How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis
Abstract
1. Introduction
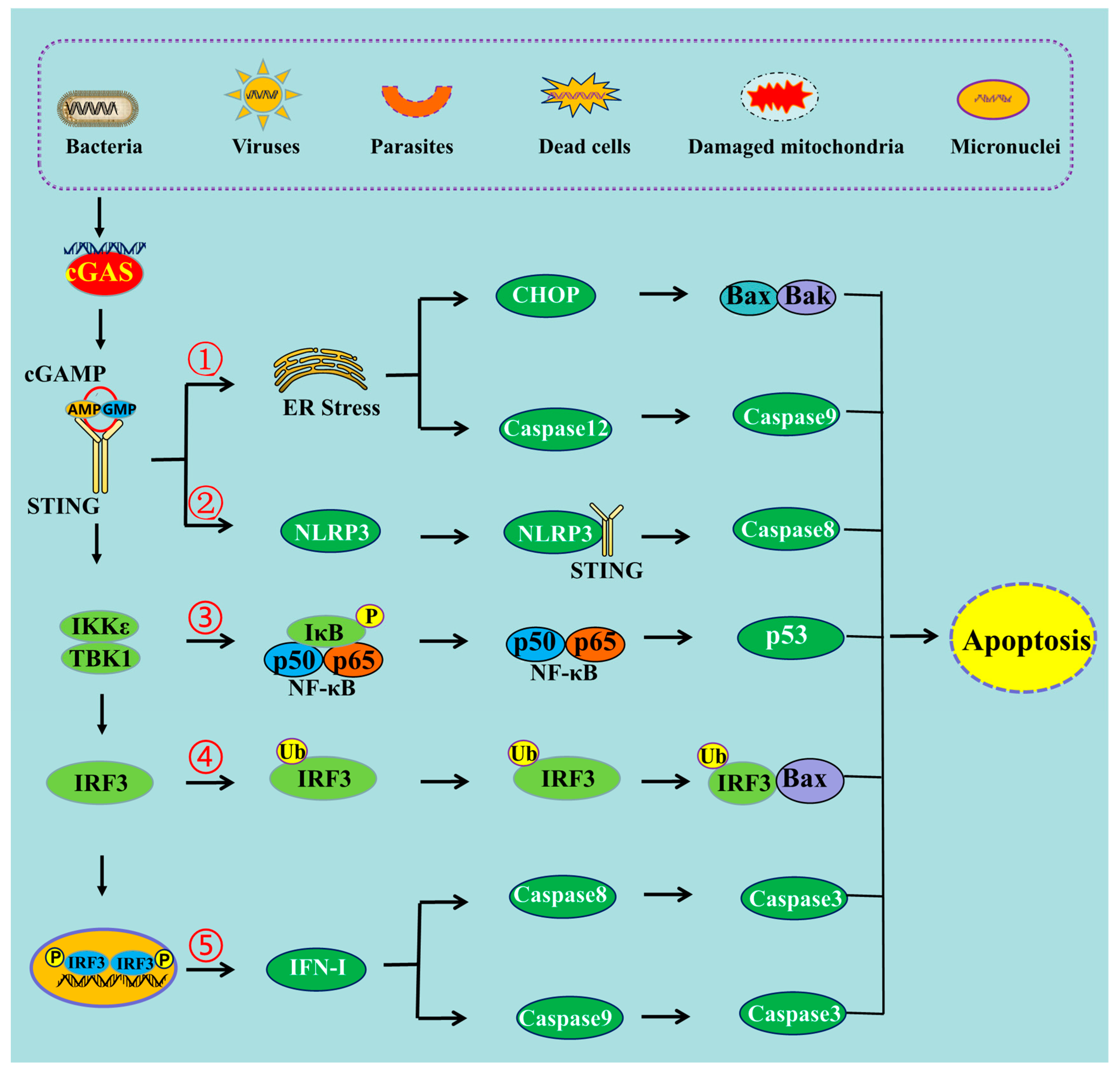
2. The Mechanisms of cGAS-STING Pathway-Induced Apoptosis
2.1. cGAS-STING Pathway Can Induce Apoptosis through Endoplasmic Reticulum Stress
2.2. cGAS-STING Pathway Can Induce Apoptosis through NLRP3 Pathway
2.3. cGAS-STING Pathway Can Induce Apoptosis through NF-κB
2.4. cGAS-STING Pathway Induces Apoptosis through IRF3-Bax Interaction
2.5. cGAS-STING Pathway Induces Apoptosis through IFN-I Production
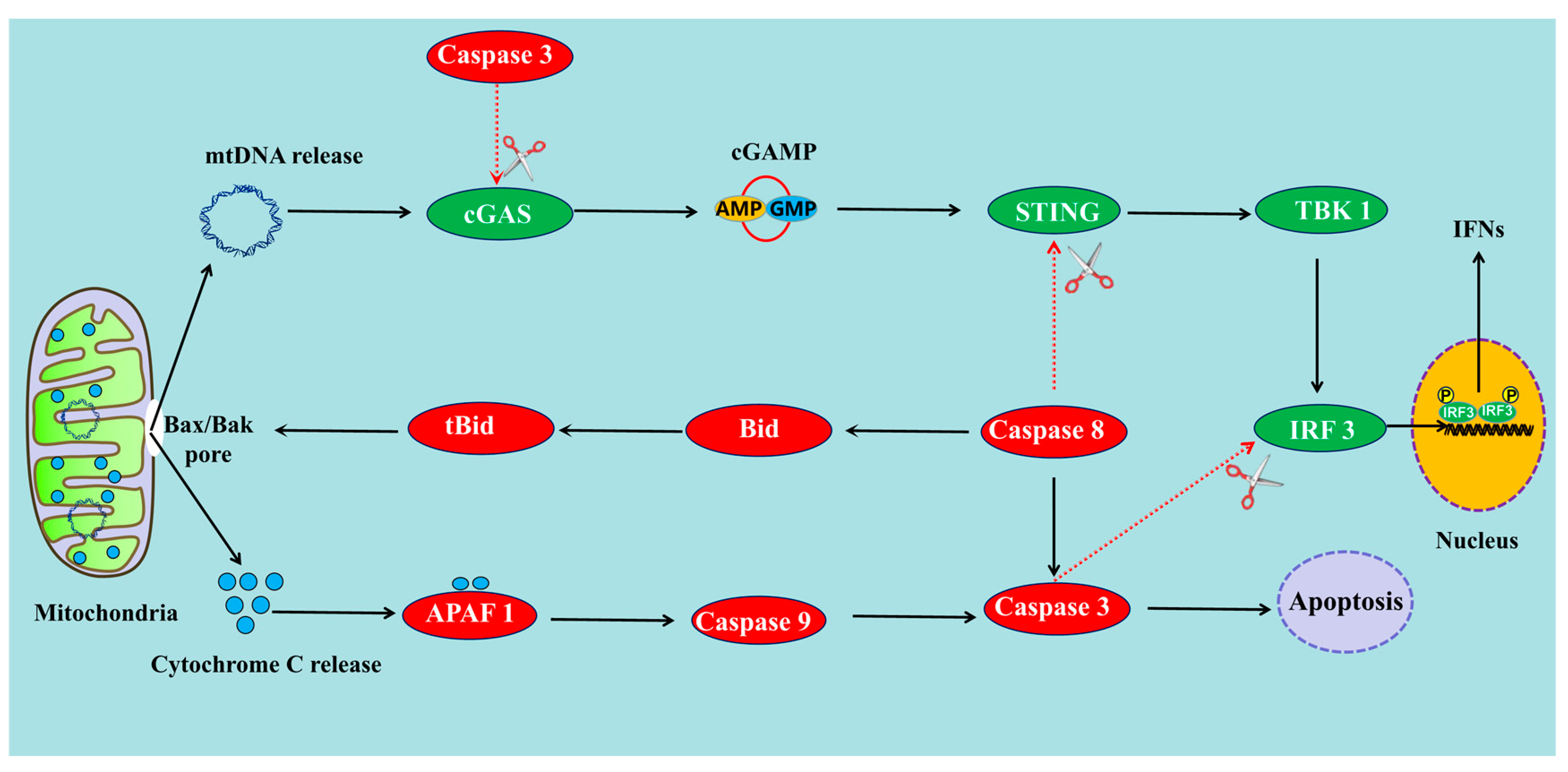
3. Does Apoptosis Promote or Inhibit the cGAS-STING Pathway?
3.1. Apoptosis Can Suppress the cGAS-STING Pathway through the Activation of Caspases
3.2. Apoptosis Can Also Promote cGAS-STING Pathway through the Release of mtDNA
4. What Are the Effects of the Apoptosis Process Induced by the cGAS-STING Pathway?
4.1. cGAS-STING-Mediated Apoptosis in Viral Pathogenesis, a Double-Edged Sword
4.2. The Effects of cGAS-STING-Mediated Apoptosis in Antitumor Immunity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayman, T.J.; Glazer, P.M. Regulation of the Cell-Intrinsic DNA Damage Response by the Innate Immune Machinery. Int. J. Mol. Sci. 2021, 22, 12761. [Google Scholar] [CrossRef]
- Ma, R.; Ortiz Serrano, T.P.; Davis, J.; Prigge, A.D.; Ridge, K.M. The cGAS-STING pathway: The role of self-DNA sensing in inflammatory lung disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 13156–13170. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Andrade-Barros, A.I.; Bernardo, J.T.G.; Balogh, E.; Quesniaux, V.F.; Ryffel, B.; Lopes-Ferreira, M. Natterin-Induced Neutrophilia Is Dependent on cGAS/STING Activation via Type I IFN Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3600. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Rashidi, A.S.; Tran, D.N.; Katzilieris-Petras, G.; Hvidt, A.K.; Gohr, M.; Fruhwurth, S.; Bodda, C.; Thomsen, M.K.; Vendelbo, M.H.; et al. Brain immune cells undergo cGAS/STING-dependent apoptosis during herpes simplex virus type 1 infection to limit type I IFN production. J. Clin. Investig. 2021, 131, e136824. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, W.W.; Wang, F.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells. Mol. Immunol. 2021, 131, 180–190. [Google Scholar] [CrossRef]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.T.; Hiscott, J.; van Grevenynghe, J. Host Restriction Factor SAMHD1 Limits Human T Cell Leukemia Virus Type 1 Infection of Monocytes via STING-Mediated Apoptosis. Cell Host Microbe 2013, 14, 422–434. [Google Scholar] [CrossRef]
- Polidarova, M.P.; Brehova, P.; Dejmek, M.; Birkus, G.; Brazdova, A. STING Agonist-Mediated Cytokine Secretion Is Accompanied by Monocyte Apoptosis. ACS Infect. Dis. 2022, 8, 463–471. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Loreto, C.; Rocca, G.L.; Anzalone, R.; Caltabiano, R.; Vespasiani, G.; Castorina, S.; Ralph, D.J.; Cellek, S.; Musumeci, G.; Giunta, S.; et al. The role of intrinsic pathway in apoptosis activation and progression in Peyronie’s disease. BioMed Res. Int. 2014, 2014, 616149. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Ma, X.-M.; Geng, K.; Law, B.; Wang, P.; Pu, Y.-L.; Chen, Q.; Xu, H.-W.; Tan, X.-Z.; Jiang, Z.-Z.; Xu, Y. Lipotoxicity-induced mtDNA release promotes diabetic cardiomyopathy by activating the cGAS-STING pathway in obesity-related diabetes. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- White, M.J.; McArthur, K.; Metcalf, D.; Lane, R.M.; Cambier, J.C.; Herold, M.J.; van Delft, M.F.; Bedoui, S.; Lessene, G.; Ritchie, M.E.; et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 2014, 159, 1549–1562. [Google Scholar] [CrossRef]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef]
- Ning, X.; Wang, Y.; Jing, M.; Sha, M.; Lv, M.; Gao, P.; Zhang, R.; Huang, X.; Feng, J.-M.; Jiang, Z. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol. Cell 2019, 74, 19–31. [Google Scholar] [CrossRef]
- Huang, R.; Shi, Q.; Zhang, S.; Lin, H.; Han, C.; Qian, X.; Huang, Y.; Ren, X.; Sun, J.; Feng, N.; et al. Inhibition of the cGAS-STING Pathway Attenuates Lung Ischemia/Reperfusion Injury via Regulating Endoplasmic Reticulum Stress in Alveolar Epithelial Type II Cells of Rats. J. Inflamm. Res. 2022, 15, 5103–5119. [Google Scholar] [CrossRef]
- Zheng, W.-L.; Xia, N.-W.; Zhang, J.-J.; Chen, N.-H.; Meurens, F.; Liu, Z.-P.; Zhu, J.-Z. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Autophagy. Int. J. Mol. Sci. 2021, 22, 13232. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.-J.; Dobbs, N.; Sakai, T.; Liou, J.; Miner, J.J.; Yan, N. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019, 216, 867–883. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.-Z.; Wang, Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed. Pharmacother. 2020, 125, 110022. [Google Scholar] [CrossRef]
- Rani, S.; Sreenivasaiah, P.K.; Kim, J.O.; Lee, M.Y.; Kang, W.S.; Kim, Y.S.; Ahn, Y.; Park, W.J.; Cho, C.; Kim, D.H. Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS ONE 2017, 12, e0176071. [Google Scholar] [CrossRef]
- Srikanth, S.; Woo, J.S.; Wu, B.-B.; El-Sherbiny, Y.M.; Leung, J.; Chupradit, K.; Rice, L.; Seo, G.J.; Calmettes, G.; Ramakrishna, C.; et al. The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat. Immunol. 2019, 20, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.J.L.; Black, M.J.; Soboloff, J.; Gill, D.L.; Dziadek, M.A.; Johnstone, L.S. Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3T3-L1 pre-adipocyte differentiation. Differentiation 2009, 77, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.M.V.; Robinson, N.; Kumar, S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differ. 2020, 27, 2989–3003. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xu, F.; Zhu, Q.; Feng, Z.; Dai, W.; Zhou, Y.; You, Q.D.; Xu, X. Medicinal chemistry perspective on cGAS-STING signaling pathway with small molecule inhibitors. Eur. J. Med. Chem. 2022, 244, 114791. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, H.; Nie, X.-Y.; Qi, Y.-J.; Shi, S.; Han, Y.-Y.; Zhou, W.-C.; He, C.-Y.; Wang, L.-T. Link between sterile inflammation and cardiovascular diseases: Focus on cGAS-STING pathway in the pathogenesis and therapeutic prospect. Front. Cardiovasc. Med. 2022, 9, 2290. [Google Scholar] [CrossRef]
- Liu, J.-J.; Zhang, X.; Wang, H. The cGAS-STING-mediated NLRP3 inflammasome is involved in the neurotoxicity induced by manganese exposure. Biomed. Pharmacother. 2022, 154, 113680. [Google Scholar] [CrossRef]
- Wang, W.-B.; Hu, D.-W.; Wu, C.-F.; Feng, Y.-Q.; Li, A.-X.; Liu, W.-Y.; Wang, Y.-C.; Chen, K.-L.; Tian, M.-F.; Xiao, F.; et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020, 16, e1008335. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124. [Google Scholar] [CrossRef]
- Tsuchiya, K. Inflammasome-associated cell death: Pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020, 64, 252–269. [Google Scholar] [CrossRef]
- Antonopoulos, C.; Russo, H.M.; El Sanadi, C.; Martin, B.N.; Li, X.; Kaiser, W.J.; Mocarski, E.S.; Dubyak, G.R. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J. Biol. Chem. 2015, 290, 20167–20184. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2021, 109, 121–141. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Wu, H.; Wu, Q.; Duan, M.; Deng, W.; Tang, Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019, 24, 101215. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-kappaB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cerikan, B.; Cortese, M.; Frankish, J.; Lee, J.Y.; Plociennikowska, A.; Heigwer, F.; Prasad, V.; Joecks, S.; Burkart, S.S.; et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-kappaB. Commun. Biol. 2022, 5, 45. [Google Scholar] [CrossRef]
- Andrade, B.; Jara-Gutierrez, C.; Paz-Araos, M.; Vazquez, M.C.; Diaz, P.; Murgas, P. The Relationship between Reactive Oxygen Species and the cGAS/STING Signaling Pathway in the Inflammaging Process. Int. J. Mol. Sci. 2022, 23, 15182. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKepsilon Act Redundantly to Mediate STING-Induced NF-kappaB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-kappaB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Zhao, B.; Du, F.; Xu, P.; Shu, C.; Sankaran, B.; Bell, S.L.; Liu, M.; Lei, Y.; Gao, X.; Fu, X.; et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 2019, 569, 718–722. [Google Scholar] [CrossRef]
- Ortis, F.; Pirot, P.; Naamane, N.; Kreins, A.Y.; Rasschaert, J.; Moore, F.; Theatre, E.; Verhaeghe, C.; Magnusson, N.E.; Chariot, A.; et al. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia 2008, 51, 1213–1225. [Google Scholar] [CrossRef]
- Yamashita, M.; Passegue, E. TNF-alpha Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 2019, 25, 357–372. [Google Scholar] [CrossRef]
- Gao, W.-L.; Gao, J.-S.; Chen, L.-Y.; Ren, Y.-D.; Ma, J.-F. Targeting XIST induced apoptosis of human osteosarcoma cells by activation of NF-kB/PUMA signal. Bioengineered 2019, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Aljenoobi, F.I.; Jan, B.L.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int. J. Biol. Macromol. 2018, 111, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, X.M.; Chen, J.X.; Zheng, G.; Xie, C.L.; Wu, H.Q.; Miao, Z.M.; Lin, Y.; Wang, X.Y.; Gao, W.Y.; et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-kappa B signaling pathway. Cell Death Dis. 2021, 12, 13. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Romieu-Mourez, R.; Solis, M.; Hernandez, E.; Mesplede, T.; Lin, R.; Leaman, D.; Hiscott, J. Transcriptional re-programming of primary macrophages reveals distinct apoptotic and anti-tumoral functions of IRF-3 and IRF-7. Eur. J. Immunol. 2009, 39, 527–540. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, D.; Sreevatsan, S.; Liu, C.; Yang, W.; Song, Z.; Yang, L.; Barrow, P.; Zhou, X. Mycobacterium bovis Induces Endoplasmic Reticulum Stress Mediated-Apoptosis by Activating IRF3 in a Murine Macrophage Cell Line. Front. Cell. Infect. Microbiol. 2016, 6, 182. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Yamashita, M.; Zhang, Y.; Sen, G.C. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J. Virol. 2011, 85, 3708–3716. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Marques, J.T.; Yamashita, M.; Peters, K.L.; Smith, K.; Desai, A.; Williams, B.R.G.; Sen, G.C. Viral apoptosis is induced by IRF-3-mediated activation of Bax. Embo J. 2010, 29, 1762–1773. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. RIG-I-like receptor-induced IRF3 mediated pathway of apoptosis (RIPA): A new antiviral pathway. Protein Cell 2017, 8, 165–168. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Kuzmanovic, T.; Zhang, Y.; Wetzel, J.L.; Sen, G.C. Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis. Immunity 2016, 44, 1151–1161. [Google Scholar] [CrossRef]
- Dedoni, S.; Olianas, M.C.; Onali, P. Interferon-beta induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK-STAT signaling and down-regulation of PI3K/Akt pathway. J. Neurochem. 2010, 115, 1421–1433. [Google Scholar] [CrossRef]
- Kato, Y.; Park, J.; Takamatsu, H.; Konaka, H.; Aoki, W.; Aburaya, S.; Ueda, M.; Nishide, M.; Koyama, S.; Hayama, Y.; et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1507–1515. [Google Scholar] [CrossRef]
- Makowska, A.; Wahab, L.; Braunschweig, T.; Kapetanakis, N.I.; Vokuhl, C.; Denecke, B.; Shen, L.; Busson, P.; Kontny, U. Interferon beta induces apoptosis in nasopharyngeal carcinoma cells via the TRAIL-signaling pathway. Oncotarget 2018, 9, 14228–14250. [Google Scholar] [CrossRef]
- Chawla-Sarkar, M.; Leaman, D.W.; Borden, E.C. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha 2: Correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin. Cancer Res. 2001, 7, 1821–1831. [Google Scholar]
- Bernardo, A.R.; Cosgaya, J.M.; Aranda, A.; Jimenez-Lara, A.M. Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell Death Dis. 2013, 4, e479. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Zheng, Q.-Y.; Li, G.-Q.; Hu, X.-B.; Feng, S.-L.; Xu, G.-L.; Zhang, K.-Q. STAT1-Mediated Down-Regulation of Bcl-2 Expression Is Involved in IFN-gamma/TNF-alpha-Induced Apoptosis in NIT-1 Cells. PLoS ONE 2015, 10, e0120921. [Google Scholar] [CrossRef]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef]
- Das, T.P.; Suman, S.; John, A.M.S.P.; Pal, D.; Edwards, A.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Activation of AKT negatively regulates the pro-apoptotic function of death-associated protein kinase 3 (DAPK3) in prostate cancer. Cancer Lett. 2016, 377, 134–139. [Google Scholar] [CrossRef]
- Silva, F.; Padin-Iruegas, M.E.; Caponio, V.C.A.; Lorenzo-Pouso, A.I.; Saavedra-Nieves, P.; Chamorro-Petronacci, C.M.; Suarez-Penaranda, J.; Perez-Sayans, M. Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 11937. [Google Scholar] [CrossRef]
- Fang, Y.; Peng, K. Regulation of innate immune responses by cell death-associated caspases during virus infection. FEBS J. 2022, 289, 4098–4111. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, Q.; Yu, X.; Zhao, Z.; Jiang, Z. Recent advances in the activation and regulation of the cGAS-STING pathway. Adv. Immunol. 2022, 156, 55–102. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.-T.; Li, B.-B.; Song, L.; Hu, C.-M.; Li, X.-Y.; Wang, D.-D.; Xiong, Y.; Zhao, P.; He, H.W.; Xia, Q.-Y.; et al. Stimulator of interferon genes (STING) provides insect antiviral immunity by promoting Dredd caspase-mediated NF-kappa B activation. J. Biol. Chem. 2018, 293, 11878–11890. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.; Kim, J.C.; Kang, T.-B.; Rajput, A.; Bogdanov, K.; Dittrich-Breiholz, O.; Kracht, M.; Brenner, O.; Wallach, D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 2009, 206, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-G.; Tang, Y.-D.; Zheng, C.-F. The crosstalk between the caspase family and the cGAS-STING signaling pathway. J. Mol. Cell Biol. 2021, 13, 739–747. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.T.; Shteinfer-Kuzmine, A.; Wang, K.N.; Zhu, J.; Yoon, H.E.; Wang, X.H.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Heimer, S.; Knoll, G.; Schulze-Osthoff, K.; Ehrenschwender, M. Raptinal bypasses BAX, BAK, and BOK for mitochondrial outer membrane permeabilization and intrinsic apoptosis. Cell Death Dis. 2019, 10, 556. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. Embo Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Webb, L.G.; Fernandez-Sesma, A. RNA viruses and the cGAS-STING pathway: Reframing our understanding of innate immune sensing. Curr. Opin. Virol. 2022, 53, 101206. [Google Scholar] [CrossRef]
- Phelan, T.; Little, M.A.; Brady, G. Targeting of the cGAS-STING system by DNA viruses. Biochem. Pharm. 2020, 174, 113831. [Google Scholar] [CrossRef]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef]
- Bryant, J.D.; Lei, Y.; VanPortfliet, J.J.; Winters, A.D.; West, A.P. Assessing Mitochondrial DNA Release into the Cytosol and Subsequent Activation of Innate Immune-related Pathways in Mammalian Cells. Curr. Protoc. 2022, 2, e372. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, N.-W.; Luo, J.; Zhang, Y.-W.; Cao, Q.; Zhang, J.-J.; Wang, Y.-N.; Zhao, Y.; Zheng, W.-L.; Chen, N.-H.; et al. The Porcine Cyclic GMP-AMP Synthase-STING Pathway Exerts an Unusual Antiviral Function Independent of Interferon and Autophagy. J. Virol. 2022, 96, e01476-22. [Google Scholar] [CrossRef]
- Cui, S.-F.; Yu, Q.-Y.; Chu, L.; Cui, Y.; Ding, M.; Wang, Q.-Y.; Wang, H.-Y.; Chen, Y.; Liu, X.; Wang, C. Nuclear cGAS Functions Non-canonically to Enhance Antiviral Immunity via Recruiting Methyltransferase Prmt5. Cell Rep. 2020, 33, 108490. [Google Scholar] [CrossRef]
- Sokolowska, O.; Nowis, D. STING Signaling in Cancer Cells: Important or Not? Arch. Immunol. Ther. Exp. 2018, 66, 125–132. [Google Scholar] [CrossRef]
- Shi, F.; Su, J.; Wang, J.; Liu, Z.; Wang, T. Activation of STING inhibits cervical cancer tumor growth through enhancing the anti-tumor immune response. Mol. Cell. Biochem. 2021, 476, 1015–1024. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2015, 42, 199. [Google Scholar] [CrossRef]
- Sato, S.; Sawada, Y.; Nakamura, M. STING Signaling and Skin Cancers. Cancers 2021, 13, 5603. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING Agonists as Cancer Therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Romo, M.R. Cell death as part of innate immunity: Cause or consequence? Immunology 2021, 163, 399–415. [Google Scholar] [CrossRef]
- Reislander, T.; Groelly, F.J.; Tarsounas, M. DNA Damage and Cancer Immunotherapy: A STING in the Tale. Mol. Cell 2020, 80, 21–28. [Google Scholar] [CrossRef]
- Pepin, G.; Gantier, M.P. cGAS-STING Activation in the Tumor Microenvironment and Its Role in Cancer Immunity. Adv. Exp. Med. Biol. 2017, 1024, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xiong, Q.; Xia, H.; Liu, W.; Dai, S.; Cai, S.; Zhu, Z.; Yan, X. Carboplatin activates the cGAS-STING pathway by upregulating the TREX-1 (three prime repair exonuclease 1) expression in human melanoma. Bioengineered 2021, 12, 6448–6458. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Liu, A.; Xia, N.; Chen, N.; Meurens, F.; Zhu, J. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis. Int. J. Mol. Sci. 2023, 24, 3029. https://doi.org/10.3390/ijms24033029
Zheng W, Liu A, Xia N, Chen N, Meurens F, Zhu J. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis. International Journal of Molecular Sciences. 2023; 24(3):3029. https://doi.org/10.3390/ijms24033029
Chicago/Turabian StyleZheng, Wanglong, Anjing Liu, Nengwen Xia, Nanhua Chen, François Meurens, and Jianzhong Zhu. 2023. "How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis" International Journal of Molecular Sciences 24, no. 3: 3029. https://doi.org/10.3390/ijms24033029
APA StyleZheng, W., Liu, A., Xia, N., Chen, N., Meurens, F., & Zhu, J. (2023). How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis. International Journal of Molecular Sciences, 24(3), 3029. https://doi.org/10.3390/ijms24033029







