Polyethylenimine-Crosslinked 3-Aminopropyltriethoxysilane-Grafted Multiwall Carbon Nanotubes for Efficient Adsorption of Reactive Yellow 2 from Water
Abstract
:1. Introduction
2. Results and Discussion
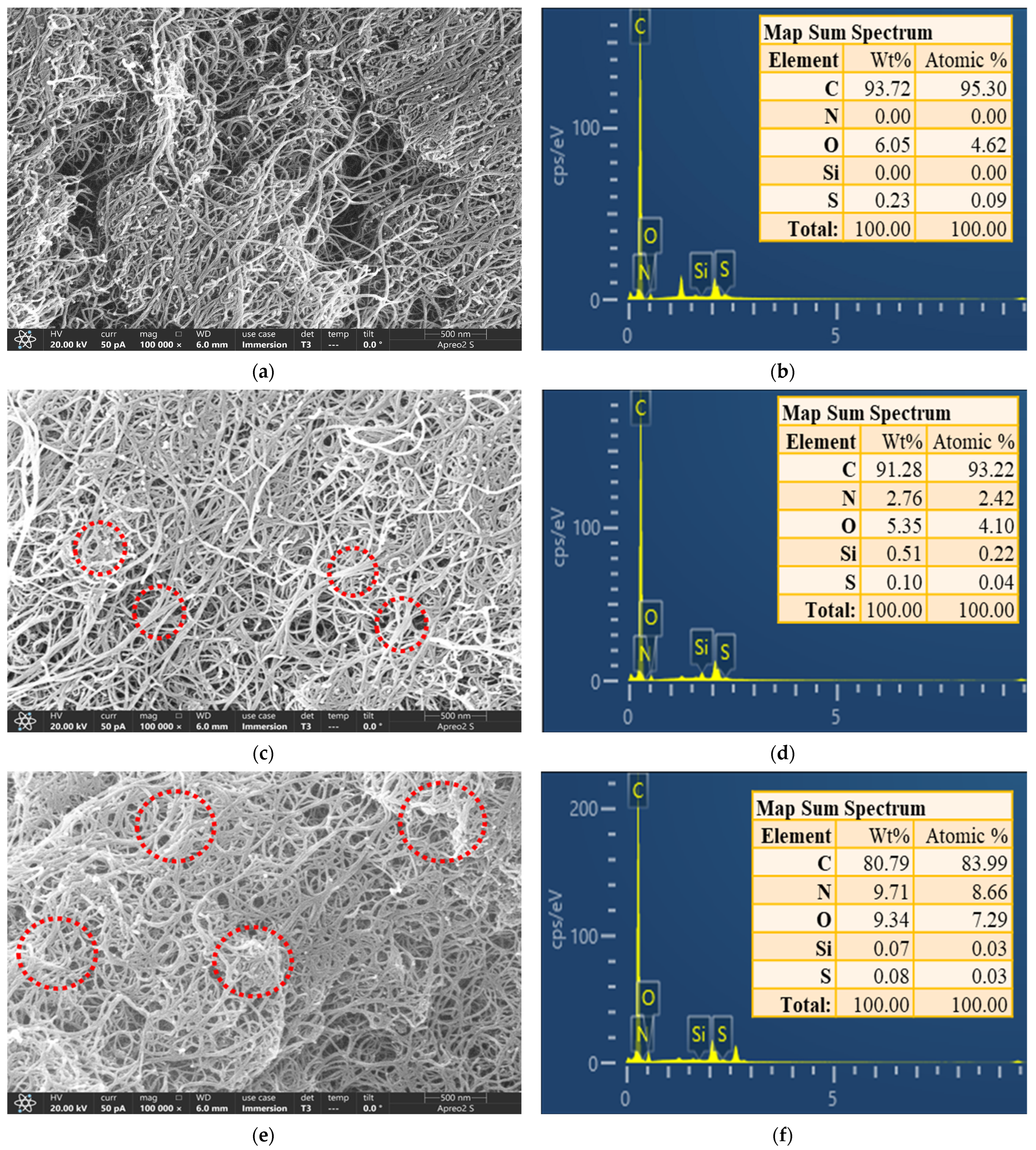
2.1. FE-SEM-EDS Analysis
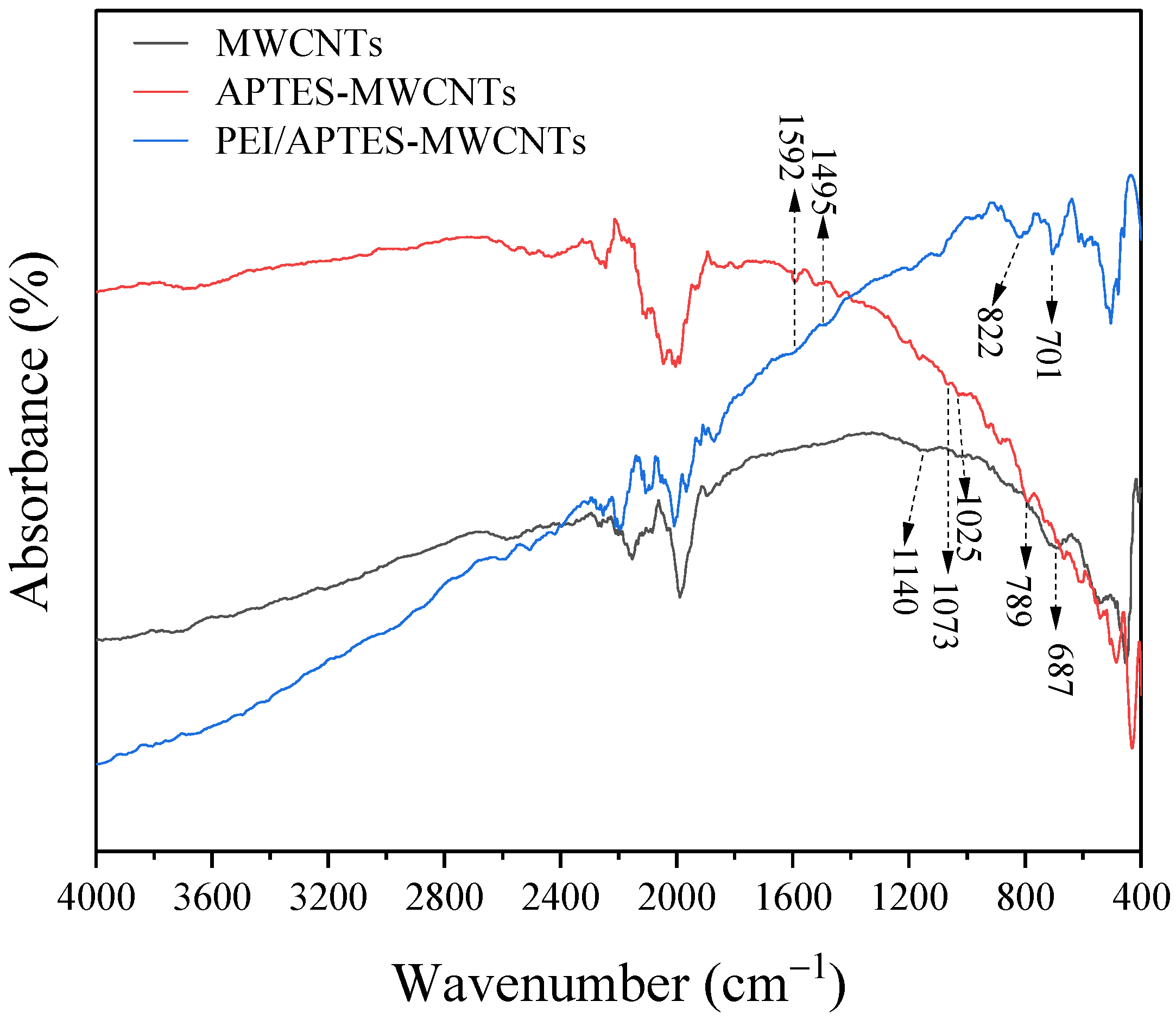
2.2. FTIR Analysis
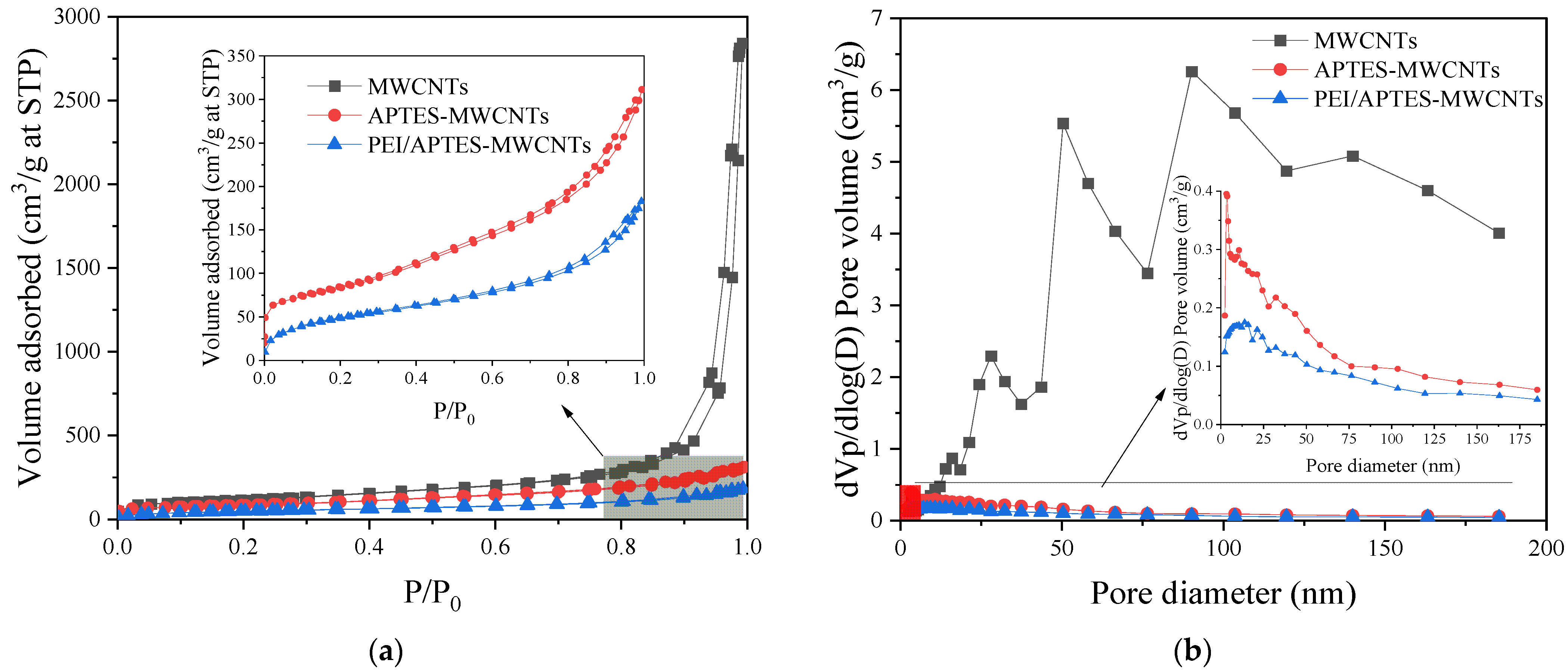
2.3. BET Analysis
2.4. Effect of pH
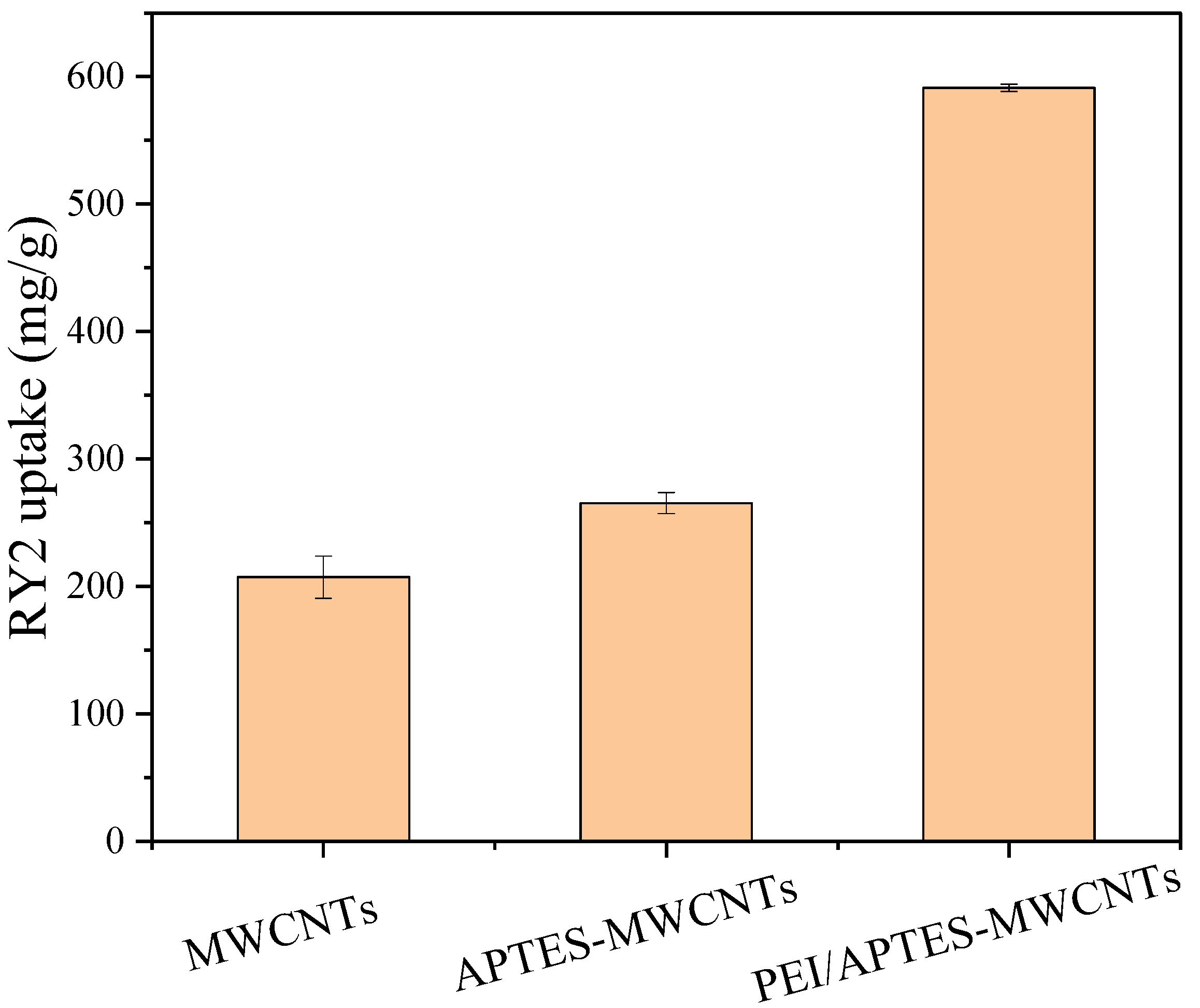
2.5. Adsorption Studies
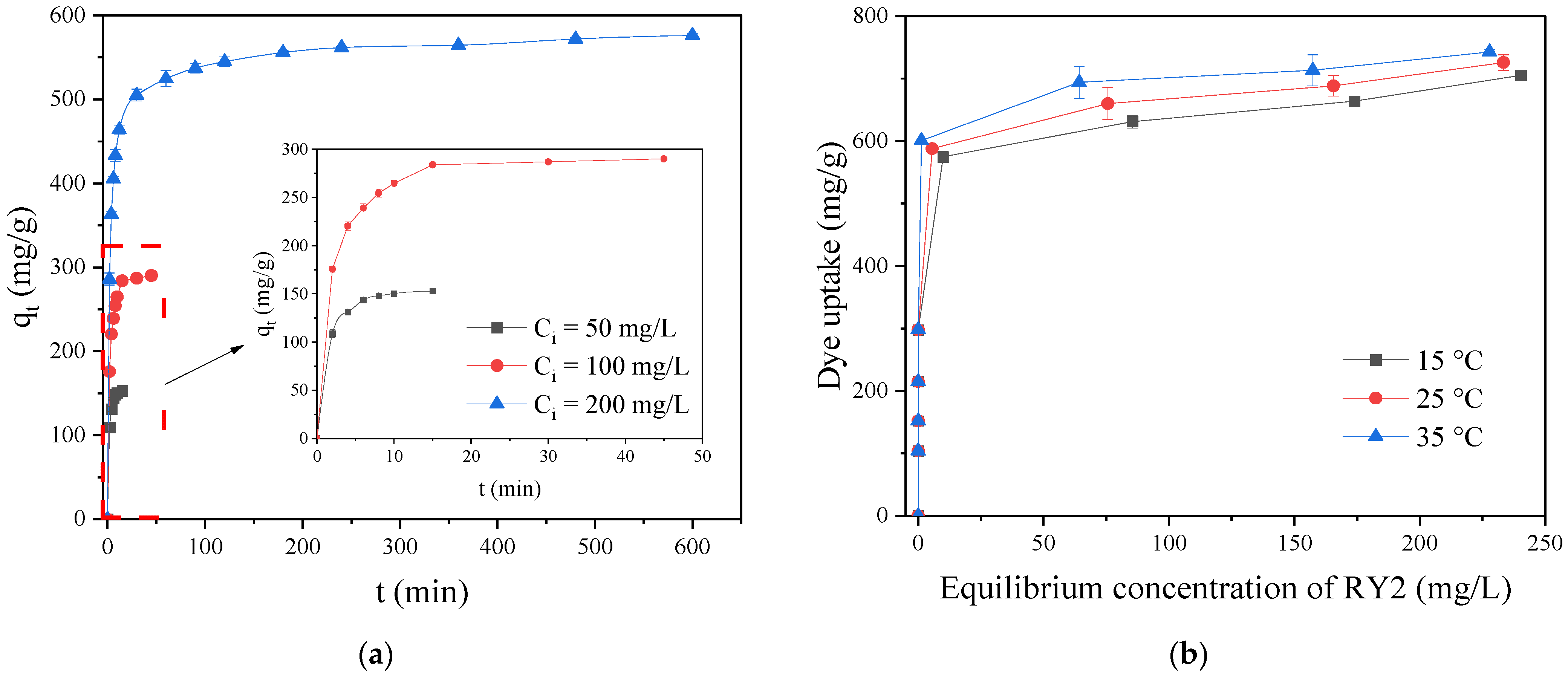
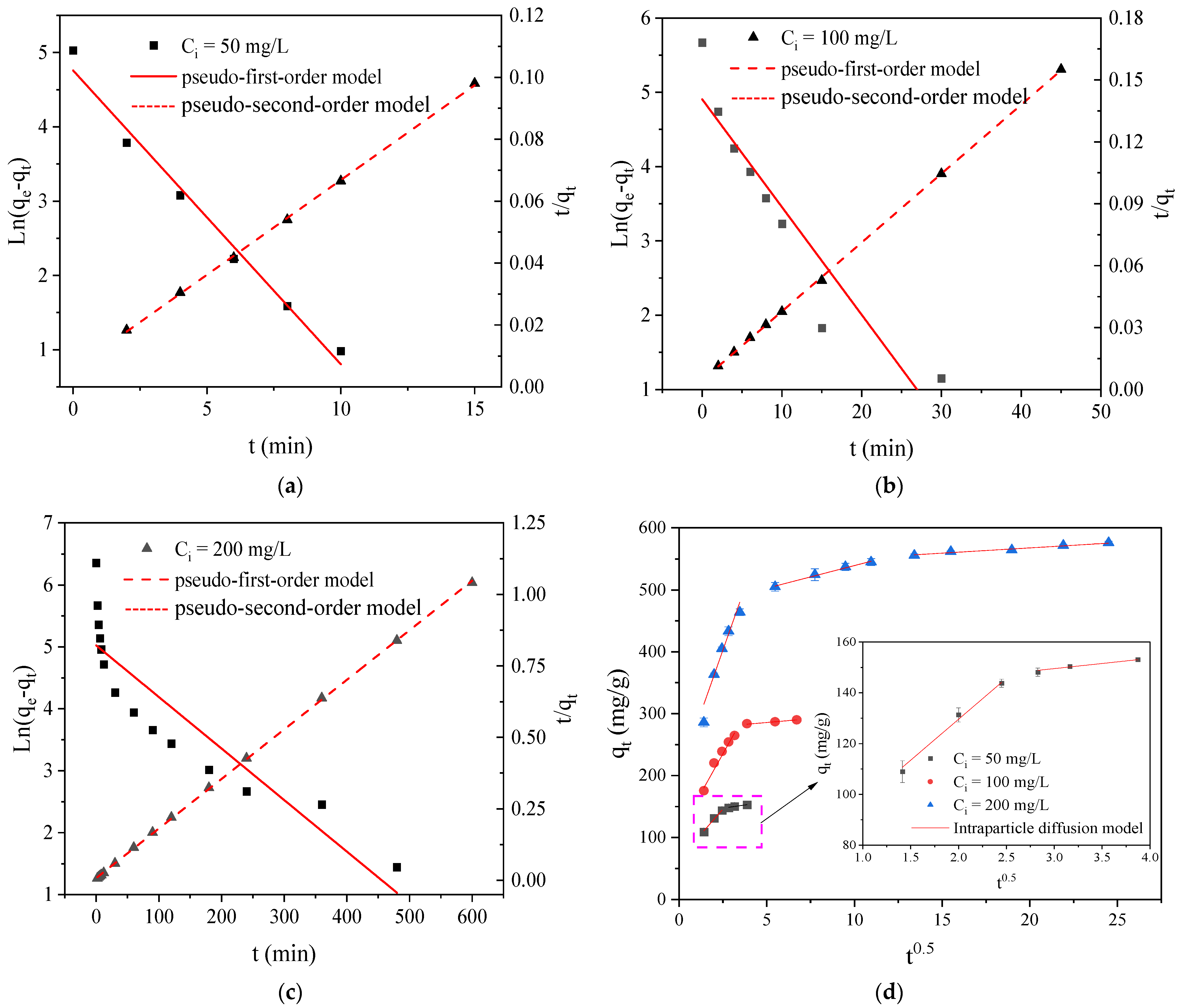
2.5.1. Adsorption Kinetics
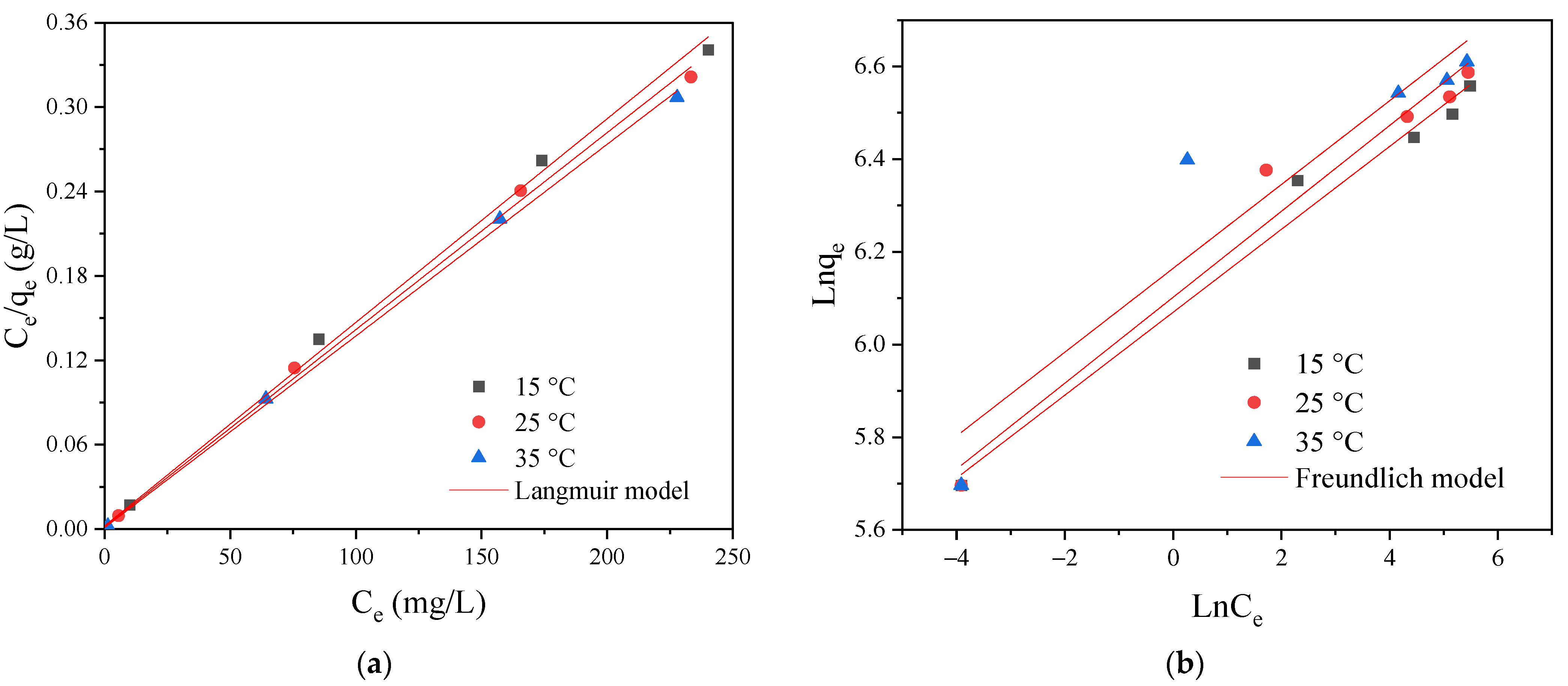
2.5.2. Adsorption Isotherms
2.5.3. Thermodynamic Analysis
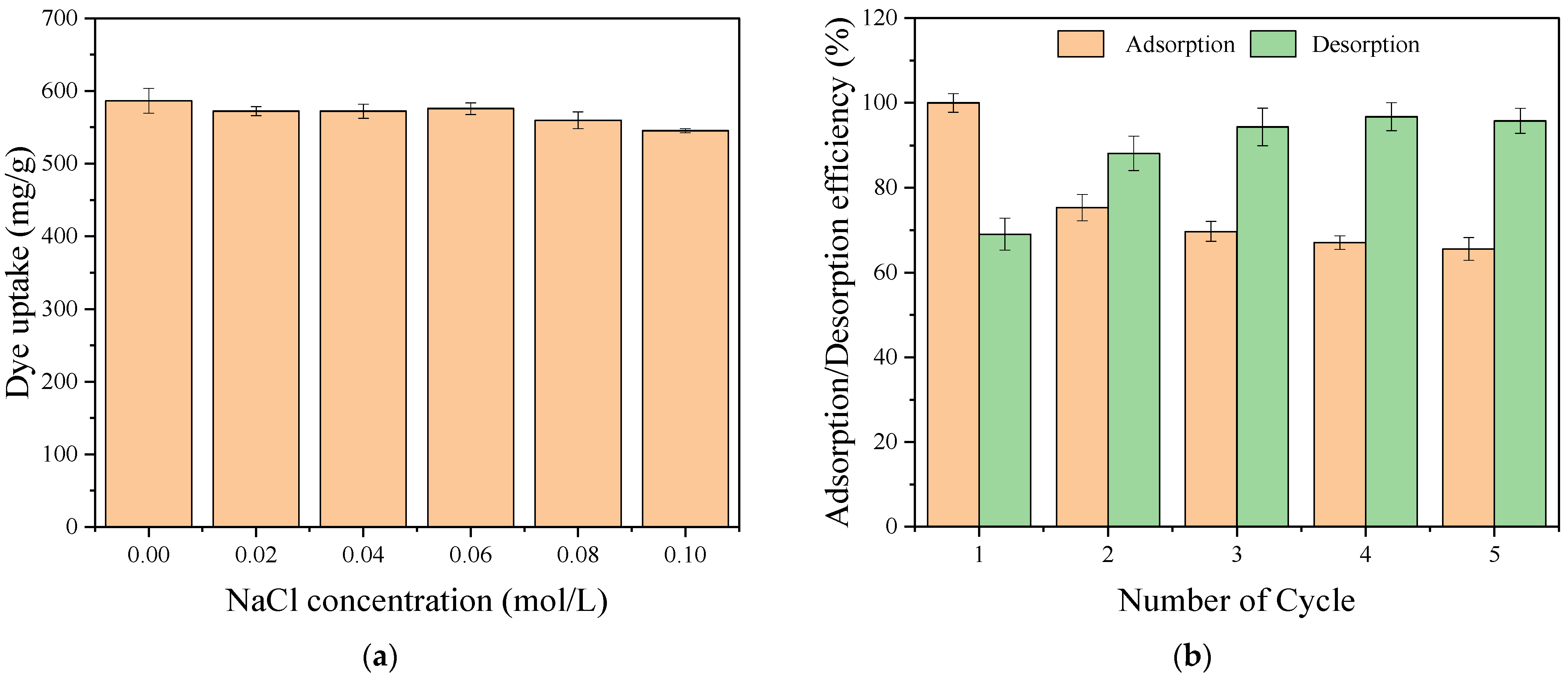
2.6. Effect of Ionic Strength
2.7. Reusability Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of PEI/APTES-MWCNTs
3.3. Characterization of Samples
3.4. Adsorption Experiments
3.5. Reusability Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Plessis, A.; du Plessis, A. Current and future water scarcity and stress. In Water as an Inescapable Risk: Current Global Water Availability, Quality and Risks with a Specific Focus on South Africa; Springer: Berlin/Heidelberg, Germany, 2019; pp. 13–25. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Angelakis, A.; Valipour, M.; Ahmed, A.; Tzanakakis, V.; Paranychianakis, N.; Krasilnikoff, J.; Drusiani, R.; Mays, L.; El Gohary, F.; Koutsoyiannis, D.; et al. Water Conflicts: From Ancient to Modern Times and in the Future. Sustainability 2021, 13, 4237. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Sun, S.; Tang, Y.; Yuan, X.; Tang, Q. Global assessment of future sectoral water scarcity under adaptive inner-basin water allocation measures. Sci. Total. Environ. 2021, 783, 146973. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.; Wang, J.; Tian, M.; Liu, S.; Quan, T.; Yang, L.; Wang, D.; Li, X.; Gao, D. A novel hydroxyl-riched covalent organic framework as an advanced adsorbent for the adsorption of anionic azo dyes. Anal. Chim. Acta 2022, 1227, 340329. [Google Scholar] [CrossRef]
- Munir, R.; Ali, K.; Naqvi, S.A.Z.; Maqsood, M.A.; Bashir, M.Z.; Noreen, S. Biosynthesis of Leucaena Leucocephala leaf mediated ZnO, CuO, MnO2, and MgO based nano-adsorbents for Reactive Golden Yellow-145 (RY-145) and Direct Red-31 (DR-31) dye removal from textile wastewater to reuse in agricultural purpose. Sep. Purif. Technol. 2023, 306, 122527. [Google Scholar] [CrossRef]
- Teshager, F.M.; Habtu, N.G.; Mequanint, K. A systematic study of cellulose-reactive anionic dye removal using a sustainable bioadsorbent. Chemosphere 2022, 303, 135024. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Azari, A.; Nabizadeh, R.; Nasseri, S.; Mahvi, A.H.; Mesdaghinia, A.R. Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 2020, 250, 126238. [Google Scholar] [CrossRef]
- Saxena, R.; Saxena, M.; Lochab, A. Recent Progress in Nanomaterials for Adsorptive Removal of Organic Contaminants from Wastewater. Chemistryselect 2020, 5, 335–353. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.; Jeevanantham, S.; Jawahar, M.J.; Neshaanthini, J. A review on synthesis methods and recent applications of nanomaterial in wastewater treatment: Challenges and future perspectives. Chemosphere 2022, 307, 135713. [Google Scholar] [CrossRef]
- Zhao, S.; Zhan, Y.; Wan, X.; He, S.; Yang, X.; Hu, J.; Zhang, G. Selective and efficient adsorption of anionic dyes by core/shell magnetic MWCNTs nano-hybrid constructed through facial polydopamine tailored graft polymerization: Insight of ad-sorption mechanism, kinetic, isotherm and thermodynamic study. J. Mol. Liq. 2020, 319, 114289. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Q.; Guo, J.; Li, H.; Wu, J.; Yang, X.; Sui, G. Effects of the amine/epoxy stoichiometry on the curing behavior and glass transition temperature of MWCNTs-NH 2/epoxy nanocomposites. Thermochim. Acta 2016, 639, 98–107. [Google Scholar] [CrossRef]
- Chu, Y.; Tang, D.; Ke, Z.; Ma, J.; Li, R. Polyethylenimine-functionalized multiwalled carbon nanotube for the adsorption of hydrogen sulfide. J. Appl. Polym. Sci. 2017, 134, 44742. [Google Scholar] [CrossRef]
- Saleh, T.A.; Elsharif, A.M.; Bin-Dahman, O.A. Synthesis of amine functionalization carbon nanotube-low symmetry porphyrin derivatives conjugates toward dye and metal ions removal. J. Mol. Liq. 2021, 340, 117024. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lin, W.-N.; Huang, Y.-L.; Tien, H.-W.; Wang, J.-Y.; Ma, C.-C.M.; Li, S.-M.; Wang, Y.-S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Kathi, J.; Rhee, K.Y. Surface modification of multi-walled carbon nanotubes using 3-aminopropyltriethoxysilane. J. Mater. Sci. 2007, 43, 33–37. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Jia, J.; Hou, C.; Hao, X.; Zhang, H. Preparation of hydroxyl and (3-aminopropyl)triethoxysilane functionalized multiwall carbon nanotubes for use as conductive fillers in the polyurethane composite. Polym. Compos. 2016, 39, 1212–1222. [Google Scholar] [CrossRef]
- Zhao, F.-Y.; An, Q.-F.; Ji, Y.-L.; Gao, C.-J. A novel type of polyelectrolyte complex/MWCNT hybrid nanofiltration membranes for water softening. J. Membr. Sci. 2015, 492, 412–421. [Google Scholar] [CrossRef]
- Wu, S.; Liu, P.; Tong, W.; Li, J.; Xu, G.; Teng, F.; Liu, J.; Feng, H.; Hu, R.; Yang, A.; et al. An ultra-sensitive core-sheath fiber strain sensor based on double strain layered structure with cracks and modified MWCNTs/silicone rubber for wearable medical electronics. Compos. Sci. Technol. 2023, 231, 109816. [Google Scholar] [CrossRef]
- Satyanarayana, N.; Xie, X.; Rambabu, B. Sol–gel synthesis and characterization of the Ag2O–SiO2 system. Mater. Sci. Eng. B 2000, 72, 7–12. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Zheng, S.; Ning, L.; Ye, Q.; Tao, M.; He, Y. Amine-functionalized low-cost industrial grade multi-walled carbon nanotubes for the capture of carbon dioxide. J. Energy Chem. 2014, 23, 111–118. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, Z.; Bin Kang, S.; Yun, H.J.; Won, S.W. Polyethylenimine-crosslinked chitin biosorbent for efficient recovery of Pd(II) from acidic solution: Characterization and adsorption mechanism. Carbohydr. Polym. Technol. Appl. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Xu, T.; Qu, R.; Zhang, Y.; Sun, C.; Wang, Y.; Kong, X.; Geng, X.; Ji, C. Preparation of bifunctional polysilsesquioxane/carbon nanotube magnetic composites and their adsorption properties for Au (III). Chem. Eng. J. 2020, 410, 128225. [Google Scholar] [CrossRef]
- Khakpour, R.; Tahermansouri, H. Synthesis, characterization and study of sorption parameters of multi-walled carbon nanotubes/chitosan nanocomposite for the removal of picric acid from aqueous solutions. Int. J. Biol. Macromol. 2018, 109, 598–610. [Google Scholar] [CrossRef]
- Hamza, M.F.; Lu, S.M.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.Z.; Vincent, T.; Guibal, E. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci. Total. Environ. 2020, 719, 137396. [Google Scholar] [CrossRef]
- Wang, Z.; Bin Kang, S.; Won, S.W. Polyethylenimine-aminated polyvinyl chloride fiber for adsorption of reactive dyes from single and binary component systems: Adsorption kinetics and isotherm studies. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 128983. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelfattah, A.M.; Tharwat, R.M.; Nabil, G.M. Adsorption of negatively charged food tartrazine and sunset yellow dyes onto positively charged triethylenetetramine biochar: Optimization, kinetics and thermodynamic study. J. Mol. Liq. 2020, 318, 114297. [Google Scholar] [CrossRef]
- Gao, M.; Xu, D.; Gao, Y.; Chen, G.; Zhai, R.; Huang, X.; Xu, X.; Wang, J.; Yang, X.; Liu, G. Mussel-inspired triple bionic adsorbent: Facile preparation of layered double hydroxide@polydopamine@metal-polyphenol networks and their selective adsorption of dyes in single and binary systems. J. Hazard. Mater. 2021, 420, 126609. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Alves, D.C.; Gonçalves, J.O.; Coseglio, B.B.; Burgo, T.A.; Dotto, G.L.; Pinto, L.A.; Cadaval, T.R. Adsorption of phenol onto chitosan hydrogel scaffold modified with carbon nanotubes. J. Environ. Chem. Eng. 2019, 7, 103460. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Safari, G.H.; Sharafi, K.; Kamani, H.; Jaafari, J. Adsorption of 4-Nitrophenol on calcium alginate-multiwall carbon nanotube beads: Modeling, kinetics, equilibriums and reusability studies. Int. J. Biol. Macromol. 2021, 185, 66–76. [Google Scholar] [CrossRef]
- Marrakchi, F.; Hameed, B.; Bouaziz, M. Mesoporous and high-surface-area activated carbon from defatted olive cake by-products of olive mills for the adsorption kinetics and isotherm of methylene blue and acid blue 29. J. Environ. Chem. Eng. 2020, 8, 104199. [Google Scholar] [CrossRef]
- Ofomaja, A.; Naidoo, E.B.; Modise, S.J. Kinetic and Pseudo-Second-Order Modeling of Lead Biosorption onto Pine Cone Powder. Ind. Eng. Chem. Res. 2010, 49, 2562–2572. [Google Scholar] [CrossRef]
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced removal of Cd2+ from water by AHP-pretreated biochar: Adsorption performance and mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Pan, D.; Wu, Y.; Ji, R.; Li, W.; Jiang, X.; Han, J. One-pot pyrolysis of a typical invasive plant into nitro-gen-doped biochars for efficient sorption of phthalate esters from aqueous solution. Chemosphere 2021, 280, 130712. [Google Scholar] [CrossRef]
- Birtane, H.; Urucu, O.A.; Yıldız, N.; Çiğil, A.B.; Kahraman, M.V. Statistical optimization and selective uptake of Au(III) from aqueous solution using carbon nanotube-cellulose based adsorbent. Mater. Today Commun. 2022, 30, 103144. [Google Scholar] [CrossRef]
- Mohammadi, A.; Veisi, P. High adsorption performance of β-cyclodextrin-functionalized multi-walled carbon nanotubes for the removal of organic dyes from water and industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 4634–4643. [Google Scholar] [CrossRef]
- Maleki, A.; Hamesadeghi, U.; Daraei, H.; Hayati, B.; Najafi, F.; McKay, G.; Rezaee, R. Amine functionalized multi-walled carbon nanotubes: Single and binary systems for high capacity dye removal. Chem. Eng. J. 2017, 313, 826–835. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, G.; Luo, C.; Zhang, L.; Guo, Y.; Yan, S. Synthesis, characterization and application of amino-functionalized multi-walled carbon nanotubes for effective fast removal of methyl orange from aqueous solution. RSC Adv. 2014, 4, 55162–55172. [Google Scholar] [CrossRef]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A. Fabrication and investigation of MnFe2O4/MWCNTs nanocomposite by hydro-thermal technique and adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2017, 419, 70–83. [Google Scholar] [CrossRef]
- Athari, M.; Fattahi, M.; Khosravi-Nikou, M.; Hajhariri, A. Adsorption of different anionic and cationic dyes by hybrid nanocomposites of carbon nanotube and graphene materials over UiO-66. Sci. Rep. 2022, 12, 20415. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van't Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2018, 273, 425–434. [Google Scholar] [CrossRef]
- Haq, F.; Farid, A.; Ullah, N.; Kiran, M.; Khan, R.U.; Aziz, T.; Mehmood, S.; Haroon, M.; Mubashir, M.; Bokhari, A.; et al. A study on the uptake of methylene blue by biodegradable and eco-friendly carboxylated starch grafted polyvinyl pyrrolidone. Environ. Res. 2022, 215, 114241. [Google Scholar] [CrossRef]
- Alaguprathana, M.; Poonkothai, M.; Ameen, F.; Bhat, S.A.; Mythili, R.; Sudhakar, C. Sodium hydroxide pre-treated Aspergillus flavus biomass for the removal of reactive black 5 and its toxicity evaluation. Environ. Res. 2022, 214, 113859. [Google Scholar] [CrossRef]
- Liu, Z.; Qiang, R.; Lin, L.; Deng, X.; Yang, X.; Zhao, K.; Yang, J.; Li, X.; Ma, W.; Xu, M. Thermally modified polyimide/SiO2 nanofiltration membrane with high permeance and selectivity for efficient dye/salt separation. J. Membr. Sci. 2022, 658, 120747. [Google Scholar] [CrossRef]
- Mostovoy, A.; Yakovlev, A.; Tseluikin, V.; Lopukhova, M. Epoxy Nanocomposites Reinforced with Functionalized Carbon Nanotubes. Polymers 2020, 12, 1816. [Google Scholar] [CrossRef] [PubMed]

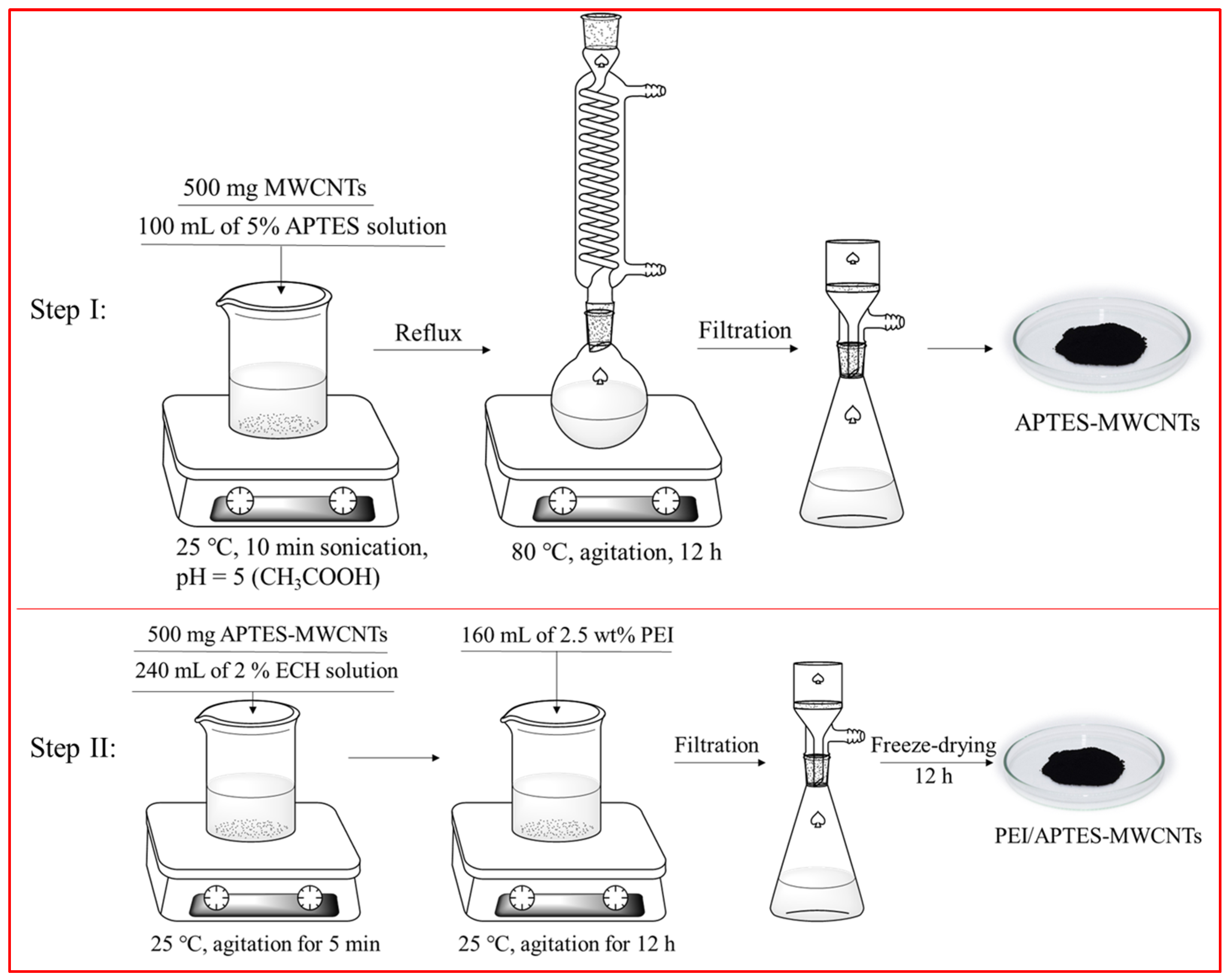
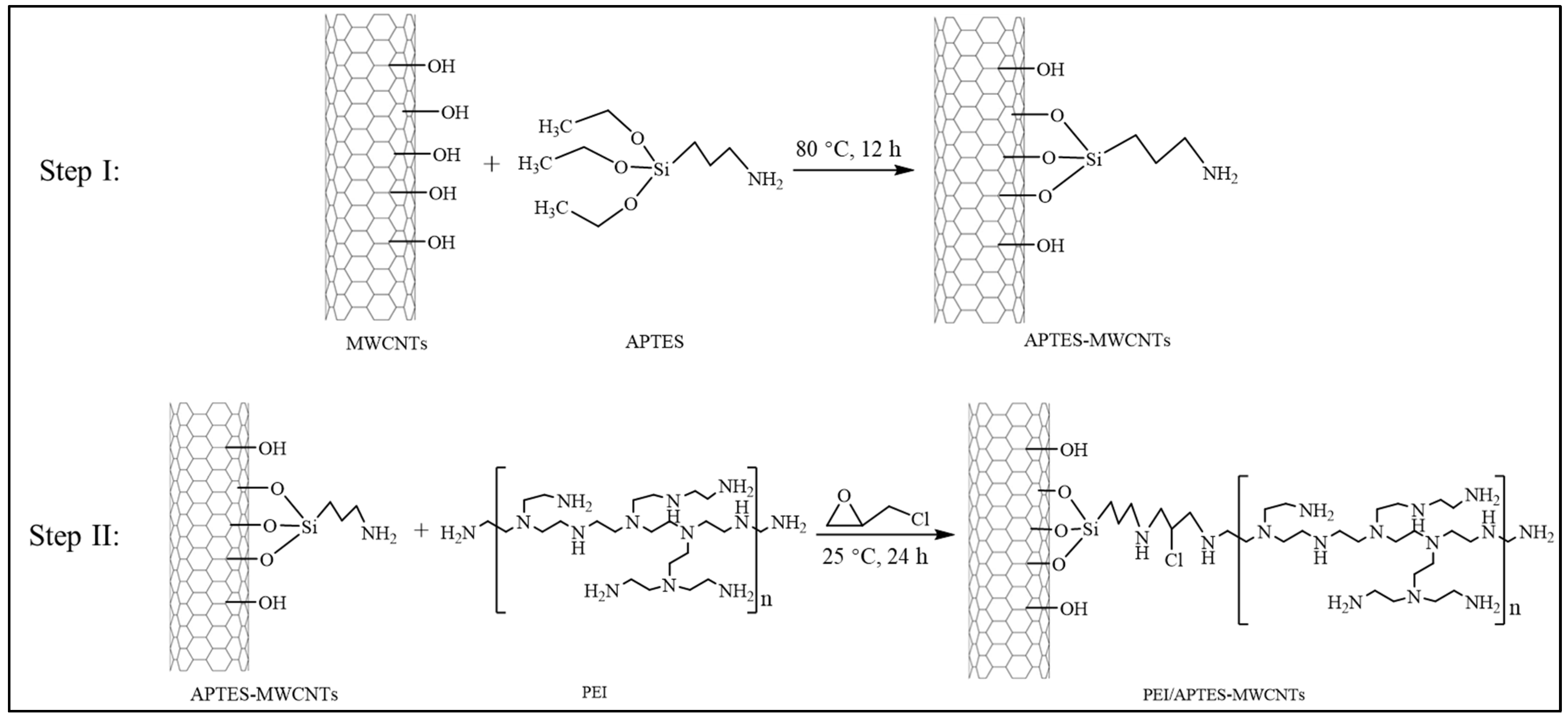
| Sample | Specific Surface Area (m2/g) | Average Pore Size (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| MWCNTs | 405.22 | 39.67 | 4.02 |
| APTES-MWCNTs | 289.65 | 6.50 | 0.47 |
| PEI/APTES-MWCNTs | 176.16 | 6.30 | 0.28 |
| Models | Parameters | Initial Concentration (mg/L) | ||
|---|---|---|---|---|
| 50 | 100 | 200 | ||
| qe,exp (mg/g) | 153.03 | 290.10 | 576.15 | |
| PFO | q1 (mg/g) | 116.68 | 135.06 | 152.11 |
| k1 (min−1) | 0.3955 | 0.1450 | 0.0083 | |
| Adj. R2 | 0.9804 | 0.8583 | 0.5054 | |
| PSO | q2 (mg/g) | 163.40 | 299.40 | 578.03 |
| k2 (g/(mg·min)) | 0.0057 | 0.0024 | 0.0004 | |
| Adj. R2 | 0.9995 | 0.9998 | 0.9999 | |
| IPD1st | ki1 (mg/(g·min0.5)) | 32.269 | 50.753 | 80.348 |
| Ci1 (mg/g) | 65.06 | 108.30 | 201.75 | |
| Adj. R2 | 0.9822 | 0.9686 | 0.9239 | |
| IPD2st | ki2 (mg/(g·min0.5)) | 4.028 | 2.478 | 7.330 |
| Ci2 (mg/g) | 137.43 | 273.47 | 466.17 | |
| Adj. R2 | 0.9781 | 0.9915 | 0.9852 | |
| IPD3st | ki3 (mg/(g·min0.5)) | — | — | 1.700 |
| Ci3 (mg/g) | 534.05 | |||
| Adj. R2 | 0.9368 | |||
| T (°C) | qexp (mg/g) | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | RL | Adj. R2 | KF (mg/g)(L/mg)1/n | n | Adj. R2 | ||
| 15 | 705.10 | 689.66 | 0.6444 | 0.0001–0.0008 | 0.9977 | 432.49 | 11.17 | 0.9776 |
| 25 | 725.84 | 714.29 | 0.8333 | 0.9984 | 446.71 | 10.79 | 0.9570 | |
| 35 | 742.43 | 735.30 | 1.3892 | 0.9992 | 475.49 | 11.05 | 0.8580 | |
| Adsorbent | Dye | qm (mg/g) | T (°C) | pH | Ref. |
|---|---|---|---|---|---|
| PEI/APTES-MWCNTs | Reactive Yellow 2 | 689.66 | 15 | 2 | This work |
| 714.29 | 25 | 2 | |||
| 735.29 | 35 | 2 | |||
| MWCNTs/Gly/β-CD | Acid Blue 113 | 172.41 | 25 | 7 | [43] |
| Methyl Orange | 96.15 | 25 | 5 | ||
| Disperse Red 1 | 500 | 25 | 7 | ||
| MWCNTs-NH2 | Acid Black 1 | 666 | 25 | 2 | [44] |
| Acid Black 25 | 714 | 25 | 2 | ||
| NH2-MWCNTs | Methyl Orange | 185.53 | 25 | 2 | [45] |
| MnFe2O4/MWCNTs | Direct Red 16 | 607.79 | 55 | 2 | [46] |
| MWCNTs-UiO-66 | Methyl Red | 105.26 | 25 | 3.61 | [47] |
| MWCNTs/Fe3O4@(PDA + PEI) | Methyl Orange | 935 | 25 | 7 | [14] |
| Congo Red | 1006 | 25 | 7 |
| T (°C) | Ke | ΔG (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol·K) | Adj. R2 |
|---|---|---|---|---|---|
| 15 | 5.36 × 105 | −31.58 | 28.21 | 207.27 | 0.9147 |
| 25 | 6.92 × 105 | −33.32 | |||
| 35 | 11.54 × 105 | −35.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Won, S.W. Polyethylenimine-Crosslinked 3-Aminopropyltriethoxysilane-Grafted Multiwall Carbon Nanotubes for Efficient Adsorption of Reactive Yellow 2 from Water. Int. J. Mol. Sci. 2023, 24, 2954. https://doi.org/10.3390/ijms24032954
Wang Z, Won SW. Polyethylenimine-Crosslinked 3-Aminopropyltriethoxysilane-Grafted Multiwall Carbon Nanotubes for Efficient Adsorption of Reactive Yellow 2 from Water. International Journal of Molecular Sciences. 2023; 24(3):2954. https://doi.org/10.3390/ijms24032954
Chicago/Turabian StyleWang, Zhuo, and Sung Wook Won. 2023. "Polyethylenimine-Crosslinked 3-Aminopropyltriethoxysilane-Grafted Multiwall Carbon Nanotubes for Efficient Adsorption of Reactive Yellow 2 from Water" International Journal of Molecular Sciences 24, no. 3: 2954. https://doi.org/10.3390/ijms24032954
APA StyleWang, Z., & Won, S. W. (2023). Polyethylenimine-Crosslinked 3-Aminopropyltriethoxysilane-Grafted Multiwall Carbon Nanotubes for Efficient Adsorption of Reactive Yellow 2 from Water. International Journal of Molecular Sciences, 24(3), 2954. https://doi.org/10.3390/ijms24032954








