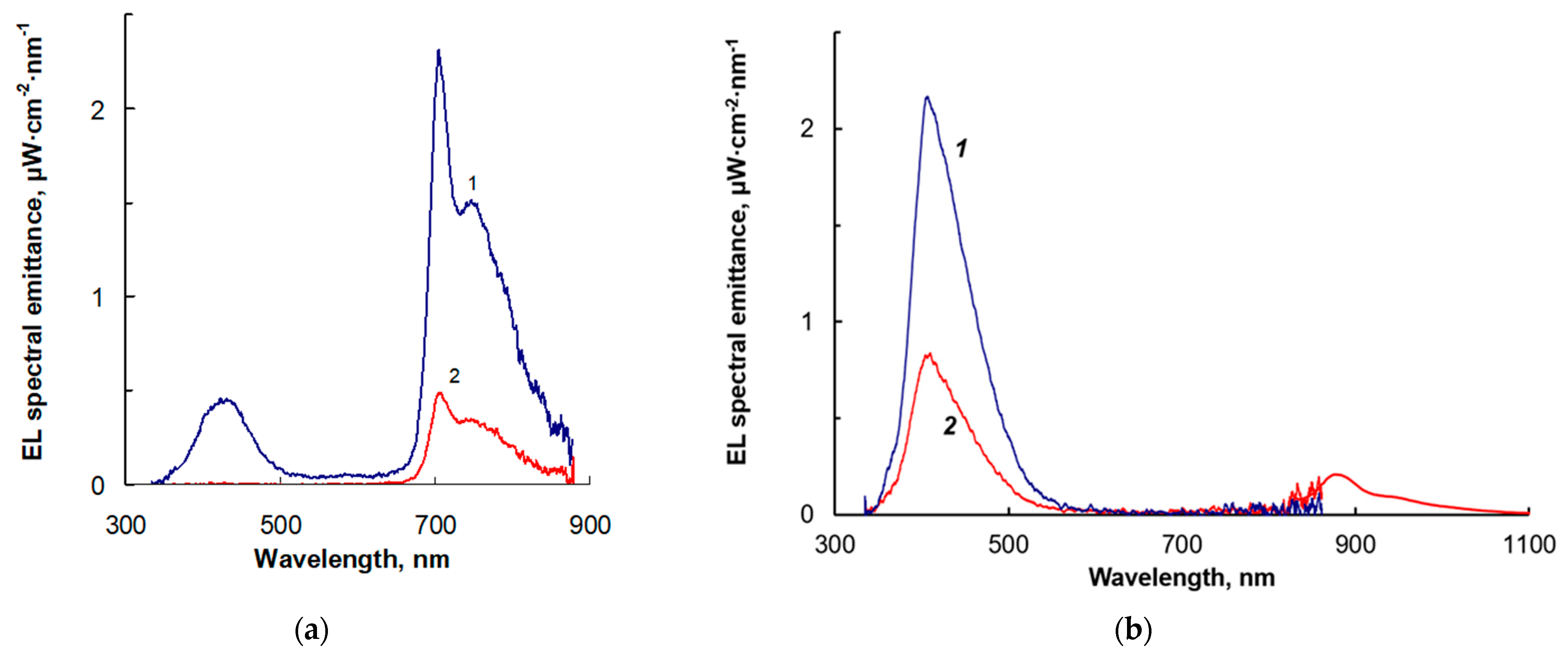
| Molecule | HOMO, eV | LUMO, eV | Gap, eV |
|---|---|---|---|
| Cu-PP-IX | −5.213 | −2.940 | 2.273 |
| Cu-C-e6 | −5.138 | −3.630 | 1.508 |
| PVK | −5.8 | −2.2 | 3.6 |
| Material | Holes | Electrons |
|---|---|---|
| Cu-PP-IX | (4.4 ± 0.4) × 10−5 | (3.2 ± 0.3) × 10−5 |
| Cu-C-e6 | (2.0 ± 0.3) × 10−5 | (4.8 ± 0.4) × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lypenko, D.A.; Aleksandrov, A.E.; Chernyadyev, A.Y.; Pozin, S.I.; Tsivadze, A.Y.; Tameev, A.R. Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6. Int. J. Mol. Sci. 2023, 24, 3178. https://doi.org/10.3390/ijms24043178
Lypenko DA, Aleksandrov AE, Chernyadyev AY, Pozin SI, Tsivadze AY, Tameev AR. Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6. International Journal of Molecular Sciences. 2023; 24(4):3178. https://doi.org/10.3390/ijms24043178
Chicago/Turabian StyleLypenko, Dmitry A., Alexey E. Aleksandrov, Andrey Yu. Chernyadyev, Sergey I. Pozin, Aslan Yu. Tsivadze, and Alexey R. Tameev. 2023. "Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6" International Journal of Molecular Sciences 24, no. 4: 3178. https://doi.org/10.3390/ijms24043178
APA StyleLypenko, D. A., Aleksandrov, A. E., Chernyadyev, A. Y., Pozin, S. I., Tsivadze, A. Y., & Tameev, A. R. (2023). Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6. International Journal of Molecular Sciences, 24(4), 3178. https://doi.org/10.3390/ijms24043178







