Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis
Abstract
1. Introduction
2. Results
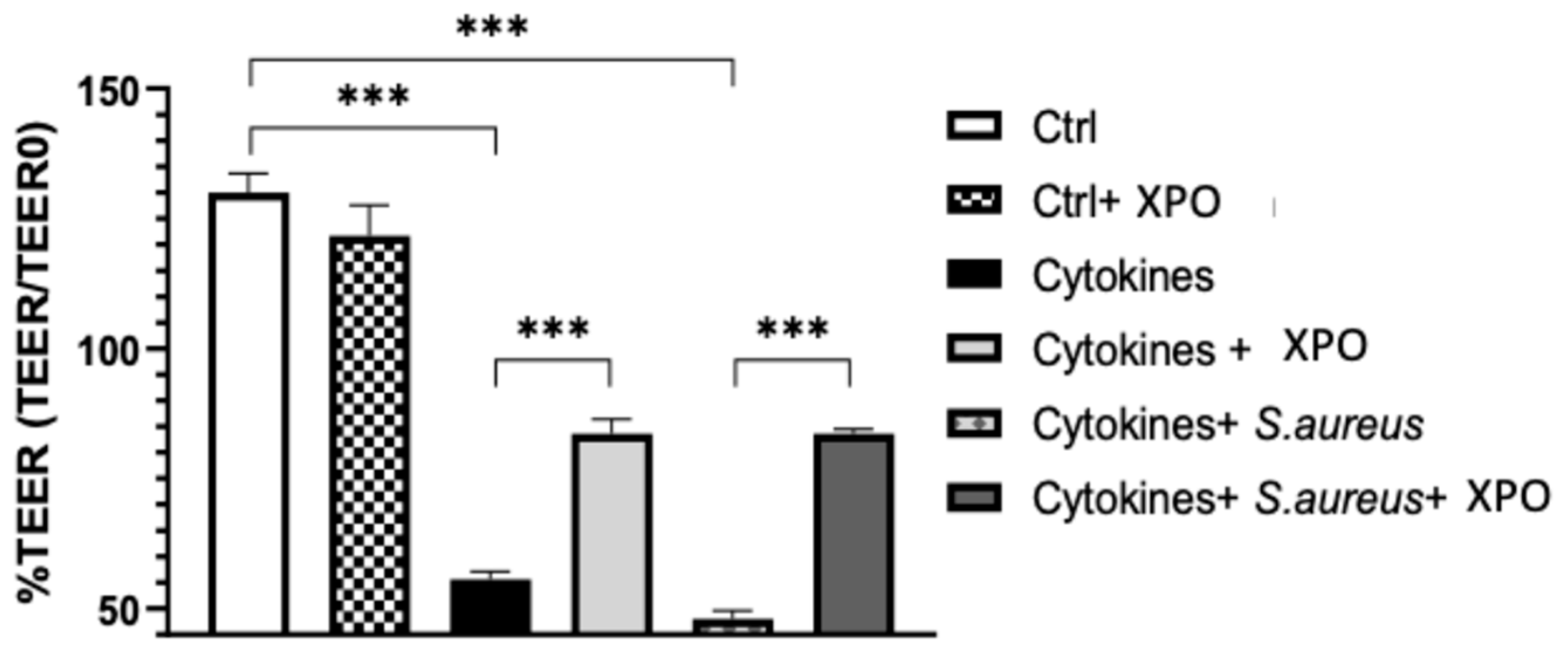
2.1. Effect of XPO on Skin Barrier Function Assessed by Trans-Epithelial Electrical Resistance (TEER) Test
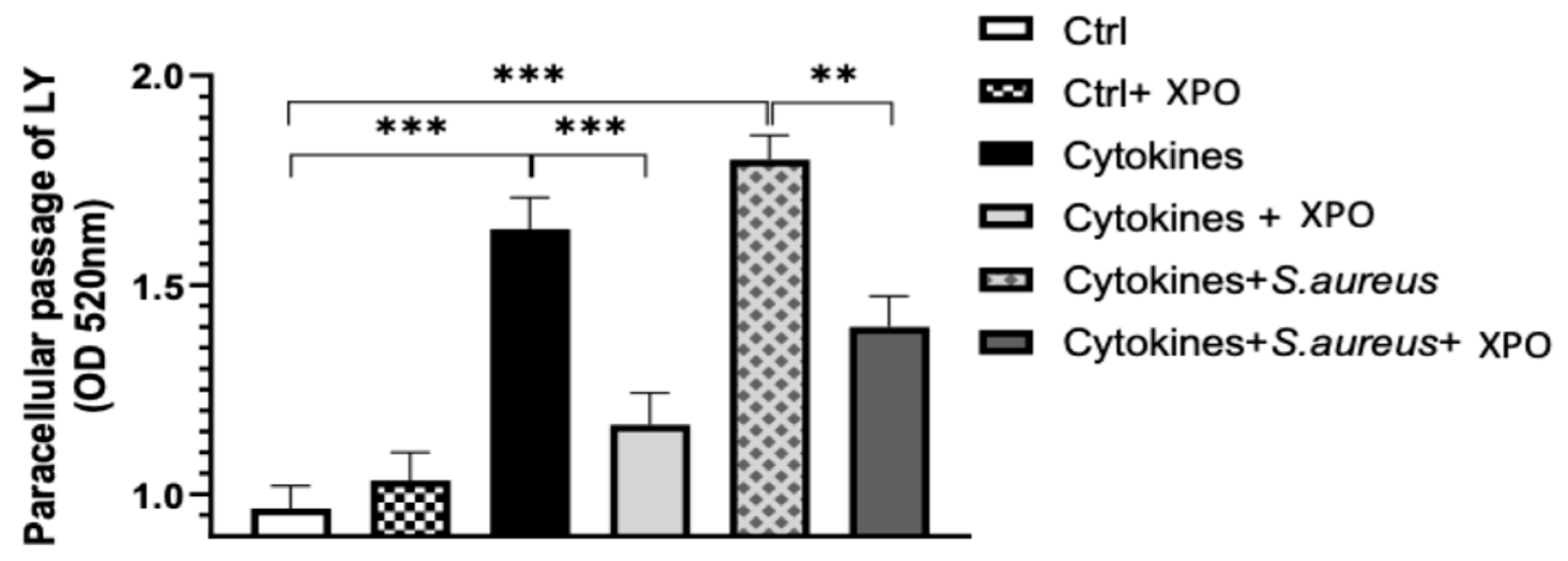
2.2. Role of XPO in Modulating Keratinocyte Membrane Integrity with Lucifer Yellow (LY) Permeation Test
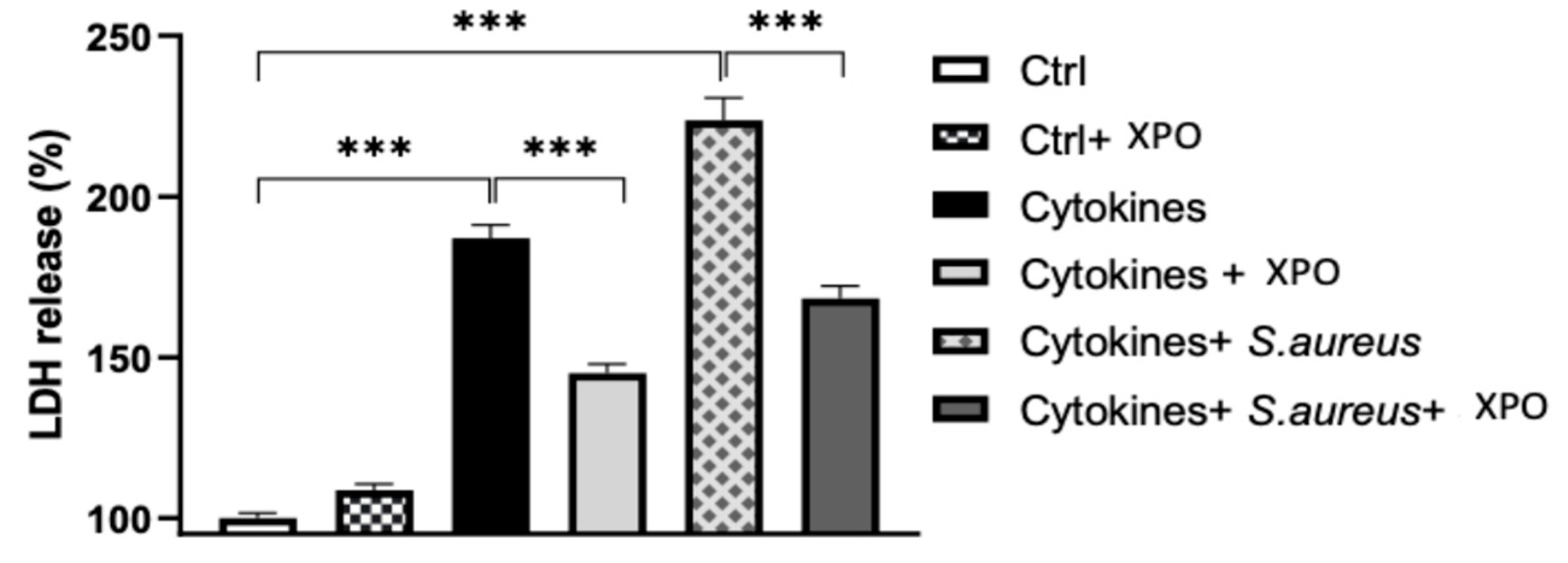
2.3. Effects of XPO Treatment on Cell Damage through Lactate Dehydrogenase (LDH) Assay
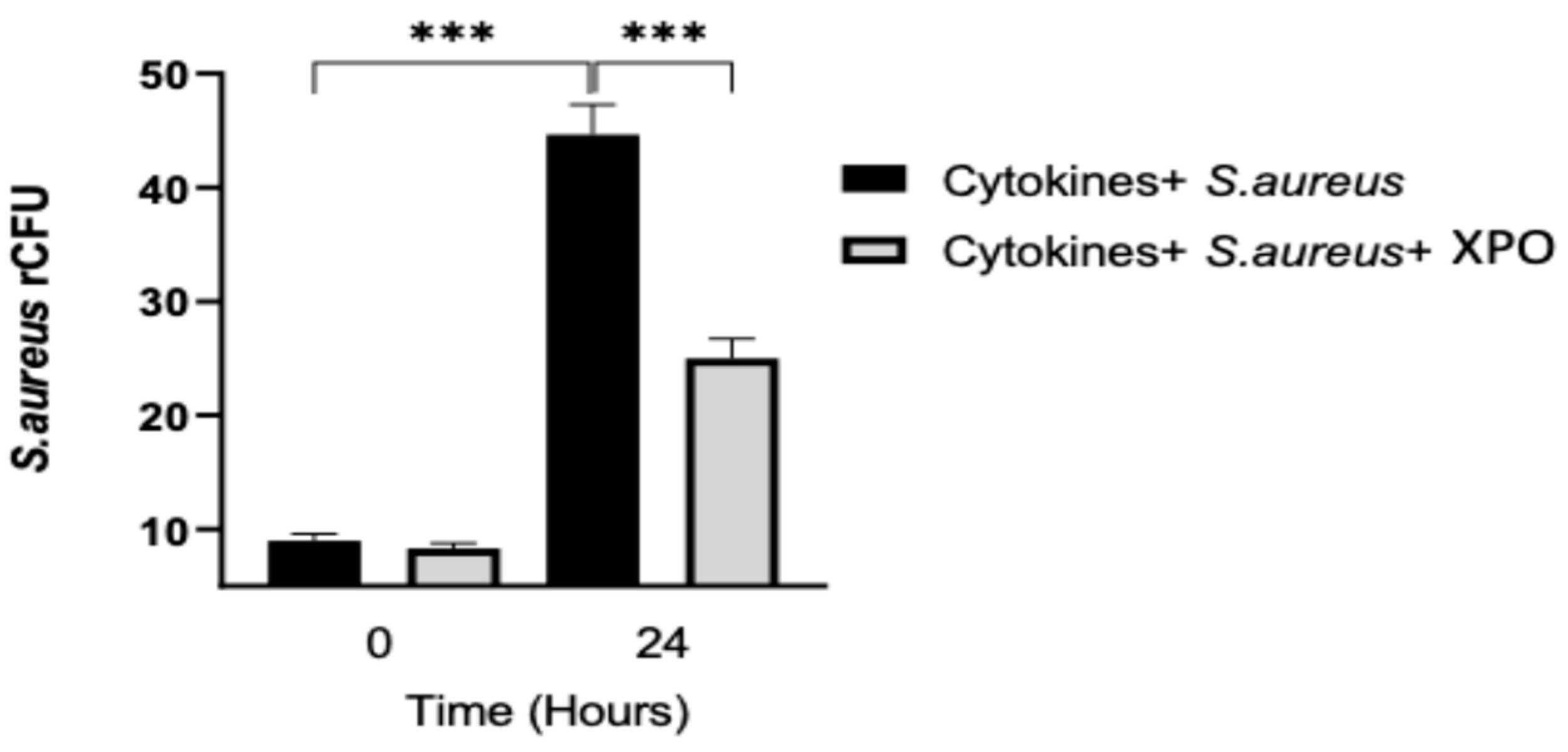
2.4. Role of XPO Treatment to Counteract Bacterial Adhesion and Invasion in Differentiated HaCaT Cells
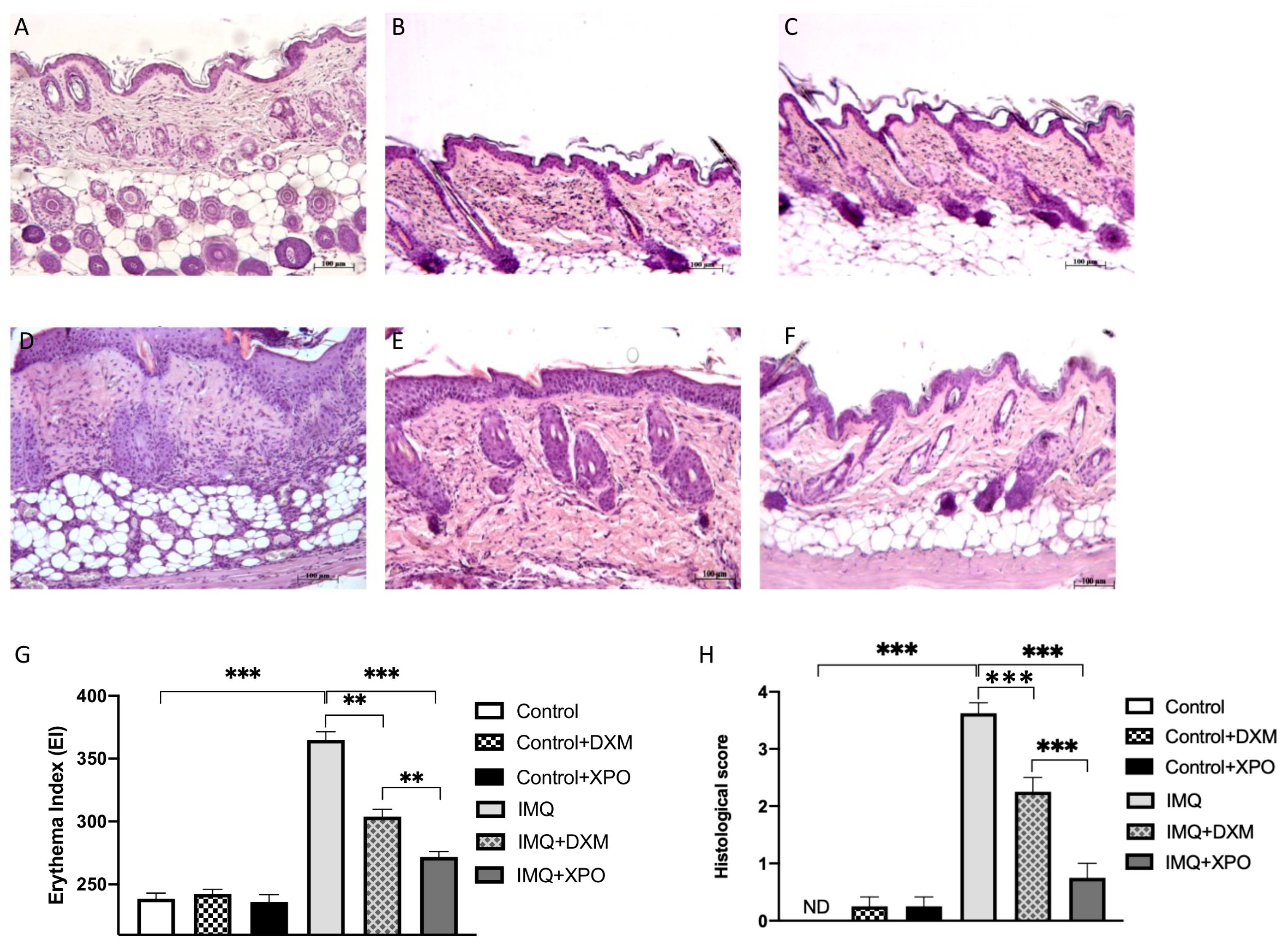
2.5. Effects of XPO Cream on Histological Changes in Mice with IMQ-Induced Psoriasis Like-Dermatitis
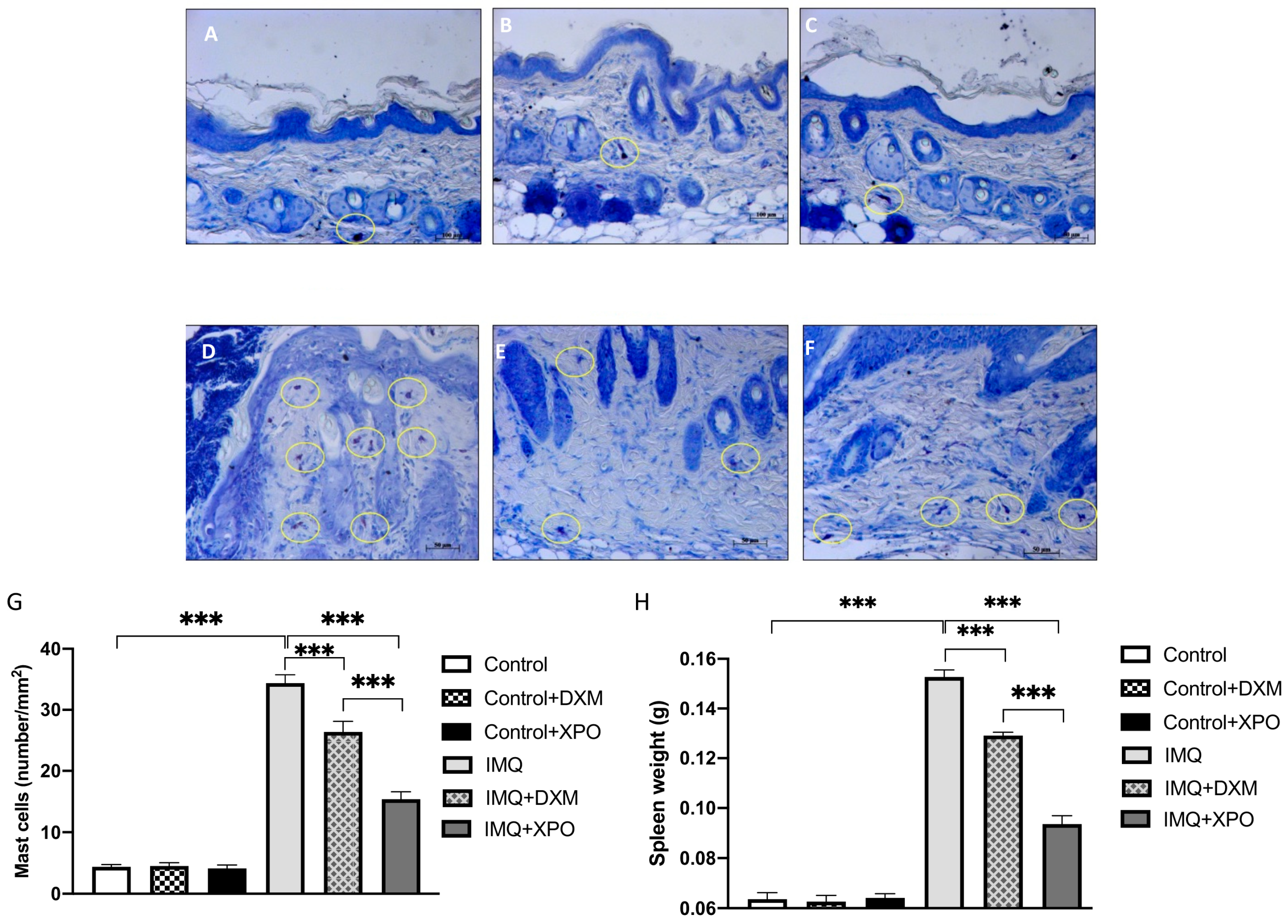
2.6. Protective Effect of XPO Cream on Mast Cell Degranulation and Spleen Weight
3. Discussion
4. Materials and Methods
4.1. In Vitro Study
4.1.1. Cell Line
4.1.2. S. aureus Culture
4.1.3. In Vitro Model of Psoriasis
4.1.4. S. aureus Infection
4.1.5. Experimental Groups
4.1.6. TEER Measurement
4.1.7. LY Permeation
4.1.8. LDH Assay
4.1.9. rCFU Evaluation
4.2. In Vivo Model of Psoriasis
4.2.1. Animals
4.2.2. IMQ-Induced Psoriasis-like Dermatitis Murine Model and Treatments
4.2.3. Experimental Groups
4.2.4. Histological Examination
4.2.5. Toluidine Blue Staining
4.2.6. Spleen Weight
4.3. Materials
4.4. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacDonald, A.; Burden, A.D. Psoriasis: Advances in pathophysiology and management. Postgrad. Med. J. 2007, 83, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Van Nuffel, E.; Beyaert, R. Immune responses and therapeutic options in psoriasis. Cell. Mol. Life Sci. 2021, 78, 2709–2727. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.D.; Bowcock, A.M. Psoriasis genetics: Breaking the barrier. Trends Genet. 2010, 26, 415–423. [Google Scholar] [CrossRef]
- Jiang, S.; Hinchliffe, T.E.; Wu, T. Biomarkers of An Autoimmune Skin Disease—Psoriasis. Genom. Proteom. Bioinform. 2015, 13, 224–233. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Hao, M.; Zhou, J.; Xie, Y.; He, Z. Plant-Derived Polysaccharides Regulated Immune Status, Gut Health and Microbiota of Broilers: A Review. Front. Vet. Sci. 2021, 8, 791371. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Czerwińska, J.; Wygonowska, E.; Kasprowicz-Furmańczyk, M.; Placek, W. D-chiro-inositol as a treatment in plaque psoriasis: A randomized placebo-controlled clinical trial. Dermatol. Ther. 2021, 34, e14538. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2021, 12, 753518. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of alpha-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Aengwanich, W.; Suttajit, M. Effect of polyphenols extracted from tamarind (Tamarindus indica L.) seed coat on pathophysiological changes and red blood cell glutathione peroxidase activity in heat-stressed broilers. Int. J. Biometeorol. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Pique, N.; Gomez-Guillen, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Ajovalasit, A.; Sabatino, M.A.; Todaro, S.; Alessi, S.; Giacomazza, D.; Picone, P.; Di Carlo, M.; Dispenza, C. Xyloglucan-based hydrogel films for wound dressing: Structure-property relationships. Carbohydr. Polym. 2018, 179, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Casili, G.; Paterniti, I.; Filippone, A.; Lanza, M.; Ardizzone, A.; Scuderi, S.A.; Cuzzocrea, S.; Esposito, E. Effect of a Product Containing Xyloglucan and Pea Protein on a Murine Model of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 3596. [Google Scholar] [CrossRef]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Fazio, E.; Filippone, A.; Paterniti, I.; Cuzzocrea, S. Efficacy of Xyloglucan against Escherichia coli Extraintestinal Urinary Tract Infection: An in vivo Study. Microb. Physiol. 2020, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Kintarak, S.; Whawell, S.A.; Speight, P.M.; Packer, S.; Nair, S.P. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 2004, 72, 5668–5675. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Zhao, W.; Han, L.; Bae, Y.; Manickam, D.S. Lucifer Yellow—A Robust Paracellular Permeability Marker in a Cell Model of the Human Blood-brain Barrier. J. Vis. Exp. 2019, 150, e58900. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Matsushima, Y.; Mizutani, K.; Yamanaka, K. The Role of Gut Microbiome in Psoriasis: Oral Administration of Staphylococcus aureus and Streptococcus danieliae Exacerbates Skin Inflammation of Imiquimod-Induced Psoriasis-Like Dermatitis. Int. J. Mol. Sci. 2020, 21, 3303. [Google Scholar] [CrossRef] [PubMed]
- Torsekar, R.; Gautam, M.M. Topical Therapies in Psoriasis. Indian Dermatol. Online J. 2017, 8, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Lv, C.; Man, G.; Song, S.; Elias, P.M.; Man, M.Q. Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis. J. Investig. Dermatol. 2014, 134, 2843–2846. [Google Scholar] [CrossRef]
- Toruniowa, B.; Jablonska, S. Mast cells in the initial stages of psoriasis. Arch. Dermatol. Res. 1988, 280, 189–193. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Wongnoppavich, A.; Panthong, A.; Khonsung, P.; Chiranthanut, N.; Soonthornchareonnon, N.; Sireeratawong, S. Antipsoriatic Effects of Wannachawee Recipe on Imiquimod-Induced Psoriasis-Like Dermatitis in BALB/c Mice. Evid. Based Complement. Altern. Med. 2018, 2018, 7931031. [Google Scholar] [CrossRef]
- Jabeen, M.; Boisgard, A.S.; Danoy, A.; El Kholti, N.; Salvi, J.P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics 2020, 12, 789. [Google Scholar] [CrossRef]
- Balci, D.D.; Duran, N.; Ozer, B.; Gunesacar, R.; Onlen, Y.; Yenin, J.Z. High prevalence of Staphylococcus aureus cultivation and superantigen production in patients with psoriasis. Eur. J. Dermatol. 2009, 19, 238–242. [Google Scholar] [CrossRef]
- Afifi, T.; de Gannes, G.; Huang, C.; Zhou, Y. Topical therapies for psoriasis: Evidence-based review. Can. Fam. Physician 2005, 51, 519–525. [Google Scholar]
- Griffiths, C.E.M.; Jo, S.J.; Naldi, L.; Romiti, R.; Guevara-Sangines, E.; Howe, T.; Pietri, G.; Gilloteau, I.; Richardson, C.; Tian, H.; et al. A multidimensional assessment of the burden of psoriasis: Results from a multinational dermatologist and patient survey. Br. J. Dermatol. 2018, 179, 173–181. [Google Scholar] [CrossRef]
- de Servi, B.; Ranzini, F.; Pique, N. Effect of Utipro((R)) (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. The role of mucoprotectants in the management of gastrointestinal disorders. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fraile, B.; Alcover, J.; Royuela, M.; Rodriguez, D.; Chaves, C.; Palacios, R.; Pique, N. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: Results of in vitro studies. Future Microbiol. 2017, 12, 721–731. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- Park, E.H.; Chun, M.J. Wound healing activity of Opuntia ficus-indica. Fitoterapia 2001, 72, 165–167. [Google Scholar] [CrossRef]
- Elias, P.M.; Choi, E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005, 14, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Pirot, F.; Guy, R.H. Homogeneous transport in a heterogeneous membrane: Water diffusion across human stratum corneum in vivo. Biophys. J. 1996, 71, 2692–2700. [Google Scholar] [CrossRef]
- Elias, P.M. Stratum corneum defensive functions: An integrated view. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef]
- Danilenko, D.M. An Overview of the Pathogenesis of Immune-mediated Skin Injury. Toxicol. Pathol. 2016, 44, 536–544. [Google Scholar] [CrossRef]
- Jiang, B.W.; Zhang, W.J.; Wang, Y.; Tan, L.P.; Bao, Y.L.; Song, Z.B.; Yu, C.L.; Wang, S.Y.; Liu, L.; Li, Y.X. Convallatoxin induces HaCaT cell necroptosis and ameliorates skin lesions in psoriasis-like mouse models. Biomed. Pharmacother. 2020, 121, 109615. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Desmet, E.; Ramadhas, A.; Lambert, J.; Van Gele, M. In vitro psoriasis models with focus on reconstructed skin models as promising tools in psoriasis research. Exp. Biol. Med. 2017, 242, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, Y.; Kajiura, T.; Yokota, K.; Kobayashi, H.; Okubo, T. Evaluation with a focus on both the antimicrobial efficacy and cumulative skin irritation potential of chlorhexidine gluconate alcohol-containing preoperative skin preparations. Am. J. Infect. Control 2012, 40, 973–978. [Google Scholar] [CrossRef]
- Visser, M.J.E.; Kell, D.B.; Pretorius, E. Bacterial Dysbiosis and Translocation in Psoriasis Vulgaris. Front. Cell. Infect. Microbiol. 2019, 9, 7. [Google Scholar] [CrossRef]
- Nikam, V.N.; Monteiro, R.C.; Dandakeri, S.; Bhat, R.M. Transepidermal Water Loss in Psoriasis: A Case-control Study. Indian Dermatol. Online J. 2019, 10, 267–271. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738. [Google Scholar] [CrossRef]
- Bracke, S.; Desmet, E.; Guerrero-Aspizua, S.; Tjabringa, S.G.; Schalkwijk, J.; Van Gele, M.; Carretero, M.; Lambert, J. Identifying targets for topical RNAi therapeutics in psoriasis: Assessment of a new in vitro psoriasis model. Arch. Dermatol. Res. 2013, 305, 501–512. [Google Scholar] [CrossRef]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.X.; Lecron, J.C.; Morel, F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins 2017, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.V.; Kintzing, M.L.; Kintzing, W.E.; Abdallah, I.M.; Brannen, A.D.; Kaddoumi, A. Plasma Rich in Growth Factors (PRGF) Disrupt the Blood-Brain Barrier Integrity and Elevate Amyloid Pathology in the Brains of 5XFAD Mice. Int. J. Mol. Sci. 2019, 20, 1489. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, I.; Leplingard, A.; Darfeuille-Michaud, A. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn’s disease patients to adhere to and to invade intestinal epithelial cells. Appl. Environ. Microbiol. 2005, 71, 2880–2887. [Google Scholar] [CrossRef]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Lanza, M.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical Delivery of Curcumin by Choline-Calix [4]arene-Based Nanohydrogel Improves Its Therapeutic Effect on a Psoriasis Mouse Model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippone, A.; Casili, G.; Lanza, M.; Scuderi, S.A.; Ardizzone, A.; Capra, A.P.; Paterniti, I.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis. Int. J. Mol. Sci. 2023, 24, 3122. https://doi.org/10.3390/ijms24043122
Filippone A, Casili G, Lanza M, Scuderi SA, Ardizzone A, Capra AP, Paterniti I, Campolo M, Cuzzocrea S, Esposito E. Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis. International Journal of Molecular Sciences. 2023; 24(4):3122. https://doi.org/10.3390/ijms24043122
Chicago/Turabian StyleFilippone, Alessia, Giovanna Casili, Marika Lanza, Sarah Adriana Scuderi, Alessio Ardizzone, Anna Paola Capra, Irene Paterniti, Michela Campolo, Salvatore Cuzzocrea, and Emanuela Esposito. 2023. "Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis" International Journal of Molecular Sciences 24, no. 4: 3122. https://doi.org/10.3390/ijms24043122
APA StyleFilippone, A., Casili, G., Lanza, M., Scuderi, S. A., Ardizzone, A., Capra, A. P., Paterniti, I., Campolo, M., Cuzzocrea, S., & Esposito, E. (2023). Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis. International Journal of Molecular Sciences, 24(4), 3122. https://doi.org/10.3390/ijms24043122












