Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients
Abstract
1. Introduction
2. Methods
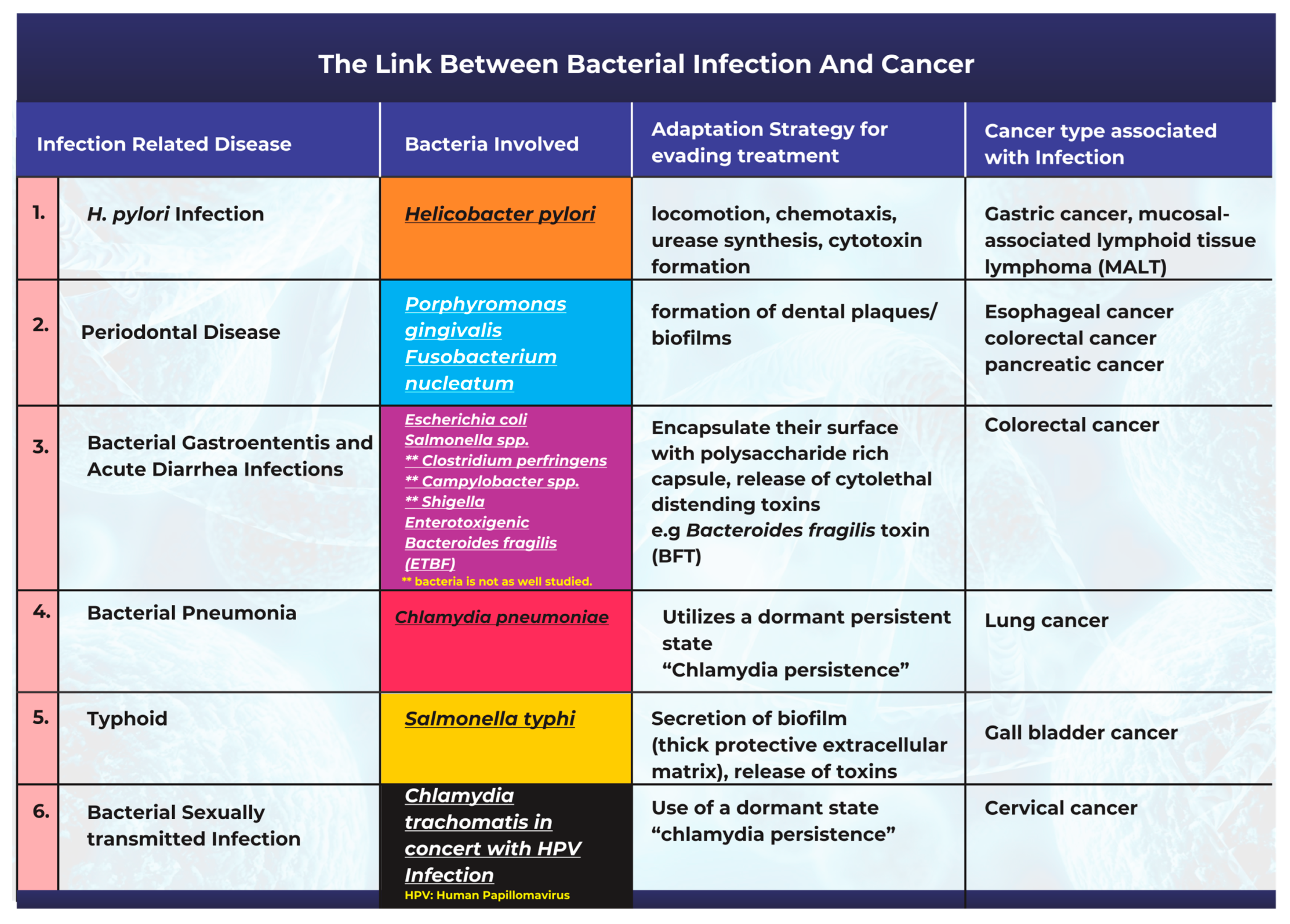
3. The Link between Bacterial Infection and the Onset of Cancer
3.1. Helicobacter pylori Infection and Gastric Cancer
3.2. Periodontal Disease Caused by Bacterial Infections Can Promote Tumorigenesis
3.3. Bacterial Pathogenesis in the Onset of Gastroenteritis, Acute Diarrhea, and Colon Cancer
3.4. Chlamydia pneumoniae Infection and Lung Cancer
3.5. Salmonella typhi Infections and Gallbladder Cancer
3.6. Bacterial Infection and Cervical Cancer
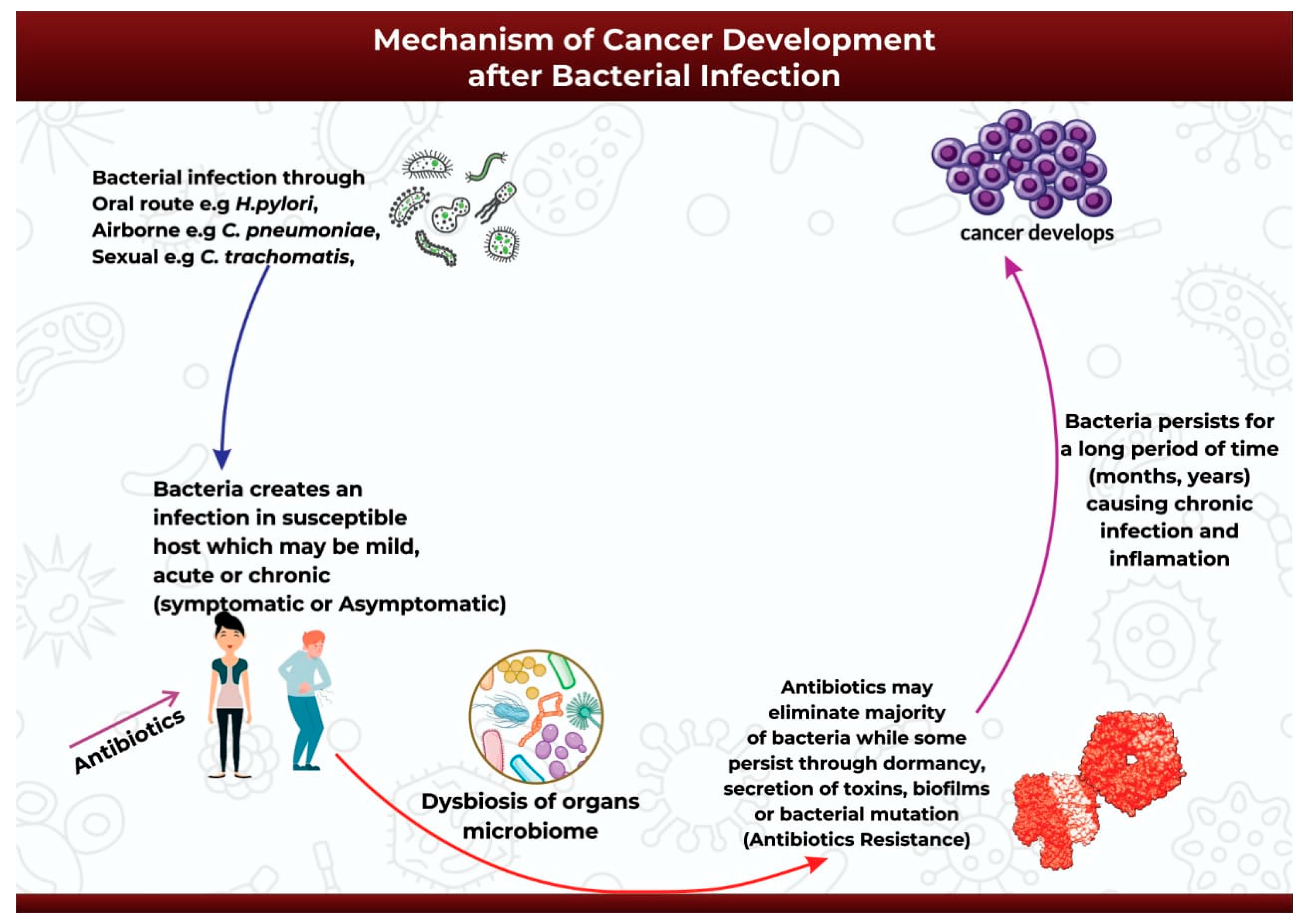
4. Mechanism of Cancer Development after Bacterial Infection
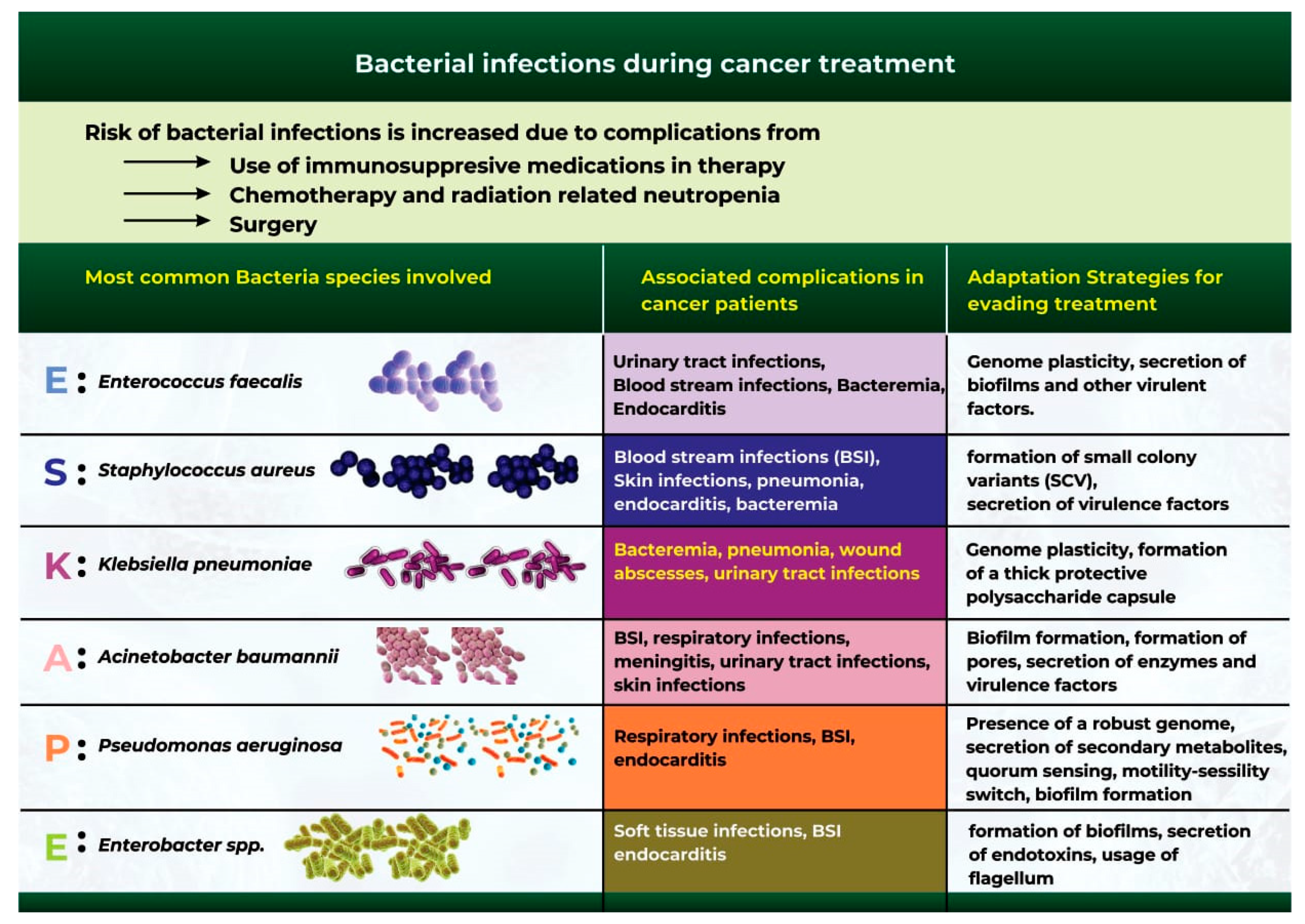
5. Bacterial Infections during Cancer Treatment
6. Antibiotic Use and Antimicrobial Resistance in Cancer Patients
Potential Approaches for Reducing Antibiotic Resistance in Cancer Patients
- Avoiding bacterial infections (prevention is always better than cure): The first stage of bacterial-driven carcinogenesis is usually an initial infection that allows the pathogen to infect the host. Hosts can prevent such infections by being vaccinated and by adopting proper hygiene measures [130]. Antibiotic prophylaxis is another common practice used for cancer patients in neutropenic settings to prevent infection and infection-related complications. According to reports, the prophylactic use of quinolones reduced the incidence of fever, infection, hospitalization, and overall mortality [104].
- The discovery and design of novel antibiotics that are pathogen-specific: Most antibiotics are designed for broad-spectrum use and can severely impact the human microbiome, leading to complications such as antibiotic-associated colitis and the rapid spread of antibiotic resistance. Utilizing more targeted approaches or using narrow-spectrum antibiotics may help address this limitation and provide additional resources for expanding the antibiotic pool, which is at risk owing to the inevitable rise in resistance [131].
- Optimizing Antibiotic Use (more does not guarantee better): Excessive antibiotic use is a significant driver of resistance [132]. To promote the effective use of antibiotics, the concept of “antimicrobial stewardship” was developed. Antimicrobial stewardship refers to the practice of establishing the antibiotic treatment that is most appropriate and then administering it for a short time and at the lowest effective dose to achieve the best possible clinical outcome in combating infection and preventing its spread [133]. In clinical settings, antimicrobial stewardship teams comprise an infectious disease doctor, an infectious disease pharmacist, and clinical microbiologists who monitor the patient’s dosage and response [104]. A research study evaluated the association between patient mortality and antibiotic stewardship outcomes in patients who developed febrile neutropenia during their cancer treatment. The study showed that adherence to antibiotic stewardship was associated with reduced mortality [134].
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hausman, D.M. What Is Cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- GBD 2019 Adolescent Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022, 23, 27–52. [Google Scholar] [CrossRef]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Dalton-Griffin, L.; Kellam, P. Infectious causes of cancer and their detection. J. Biol. 2009, 8, 67. [Google Scholar] [CrossRef]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Sperandio, V. A complex relationship: The interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol. 2011, 4, 133–138. [Google Scholar] [CrossRef]
- Conrad, R.; Vlassov, A.S. The Human Microbiota: Composition, Functions, and Therapeutic Potential. Med. Sci. Rev. 2015, 2, 92–103. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Khatun, S.; Appidi, T.; Rengan, A.K. The role played by bacterial infections in the onset and metastasis of cancer. Curr. Res. Microb. Sci. 2021, 2, 100078. [Google Scholar] [CrossRef]
- Laliani, G.; Sorboni, S.G.; Lari, R.; Yaghoubi, A.; Soleimanpour, S.; Khazaei, M.; Hasanian, S.M.; Avan, A. Bacteria and cancer: Different sides of the same coin. Life Sci. 2020, 246, 117398. [Google Scholar] [CrossRef]
- Lax, A.J. Opinion: Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Parsonnet, J. Bacterial infection as a cause of cancer. Environ. Health Perspect. 1995, 103 (Suppl. 8), 263–268. [Google Scholar] [CrossRef]
- Song, S.; Vuai, M.S.; Zhong, M. The role of bacteria in cancer therapy—Enemies in the past, but allies at present. Infect. Agent Cancer 2018, 13, 9. [Google Scholar] [CrossRef]
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Asaka, M.; Sepulveda, A.R.; Sugiyama, T.; Graham, D.Y. Chapter 40: Gastric Cancer. In Helicobacter pylori: Physiology and Genetics; Mobley, H.L.T., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001. [Google Scholar]
- Khosravi, Y.; Dieye, Y.; Poh, B.H.; Ng, C.G.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Culturable bacterial microbiota of the stomach of Helicobacter pylori positive and negative gastric disease patients. Sci. World J. 2014, 2014, 610421. [Google Scholar] [CrossRef] [PubMed]
- Elsalem, L.; Jum’ah, A.A.; Alfaqih, M.A.; Aloudat, O. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin. Exp. Gastroenterol. 2020, 13, 151–185. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011, 5, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Elagan, S.K.; Almalki, S.J.; Alharthi, M.R.; Mohamed, M.S.; El-Badawy, M.F. Role of Bacteria in the Incidence of Common GIT Cancers: The Dialectical Role of Integrated Bacterial DNA in Human Carcinogenesis. Infect. Drug Resist. 2021, 14, 2003–2014. [Google Scholar] [CrossRef]
- Vogiatzi, P.; Cassone, M.; Luzzi, I.; Lucchetti, C.; Otvos, L.; Giordano, A. Helicobacter pylori as a class I carcinogen: Physiopathology and management strategies. J. Cell. Biochem. 2007, 102, 264–273. [Google Scholar] [CrossRef]
- Blaser, M.J.; Berg, D.E. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 2001, 107, 767–773. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Cover, T.L. Helicobacter pylori Diversity and Gastric Cancer Risk. mBio 2016, 7, e01869-15. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter pylori—Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Blanke, S.R. Remodeling the host environment: Modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA). Front. Cell. Infect. Microbiol. 2012, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Ikeda, Y.; Ikeda, E.; Takahashi, M.; Tanaka, D.; Nakajima, Y.; Arakawa, S.; Izumi, Y.; Miyake, S. Oral infectious bacteria in dental plaque and saliva as risk factors in patients with esophageal cancer. Cancer 2021, 127, 512–519. [Google Scholar] [CrossRef]
- Michaud, D.S.; Lu, J.; Peacock-Villada, A.Y.; Barber, J.R.; Joshu, C.E.; Prizment, A.E.; Beck, J.D.; Offenbacher, S.; Platz, E.A. Periodontal Disease Assessed Using Clinical Dental Measurements and Cancer Risk in the ARIC Study. J. Natl. Cancer Inst. 2018, 110, 843–854. [Google Scholar] [CrossRef]
- Öğrendik, M. Periodontal Pathogens in the Etiology of Pancreatic Cancer. Gastrointest. Tumors 2017, 3, 125–127. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral bacteria and cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Duan, X.; Liu, K.; Mohammed, M.; Gu, Z.; Ren, J.; Yakoumatos, L.; Yuan, X.; Lu, L.; et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br. J. Cancer 2021, 125, 433–444. [Google Scholar] [CrossRef]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Sayehmiri, F.; Sayehmiri, K.; Asadollahi, K.; Soroush, S.; Bogdanovic, L.; Jalilian, F.A.; Emaneini, M.; Taherikalani, M. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol. 2015, 28, 160–167. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Periodontal disease: The portrait of an epidemic. J. Public Health Emerg. 2020, 4–10. [Google Scholar] [CrossRef]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Printz, C. Study adds evidence to link between gum disease and cancer risk: Researchers observe connection with gastric, esophageal cancer. Cancer 2021, 127, 495–496. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Lovegrove, J.M. Dental plaque revisited: Bacteria associated with periodontal disease. J. N. Z. Soc. Periodontol. 2004, 87, 7–21. [Google Scholar]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. Npj Aging Mech. Dis. 2017, 3, 15. [Google Scholar] [CrossRef]
- Sattar, S.B.A.; Singh, S. Bacterial Gastroenteritis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Sun, J. Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin. Med. J. 2022, 135, 400–408. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, F.; Yang, C.; Deng, Y.; Kwan, P.S.L.; Li, Y.; Lin, Y.; Qiu, Y.; Shi, X.; Chen, H.; et al. Whole-Genome Analysis of Salmonella enterica Serovar Enteritidis Isolates in Outbreak Linked to Online Food Delivery, Shenzhen, China, 2018. Emerg. Infect. Dis. 2020, 26, 789–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Zhang, Z.; Tian, S.; Liu, M.; Li, X.; Han, Y.; Zhu, K.; Liu, H.; Yang, C.; et al. A Severe Gastroenteritis Outbreak of Salmonella enterica Serovar Enteritidis Linked to Contaminated Egg Fried Rice, China, 2021. Front. Microbiol. 2021, 12, 779749. [Google Scholar] [CrossRef] [PubMed]
- Ternhag, A.; Törner, A.; Svensson, A.; Ekdahl, K.; Giesecke, J. Short- and long-term effects of bacterial gastrointestinal infections. Emerg. Infect. Dis. 2008, 14, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; Pelt, W.V.; Neefjes, J. Increased colon cancer risk after severe Salmonella infection. PLoS ONE 2018, 13, e0189721. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wu, S.; Zhang, Y.-G.; Xia, Y.; Zhou, Z.; Kato, I.; Dong, H.; Bissonnette, M.; Sun, J. Salmonella Protein AvrA Activates the STAT3 Signaling Pathway in Colon Cancer. Neoplasia 2016, 18, 307–316. [Google Scholar] [CrossRef]
- Hernández-Luna, M.A.; López-Briones, S.; Luria-Pérez, R. The Four Horsemen in Colon Cancer. J. Oncol. 2019, 2019, 5636272. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Huber, A.R.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 2018, 44, 619–632. [Google Scholar] [CrossRef]
- Veziant, J.; Gagnière, J.; Jouberton, E.; Bonnin, V.; Sauvanet, P.; Pezet, D.; Barnich, N.; Miot-Noirault, E.; Bonnet, M. Association of colorectal cancer with pathogenic Escherichia coli: Focus on mechanisms using optical imaging. World J. Clin. Oncol. 2016, 7, 293–301. [Google Scholar] [CrossRef]
- Sears, C.L.; Islam, S.; Saha, A.; Arjumand, M.; Alam, N.H.; Faruque, A.S.; Salam, M.A.; Shin, J.; Hecht, D.; Weintraub, A.; et al. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin. Infect. Dis. 2008, 47, 797–803. [Google Scholar] [CrossRef]
- Elsaghir, H.; Reddivari, A.K.R. Bacteroides Fragilis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Cardemil, C.V.; Balachandran, N.; Kambhampati, A.; Grytdal, S.; Dahl, R.M.; Rodriguez-Barradas, M.C.; Vargas, B.; Beenhouwer, D.O.; Evangelista, K.V.; Marconi, V.C.; et al. Incidence, Etiology, and Severity of Acute Gastroenteritis Among Prospectively Enrolled Patients in 4 Veterans Affairs Hospitals and Outpatient Centers, 2016–2018. Clin. Infect. Dis. 2021, 73, e2729–e2738. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Felisberto-Rodrigues, C.; Meir, A.; Prevost, M.S.; Redzej, A.; Trokter, M.; Waksman, G. Secretion systems in Gram-negative bacteria: Structural and mechanistic insights. Nat. Rev. Microbiol. 2015, 13, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.R.; Chang, Y.F.; Ma, J.; Chiu, C.H.; Kuo, M.L.; Lai, C.H. From DNA Damage to Cancer Progression: Potential Effects of Cytolethal Distending Toxin. Front. Immunol. 2021, 12, 760451. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef]
- Premachandra, N.M.; Jayaweera, J.A.A.S. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agents Cancer 2022, 17, 11. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Xiong, W.; Qiu, M.; Kang, S.; Xu, Q.; Cai, L.; He, F. Combined and interaction effect of Chlamydia pneumoniae infection and smoking on lung cancer: A case-control study in Southeast China. BMC Cancer 2020, 20, 903. [Google Scholar] [CrossRef]
- Laurila, A.L.; Anttila, T.; Läärä, E.; Bloigu, A.; Virtamo, J.; Albanes, D.; Leinonen, M.; Saikku, P. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. Int. J. Cancer 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Littman, A.J.; Jackson, L.A.; Vaughan, T.L. Chlamydia pneumoniae and lung cancer: Epidemiologic evidence. Cancer Epidemiol. Biomark. Prev. 2005, 14, 773–778. [Google Scholar] [CrossRef]
- Zhan, P.; Suo, L.J.; Qian, Q.; Shen, X.K.; Qiu, L.X.; Yu, L.K.; Song, Y. Chlamydia pneumoniae infection and lung cancer risk: A meta-analysis. Eur. J. Cancer 2011, 47, 742–747. [Google Scholar] [CrossRef]
- Gautam, J.; Krawiec, C. Chlamydia Pneumonia; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Cummins, J.; Tangney, M. Bacteria and tumours: Causative agents or opportunistic inhabitants? Infect. Agents Cancer 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Persistence: A Survival Strategy to Evade Antimicrobial Effects. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef] [PubMed]
- Ashurst, J.V.; Truong, J.; Woodbury, B. Salmonella Typhi; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Di Domenico, E.G.; Cavallo, I.; Pontone, M.; Toma, L.; Ensoli, F. Biofilm Producing Salmonella typhi: Chronic Colonization and Development of Gallbladder Cancer. Int. J. Mol. Sci. 2017, 18, 1887. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, A.; Pal, D.; Kumar, A. Salmonella typhi induced oncogenesis in gallbladder cancer: Co-relation and progression. Adv. Cancer Biol. Metastasis 2022, 4, 100032. [Google Scholar] [CrossRef]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef]
- Gonzalez-Escobedo, G.; La Perle, K.M.; Gunn, J.S. Histopathological analysis of Salmonella chronic carriage in the mouse hepatopancreatobiliary system. PLoS ONE 2013, 8, e84058. [Google Scholar] [CrossRef]
- Shukla, R.; Shukla, P.; Behari, A.; Khetan, D.; Chaudhary, R.K.; Tsuchiya, Y.; Ikoma, T.; Asai, T.; Nakamura, K.; Kapoor, V.K. Roles of Salmonella typhi and Salmonella paratyphi in Gallbladder Cancer Development. Asian Pac. J. Cancer Prev. 2021, 22, 509–516. [Google Scholar] [CrossRef]
- Nath, G.; Singh, Y.K.; Kumar, K.; Gulati, A.K.; Shukla, V.K.; Khanna, A.K.; Tripathi, S.K.; Jain, A.K.; Kumar, M.; Singh, T.B. Association of carcinoma of the gallbladder with typhoid carriage in a typhoid endemic area using nested PCR. J. Infect. Dev. Ctries 2008, 2, 302–307. [Google Scholar] [CrossRef]
- Gunn, J.S.; Marshall, J.M.; Baker, S.; Dongol, S.; Charles, R.C.; Ryan, E.T. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014, 22, 648–655. [Google Scholar] [CrossRef]
- Franchini, A.P.A.; Iskander, B.; Anwer, F.; Oliveri, F.; Fotios, K.; Panday, P.; Hamid, P. The Role of Chlamydia trachomatis in the Pathogenesis of Cervical Cancer. Cureus 2022, 14, e21331. [Google Scholar] [CrossRef]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Yang, X.; Siddique, A.; Khan, A.A.; Wang, Q.; Malik, A.; Jan, A.T.; Rudayni, H.A.; Chaudhary, A.A.; Khan, S. Infection: Their potential implication in the Etiology of Cervical Cancer. J. Cancer 2021, 12, 4891–4900. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Z.; Luo, H.; Zhang, W.; Zhu, X. Chlamydia trachomatis Infection-Associated Risk of Cervical Cancer: A Meta-Analysis. Medicine 2016, 95, e3077. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sah, R.; Muhammad, K.; Waheed, Y. Tracking HPV Infection, Associated Cancer Development, and Recent Treatment Efforts—A Comprehensive Review. Vaccines 2023, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Grant, S.S.; Hung, D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef]
- Pham, T.A.; Lawley, T.D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 2014, 17, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Rolston, K.V. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Bhat, K.A.; Kotian, K.H. Bacterial Infection among Cancer Patients: Analysis of Isolates and Antibiotic Sensitivity Pattern. Int. J. Microbiol. 2021, 2021, 8883700. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Peterson, L.E.; Musser, J.M. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J. Infect. 2014, 69, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gedik, H.; Simşek, F.; Kantürk, A.; Yildirmak, T.; Arica, D.; Aydin, D.; Demirel, N.; Yokuş, O. Bloodstream infections in patients with hematological malignancies: Which is more fatal—Cancer or resistant pathogens? Ther. Clin. Risk Manag. 2014, 10, 743–752. [Google Scholar] [CrossRef]
- Moreno-Sanchez, F.; Gomez-Gomez, B. Antibiotic Management of Patients with Hematologic Malignancies: From Prophylaxis to Unusual Infections. Curr. Oncol. Rep. 2022, 24, 835–842. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, H.; Lee, R.; Cho, S.Y.; Lee, D.G. Bloodstream Infections in Patients with Hematologic Diseases: Causative Organisms and Factors Associated with Resistance. Infect. Chemother. 2022, 54, 340–352. [Google Scholar] [CrossRef]
- Marin, M.; Gudiol, C.; Ardanuy, C.; Garcia-Vidal, C.; Calvo, M.; Arnan, M.; Carratalà, J. Bloodstream infections in neutropenic patients with cancer: Differences between patients with haematological malignancies and solid tumours. J. Infect. 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Royo-Cebrecos, C.; Laporte-Amargós, J.; Peña, M.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; Montejo, M.; Mikulska, M.; et al. Bloodstream Infections in Patients with Cancer: Differences between Patients with Hematological Malignancies and Solid Tumors. Pathogens 2022, 11, 1132. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- El-Mahallawy, H.A.; Hassan, S.S.; El-Wakil, M.; Moneer, M.M. Bacteremia due to ESKAPE pathogens: An emerging problem in cancer patients. J. Egypt. Natl. Cancer Inst. 2016, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Gudiol, C.; Garcia-Vidal, C.; Tubau, F.; Contra, A.; Boix, L.; Domingo-Domenech, E.; Calvo, M.; Carratalà, J. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support. Care Cancer 2014, 22, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.; Fowler, V.G.; Shelburne, S.A. Invasive gram-positive bacterial infection in cancer patients. Clin. Infect. Dis. 2014, 59 (Suppl. 5), S331–S334. [Google Scholar] [CrossRef] [PubMed]
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus Infections; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Awadh, H.; Chaftari, A.M.; Khalil, M.; Fares, J.; Jiang, Y.; Deeba, R.; Ali, S.; Hachem, R.; Raad, I.I. Management of enterococcal central line-associated bloodstream infections in patients with cancer. BMC Infect. Dis. 2021, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.B. Enterococci: An Important Nosocomial Pathogen. In Pathogenic Bacteria; IntechOpen: London, UK, 2019. [Google Scholar]
- Montassier, E.; Batard, E.; Gastinne, T.; Potel, G.; de La Cochetière, M.F. Recent changes in bacteremia in patients with cancer: A systematic review of epidemiology and antibiotic resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Torres, M.T.; Pedron, C.N.; Andrade, V.B.; Silva, P.I.; Silva, F.D.; de la Fuente-Nunez, C.; Oliveira, V.X. Synthetic Peptide Derived from Scorpion Venom Displays Minimal Toxicity and Anti-infective Activity in an Animal Model. ACS Infect. Dis. 2021, 7, 2736–2745. [Google Scholar] [CrossRef]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Manipulates Innate Immunity through Own and Host-Expressed Proteases. Front. Cell. Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef]
- Goldmann, O.; Medina, E. Staphylococcus aureus strategies to evade the host acquired immune response. Int. J. Med. Microbiol. 2018, 308, 625–630. [Google Scholar] [CrossRef]
- Worku, M.; Belay, G.; Tigabu, A. Bacterial profile and antimicrobial susceptibility patterns in cancer patients. PLoS ONE 2022, 17, e0266919. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Pessoa, J.S. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef]
- Gomez-Simmonds, A.; Uhlemann, A.C. Clinical Implications of Genomic Adaptation and Evolution of Carbapenem-Resistant Klebsiella pneumoniae. J. Infect. Dis. 2017, 215, S18–S27. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. Clinical outcomes and nephrotoxicity of colistin loading dose for treatment of extensively drug-resistant. Infect. Drug Resist. 2017, 10, 293–298. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Halat, D.H. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wei, Q.; Zhao, T.; Guo, Y.; Ma, L.Z. A Survival Strategy for Pseudomonas aeruginosa that Uses Exopolysaccharides to Sequester and Store Iron To Stimulate Psl-Dependent Biofilm Formation. Appl. Environ. Microbiol. 2016, 82, 6403–6413. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Giron, M. Enterobacter Infections; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Davin-Regli, A.; Lavigne, J.P.; Pagès, J.M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Pandey, K.; Umar, S. Microbiome in drug resistance to colon cancer. Curr. Opin. Physiol. 2021, 23, 100472. [Google Scholar] [CrossRef]
- Casals-Pascual, C.; Vergara, A.; Vila, J. Intestinal microbiota and antibiotic resistance: Perspectives and solutions. Hum. Microbiome J. 2018, 9, 11–15. [Google Scholar] [CrossRef]
- Baron, S.A.; Diene, S.M.; Rolain, J.-M. Human microbiomes and antibiotic resistance. Hum. Microbiome J. 2018, 10, 43–52. [Google Scholar] [CrossRef]
- Gargiullo, L.; Del Chierico, F.; D’Argenio, P.; Putignani, L. Gut Microbiota Modulation for Multidrug-Resistant Organism Decolonization: Present and Future Perspectives. Front. Microbiol. 2019, 10, 1704. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Bacterial Infections: Overview. In International Encyclopedia of Public Health; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 273–282. [Google Scholar]
- Maxson, T.; Mitchell, D.A. Targeted Treatment for Bacterial Infections: Prospects for Pathogen-Specific Antibiotics Coupled with Rapid Diagnostics. Tetrahedron 2016, 72, 3609–3624. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Antonara, S.; Barton, T. Prevention Strategies to Combat Antimicrobial Resistance in Children in Resource-Limited Settings. Curr. Trop. Med. Rep. 2018, 5, 5–15. [Google Scholar] [CrossRef]
- Doron, S.; Davidson, L.E. Antimicrobial stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef]
- Rosa, R.G.; Goldani, L.Z.; dos Santos, R.P. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropaenia: A prospective cohort study. BMC Infect. Dis. 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. https://doi.org/10.3390/ijms24043110
Yusuf K, Sampath V, Umar S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. International Journal of Molecular Sciences. 2023; 24(4):3110. https://doi.org/10.3390/ijms24043110
Chicago/Turabian StyleYusuf, Kafayat, Venkatesh Sampath, and Shahid Umar. 2023. "Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients" International Journal of Molecular Sciences 24, no. 4: 3110. https://doi.org/10.3390/ijms24043110
APA StyleYusuf, K., Sampath, V., & Umar, S. (2023). Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. International Journal of Molecular Sciences, 24(4), 3110. https://doi.org/10.3390/ijms24043110






