Evolution of Antiretroviral Drug Rilpivirine and Approach to Oncology
Abstract
1. Introduction
2. Rilpivirine
3. Repurposing of Rilpivirine
4. Possible Anti-Cancer Pathways of Rilpivirine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauci, A.S. The acquired immune deficiency syndrome. The ever-broadening clinical spectrum. JAMA 1983, 249, 2375–2376. [Google Scholar] [CrossRef]
- De Cock, K.M.; Jaffe, H.W.; Curran, J.W. Reflections on 40 Years of AIDS. Emerg. Infect. Dis. 2021, 27, 1553–1560. [Google Scholar] [CrossRef]
- Fanales-Belasio, E.; Raimondo, M.; Suligoi, B.; Buttò, S. HIV virology and pathogenetic mechanisms of infection: A brief overview. Ann. Ist. Super Sanita 2010, 46, 5–14. [Google Scholar] [CrossRef]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef]
- EMA. Edurant. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/edurant (accessed on 15 November 2022).
- FDA. Prescribing Information: EDURANT (Rilpivirine) Tablets for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202022s011lbl.pdf (accessed on 15 November 2022).
- Adams, J.; Patel, N.; Mankaryous, N.; Tadros, M.; Miller, C.D. Nonnucleoside reverse transcriptase inhibitor resistance and the role of the second-generation agents. Ann. Pharmacother. 2010, 44, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.G.; Zila, V.; Peters, K.; Schifferdecker, S.; Stanic, M.; Lucic, B.; Laketa, V.; Lusic, M.; Müller, B.; Kräusslich, H.G. HIV-1 uncoating by release of viral cDNA from capsid-like structures in the nucleus of infected cells. eLife 2021, 10, e64776. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bauman, J.D.; Clark, A.D., Jr.; Frenkel, Y.V.; Lewi, P.J.; Shatkin, A.J.; Hughes, S.H.; Arnold, E. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: Strategic flexibility explains potency against resistance mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Saravolatz, L.D. Rilpivirine: A new non-nucleoside reverse transcriptase inhibitor. J. Antimicrob. Chemother. 2013, 68, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M. Rilpivirine. Drugs 2012, 72, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, H.M.; van Heeswijk, R.P.G.; Kestens, D.; Stevens, M.; Buelens, A.; Boven, K.; Hoetelmans, R.M.W. The pharmacokinetic (PK) interaction between omeprazole and TMC278, an investigational non-nucleoside reverse transcriptase inhibitor (NNRTI). J. Int. AIDS Soc. 2008, 11, P239. [Google Scholar] [CrossRef]
- Online, D. Rilpivirine. Available online: https://go.drugbank.com/drugs/DB08864 (accessed on 18 March 2022).
- Weiss, J.; Haefeli, W.E. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int. J. Antimicrob. Agents 2013, 41, 484–487. [Google Scholar] [CrossRef]
- Engelman, K.D.; Engelman, A.N. Long-Acting Cabotegravir for HIV/AIDS Prophylaxis. Biochemistry 2021, 60, 1731–1740. [Google Scholar] [CrossRef]
- Liegeon, G.; Ghosn, J. Long-acting injectable cabotegravir for PrEP: A game-changer in HIV prevention? HIV Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- EMA. Vocabria. Available online: https://www.ema.europa.eu/en/documents/product-information/vocabria-epar-product-information_en.pdf (accessed on 2 January 2023).
- Markham, A. Cabotegravir Plus Rilpivirine: First Approval. Drugs 2020, 80, 915–922. [Google Scholar] [CrossRef] [PubMed]
- FDA. CABENUVA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212888s000lbl.pdf (accessed on 2 January 2023).
- Margolis, D.A.; Brinson, C.C.; Smith, G.H.R.; de Vente, J.; Hagins, D.P.; Eron, J.J.; Griffith, S.K.; Clair, M.H.S.; Stevens, M.C.; Williams, P.E.; et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): A randomised, phase 2b, dose-ranging trial. Lancet Infect. Dis. 2015, 15, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.A.; Gonzalez-Garcia, J.; Stellbrink, H.J.; Eron, J.J.; Yazdanpanah, Y.; Podzamczer, D.; Lutz, T.; Angel, J.B.; Richmond, G.J.; Clotet, B.; et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017, 390, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.H.R.; Henry, W.K.; Podzamczer, D.; Masiá, M.D.M.; Bettacchi, C.J.; Arasteh, K.; Jaeger, H.; Khuong-Josses, M.A.; Montes-Ramírez, M.L.; Stellbrink, H.J.; et al. Efficacy, Safety, and Durability of Long-Acting Cabotegravir and Rilpivirine in Adults With Human Immunodeficiency Virus Type 1 Infection: 5-Year Results From the LATTE-2 Study. Open Forum. Infect. Dis. 2021, 8, ofab439. [Google Scholar] [CrossRef]
- Orkin, C.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Overton, E.T.; Girard, P.M.; Oka, S.; Walmsley, S.; Bettacchi, C.; Brinson, C.; et al. Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. N. Engl. J. Med. 2020, 382, 1124–1135. [Google Scholar] [CrossRef]
- Swindells, S.; Andrade-Villanueva, J.F.; Richmond, G.J.; Rizzardini, G.; Baumgarten, A.; Masiá, M.; Latiff, G.; Pokrovsky, V.; Bredeek, F.; Smith, G.; et al. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N. Engl. J. Med. 2020, 382, 1112–1123. [Google Scholar] [CrossRef]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Jaeger, H.; Orrell, C.; Nagimova, F.; Bredeek, F.; García Deltoro, M.; Swindells, S.; Andrade-Villanueva, J.F.; et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: A randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021, 396, 1994–2005. [Google Scholar] [CrossRef]
- Healthcare, V. ViiV Healthcare Announces the Marketing Authorisation of the First Complete Long-Acting Injectable HIV Treatment in Europe. Available online: https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2020/december/viiv-healthcare-announces-the-marketing-authorisation/#1 (accessed on 4 January 2023).
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika Virus Infection in the Brain by the Antiretroviral Drug Rilpivirine. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef]
- Makarasen, A.; Patnin, S.; Vijitphan, P.; Reukngam, N.; Khlaychan, P.; Kuno, M.; Intachote, P.; Saimanee, B.; Sengsai, S.; Techasakul, S. Structural Basis of 2-Phenylamino-4-phenoxyquinoline Derivatives as Potent HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors. Molecules 2022, 27, 461. [Google Scholar] [CrossRef]
- Hecht, M.; Erber, S.; Harrer, T.; Klinker, H.; Roth, T.; Parsch, H.; Fiebig, N.; Fietkau, R.; Distel, L.V. Efavirenz Has the Highest Anti-Proliferative Effect of Non-Nucleoside Reverse Transcriptase Inhibitors against Pancreatic Cancer Cells. PLoS ONE 2015, 10, e0130277. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Teo, T.; Kumarasiri, M.; Slater, M.; Martin, J.H.; Wang, S.; Head, R. Combined In Silico and In Vitro Evidence Supporting an Aurora A Kinase Inhibitory Role of the Anti-Viral Drug Rilpivirine and an Anti-Proliferative Influence on Cancer Cells. Pharmaceuticals 2022, 15, 1186. [Google Scholar] [CrossRef]
- Yan, M.; Wang, C.; He, B.; Yang, M.; Tong, M.; Long, Z.; Liu, B.; Peng, F.; Xu, L.; Zhang, Y.; et al. Aurora-A Kinase: A Potent Oncogene and Target for Cancer Therapy. Med. Res. Rev. 2016, 36, 1036–1079. [Google Scholar] [CrossRef]
- Tavernier, N.; Sicheri, F.; Pintard, L. Aurora A kinase activation: Different means to different ends. J. Cell Biol. 2021, 220, e202106128. [Google Scholar] [CrossRef]
- Mou, P.K.; Yang, E.J.; Shi, C.; Ren, G.; Tao, S.; Shim, J.S. Aurora kinase A, a synthetic lethal target for precision cancer medicine. Exp. Mol. Med. 2021, 53, 835–847. [Google Scholar] [CrossRef]
- Carpinelli, P.; Moll, J. Aurora kinase inhibitors: Identification and preclinical validation of their biomarkers. Expert. Opin. Ther. Targets 2008, 12, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Demaria, M.; Giorgi, C.; Lebiedzinska, M.; Esposito, G.; D’Angeli, L.; Bartoli, A.; Gough, D.J.; Turkson, J.; Levy, D.E.; Watson, C.J.; et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging 2010, 2, 823–842. [Google Scholar] [CrossRef]
- Wang, S.; Koromilas, A.E. STAT1-mediated translational control in tumor suppression and antitumor therapies. Mol. Cell Oncol. 2016, 3, e1055049. [Google Scholar] [CrossRef]
- Martí-Rodrigo, A.; Alegre, F.; Moragrega, Á.B.; García-García, F.; Martí-Rodrigo, P.; Fernández-Iglesias, A.; Gracia-Sancho, J.; Apostolova, N.; Esplugues, J.V.; Blas-García, A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut 2020, 69, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Tortora, G.; Melisi, D.; Ciardiello, F. Angiogenesis: A target for cancer therapy. Curr. Pharm. Des. 2004, 10, 11–26. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, N.; Jeldres, C.; Patard, J.J.; Perrotte, P.; Suardi, N.; Hutterer, G.; Patenaude, F.; Oudard, S.; Karakiewicz, P.I. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur. Urol. 2008, 53, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Pang, X.; Lian, W.; Xu, L.; Wang, J.; Jia, H.; Zhang, B.; Liu, A.-L.; Du, G.-H. Discovery of VEGFR2 inhibitors by integrating naïve Bayesian classification, molecular docking and drug screening approaches. RSC Adv. 2018, 8, 5286–5297. [Google Scholar] [CrossRef] [PubMed]
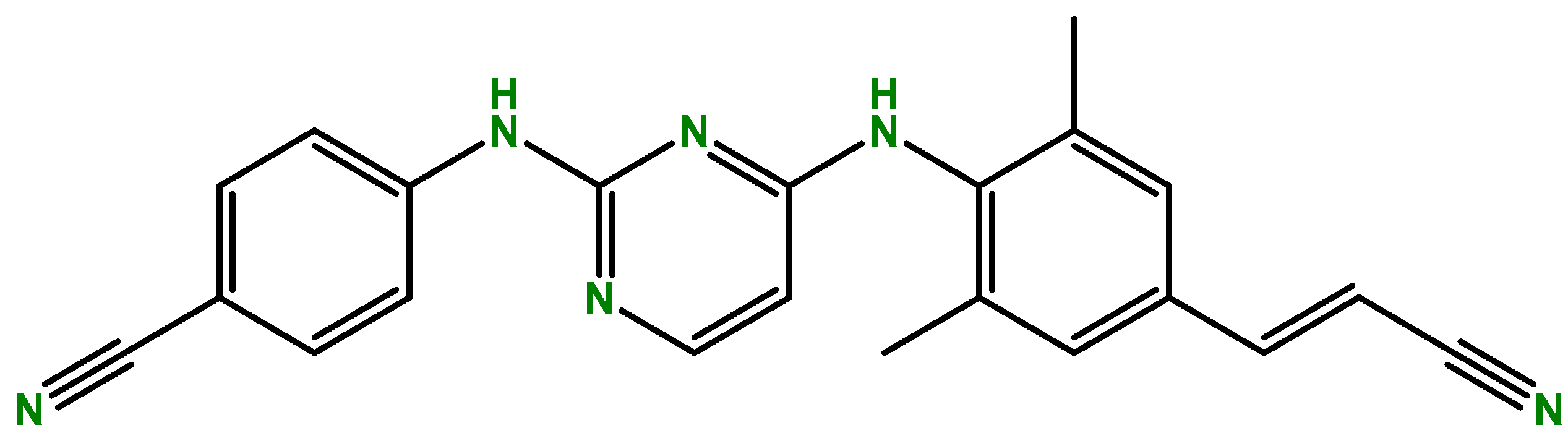
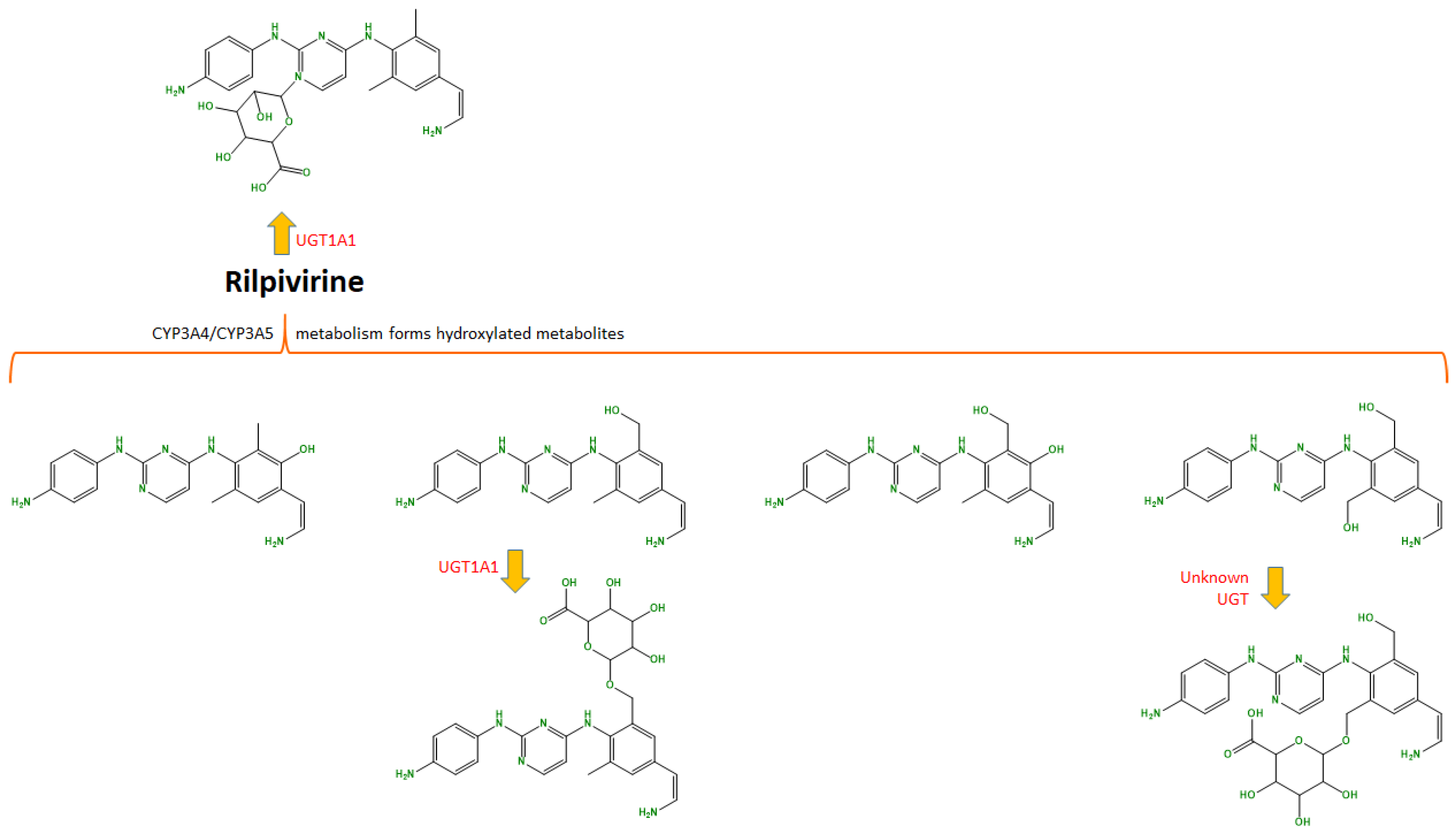

| Cancer Type | Model | Results | Ref |
|---|---|---|---|
| Lymphoblastic leukemia | MOLT-3 cells | Antineoplastic effect of rilpivirine in cancer cells with IC50 ranging from 4.3–57.1 μM at 48 h | [31] |
| Cervical cancer | HeLa cells | ||
| Promyeloblast | HL-60 cells | ||
| Breast cancer | T47-D cells | ||
| Lung carcinoma | H69AR cells | ||
| Pancreatic cancer | BxPC-3 and Panc-1 cells | Cytotoxic at low concentrations in BxPC-3 cells, with the promotion of adverse effects in colony formation at 16.2 μM and apoptosis at 24.4 μM at 72 h. Cytotoxicity in Panc-1 cells at 294 μM at 72 h | [32] |
| Acute myeloid leukemia | Cell lines | IC50 values between 3.045–9.422 µM in cell lines tested at 72 h. Inhibitory effect in aurora A kinase at 0.116 µM | [33] |
| Colorectal carcinoma | |||
| Pancreatic cancer | |||
| Ovarian cancer | |||
| Breast cancer | T47-D cells | Decreased autophosphorylation of Aurora A, leading to its inhibition. Cell cycle arrest at the G2/M stage and induced apoptosis in a time and concentration-dependent manner |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Vale, N. Evolution of Antiretroviral Drug Rilpivirine and Approach to Oncology. Int. J. Mol. Sci. 2023, 24, 2890. https://doi.org/10.3390/ijms24032890
Pereira M, Vale N. Evolution of Antiretroviral Drug Rilpivirine and Approach to Oncology. International Journal of Molecular Sciences. 2023; 24(3):2890. https://doi.org/10.3390/ijms24032890
Chicago/Turabian StylePereira, Mariana, and Nuno Vale. 2023. "Evolution of Antiretroviral Drug Rilpivirine and Approach to Oncology" International Journal of Molecular Sciences 24, no. 3: 2890. https://doi.org/10.3390/ijms24032890
APA StylePereira, M., & Vale, N. (2023). Evolution of Antiretroviral Drug Rilpivirine and Approach to Oncology. International Journal of Molecular Sciences, 24(3), 2890. https://doi.org/10.3390/ijms24032890









