Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation
Abstract
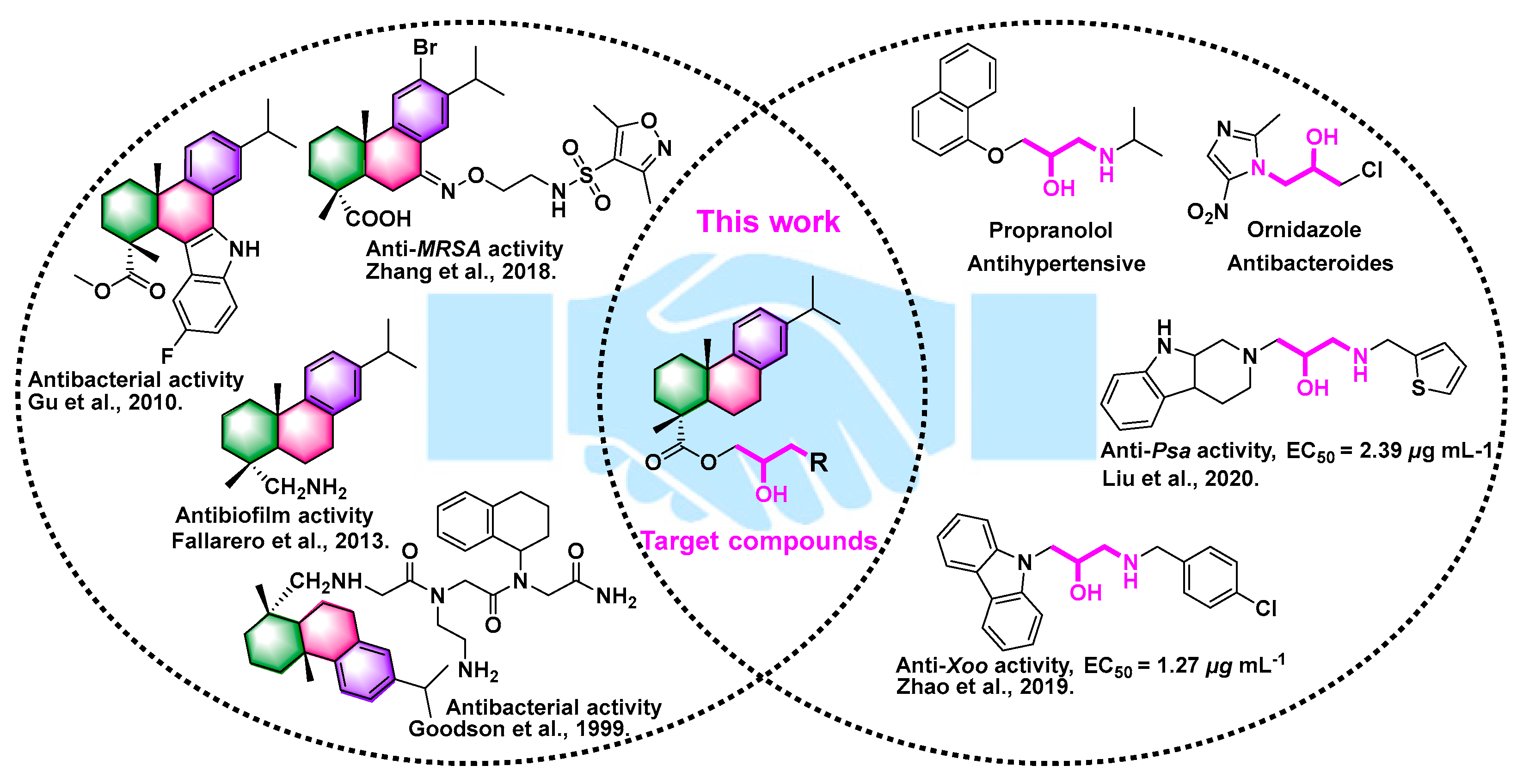
1. Introduction
2. Results and Discussion
2.1. Synthesis of DAA Derivatives
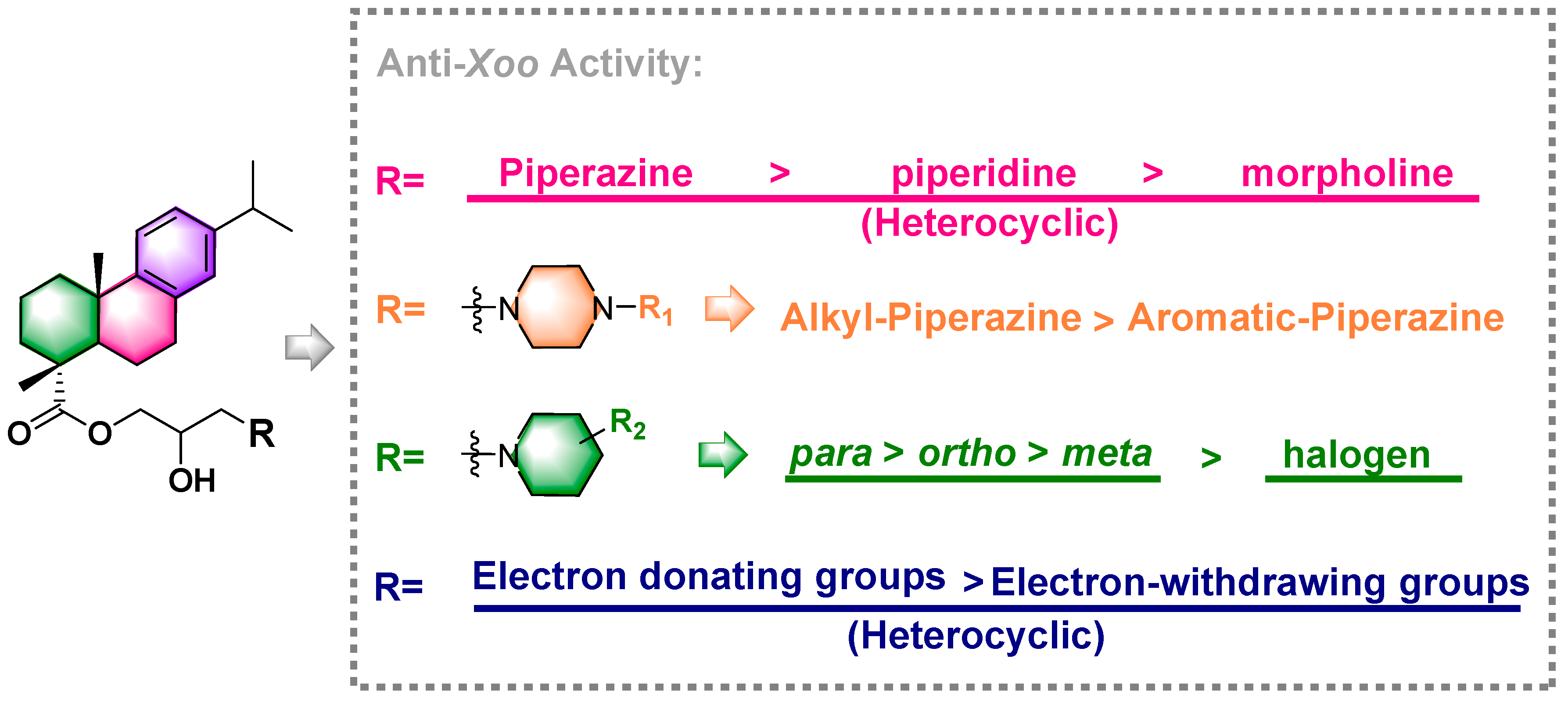
2.2. Antibacterial Activities Evaluation of Target Compounds
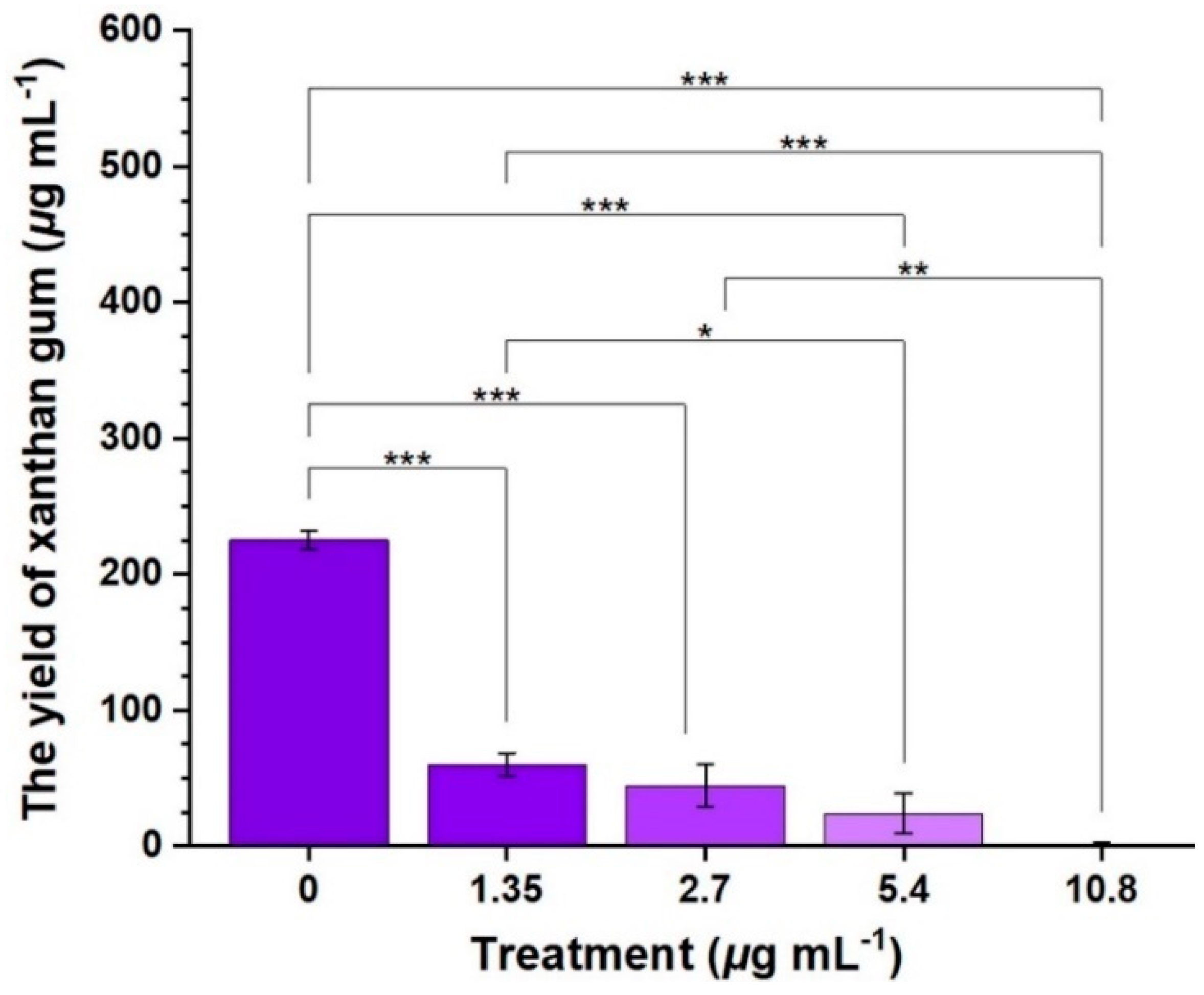
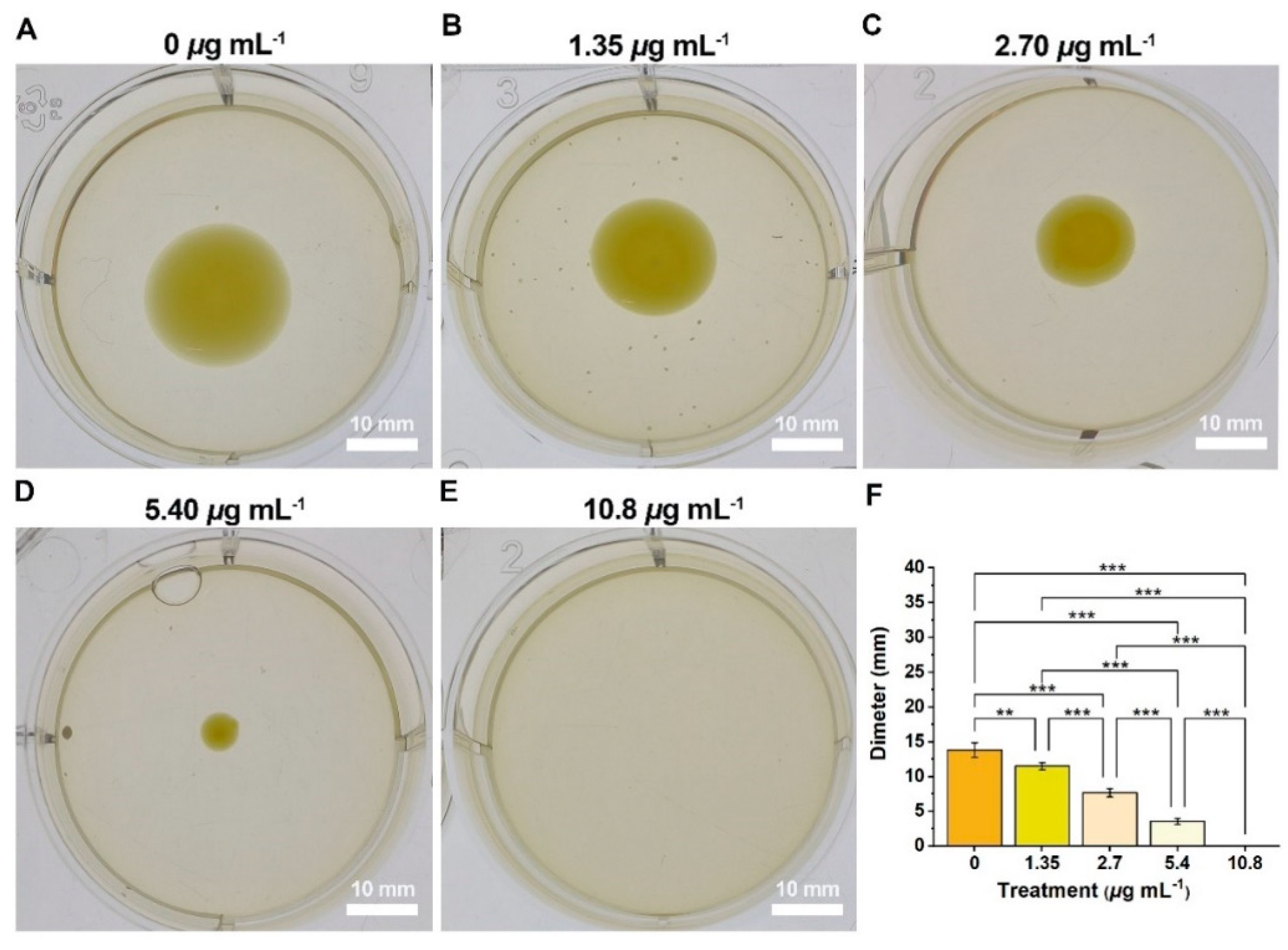
2.3. Inhibitory Effects of Compound 2b on the Xoo-Biofilm Formation and EPS Production
2.4. The Inhibition Effect of Swimming Motility
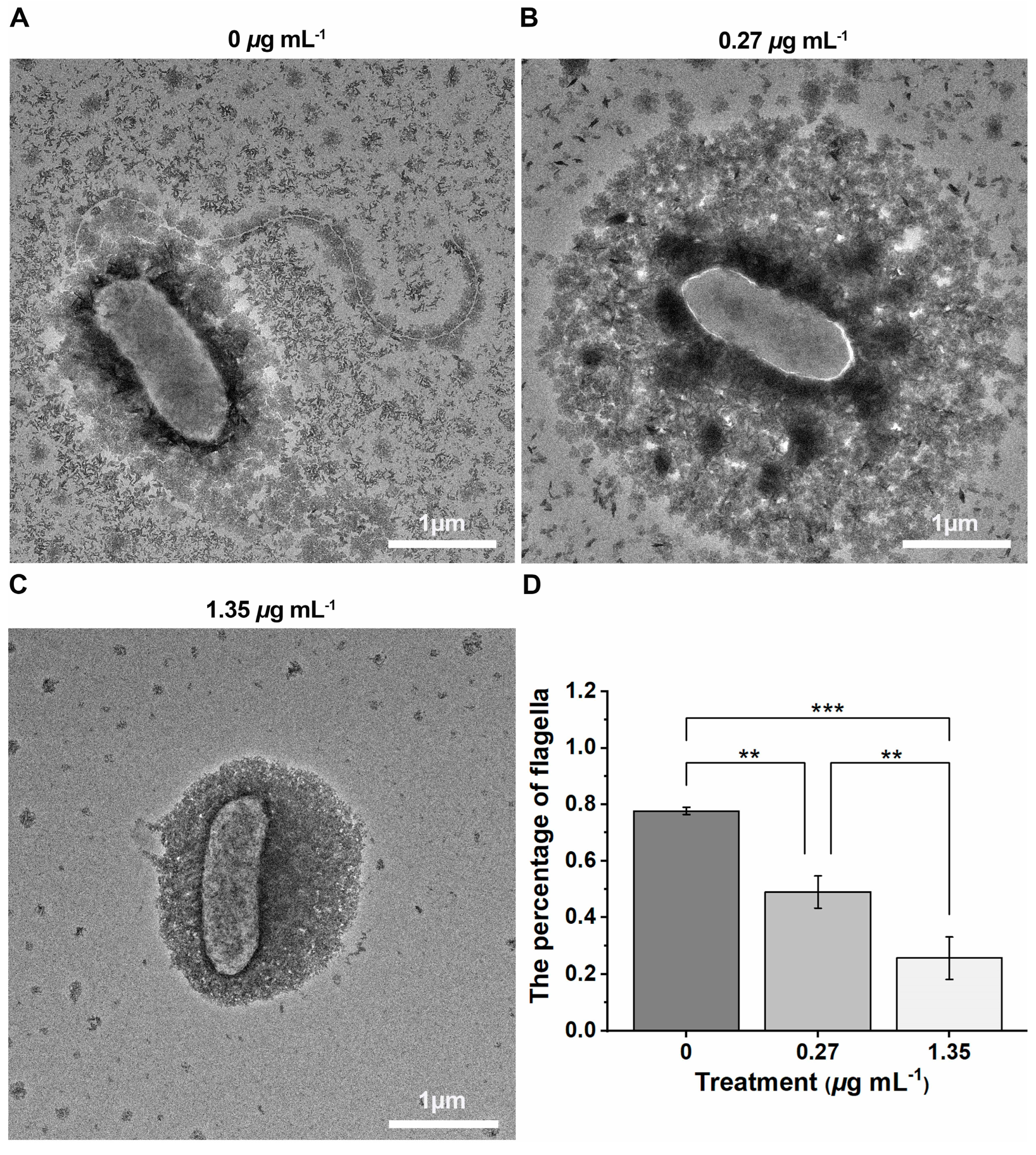
2.5. The Inhibition Effect of Xoo-Flagellum Assembly
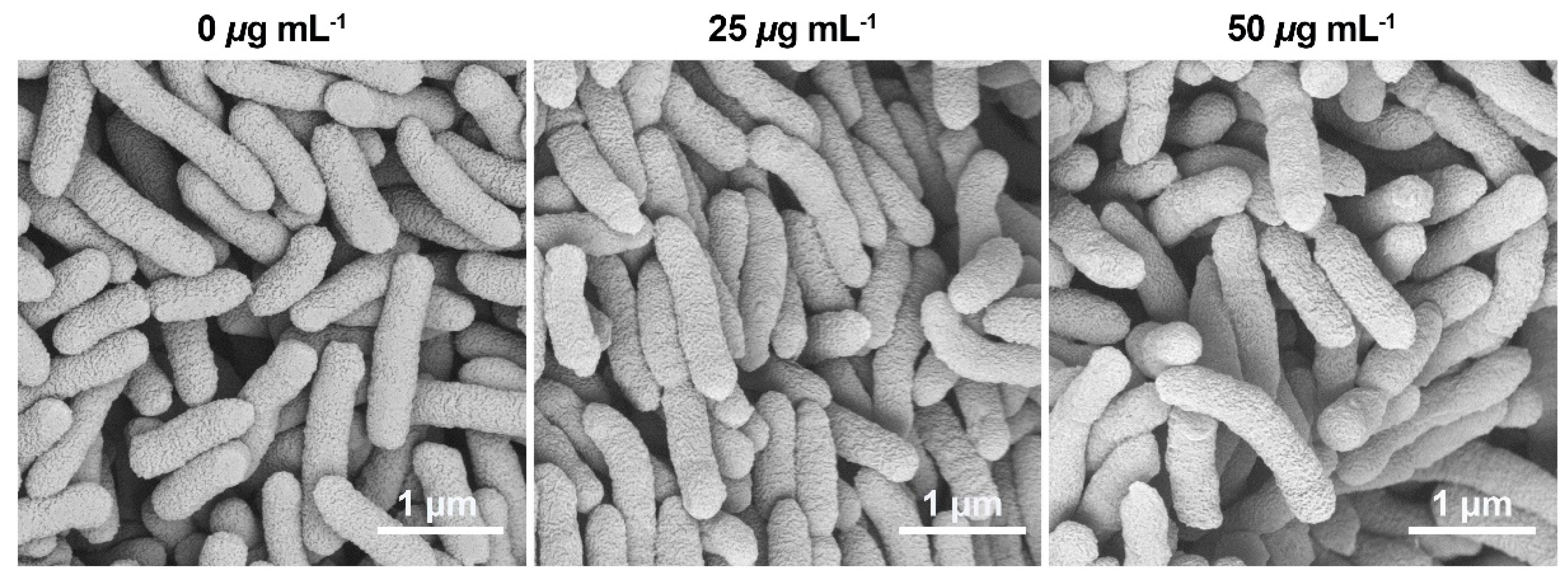
2.6. Cell Membrane Morphology Analysis
2.7. Pathogenicity of the Xoo Interacted with Compound 2b
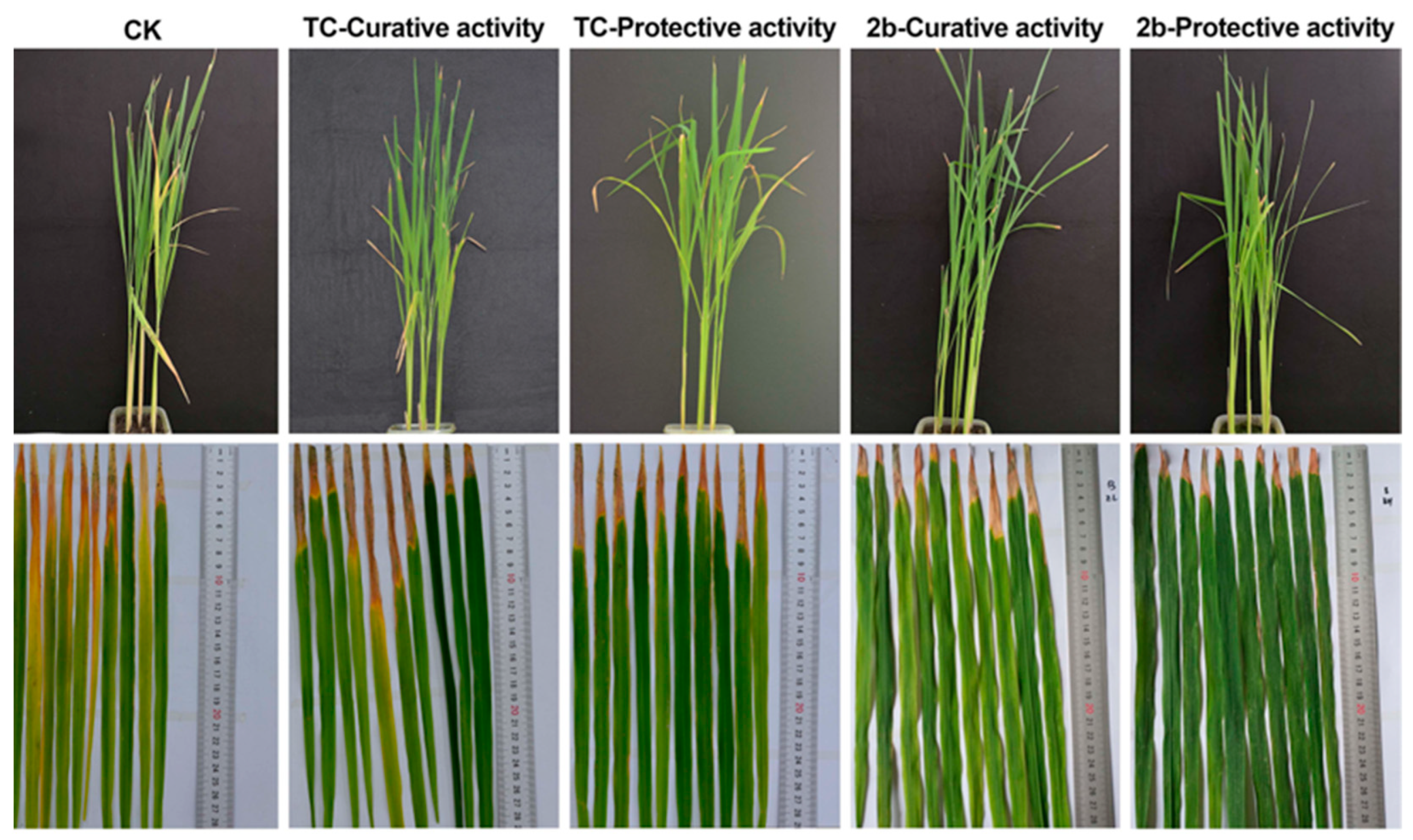
2.8. In Vivo Anti-Xoo Effect of Compound 2b Controlling Bacterial Disease at 200 μg mL−1
2.9. The Toxicity Evaluation of Compound 2b on Rice Leaves at 0, 200, and 500 μg mL−1
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Antibacterial Activity Evaluation In Vitro and In Vivo
3.3. Xoo-Biofilm Formation and EPS Production Analysis
3.4. Swimming Motility Assay
3.5. Morphology Observation of TEM
3.6. Morphology Observation of Scanning Electron Microscope (SEM)
3.7. Pathogenicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dehydroabietic acid | DAA |
| Xanthomonas oryzae pv. oryzae | Xoo |
| Xanthomonas. axonopodis pv citri | Xac |
| Pseudomonas syringae pv. actinidiae | Psa |
| Bacterial leaf blight | BLB |
| Extracellular polysaccharide | EPS |
| Virulence factors | VFs |
| Effective concentration for 50% of maximal effect | EC50 |
| Thiodiazole copper | TC |
References
- Doucoure, H.; Perez-Quintero, A.L.; Reshetnyak, G.; Tekete, C.; Auguy, F.; Thomas, E.; Koebnik, R.; Szurek, B.; Koita, O.; Verdier, V.; et al. Functional and Genome Sequence-Driven Characterization of tal Effector Gene Repertoires Reveals Novel Variants With Altered Specificities in Closely Related Malian Xanthomonas oryzae pv. oryzae Strains. Front. Microbiol. 2018, 9, 1657. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Wang, L.; Wang, L.Y.; Liu, L.M.; Li, L.M.; Sun, L.; Rao, Q.; Zhang, J.; Huang, S.W. JMJ704 positively regulates rice defense response against Xanthomonas oryzae pv. oryzae infection via reducing H3K4me2/3 associated with negative disease resistance regulators. BMC Plant Biol. 2015, 15, 286. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Venturi, V.; He, Y.W.; Kojima, M.; Sakakibari, H.; Hofte, M.; De Vleesschauwer, D. Phytohormone-mediated interkingdom signaling shapes the outcome of rice-Xanthomonas oryzae pv. oryzae interactions. BMC Plant. Biol. 2015, 15, 10. [Google Scholar] [CrossRef]
- Marimon, O.; Teixeira, J.M.; Cordeiro, T.N.; Soo, V.W.; Wood, T.L.; Mayzel, M.; Amata, I.; Garcia, J.; Morera, A.; Gay, M.; et al. An oxygen-sensitive toxin-antitoxin system. Nat. Commun. 2016, 7, 13634. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Durgadevi, R.; Abirami, G.; Alexpandi, R.; Nandhini, K.; Kumar, P.; Prakash, S.; Veera Ravi, A. Explication of the potential of 2-hydroxy-4-methoxybenzaldehyde in hampering uropathogenic proteus mirabilis crystalline biofilm and virulence. Front. Microbiol. 2019, 10, 2804. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.F.; Liu, J.; Wang, Z.Q.; Chen, W.M.; Lin, J. Fluorescent Detection of the Ubiquitous Bacterial Messenger 3’,5’ Cyclic Diguanylic Acid by Using a Small Aromatic Molecule. Front. Microbiol. 2019, 10, 3163. [Google Scholar] [CrossRef] [PubMed]
- Camara-Almiron, J.; Navarro, Y.; Diaz-Martinez, L.; Magno-Perez-Bryan, M.C.; Molina-Santiago, C.; Pearson, J.R.; de Vicente, A.; Perez-Garcia, A.; Romero, D. Dual functionality of the amyloid protein TasA in Bacillus physiology and fitness on the phylloplane. Nat. Commun. 2020, 11, 1859. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Uchida, K.; Dono, K.; Aizawa, S. Length control of the flagellar hook in a temperature-sensitive flgE mutant of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2013, 195, 3590–3595. [Google Scholar] [CrossRef]
- Jackson, K.M.; Schwartz, C.; Wachter, J.; Rosa, P.A.; Stewart, P.E. A widely conserved bacterial cytoskeletal component influences unique helical shape and motility of the spirochete Leptospira biflexa. Mol. Microbiol. 2018, 108, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Weller-Stuart, T.; Toth, I.; De Maayer, P.; Coutinho, T. Swimming and twitching motility are essential for attachment and virulence of Pantoea ananatis in onion seedlings. Mol. Plant Pathol. 2017, 18, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Hoeflinger, J.L.; Miller, M.J. Cronobacter sakazakii ATCC 29544 Autoaggregation Requires FliC Flagellation, Not Motility. Front. Microbiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.M.; Qi, P.Y.; Xiao, W.L.; Zhang, T.H.; Zhou, X.; Liu, L.W.; Yang, S. Fabrication of Isopropanolamine-Decorated Coumarin Derivatives as Novel Quorum Sensing Inhibitors to Suppress Plant Bacterial Disease. J. Agric. Food Chem. 2022, 70, 6037–6049. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Ji, Q.T.; Ren, G.G.; Wang, F.; Su, F.; Wang, P.Y.; Zhou, X.; Wu, Z.B.; Li, Z.; Yang, S. ntibacterial Functions and Proposed Modes of Action of Novel 1,2,3,4-Tetrahydro-beta-carboline Derivatives that Possess an Attractive 1,3-Diaminopropan-2-ol Pattern against Rice Bacterial Blight, Kiwifruit Bacterial Canker, and Citrus Bacterial Canker. J. Agric. Food Chem. 2020, 68, 12558–12568. [Google Scholar] [CrossRef] [PubMed]
- Vankova, E.; Paldrychova, M.; Kasparova, P.; Lokocova, K.; Kodes, Z.; Matatkova, O.; Kolouchova, I.; Masak, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef]
- Berger, M.; Roller, A.; Maulide, N. Synthesis and antimicrobial evaluation of novel analogues of dehydroabietic acid prepared by C-H-Activation. Eur. J. Med. Chem. 2017, 126, 937–943. [Google Scholar] [CrossRef]
- Goodson, B.; Ehrhardt, A.; Ng, S.; Nuss, J.; Johnson, K.; Giedlin, M.; Yamamoto, R.; Moos, W.H.; Krebber, A.; Ladner, M.; et al. Characterization of Novel Antimicrobial Peptoids. Antimicrob. Agents Chemother. 1999, 43, 1429–1434. [Google Scholar] [CrossRef]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar] [CrossRef]
- Gu, W.; Wang, S.F. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yao, Y.; Yang, T.; Wang, X.Y.; Zhu, Z.Y.; Xu, W.T.; Lin, H.X.; Gao, Z.B.; Zhou, H.; Yang, C.G.; et al. The synthesis and antistaphylococcal activity of N-sulfonaminoethyloxime derivatives of dehydroabietic acid. Bioorg. Med. Chem. Lett. 2018, 28, 1943–1948. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.W.; Long, Z.Q.; Li, Z.X.; Zhu, J.J.; Wang, P.Y.; Qi, P.Y.; Liu, L.W.; Yang, S. Rational Optimization of 1,2,3-Triazole-Tailored Carbazoles As Prospective Antibacterial Alternatives with Significant In Vivo Control Efficiency and Unique Mode of Action. J. Agric. Food Chem. 2021, 69, 4615–4627. [Google Scholar] [CrossRef]
- Xiang, M.; Song, Y.L.; Ji, J.; Zhou, X.; Liu, L.W.; Wang, P.Y.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of novel 18β-glycyrrhetinic piperazine amides displaying significant in vitro and in vivo antibacterial activities against intractable plant bacterial diseases. Pest Manag. Sci. 2020, 76, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Huang, X.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Tao, Q.Q.; Li, Z.; Yang, S. Identification of Racemic and Chiral Carbazole Derivatives Containing an Isopropanolamine Linker as Prospective Surrogates against Plant Pathogenic Bacteria: In Vitro and In Vivo Assays and Quantitative Proteomics. J. Agric. Food Chem. 2019, 67, 7512–7525. [Google Scholar] [CrossRef]
- Brown, L.R.; Caulkins, R.C.; Schartel, T.E.; Rosch, J.W.; Honsa, E.S.; Schultz-Cherry, S.; Meliopoulos, V.A.; Cherry, S.; Thornton, J.A. Increased Zinc Availability Enhances Initial Aggregation and Biofilm Formation of Streptococcus pneumoniae. Front. Cell Infect. Microbiol. 2017, 7, 233. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhang, L.; Gu, X.; Liu, G.; Shahid, M.; Gao, J.; Ali, T.; Han, B. Nocardia cyriacigeogica from Bovine Mastitis Induced In vitro Apoptosis of Bovine Mammary Epithelial Cells via Activation of Mitochondrial-Caspase Pathway. Front. Cell Infect. Microbiol. 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Seijsing, F.; Nileback, L.; Ohman, O.; Pasupuleti, R.; Stahl, C.; Seijsing, J.; Hedhammar, M. Recombinant spider silk coatings functionalized with enzymes targeting bacteria and biofilms. Microbiologyopen 2020, 9, e993. [Google Scholar] [CrossRef]
- Liu, Z.D.; Schade, R.; Luthringer, B.; Hort, N.; Rothe, H.; Muller, S.; Liefeith, K.; Willumeit-Romer, R.; Feyerabend, F. Influence of the Microstructure and Silver Content on Degradation, Cytocompatibility, and Antibacterial Properties of Magnesium-Silver Alloys In Vitro. Oxid. Med. Cell Longev. 2017, 2017, 8091265. [Google Scholar] [CrossRef]
- Zeng, X.H.; She, P.F.; Zhou, L.Y.; Li, S.J.; Hussain, Z.; Chen, L.H.; Wu, Y. Drug repurposing: Antimicrobial and antibiofilm effects of penfluridol against Enterococcus faecalis. Microbiologyopen 2021, 10, e1148. [Google Scholar] [CrossRef]
- Zheng, J.X.; Lin, Z.W.; Chen, C.; Chen, Z.; Lin, F.J.; Wu, Y.; Yang, S.Y.; Sun, X.; Yao, W.M.; Li, D.Y.; et al. Biofilm Formation in Klebsiella pneumoniae Bacteremia Strains Was Found to be Associated with CC23 and the Presence of wcaG. Front. Cell Infect. Microbiol. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Chen, X.; Gueydan, C.; Han, J.H. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Wanyi Tai, P.Z.; Gao, X.H. Cytosolic delivery of proteins by cholesterol tagging. Sci. Adv. 2020, 6, eabb0310. [Google Scholar]
- Costa, O.Y.A.; de Hollander, M.; Pijl, A.; Liu, B.; Kuramae, E.E. Cultivation-independent and cultivation-dependent metagenomes reveal genetic and enzymatic potential of microbial community involved in the degradation of a complex microbial polymer. Microbiome 2020, 8, 76. [Google Scholar] [CrossRef]
- Rajput, A.; Thakur, A.; Sharma, S.; Kumar, M. aBiofilm: A resource of anti-biofilm agents and their potential implications in targeting antibiotic drug resistance. Nucleic Acids Res. 2018, 46, D894–D900. [Google Scholar] [CrossRef]
- Yao, J.; Allen, C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J. Bacteriol. 2006, 188, 3697–3708. [Google Scholar] [CrossRef]
- Kurre, R.; Maier, B. Oxygen Depletion Triggers Switching between Discrete Speed Modes of Gonococcal Type IV Pili. Biophys. J. 2012, 102, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Mideros-Mora, C.; Miguel-Romero, L.; Felipe-Ruiz, A.; Casino, P.; Marina, A. Revisiting the pH-gated conformational switch on the activities of HisKA-family histidine kinases. Nat. Commun. 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.W.; Nishikino, T.; Takekawa, N.; Terashima, H.; Kojima, S.; Imada, K.; Homma, M.; Liu, J. In Situ Structure of the Vibrio Polar Flagellum Reveals a Distinct Outer Membrane Complex and Its Specific Interaction with the Stator. J. Bacteriol. 2020, 202, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Huang, Q.; Cui, A.L.; Liu, X.L.; Hou, B.; Zhang, L.Y.; Liu, M.; Meng, X.R.; Li, S.W. Overexpression of Outer Membrane Protein X (OmpX) Compensates for the Effect of TolC Inactivation on Biofilm Formation and Curli Production in Extraintestinal Pathogenic Escherichia coli (ExPEC). Front. Cell Infect. Microbiol. 2018, 8, 208. [Google Scholar] [CrossRef]
- Sun, W.X.; Dunning, F.M.; Pfund, C.; Weingarten, R.; Bent, A.F. Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 2006, 18, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.H.; An, L.; Wu, Y.; Long, J.; Song, L.Y.; Fang, R.X.; Jia, Y.T. A dual role for proline iminopeptidase in the regulation of bacterial motility and host immunity. Mol. Plant Pathol. 2018, 19, 2011–2024. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.W.; Mangalea, M.R.; Purcell, E.B.; Borchardt, E.K.; Tamayo, R. The second messenger cyclic Di-GMP regulates Clostridium difficile toxin production by controlling expression of sigD. J. Bacteriol. 2013, 195, 5174–5185. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, E.; Paccagnini, E.; Cantele, F.; Gentile, M.; Dini, D.; Fino, F.; Diener, D.; Mencarelli, C.; Lupetti, P. Two classes of short intraflagellar transport train with different 3D structures are present in Chlamydomonas flagella. J. Cell Sci. 2016, 129, 2064–2074. [Google Scholar]
- Agarwal, A.; Guthrie, K.M.; Czuprynski, C.J.; Schurr, M.J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Polymeric Multilayers that contain Silver Nanoparticles can be Stamped onto Biological Tissues to Provide Antibacterial Activity. Adv. Funct. Mater. 2011, 21, 1863–1873. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Sims, K.R.; Liu, Y.; Hwang, G.; Jung, H.I.; Koo, H.; Benoit, D.S.W. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale 2018, 11, 219–236. [Google Scholar] [CrossRef]
- Wu, G.H.; Majewski, J.; Ege, C.; Kjaer, K.; Weygand, M.J.; Lee, K.Y. Interaction between lipid monolayers and poloxamer 188: An X-ray reflectivity and diffraction study. Biophys. J. 2005, 89, 3159–3173. [Google Scholar] [CrossRef]
- Raguz, M.; Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups. Exp. Eye Res. 2015, 132, 78–90. [Google Scholar] [CrossRef]
- Pang, R.; Xie, T.F.; Wu, Q.P.; Li, Y.P.; Lei, T.; Zhang, J.M.; Ding, Y.; Wang, J.; Xue, L.; Chen, M.T.; et al. Comparative Genomic Analysis Reveals the Potential Risk of Vibrio parahaemolyticus Isolated From Ready-To-Eat Foods in China. Front. Microbiol. 2019, 10, 186. [Google Scholar] [CrossRef]
- Imperi, F.; Leoni, L.; Visca, P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Ueda, M.; Egoshi, S.; Dodo, K.; Ishimaru, Y.; Yamakoshi, H.; Nakano, T.; Takaoka, Y.; Tsukiji, S.; Sodeoka, M. Noncanonical Function of a Small-Molecular Virulence Factor Coronatine against Plant Immunity: An In Vivo Raman Imaging Approach. ACS Cent. Sci. 2017, 3, 462–472. [Google Scholar] [CrossRef]
- Taguchi, F.; Suzuki, T.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is an intrinsic virulence factor in host tobacco infection. J. Bacteriol. 2010, 192, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mahamat Abdelrahim, A.; Radomski, N.; Delannoy, S.; Djellal, S.; Le Negrate, M.; Hadjab, K.; Fach, P.; Hennekinne, J.A.; Mistou, M.Y.; Firmesse, O. Large-Scale Genomic Analyses and Toxinotyping of Clostridium perfringens Implicated in Foodborne Outbreaks in France. Front. Microbiol. 2019, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Wang, X.X.; Wu, L.; Huang, L.; Qin, Q.J.; Yao, J.L.; Lu, G.T.; Tang, J.L. Xanthomonas campestris sensor kinase HpaS co-opts the orphan response regulator VemR to form a branched two-component system that regulates motility. Mol. Plant Pathol. 2020, 21, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.F.; Yin, W.F.; Song, S.H.; Zhang, Z.H.; Ye, P.Y.; Zhang, Y.; Zhou, J.N.; He, F.; Li, P.; Deng, Y.Y. Ralstonia solanacearum promotes pathogenicity by utilizing L-glutamic acid from host plants. Mol. Plant Pathol. 2020, 21, 1099–1110. [Google Scholar] [CrossRef]
- Hamdoun, S.; Gao, M.; Gill, M.; Kwon, A.; Norelli, J.L.; Lu, H. Signalling requirements for Erwinia amylovora-induced disease resistance, callose deposition and cell growth in the non-host Arabidopsis thaliana. Mol. Plant Pathol. 2018, 19, 1090–1103. [Google Scholar] [CrossRef]
- Wei, Y.; Caceres-Moreno, C.; Jimenez-Gongora, T.; Wang, K.K.; Sang, Y.Y.; Lozano-Duran, R.; Macho, A.P. The Ralstonia solanacearum csp22 peptide, but not flagellin-derived peptides, is perceived by plants from the Solanaceae family. Plant Biotechnol. J. 2018, 16, 1349–1362. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Fleitas Martinez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef]
- Ferro, T.A.; Araujo, J.M.; Dos Santos Pinto, B.L.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.; Colares, V.L.; Novais, T.M.; Filho, C.M.; Struve, C.; et al. Cinnamaldehyde Inhibits Staphylococcus aureus Virulence Factors and Protects against Infection in a Galleria mellonella Model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef]
- Wang, G.G.; Gao, Y.W.; Wu, X.H.; Gao, X.E.; Zhang, M.; Liu, H.M.; Fang, T.Q. Inhibitory Effect of Piceatannol on Streptococcus suis Infection Both in vitro and in vivo. Front. Microbiol. 2020, 11, 593588. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.Y.; Zhang, T.H.; Feng, Y.M.; Wang, M.W.; Shao, W.B.; Zeng, D.; Jin, L.H.; Wang, P.Y.; Zhou, X.; Yang, S. Exploring an Innovative Strategy for Suppressing Bacterial Plant Disease: Excavated Novel Isopropanolamine-Tailored Pterostilbene Derivatives as Potential Antibiofilm Agents. J. Agric. Food Chem. 2022, 70, 4899–4911. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Liu, S.S.; Yang, J.; Xie, J.; Zhou, X.; Wu, Z.B.; Liu, L.W.; Wang, P.Y.; Yang, S. Discovery of Epipodophyllotoxin-Derived B2 as Promising XooFtsZ Inhibitor for Controlling Bacterial Cell Division: Structure-Based Virtual Screening, Synthesis, and SAR Study. Int. J. Mol. Sci. 2022, 23, 9119. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, B.X.; Zhang, T.H.; Tao, Q.Q.; Zhou, X.; Wang, P.Y.; Yang, S. Novel 1,3,4-oxadiazole thioether and sulfone derivatives bearing a flexible N-heterocyclic moiety: Synthesis, characterization, and anti-microorganism activity. Arabian J. Chem. 2023, 16, 2. [Google Scholar] [CrossRef]




| Compds | Inhibition Ratio (%) | |||||
|---|---|---|---|---|---|---|
| Xoo | Xac | Psa | ||||
| 100 μg mL−1 | 50 μg mL−1 | 100 μg mL−1 | 50 μg mL−1 | 100 μg mL−1 | 50 μg mL−1 | |
| DAA | 43.9 ± 2.7 | 38.4 ± 3.4 | 56.9 ± 3.4 | 44.1 ± 12.6 | 39.8 ± 9.5 | 34.5 ± 10.2 |
| 2a | 91.7 ± 0.8 | 91.6 ± 0.3 | 88.6 ± 1.5 | 87.5 ± 0.4 | 48.3 ± 6.8 | 41.2 ± 1.6 |
| 2b | 92.3 ± 0.3 | 91.0 ± 0.3 | 89.7 ± 0.2 | 89.4 ± 0.3 | 40.6 ± 5.1 | 38.1 ± 7.5 |
| 2c | 90.7 ± 0.3 | 89.2 ± 1.4 | 89.0 ± 1.3 | 85.9 ± 1.5 | 54.3 ± 7.5 | 49.6 ± 6.8 |
| 2d | 0 | 0 | 0 | 0 | 0 | 0 |
| 2e | 0 | 0 | 0 | 0 | 0 | 0 |
| 2f | 0 | 0 | 86.7 ± 0.1 | 85.9 ± 0.1 | 17.7 ± 3.1 | 16.8 ± 7.7 |
| 2g | 10.7 ± 3.8 | 0 | 0 | 0 | 0 | 0 |
| 2h | 65.9 ± 0.5 | 64.2 ± 0.2 | 41.8 ± 7.0 | 37.2 ± 5.2 | 0 | 0 |
| 2i | 88.2 ± 0.7 | 79.0 ± 0.8 | 83.5 ± 1.1 | 79.0 ± 2.9 | 53.2 ± 7.1 | 50.1 ± 5.5 |
| 2j | 85.6 ± 0.4 | 84.3 ± 0.3 | 87.4 ± 1.8 | 84.0 ± 0.5 | 58.1 ± 0.3 | 52.6 ± 4.2 |
| 2k | 91.4 ± 0.1 | 88.9 ± 0.4 | 83.3 ± 0.8 | 82.6 ± 0.9 | 36.0 ± 1.6 | 7.3 ± 5.0 |
| 2l | 89.6 ± 3.1 | 87.5 ± 1.2 | 85.1 ± 2.4 | 82.1 ± 1.4 | 55.2 ± 1.5 | 50.1 ± 0.7 |
| 2m | 89.7 ± 0.6 | 89.4 ± 1.2 | 47.2 ± 5.4 | 44.3 ± 0.7 | 47.5 ± 9.9 | 46.6 ± 1.6 |
| 2n | 11.3 ± 2.5 | 0 | 48.4 ± 2.7 | 45.0 ± 1.2 | 45.9 ± 2.8 | 40.8 ± 8.5 |
| 2o | 91.9 ± 0.9 | 91.8 ± 0.3 | 89.1 ± 1.5 | 88.5 ± 0.5 | 51.0 ± 5.8 | 48.2 ± 5.5 |
| 2p | 62.2 ± 3.5 | 54.5 ± 1.2 | 48.7 ± 9.2 | 40.0 ± 3.9 | 30.4 ± 1.2 | 21.8 ± 1.3 |
| TC | 85.1 ± 5.3 | 46.8 ± 2.2 | 56.3 ± 3.2 | 32.3 ± 2.1 | 63.1 ± 6.2 | 33.6 ± 2.2 |
| Compds | Regression Equation | R2 | EC50 (μg mL−1) | EC50′ (μM) |
|---|---|---|---|---|
| 2a | y = 2.6697x + 2.7486 | 0.9182 | 7.0 ± 0.5 | 15.3 |
| 2b | y = 2.0052x + 4.1343 | 0.9558 | 2.7 ± 0.3 | 5.7 |
| 2c | y = 1.4267x + 4.5965 | 0.9369 | 3.2 ± 0.9 | 6.4 |
| 2d | y = 2.3605x + 2.3735 | 0.8867 | 13.0 ± 1.7 | 25.1 |
| 2e | >100 | |||
| 2f | >100 | |||
| 2g | >100 | |||
| 2h | y = 1.0065x + 4.2365 | 0.9829 | 5.8 ± 0.7 | 10.5 |
| 2i | y = 2.0242x + 3.9693 | 0.9066 | 3.2 ± 0.7 | 7.2 |
| 2j | y = 1.8918x + 3.9619 | 0.8840 | 3.6 ± 0.5 | 8.1 |
| 2k | y = 1.9734x + 3.7912 | 0.9149 | 4.1 ± 0.8 | 9.2 |
| 2l | y = 1.4200x + 4.5751 | 0.9630 | 3.0 ± 0.4 | 6.7 |
| 2m | y = 1.5424x + 3.8815 | 0.9288 | 5.3 ± 0.8 | 10.2 |
| 2n | >100 | |||
| 2o | y = 4.5670x + 1.5782 | 0.9943 | 5.7 ± 0.5 | 12.5 |
| 2p | y = 0.6191x + 4.1400 | 0.9510 | 24.4 ± 1.2 | 55.0 |
| TC | y = 5.4033x − 2.3402 | 0.9621 | 61.2 ± 5.2 | 186.6 |
| Treatment | Curative Activity (14 Days after Spraying) | Protection Activity (14 Days after Spraying) | ||||
|---|---|---|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) | Morbidity (%) | Disease Index (%) | Control Efficiency (%) | |
| 2b | 100 | 40.00 | 48.57 | 100 | 30.00 | 61.43 |
| TC | 100 | 57.78 | 25.72 | 100 | 62.22 | 20.00 |
| CK | 100 | 77.78 | / | 100 | 77.78 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, P.; Wang, N.; Zhang, T.; Feng, Y.; Zhou, X.; Zeng, D.; Meng, J.; Liu, L.; Jin, L.; Yang, S. Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. Int. J. Mol. Sci. 2023, 24, 2897. https://doi.org/10.3390/ijms24032897
Qi P, Wang N, Zhang T, Feng Y, Zhou X, Zeng D, Meng J, Liu L, Jin L, Yang S. Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. International Journal of Molecular Sciences. 2023; 24(3):2897. https://doi.org/10.3390/ijms24032897
Chicago/Turabian StyleQi, Puying, Na Wang, Taihong Zhang, Yumei Feng, Xiang Zhou, Dan Zeng, Jiao Meng, Liwei Liu, Linhong Jin, and Song Yang. 2023. "Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation" International Journal of Molecular Sciences 24, no. 3: 2897. https://doi.org/10.3390/ijms24032897
APA StyleQi, P., Wang, N., Zhang, T., Feng, Y., Zhou, X., Zeng, D., Meng, J., Liu, L., Jin, L., & Yang, S. (2023). Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. International Journal of Molecular Sciences, 24(3), 2897. https://doi.org/10.3390/ijms24032897






