Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer
Abstract
1. Introduction
2. Results
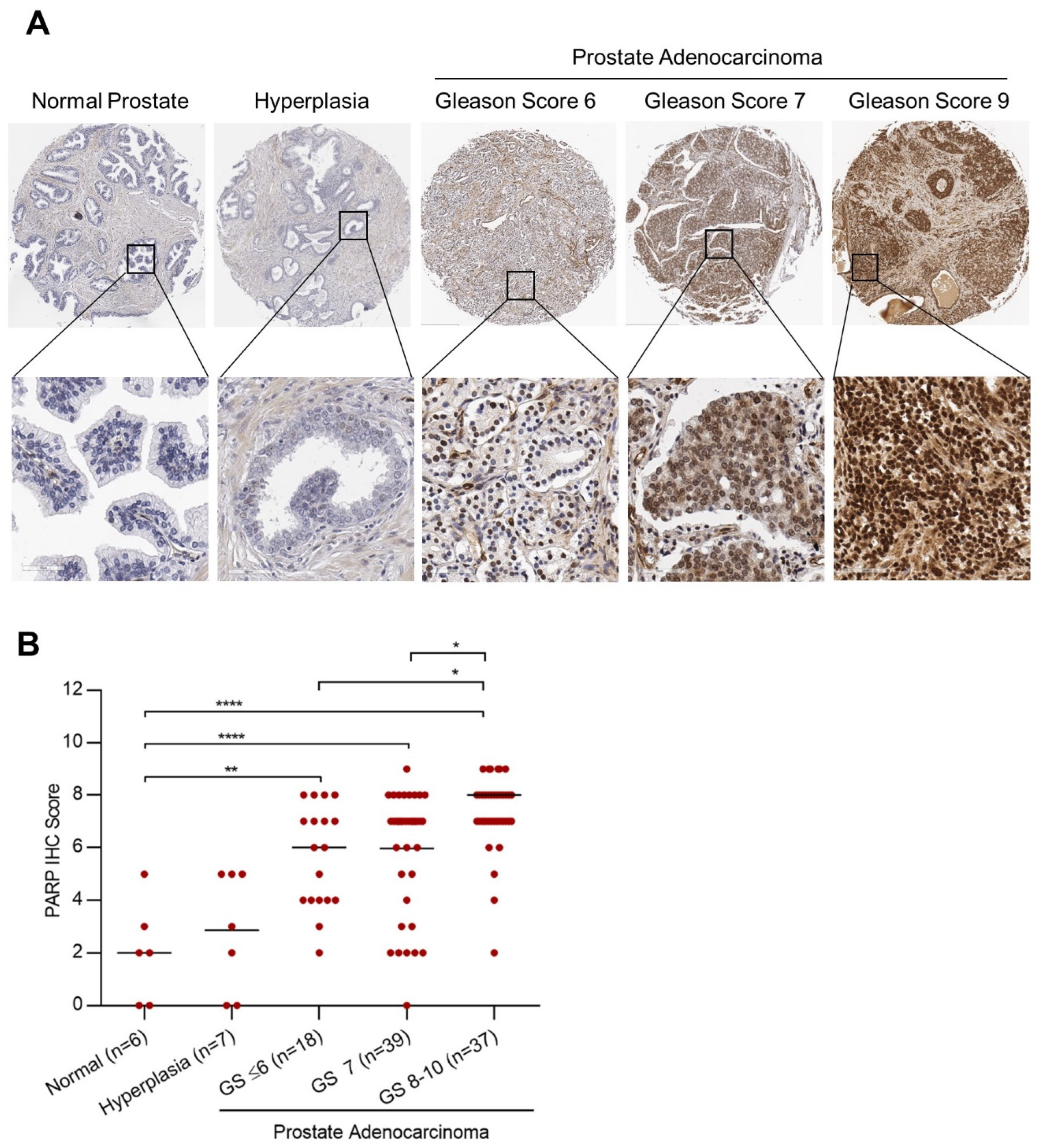
2.1. PARP-1 Expression in Prostate Cancer and Correlation with Gleason Score
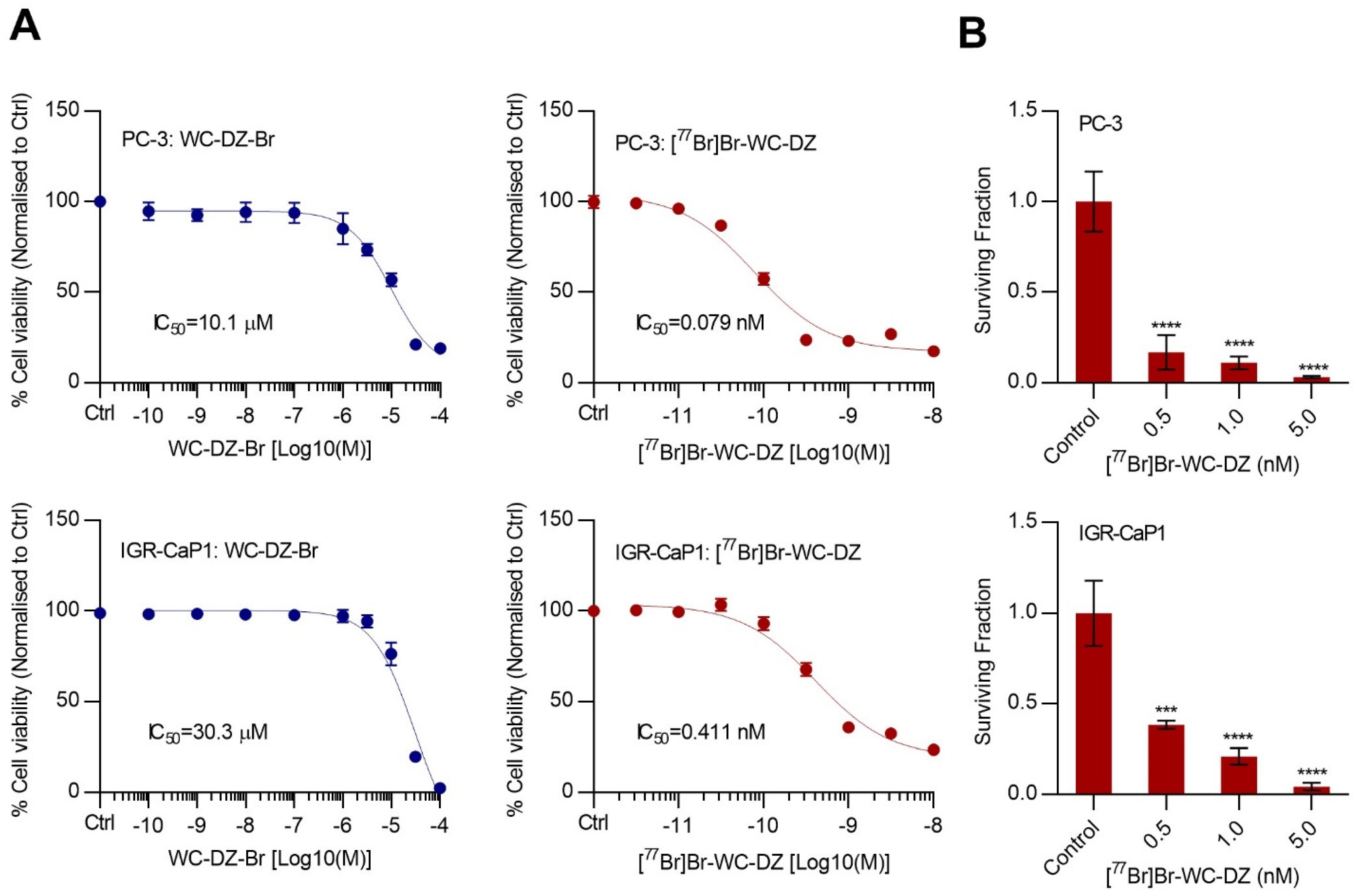
2.2. Synthesis, Purification, and PARP Binding Affinity of [77Br]Br-WC-DZ
2.3. The [77Br]Br-WC-DZ-Induced Cytotoxicity and Inhibited the Colony Formation of Prostate Cancer Cells
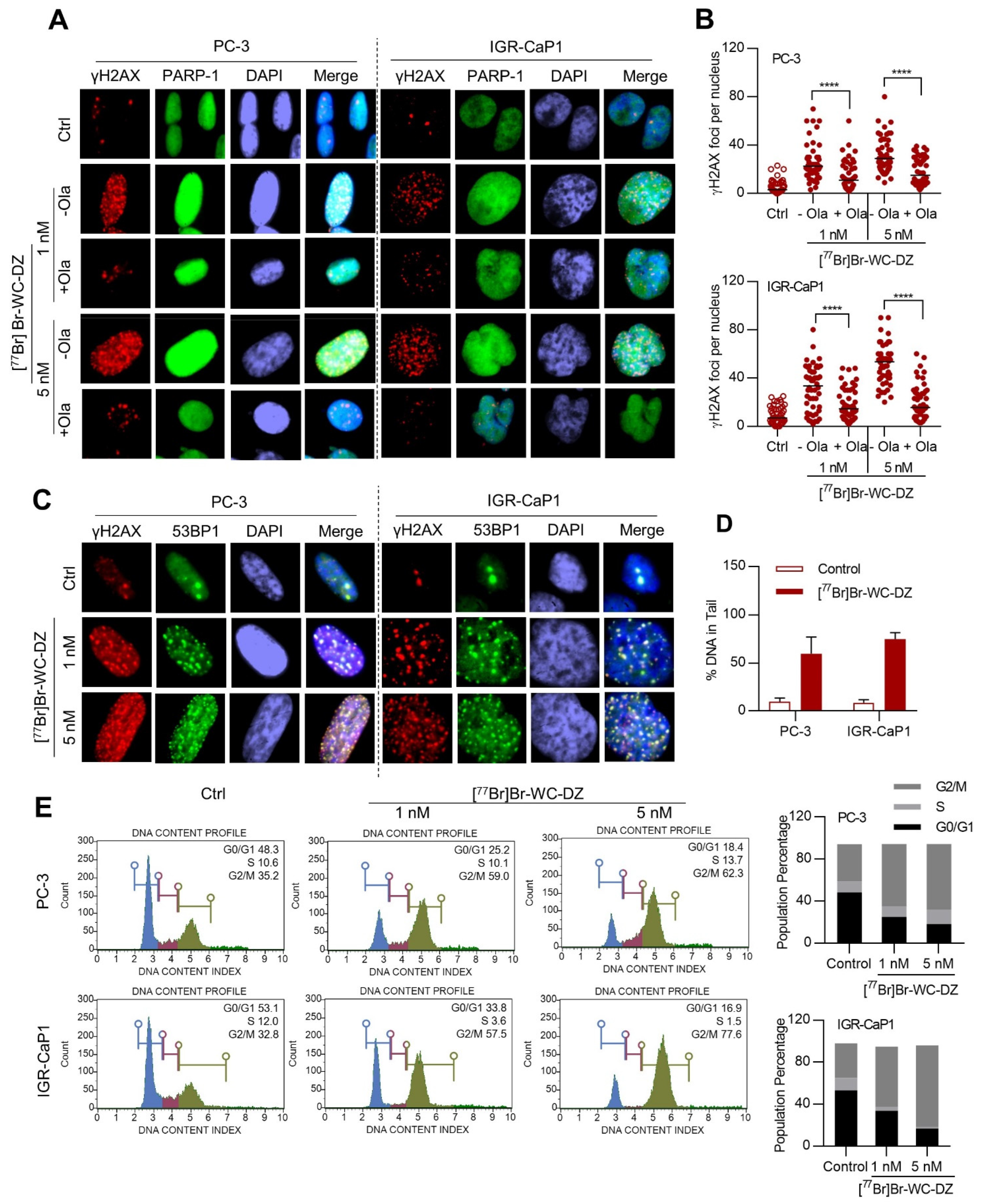
2.4. [77Br]Br-WC-DZ Induce PARP-1 Dependent DNA Damage
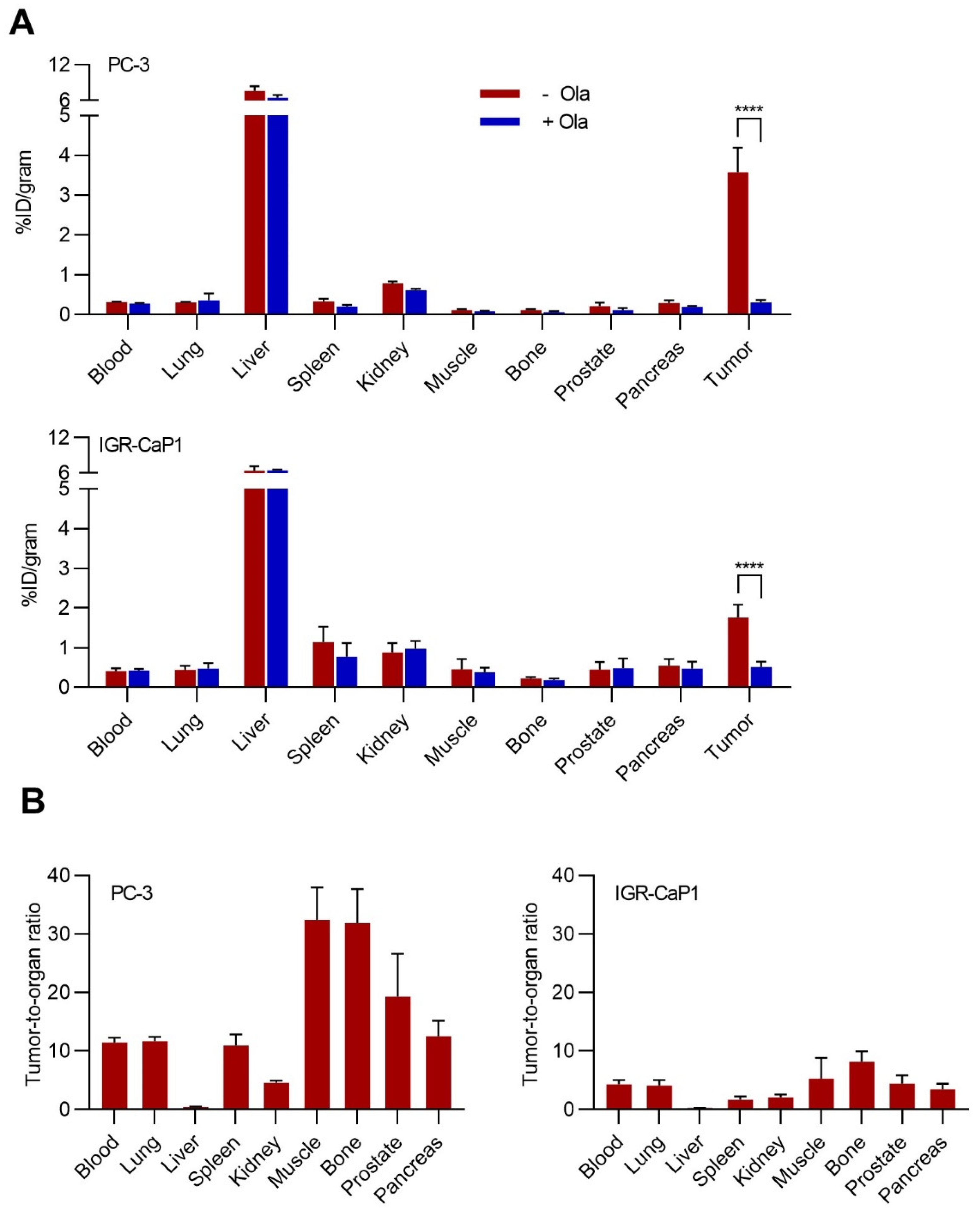
2.5. Biodistribution of [77Br]Br-WC-DZ in Vivo
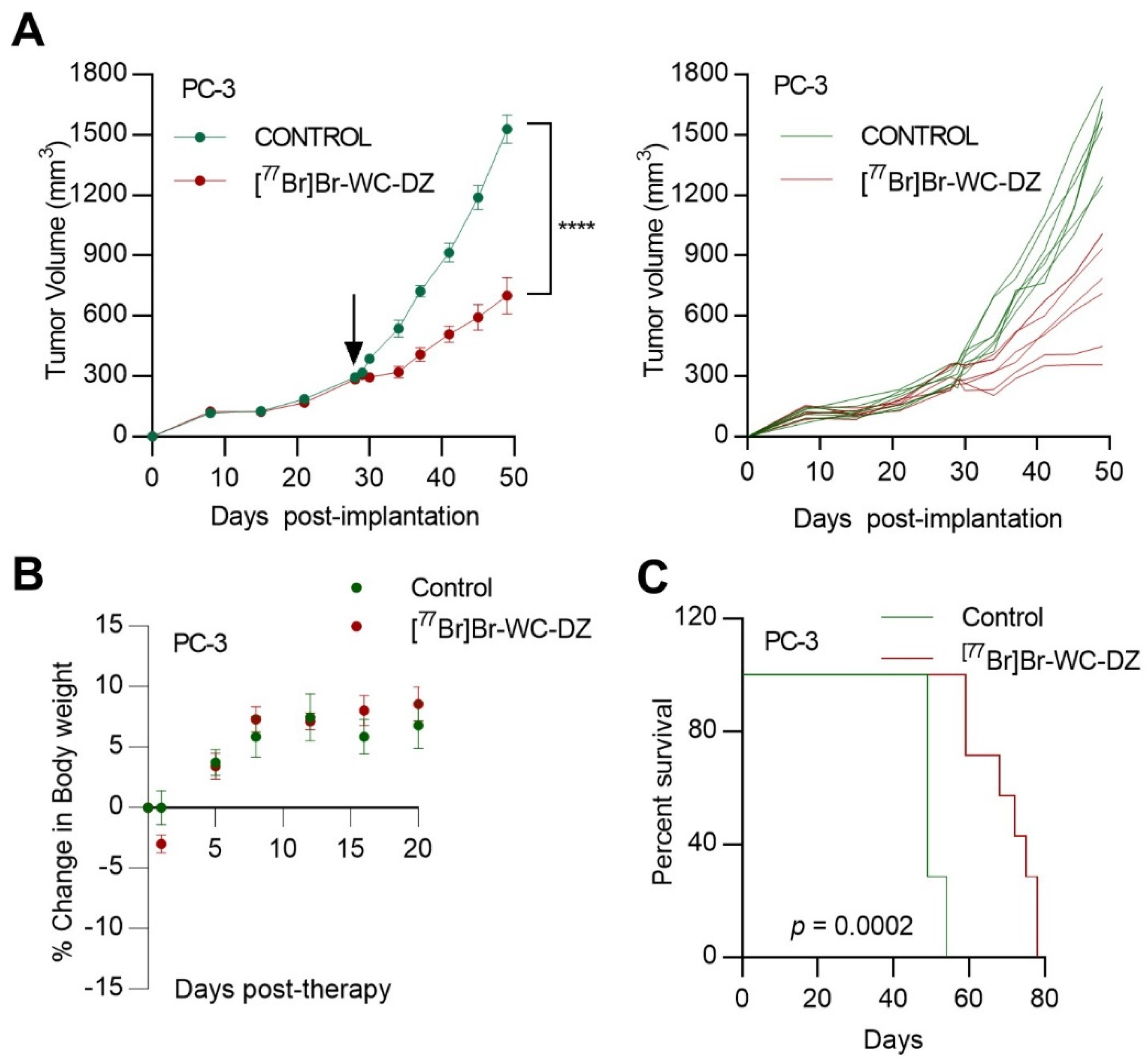
2.6. [77Br]Br-WC-DZ Delayed Prostate Cancer Xenograft Tumor Growth
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. PARP-1 Expression in Prostate Cancer Tissue Microarray
4.3. Chemistry and Radiochemistry
4.4. In Vitro Cytotoxicity
4.5. Clonogenic Assay
4.6. Immunofluorescence
4.7. DNA Damage Assessment by Comet Assay
4.8. Cell-Cycle Analysis by Flow Cytometry
4.9. In vivo Biodistribution and Antitumor Activity Studies
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual Report to the Nation on the Status of Cancer, Part I: National Cancer Statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Powers, E.; Karachaliou, G.S.; Kao, C.; Harrison, M.R.; Hoimes, C.J.; George, D.J.; Armstrong, A.J.; Zhang, T. Novel Therapies Are Changing Treatment Paradigms in Metastatic Prostate Cancer. J. Hematol. Oncol. 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- do Pazo, C.; Webster, R.M. The Prostate Cancer Drug Market. Nat. Rev. Drug Discov. 2021, 20, 663–664. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Evans, C.P.; Higano, C.S.; Keane, T.; Andriole, G.; Saad, F.; Iversen, P.; Miller, K.; Kim, C.S.; Kimura, G.; Armstrong, A.J.; et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-Treated Men with Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2016, 70, 675–683. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.A.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone Acetate plus Prednisone versus Placebo plus Prednisone in Chemotherapy-Naive Men with Metastatic Castration-Resistant Prostate Cancer (COU-AA-302): Final Overall Survival Analysis of a Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Meisel, A.; Von Felten, S.; Vogt, D.R.; Liewen, H.; De Wit, R.; De Bono, J.; Sartor, O.; Stenner-Liewen, F. Severe Neutropenia during Cabazitaxel Treatment Is Associated with Survival Benefit in Men with Metastatic Castration-Resistant Prostate Cancer (MCRPC): A Post-Hoc Analysis of the TROPIC Phase III Trial. Eur. J. Cancer 2016, 56, 93–100. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and Safety of Radium-223 Dichloride in Patients with Castration-Resistant Prostate Cancer and Symptomatic Bone Metastases, with or without Previous Docetaxel Use: A Prespecified Subgroup Analysis from the Randomised, Double-Blind, Phase 3 ALSYMPCA Trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [PubMed]
- Sridhar, S.S.; Freedland, S.J.; Gleave, M.E.; Higano, C.; Mulders, P.; Parker, C.; Sartor, O.; Saad, F. Castration-Resistant Prostate Cancer: From New Pathophysiology to New Treatment. Eur. Urol. 2014, 65, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.R.; Pond, G.R.; Soban, F.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic Small-Molecule Prodrug Conjugates for Targeted Delivery and Controlled Release of Toll-like Receptor 7 Agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, N.; Wu, C.Y.; Li, S.; Chen, Y.A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D.; et al. Validation of Sv2a-Targeted Pet Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 13085. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Ko, H.L.; Ren, E.C. Functional Aspects of PARP1 in DNA Repair and Transcription. Biomoleculs 2012, 2, 524–548. [Google Scholar] [CrossRef]
- Leung, A.K. Non-cortical magnitude coding of space and time by pigeons. Curr. Biol. 2017, 27, R1249–R1267. [Google Scholar] [CrossRef]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP Inhibition: PARP1 and Beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, D.V. Evolution of Poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors. From Concept to Clinic. J. Med. Chem. 2010, 53, 4561–4584. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Galia, A.; Fraggetta, F.; Corte, C.L.; Pepe, P.; Vignera, S.L.; Improta, G.; Bosco, P.; Calogero, A.E. Poly (ADP-Ribose) Polymerase 1 Protein Expression in Normal and Neoplastic Prostatic Tissue. Eur. J. Histochem. 2013, 57, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhu, H.; Liang, Y.; Kong, Z.; Duan, X.; Li, S.; Zhao, Z.; Yang, D.; Zeng, G. Expression of PARP-1 and Its Active Polymer PAR in Prostate Cancer and Benign Prostatic Hyperplasia in Chinese Patients. Int. Urol. Nephrol. 2014, 46, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Bono, J.D.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Marshall, C.H.; Sokolova, A.O.; McNatty, A.L.; Cheng, H.H.; Eisenberger, M.A.; Bryce, A.H.; Schweizer, M.T.; Antonarakis, E.S. Differential Response to Olaparib Treatment Among Men with Metastatic Castration-Resistant Prostate Cancer Harboring BRCA1 or BRCA2 Versus ATM Mutations. Eur. Urol. 2019, 76, 452–458. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Gomella, L.G.; Petrylak, D.P. When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials. Eur. Urol. Oncol. 2020, 3, 594–611. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Nicolosi, P.; Ledet, E.; Yang, S.; Michalski, S.; Freschi, B.; O’Leary, E.; Esplin, E.D.; Nussbaum, R.L.; Sartor, O. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019, 5, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.A.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 Targeted Nuclear Imaging and Theranostic Probes. J. Clin. Med. 2020, 9, 2130. [Google Scholar] [CrossRef] [PubMed]
- Puentes, L.N.; Makvandi, M.; Mach, R.H. Molecular Imaging: Parp-1 and Beyond. J. Nucl. Med. 2021, 62, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.; Carlucci, G.; Salinas, B.; Di Gialleonardo, V.; Kossatz, S.; Vansteene, A.; Longo, V.A.; Bolaender, A.; Chiosis, G.; Keshari, K.R.; et al. Non-Invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol. Imaging Biol. 2016, 18, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chu, W.; Xu, J.; Jones, L.A.; Peng, X.; Li, S.; Chen, D.L.; Mach, R.H. Synthesis, [18F] Radiolabeling, and Evaluation of Poly (ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors for in Vivo Imaging of PARP-1 Using Positron Emission Tomography. Bioorg. Med. Chem. 2014, 22, 1700–1707. [Google Scholar] [CrossRef]
- Riad, A.; Gitto, S.B.; Lee, H.; Winters, H.D.; Martorano, P.M.; Hsieh, C.-J.; Xu, K.; Omran, D.K.; Powell, D.J., Jr.; Mach, R.H.; et al. PARP Theranostic Auger Emitters Are Cytotoxic in BRCA Mutant Ovarian Cancer and Viable Tumors from Ovarian Cancer Patients Enable Ex-Vivo Screening of Tumor Response. Molecules 2020, 25, 6029. [Google Scholar] [CrossRef]
- Salinas, B.; Irwin, C.P.; Kossatz, S.; Bolaender, A.; Chiosis, G.; Pillarsetty, N.; Weber, W.A.; Reiner, T. Radioiodinated PARP1 Tracers for Glioblastoma Imaging. EJNMMI Res. 2015, 5, 46. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1–Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef]
- Lee, H.; Riad, A.; Martorano, P.; Mansfield, A.; Samanta, M.; Batra, V.; Mach, R.H.; Maris, J.M.; Pryma, D.A.; Makvandi, M. PARP-1-Targeted Auger Emitters Display High-LET Cytotoxic Properties In Vitro but Show Limited Therapeutic Utility in Solid Tumor Models of Human Neuroblastoma. J. Nucl. Med. 2020, 61, 850–856. [Google Scholar] [CrossRef]
- Makvandi, M.; Lee, H.; Puentes, L.N.; Reilly, S.W.; Rathi, K.S.; Weng, C.-C.; Chan, H.S.; Hou, C.; Raman, P.; Martinez, D.; et al. Targeting PARP-1 with Alpha-Particles Is Potently Cytotoxic to Human Neuroblastoma in Preclinical Models. Mol. Cancer Ther. 2019, 18, 1195–1204. [Google Scholar] [CrossRef]
- Dabagian, H.; Taghvaee, T.; Martorano, P.; Martinez, D.; Samanta, M.; Watkins, C.M.; Chai, R.; Mansfield, A.; Graham, T.J.; Maris, J.M.; et al. PARP Targeted Alpha-Particle Therapy Enhances Response to PD-1 Immune-Checkpoint Blockade in a Syngeneic Mouse Model of Glioblastoma. Cite This ACS Pharmacol. Transl. Sci 2021, 2021, 351. [Google Scholar] [CrossRef] [PubMed]
- Buchegger, F.; Perillo-Adamer, F.; Dupertuis, Y.M.; Bischof Delaloye, A. Auger Radiation Targeted into DNA: A Therapy Perspective. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger Electrons for Cancer Therapy—A Review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted Radionuclide Therapy of Prostate Cancer—From Basic Research to Clinical Perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [PubMed]
- Bavelaar, B.M.; Lee, B.Q.; Gill, M.R.; Falzone, N.; Vallis, K.A. Subcellular Targeting of Theranostic Radionuclides. Front. Pharmacol. 2018, 9, 996. [Google Scholar] [CrossRef]
- Pirovano, G.; Jannetti, S.A.; Carter, L.M.; Sadique, A.; Kossatz, S.; Guru, N.; França, P.D.D.S.; Maeda, M.; Zeglis, B.M.; Lewis, J.S.; et al. Targeted Brain Tumor Radiotherapy Using an Auger Emitter. Clin. Cancer Res. 2020, 26, 2871–2881. [Google Scholar] [CrossRef]
- Wilson, T.; Pirovano, G.; Xiao, G.; Samuels, Z.; Roberts, S.; Viray, T.; Guru, N.; Zanzonico, P.; Gollub, M.; Pillarsetty, N.V.K.; et al. PARP-Targeted Auger Therapy in P53 Mutant Colon Cancer Xenograft Mouse Models. Mol. Pharm. 2021, 18, 3418–3428. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.A.; Peil, J.; Vogg, A.T.J.; Bolm, C.; Terhorst, S.; Classen, A.; Bauwens, M.; Maurer, J.; Mottaghy, F.; Morgenroth, A. Auger Emitter Conjugated PARP Inhibitor for Therapy in Triple Negative Breast Cancers: A Comparative In-Vitro Study. Cancers 2022, 14, 230. [Google Scholar] [CrossRef]
- Shen, C.J.; Minn, I.; Hobbs, R.F.; Chen, Y.; Josefsson, A.; Brummet, M.; Banerjee, S.R.; Brayton, C.F.; Mease, R.C.; Pomper, M.G.; et al. Auger Radiopharmaceutical Therapy Targeting Prostate-Specific Membrane Antigen in a Micrometastatic Model of Prostate Cancer. Theranostics 2020, 10, 2888–2896. [Google Scholar] [CrossRef]
- Rowland, D.J.; McCarthy, T.J.; Welch, M.J. Radiobromine for Imaging and Therapy. Handb. Radiopharm. 2003, 14, 441–465. [Google Scholar]
- Makvandi, M.; Xu, K.; Lieberman, B.P.; Anderson, R.C.; Effron, S.S.; Winters, H.D.; Zeng, C.; McDonald, E.S.; Pryma, D.A.; Greenberg, R.A.; et al. A Radiotracer Strategy to Quantify PARP-1 Expression In Vivo Provides a Biomarker That Can Enable Patient Selection for PARP Inhibitor Therapy. Cancer Res. 2016, 76, 4516. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Steurer, S.; Clauditz, T.S.; Krech, T.; Wittmer, C.; Lutz, F.; Lennartz, M.; Janssen, T.; Hakimi, N.; Simon, R.; et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. Eur. Urol. 2016, 69, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Barakzai, M.A. Prostatic Adenocarcinoma: A Grading from Gleason to the New Grade-Group System: A Historical and Critical Review. Asian Pac. J. Cancer Prev. 2019, 20, 661. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, S.; Marinaki, S.; Liapis, G.; Kalaitzakis, E.; Fragkioudaki, S.; Kalogeropoulos, P.; Michelakis, I.; Goules, A.; Tzioufas, A.G.; Boletis, J.N. Production and Supply of High Specific Activity Radioisotopes for Radiotherapy Applications. Rev. Med. Nucl. Alasbimn J 2003, 6, 2425–2435. [Google Scholar]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and Characterization of a Human Prostatic Carcinoma Cell Line (PC-3). Invest. Urol. 1979, 17, 16–23. [Google Scholar]
- Lu, Z.H.; Kaliberov, S.; Sohn, R.E.; Kaliberova, L.; Du, Y.; Prior, J.L.; Leib, D.J.; Chauchereau, A.; Sehn, J.K.; Curiel, D.T.; et al. A New Model of Multi-Visceral and Bone Metastatic Prostate Cancer with Perivascular Niche Targeting by a Novel Endothelial Specific Adenoviral Vector. Oncotarget 2017, 8, 12272–12289. [Google Scholar] [CrossRef]
- Chauchereau, A.; Al Nakouzi, N.; Gaudin, C.; Le Moulec, S.; Compagno, D.; Auger, N.; Bénard, J.; Opolon, P.; Rozet, F.; Validire, P.; et al. Stemness Markers Characterize IGR-CaP1, a New Cell Line Derived from Primary Epithelial Prostate Cancer. Exp. Cell Res. 2011, 317, 262–275. [Google Scholar] [CrossRef]
- Smith, R.; Liu, M.; Liby, T.; Bayani, N.; Bucher, E.; Chiotti, K.; Derrick, D.; Chauchereau, A.; Heiser, L.; Alumkal, J.; et al. Enzalutamide Response in a Panel of Prostate Cancer Cell Lines Reveals a Role for Glucocorticoid Receptor in Enzalutamide Resistant Disease. Sci. Rep. 2020, 10, 27520. [Google Scholar] [CrossRef]
- Popp, H.D.; Brendel, S.; Hofmann, W.K.; Fabarius, A. Immunofluorescence Microscopy of ΓH2AX and 53BP1 for Analyzing the Formation and Repair of DNA Double-Strand Breaks. J. Vis. Exp. 2017, 2017, 56617. [Google Scholar]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, A.; Graff, J.R.; Akcakanat, A.; Do, K.-A.; Lluch, A.; Hennessy, B.T.; Hortobagyi, G.N.; Mills, G.B.; Gonzalez-Angulo, A.M.; De Benedetti, A.; et al. EIF-4E Expression and Its Role in Malignancies and Metastases. Oncogene 2004, 23, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xu, J.; Mpoy, C.; Chu, W.; Kim, S.H.; Li, H.; Rogers, B.E.; Katzenellenbogen, J.A. Preliminary Evaluation of a Novel 18F-Labeled PARP-1 Ligand for PET Imaging of PARP-1 Expression in Prostate Cancer. Nucl. Med. Biol. 2018, 66, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ellison, P.A.; Olson, A.P.; Barnhart, T.E.; Hoffman, S.L.V.; Reilly, S.W.; Makvandi, M.; Bartels, J.L.; Murali, D.; DeJesus, O.T.; Lapi, S.E.; et al. Improved Production of 76Br, 77Br and 80mBr via CoSe Cyclotron Targets and Vertical Dry Distillation. Nucl. Med. Biol. 2020, 80–81, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chu, W.; Voller, T.; Katzenellenbogen, J.A. Copper-Mediated Nucleophilic Radiobromination of Aryl Boron Precursors: Convenient Preparation of a Radiobrominated PARP-1 Inhibitor. Tetrahedron Lett. 2018, 59, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hampton, O.A.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Gorlick, R.; Kolb, E.A.; Lock, R.; Carol, H.; Keir, S.T.; et al. Initial Testing (Stage 1) of the PARP Inhibitor BMN 673 by the Pediatric Preclinical Testing Program: PALB2 Mutation Predicts Exceptional In Vivo Response to BMN 673. Pediatr. Blood Cancer 2015, 62, 91–98. [Google Scholar] [CrossRef]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Giri, D.; Schmidt, K.; Podsypanina, K.; Parsons, R.; Greenberg, N.; Ittmann, M. Haploinsufficiency of the Pten Tumor Suppressor Gene Promotes Prostate Cancer Progression. Proc. Natl. Acad. Sci. USA. 2001, 98, 11563. [Google Scholar] [CrossRef]
- Ferraris, D.V.; Li, J.-H.; Kalish, J.; Zhang, J. Benzoazepine and Benzodiazepine Derivatives and Their Use as PARP Inhibitors. US Patent WO/2002/044183, 30 November 2001. [Google Scholar]
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed [211At] Astatination and [125I] Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755. [Google Scholar] [CrossRef]
- Tang, L. Radionuclide Production and Yields at Washington University School of Medicine. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 121–133. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreekumar, S.; Zhou, D.; Mpoy, C.; Schenk, E.; Scott, J.; Arbeit, J.M.; Xu, J.; Rogers, B.E. Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 3083. https://doi.org/10.3390/ijms24043083
Sreekumar S, Zhou D, Mpoy C, Schenk E, Scott J, Arbeit JM, Xu J, Rogers BE. Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer. International Journal of Molecular Sciences. 2023; 24(4):3083. https://doi.org/10.3390/ijms24043083
Chicago/Turabian StyleSreekumar, Sreeja, Dong Zhou, Cedric Mpoy, Elsa Schenk, Jalen Scott, Jeffrey M. Arbeit, Jinbin Xu, and Buck E. Rogers. 2023. "Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer" International Journal of Molecular Sciences 24, no. 4: 3083. https://doi.org/10.3390/ijms24043083
APA StyleSreekumar, S., Zhou, D., Mpoy, C., Schenk, E., Scott, J., Arbeit, J. M., Xu, J., & Rogers, B. E. (2023). Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer. International Journal of Molecular Sciences, 24(4), 3083. https://doi.org/10.3390/ijms24043083






