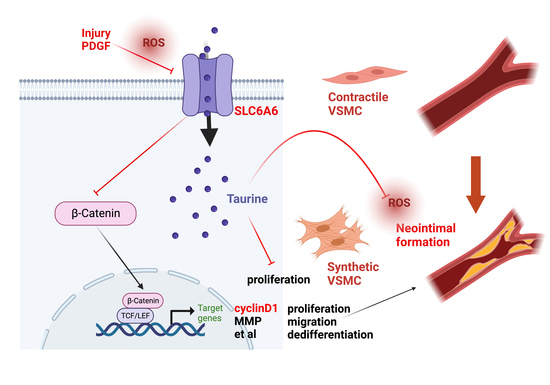
Ant-Neointimal Formation Effects of SLC6A6 in Preventing Vascular Smooth Muscle Cell Proliferation and Migration via Wnt/β-Catenin Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Cell Culture
2.3. SLC6A6 Knockdown or Overexpression In Vitro
2.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Western Blotting
2.6. VSMC Proliferation Assay
2.7. VSMC Migration Assay
2.8. ROS Mearsurement
2.9. Human Artery Samples Collection
2.10. Histological and Morphometric Analyses
2.11. Immunohistochemical and Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
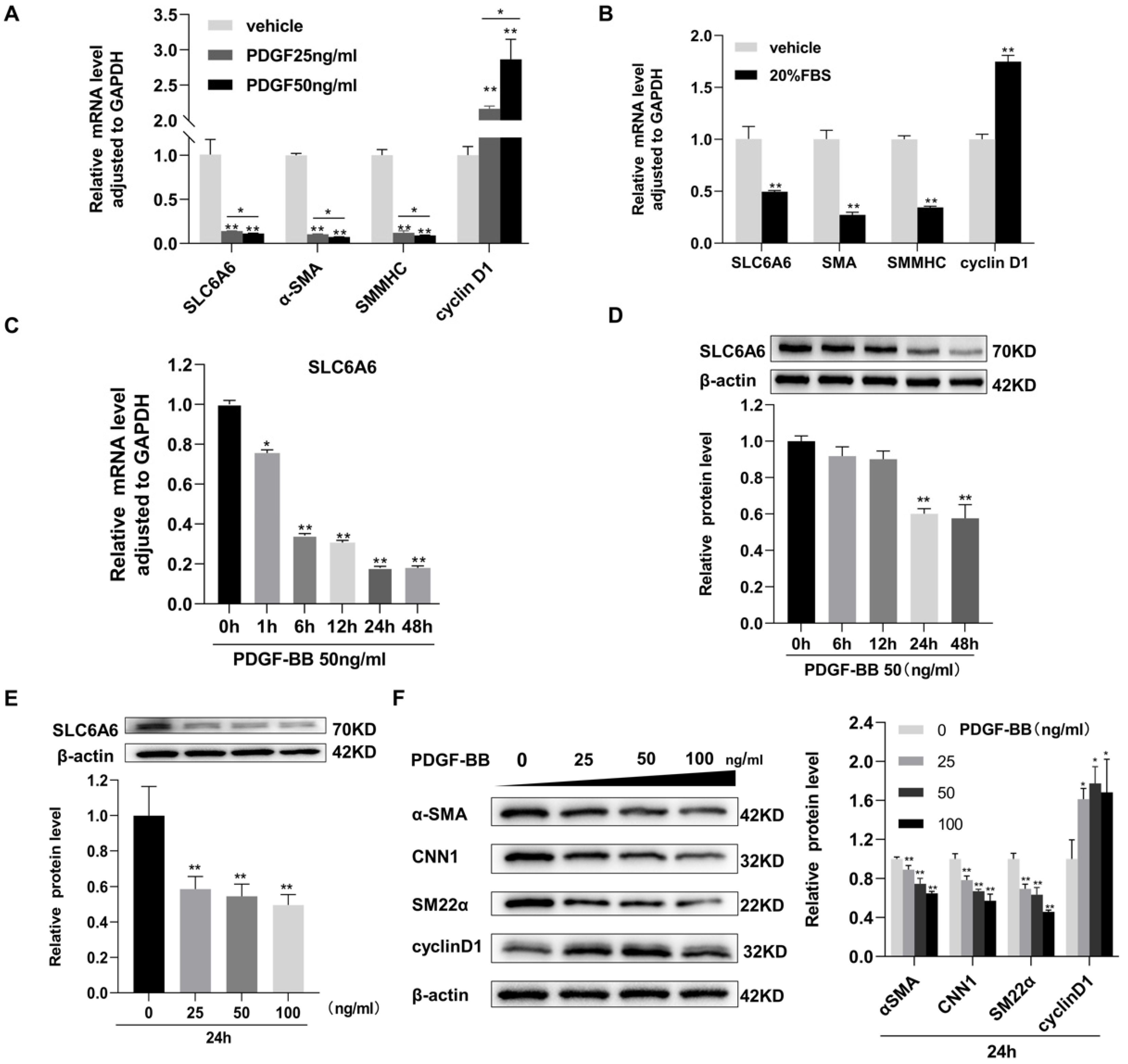
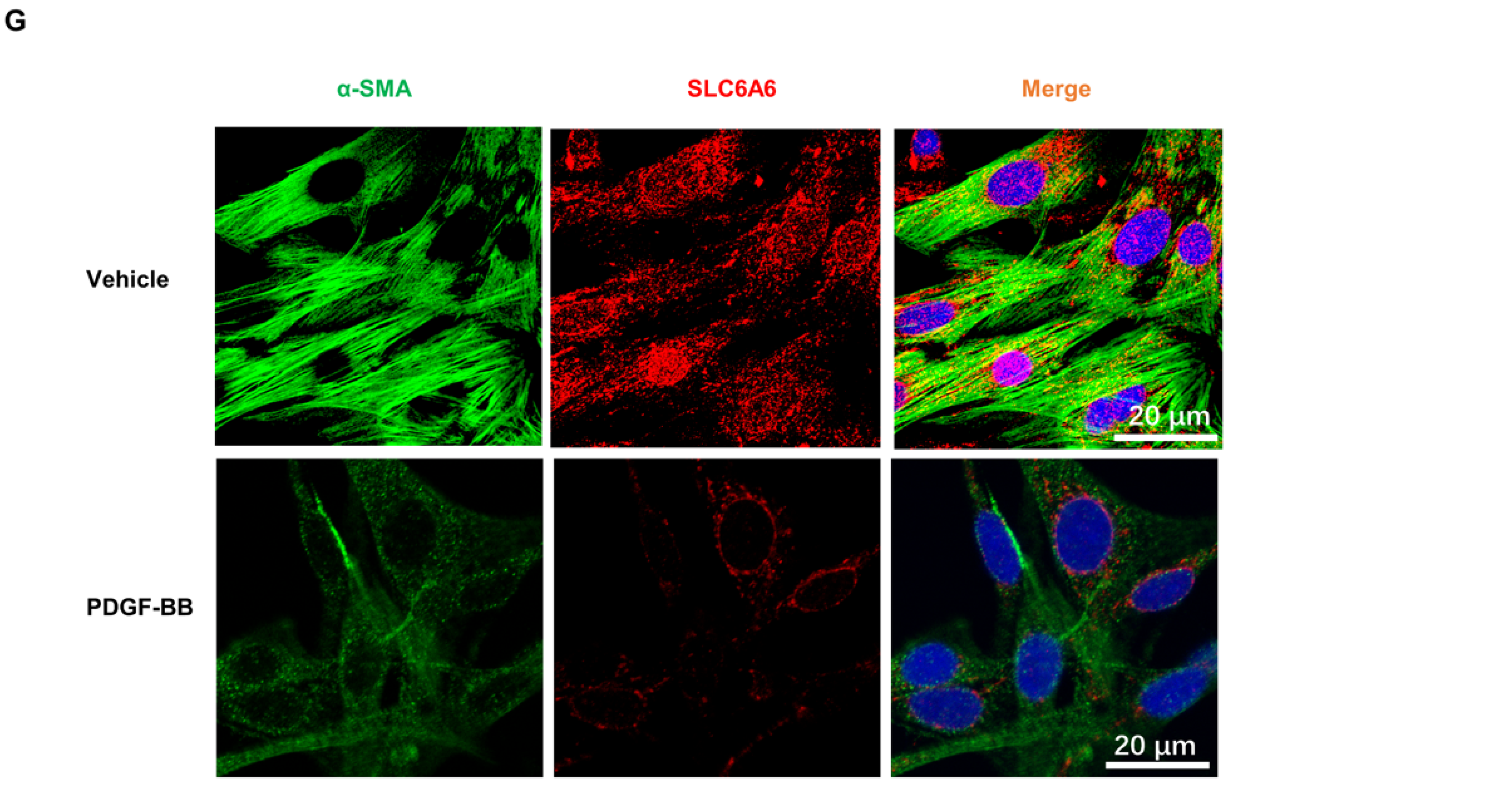
3.1. SLC6A6 Decreased during VSMCs Dedifferentiation
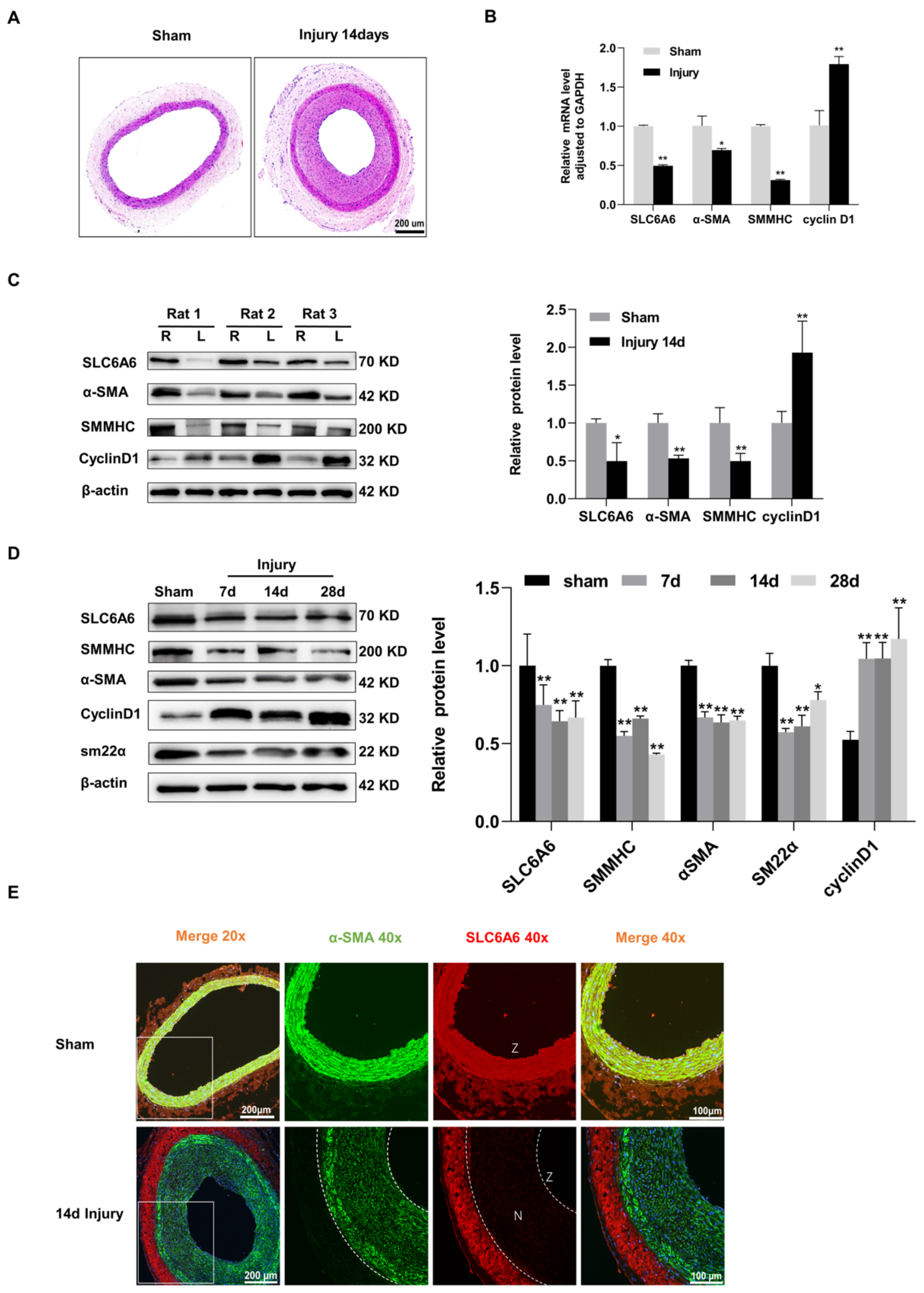
3.2. SLC6A6 Downregulated during Neointimal Formation
3.3. SLC6A6 Decreased in Atherosclerosis
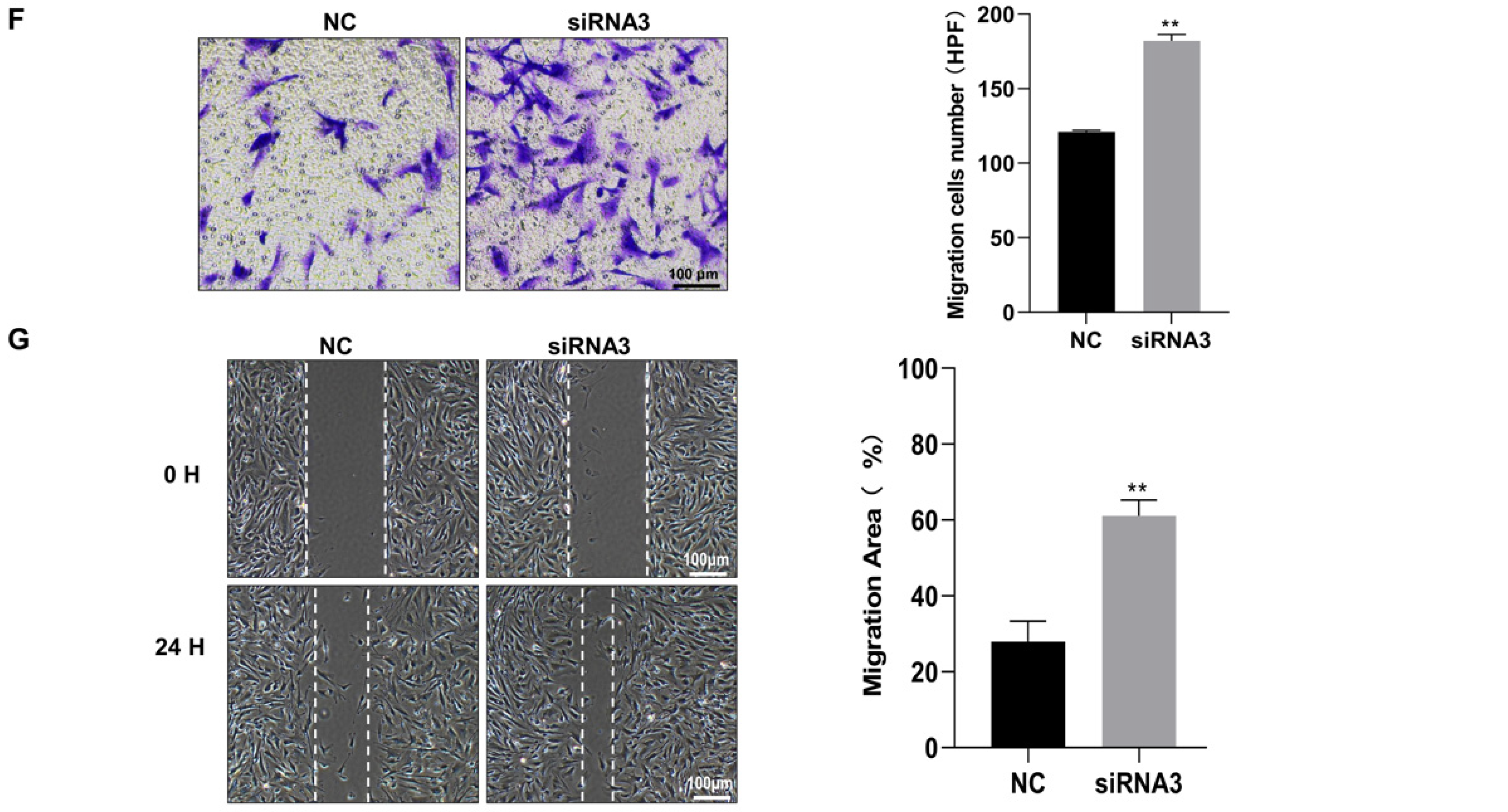
3.4. SLC6A6 Knockdown Promoted VSMCs Dedifferentiation, Proliferation, and Migration
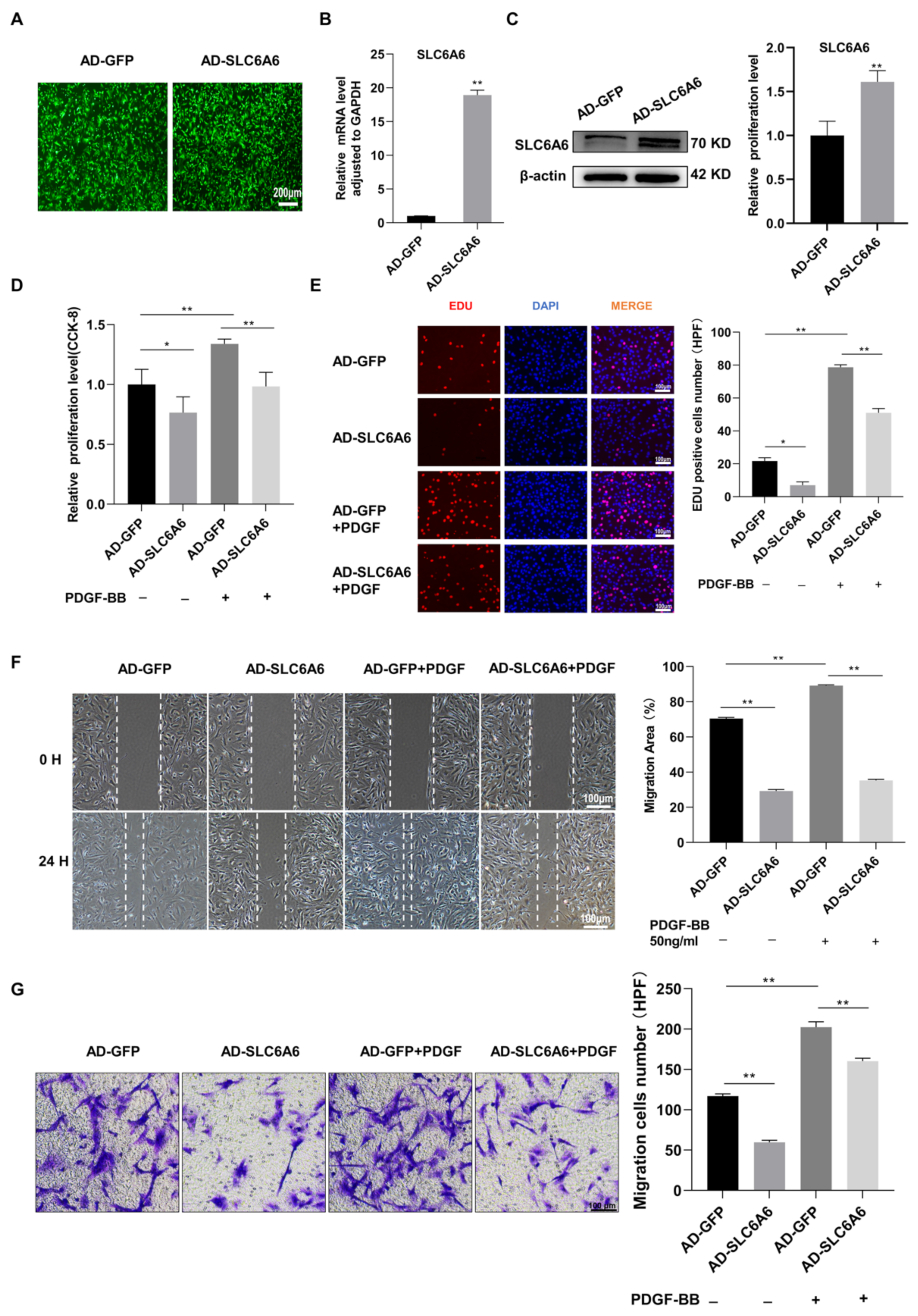
3.5. SLC6A6 Overexpression Reduced PDGF-BB-Induced VSMC Proliferation and Migration and Dedifferentiation
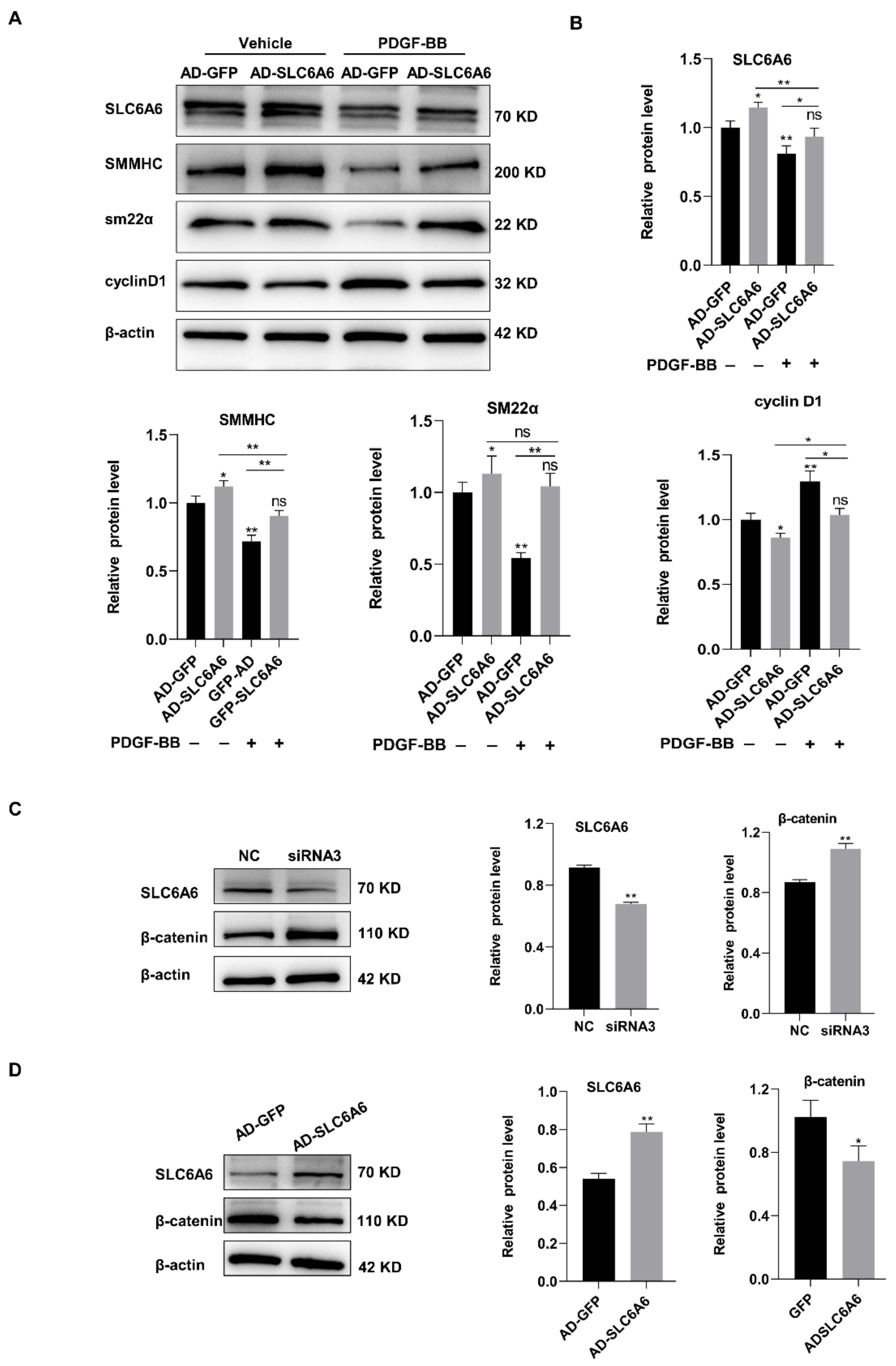
3.6. SLC6A6 Regulated the Functions of VSMCs via β-Catenin Pathway
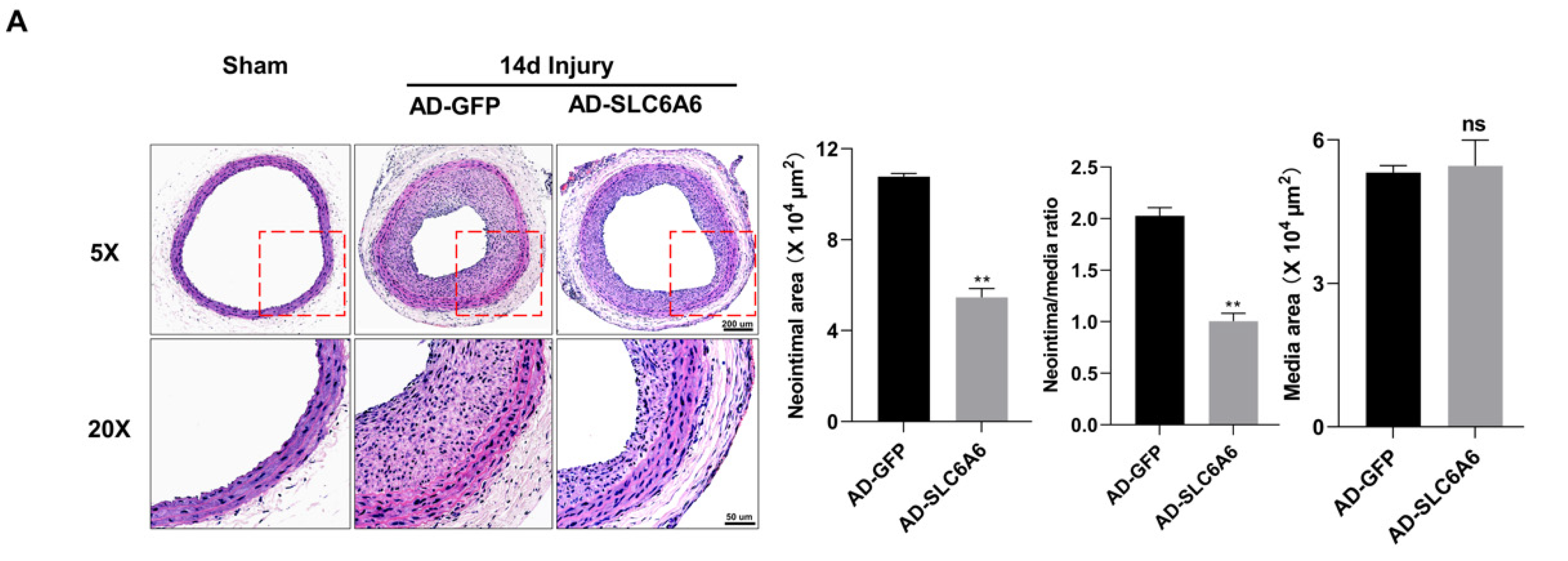
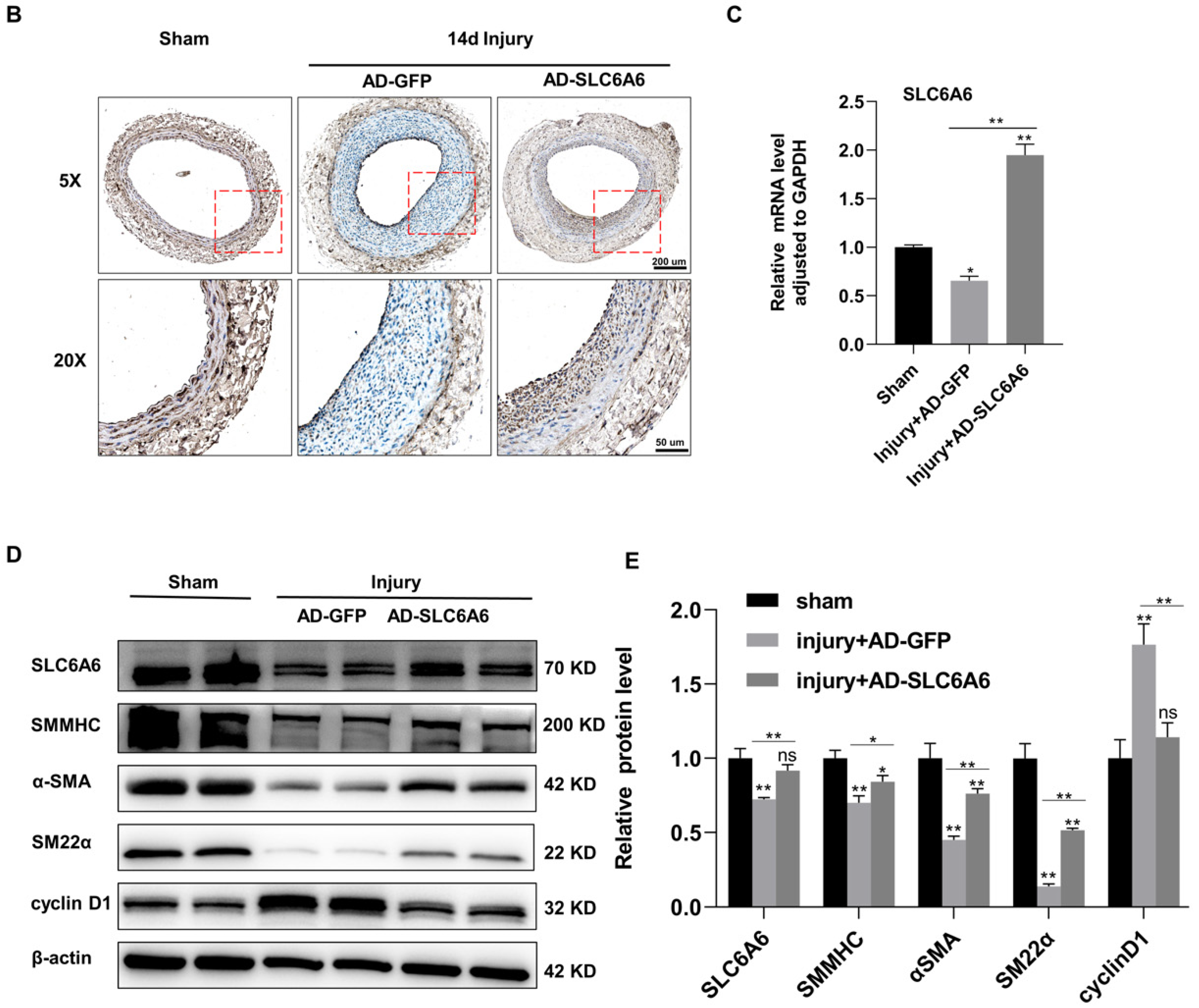
3.7. SLC6A6 Ameliorated Neointimal Formation after Vascular Injury
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Byrne, R.A.; Stone, G.W.; Ormiston, J.; Kastrati, A. Coronary balloon angioplasty, stents, and scaffolds. Lancet 2017, 390, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, C. Neointimal hyperplasia, vein graft remodeling, and long-term patency. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1194–H1195. [Google Scholar] [CrossRef] [PubMed]
- Rymer, J.A.; Jones, W.S. Femoropopliteal In-Stent Restenosis. Circ. Cardiovasc. Interv. 2018, 11, e007559. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Li, Y.; Zhou, Y.F.; Haslam, J.; Elvis, O.N.; Mao, L.; Xia, Y.P.; Hu, B. Semaphorin-3E attenuates neointimal formation via suppressing VSMCs migration and proliferation. Cardiovasc. Res. 2017, 113, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L.; et al. miR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases. Circ. Res. 2020, 126, e120–e135. [Google Scholar] [CrossRef]
- Chappell, J.; Harman, J.L.; Narasimhan, V.M.; Yu, H.; Foote, K.; Simons, B.D.; Bennett, M.R.; Jørgensen, H.F. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016, 119, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Suna, G.; Wojakowski, W.; Lynch, M.; Barallobre-Barreiro, J.; Yin, X.; Mayr, U.; Baig, F.; Lu, R.; Fava, M.; Hayward, R.; et al. Extracellular Matrix Proteomics Reveals Interplay of Aggrecan and Aggrecanases in Vascular Remodeling of Stented Coronary Arteries. Circulation 2018, 137, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yang, B.; Zhou, X.M.; Song, F.L.; Li, J.M.; Zhou, K.; Hu, W.; Peng, Y.Q.; Tang, S.Y.; Yuan, L.Q.; et al. L-carnitine and taurine synergistically inhibit the proliferation and osteoblastic differentiation of vascular smooth muscle cells. Acta Pharmacol. Sin. 2010, 31, 289–296. [Google Scholar] [PubMed]
- Desforges, M.; Parsons, L.; Westwood, M.; Sibley, C.P.; Greenwood, S.L. Taurine transport in human placental trophoblast is important for regulation of cell differentiation and survival. Cell Death Dis. 2013, 4, e559. [Google Scholar] [CrossRef]
- Liao, X.B.; Peng, Y.Q.; Zhou, X.M.; Yang, B.; Zheng, Z.; Liu, L.M.; Song, F.L.; Li, J.M.; Zhou, K.; Meng, J.C.; et al. Taurine restores Axl/Gas6 expression in vascular smooth muscle cell calcification model. Amino Acids 2010, 39, 375–383. [Google Scholar] [CrossRef]
- Warskulat, U.; Borsch, E.; Reinehr, R.; Heller-Stilb, B.; Roth, C.; Witt, M.; Häussinger, D. Taurine deficiency and apoptosis: Findings from the taurine transporter knockout mouse. Arch. Biochem. Biophys. 2007, 462, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, X.J.; Wu, K.C.; Zhang, C.J.; Zhou, G.H.; Lv, J.N.; Sun, L.F.; Cheng, F.F.; Cai, X.B.; Jin, Z.B. miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance. Proc. Natl. Acad. Sci. USA 2017, 114, 6376–6381. [Google Scholar] [CrossRef] [PubMed]
- Preising, M.N.; Görg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmüller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 11507–11527. [Google Scholar] [CrossRef]
- Ito, T.; Yamamoto, N.; Nakajima, S.; Schaffer, S.W. Beta-Catenin and SMAD3 Are Associated with Skeletal Muscle Aging in the Taurine Transpoeter Knockout Mouse. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 497–502. [Google Scholar] [PubMed]
- Han, X.; Patters, A.B.; Ito, T.; Azuma, J.; Schaffer, S.W.; Chesney, R.W. Knockout of the TauT gene predisposes C57BL/6 mice to streptozotocin-induced diabetic nephropathy. PLoS ONE 2015, 10, e0117718. [Google Scholar] [CrossRef]
- Qvartskhava, N.; Jin, C.J.; Buschmann, T.; Albrecht, U.; Bode, J.G.; Monhasery, N.; Oenarto, J.; Bidmon, H.J.; Görg, B.; Häussinger, D. Taurine transporter (TauT) deficiency impairs ammonia detoxification in mouse liver. Proc. Natl. Acad. Sci. USA 2019, 116, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Garnier, S.; Harakalova, M.; Weiss, S.; Mokry, M.; Regitz-Zagrosek, V.; Hengstenberg, C.; Cappola, T.P.; Isnard, R.; Arbustini, E.; Cook, S.A.; et al. Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23. Eur. Heart J. 2021, 42, 2000–2011. [Google Scholar] [CrossRef]
- Yang, Y.J.; Han, Y.Y.; Chen, K.; Zhang, Y.; Liu, X.; Li, S.; Wang, K.Q.; Ge, J.B.; Liu, W.; Zuo, J. TonEBP modulates the protective effect of taurine in ischemia-induced cytotoxicity in cardiomyocytes. Cell Death Dis. 2015, 6, e2025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ito, T.; Kimura, Y.; Uozumi, Y.; Takai, M.; Muraoka, S.; Matsuda, T.; Ueki, K.; Yoshiyama, M.; Ikawa, M.; Okabe, M.; et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J. Mol. Cell. Cardiol. 2008, 44, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Yang, J.; Feng, T.; Chen, Y.; Feng, R.; Wang, H.; Qian, Y. Taurine and its transporter TAUT positively affect male reproduction and early embryo development. Hum. Reprod. 2022, 37, 1229–1243. [Google Scholar] [CrossRef]
- Ramila, K.C.; Jong, C.J.; Pastukh, V.; Ito, T.; Azuma, J.; Schaffer, S.W. Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H232–H239. [Google Scholar] [CrossRef]
- Jong, C.J.; Ito, T.; Prentice, H.; Wu, J.Y.; Schaffer, S.W. Role of Mitochondria and Endoplasmic Reticulum in Taurine-Deficiency-Mediated Apoptosis. Nutrients 2017, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshikawa, N.; Inui, T.; Miyazaki, N.; Schaffer, S.W.; Azuma, J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS ONE 2014, 9, e107409. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Sakurai, T.; Toda, Y.; Morito, A.; Sakono, M.; Fukuda, N. Prevention of neointima formation by taurine ingestion after carotid balloon injury. Vasc. Pharmacol. 2010, 53, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Toda, Y.; Kitajima, H.; Oda, H.; Nagate, T.; Kameo, K.; Murakami, S. Taurine inhibits development of atherosclerotic lesions in apolipoprotein E-deficient mice. Clin. Exp. Pharmacol. Physiol. 2001, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.B.; Zhou, X.M.; Li, J.M.; Tan, Z.P.; Liu, L.M.; Zhang, W.; Tan, H.; Lu, Y.; Yuan, L.Q. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids 2007, 33, 639–643. [Google Scholar] [CrossRef]
- Rong, Z.H.; Chang, N.B.; Yao, Q.P.; Li, T.; Zhu, X.L.; Cao, Y.; Jiang, M.J.; Cheng, Y.S.; Jiang, R.; Jiang, J. Suppression of circDcbld1 Alleviates Intimal Hyperplasia in Rat Carotid Artery by Targeting miR-145-3p/Neuropilin-1. Mol. Ther. Nucleic Acids 2019, 18, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Taherabadi, S.J. The effects of exercise training on Kinesin and GAP-43 expression in skeletal muscle fibers of STZ-induced diabetic rats. Sci. Rep. 2021, 11, 9535. [Google Scholar] [CrossRef] [PubMed]
- Bostani, M.; Rahmati, M.; Mard, S.A. The effect of endurance training on levels of LINC complex proteins in skeletal muscle fibers of STZ-induced diabetic rats. Sci. Rep. 2020, 10, 8738. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, Z.; Ding, F.; He, Y.; Wang, P.; Liu, X.; Xu, F.; Wang, J.; Yang, Y. Taurine Transporter Regulates Adipogenic Differentiation of Human Adipose-Derived Stem Cells through Affecting Wnt/β-catenin Signaling Pathway. Int. J. Biol. Sci. 2019, 15, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Quasnichka, H.; Slater, S.C.; Beeching, C.A.; Boehm, M.; Sala-Newby, G.B.; George, S.J. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ. Res. 2006, 99, 1329–1337. [Google Scholar] [CrossRef]
- Jia, Y.; Mao, C.; Ma, Z.; Huang, J.; Li, W.; Ma, X.; Zhang, S.; Li, M.; Yu, F.; Sun, Y.; et al. PHB2 Maintains the Contractile Phenotype of VSMCs by Counteracting PKM2 Splicing. Circ. Res. 2022, 131, 807–824. [Google Scholar] [PubMed]
- Han, X.; Chesney, R.W. Stress-responsive gene TauT and acute kidney injury. J. Biomed. Sci. 2010, 17, S28. [Google Scholar] [CrossRef] [PubMed]
- Ando, D.; Kubo, Y.; Akanuma, S.; Yoneyama, D.; Tachikawa, M.; Hosoya, K. Function and regulation of taurine transport in Müller cells under osmotic stress. Neurochem. Int. 2012, 60, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Lari, R.; Anichini, R.; De Bellis, A.; Berti, A.; Napoli, Z.; Seghieri, G.; Franconi, F. Taurine transporter gene expression in peripheral mononuclear blood cells of type 2 diabetic patients. Amino Acids 2012, 42, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019, 21, 101063. [Google Scholar] [CrossRef]
- Mele, A.; Mantuano, P.; De Bellis, M.; Rana, F.; Sanarica, F.; Conte, E.; Morgese, M.G.; Bove, M.; Rolland, J.F.; Capogrosso, R.F.; et al. A long-term treatment with taurine prevents cardiac dysfunction in mdx mice. Transl. Res. J. Lab. Clin. Med. 2019, 204, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tenner, T.E., Jr.; Lombardini, J.B. Inhibition of rat vascular smooth muscle cell proliferation by taurine and taurine analogues. Biochem. Pharmacol. 1999, 57, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Kim, K.Y.; Lee, N.Y.; Kang, Y.S.; Hwang, Y.J.; Kim, Y.; Sung, J.J.; McKee, A.; Kowall, N.; Lee, J.; et al. Expression of taurine transporter (TauT) is modulated by heat shock factor 1 (HSF1) in motor neurons of ALS. Mol. Neurobiol. 2013, 47, 699–710. [Google Scholar] [CrossRef]
- Kempf, E.; Ebel, A.; Wendling, S.; Bollack, C.; Mandel, P. The distribution of free amino acids in arterial walls and their modifications during aging. Rev. Eur. D’etudes Clin. Biol. Eur. J. Clin. Biol. Res. 1970, 15, 857–861. [Google Scholar]
- An, W.; Luong, L.A.; Bowden, N.P.; Yang, M.; Wu, W.; Zhou, X.; Liu, C.; Niu, K.; Luo, J.; Zhang, C.; et al. Cezanne is a critical regulator of pathological arterial remodelling by targeting β-catenin signalling. Cardiovasc. Res. 2022, 118, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Mou, Y.; Zhao, Y.; Blankesteijn, W.M.; Hall, J.L. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ. Res. 2002, 90, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Cai, Z.; Chu, S.; Sun, Z.; Wang, X.; Hu, L.; Yi, J.; Shen, L.; He, B. Orphan Nuclear Receptor Nur77 Inhibits Angiotensin II-Induced Vascular Remodeling via Downregulation of β-Catenin. Hypertension 2016, 67, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, A.; Williams, H.; Lyon, C.A.; Taylor, V.; Swain, A.; Johnson, J.L.; George, S.J. Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ. Res. 2011, 108, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, Y.; Ito, T.; Hoshino, Y.; Mohri, T.; Maeda, M.; Takahashi, K.; Fujio, Y.; Azuma, J. Myogenic differentiation induces taurine transporter in association with taurine-mediated cytoprotection in skeletal muscles. Biochem. J. 2006, 394, 699–706. [Google Scholar] [CrossRef]
- Meng, L.; Lu, C.; Wu, B.; Lan, C.; Mo, L.; Chen, C.; Wang, X.; Zhang, N.; Lan, L.; Wang, Q.; et al. Taurine Antagonizes Macrophages M1 Polarization by Mitophagy-Glycolysis Switch Blockage via Dragging SAM-PP2Ac Transmethylation. Front. Immunol. 2021, 12, 648913. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; van Eys, G.; Bochaton-Piallat, M.L.; Proudfoot, D.; Biessen, E.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Z.; Li, F.; Zhang, R.; Niu, S.; Di, X.; Ni, L.; Liu, C. Ant-Neointimal Formation Effects of SLC6A6 in Preventing Vascular Smooth Muscle Cell Proliferation and Migration via Wnt/β-Catenin Signaling. Int. J. Mol. Sci. 2023, 24, 3018. https://doi.org/10.3390/ijms24033018
Rong Z, Li F, Zhang R, Niu S, Di X, Ni L, Liu C. Ant-Neointimal Formation Effects of SLC6A6 in Preventing Vascular Smooth Muscle Cell Proliferation and Migration via Wnt/β-Catenin Signaling. International Journal of Molecular Sciences. 2023; 24(3):3018. https://doi.org/10.3390/ijms24033018
Chicago/Turabian StyleRong, Zhihua, Fengshi Li, Rui Zhang, Shuai Niu, Xiao Di, Leng Ni, and Changwei Liu. 2023. "Ant-Neointimal Formation Effects of SLC6A6 in Preventing Vascular Smooth Muscle Cell Proliferation and Migration via Wnt/β-Catenin Signaling" International Journal of Molecular Sciences 24, no. 3: 3018. https://doi.org/10.3390/ijms24033018
APA StyleRong, Z., Li, F., Zhang, R., Niu, S., Di, X., Ni, L., & Liu, C. (2023). Ant-Neointimal Formation Effects of SLC6A6 in Preventing Vascular Smooth Muscle Cell Proliferation and Migration via Wnt/β-Catenin Signaling. International Journal of Molecular Sciences, 24(3), 3018. https://doi.org/10.3390/ijms24033018