Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas
Abstract
1. Introduction
2. Results
2.1. 2%HPL Led to a Lower Endothelial Cell Loss Than 2%FBS
2.2. 2%HPL and 2%FBS Did Not Influence Endothelial Cell Morphology
2.3. NGS Indicated a More Extensive and Robust Differential Gene Regulation in Endothelial Compared to Stromal Corneal Cells
2.4. Differential 2%HPL and 2%FBS Culture Did Not Influence the Expression Levels of Known Corneal Stromal Cell Markers, but Caused Mild Alterations among Corneal Endothelial Cell Markers
2.5. Category Netplots Visualize That Gene Expression Alterations following 2%HPL vs. 2%FBS Cornea Culture Influenced Different Areas of Cell Function in Stromal Compared to Corneal Endothelial Cells
2.6. GSEA (Gene Set Enrichment Analysis) of Hallmark Gene Sets and Associated DGEA (Differential Gene Expression Analysis) Showed Differential Expression Patterns Predominantly in the Corneal Endothelial Cells
3. Discussion
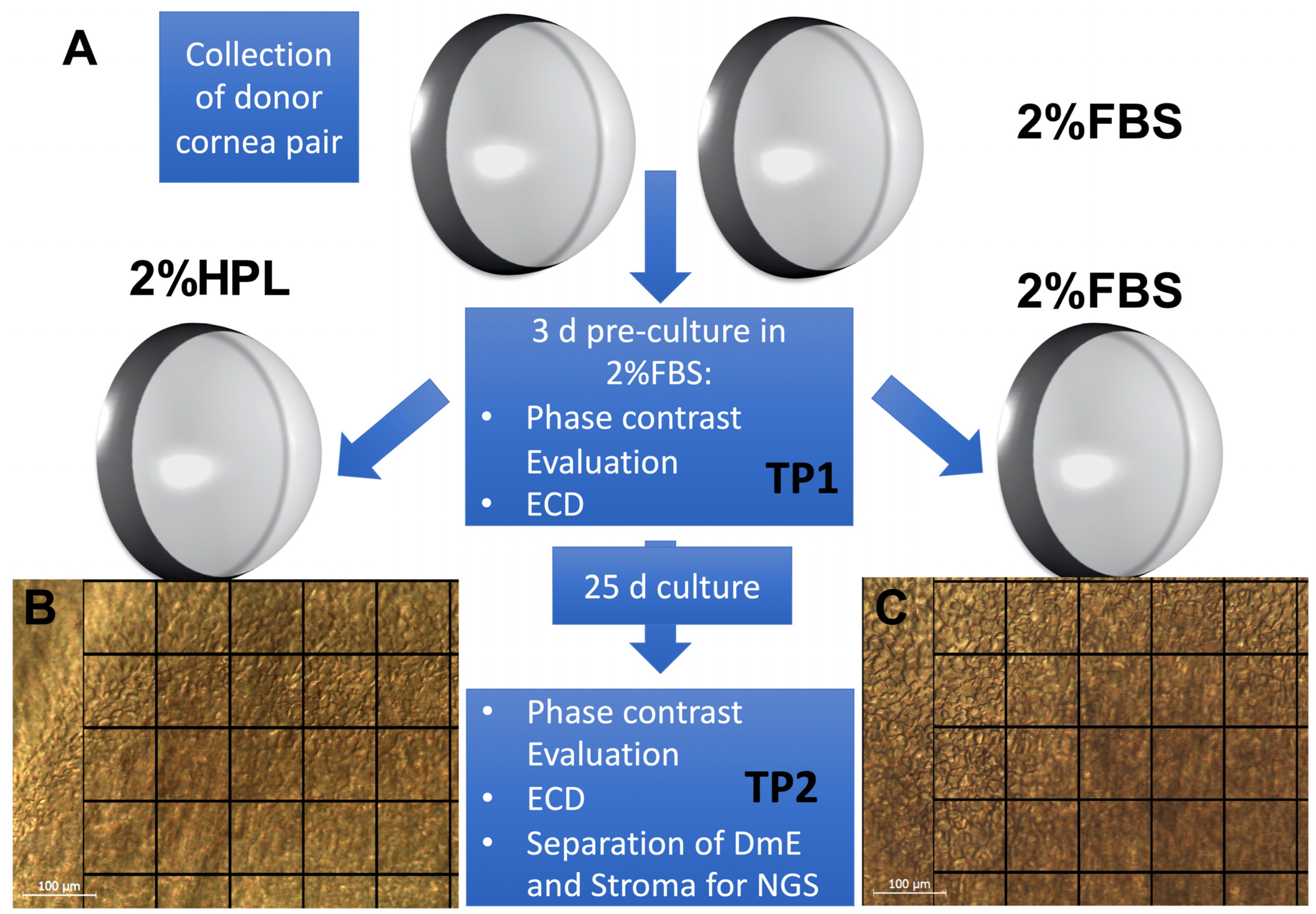
4. Material and Methods
4.1. Organ Culture and Cornea Processing
4.2. Endothelial Cell Density and Morphology Evaluation
4.3. Cornea Processing for NGS
4.4. Next Generation Sequencing
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuest, M.; Liu, Y.C.; Arundhati, A.; Li, L.; Tan, D.; Mehta, J.S. Long-term outcomes of hemi-automated lamellar keratoplasty. Clin. Exp. Ophthalmol. 2018, 46, 1017–1027. [Google Scholar] [CrossRef]
- Fuest, M.; Ang, M.; Htoon, H.M.; Tan, D.; Mehta, J.S. Long-term Visual Outcomes Comparing Descemet Stripping Automated Endothelial Keratoplasty and Penetrating Keratoplasty. Am. J. Ophthalmol. 2017, 182, 62–71. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Parekh, M.; Borroni, D.; Ruzza, A.; Levis, H.J.; Ferrari, S.; Ponzin, D.; Romano, V. A comparative study on different Descemet membrane endothelial keratoplasty graft preparation techniques. Acta Ophthalmol. 2018, 96, e718–e726. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Mehta, J.S. Strategies for deep anterior lamellar keratoplasty after hydrops in keratoconus. Eye Contact Lens 2018, 44, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Levis, H.J.; Gallon, P.; Lace, R.; Borroni, D.; Ponzin, D.; Ruzza, A.; Kaye, S.B.; Ferrari, S.; Parekh, M. Biobanking of Dehydrated Human Donor Corneal Stroma to Increase the Supply of Anterior Lamellar Grafts. Cornea 2019, 38, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Flockerzi, E.; Maier, P.; Böhringer, D.; Reinshagen, H.; Kruse, F.; Cursiefen, C.; Reinhard, T.; Geerling, G.; Torun, N.; Seitz, B. Trends in Corneal Transplantation from 2001 to 2016 in Germany: A Report of the DOG-Section Cornea and its Keratoplasty Registry. Am. J. Ophthalmol. 2018, 188, 91–98. [Google Scholar] [CrossRef]
- Pels, E.; Beele, H.; Claerhout, I. Eye bank issues: II. Preservation techniques: Warm versus cold storage. Int. Ophthalmol. 2008, 28, 155–163. [Google Scholar] [CrossRef]
- Fuest, M.; Plum, W.; Salla, S.; Walter, P.; Hermel, M. Conjunctival and intraocular swabs for the microbiological assessment of donor corneas. Acta Ophthalmol. 2016, 94, 70–75. [Google Scholar] [CrossRef]
- Hermel, M.; Salla, S.; Fuest, M.; Walter, P. The role of corneal endothelial morphology in graft assessment and prediction of endothelial cell loss during organ culture of human donor corneas. Acta Ophthalmol. 2017, 95, 205–210. [Google Scholar] [CrossRef]
- Fuest, M.; Boor, P.; Knuechel, R.; Walter, P.; Salla, S. Postmortem conjunctival and nasopharyngeal swabs in SARS-CoV-2 infected and uninfected patients. Acta Ophthalmol. 2021, 99, e615–e617. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J. Preservation of Human Cornea. Transfus. Med. Hemother. 2011, 38, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, A.; Drager, R.; Schlesier, M.; Mertelsmann, R.; Lindemann, A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol. Immunother. 2000, 49, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Reyes, E.; Whitney, M.J.; Spees, J.L. Enhanced engraftment of mesenchymal stem cells in a cutaneous wound model by culture in allogenic species-specific serum and administration in fibrin constructs. Stem. Cells 2006, 24, 2232–2243. [Google Scholar] [CrossRef]
- Selvaggi, T.A.; Walker, R.E.; Fleisher, T.A. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 1997, 89, 776–779. [Google Scholar] [CrossRef]
- Tuschong, L.; Soenen, S.L.; Blaese, R.M.; Candotti, F.; Muul, L.M. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene 2002, 13, 1605–1610. [Google Scholar] [CrossRef]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef]
- Kirikae, T.; Tamura, H.; Hashizume, M.; Kirikae, F.; Uemura, Y.; Tanaka, S.; Yokochi, T.; Nakano, M. Endotoxin contamination in fetal bovine serum and its influence on tumor necrosis factor production by macrophage-like cells J774.1 cultured in the presence of the serum. Int. J. Immunopharmacol. 1997, 19, 255–262. [Google Scholar] [CrossRef]
- Seidelmann, N.; Duarte Campos, D.F.; Rohde, M.; Johnen, S.; Salla, S.; Yam, G.H.; Mehta, J.S.; Walter, P.; Fuest, M. Human platelet lysate as a replacement for fetal bovine serum in human corneal stromal keratocyte and fibroblast culture. J. Cell Mol. Med. 2021, 25, 9647–9659. [Google Scholar] [CrossRef]
- Binte, M.Y.N.Z.; Riau, A.K.; Yam, G.H.F.; Binte Halim, N.S.H.; Mehta, J.S. Isolation and Propagation of Human Corneal Stromal Keratocytes for Tissue Engineering and Cell Therapy. Cells 2022, 11, 178. [Google Scholar] [CrossRef]
- Talpan, D.; Salla, S.; Seidelmann, N.; Walter, P.; Fuest, M. Antifibrotic Effects of Caffeine, Curcumin and Pirfenidone in Primary Human Keratocytes. Int. J. Mol. Sci. 2023, 24, 1461. [Google Scholar] [CrossRef] [PubMed]
- Jabbarpour, Z.; Aghayan, S.; Arjmand, B.; Fallahzadeh, K.; Alavi-Moghadam, S.; Larijani, B.; Aghayan, H.R. Xeno-free protocol for GMP-compliant manufacturing of human fetal pancreas-derived mesenchymal stem cells. Stem. Cell Res. Ther. 2022, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme. Guide to Good Manufacturing Practice for Medicinal Products. Annex 2A Manufacture of Advanced Therapy Medicinal Products for Human Use. Geneva: PIC/S; Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-Operation Scheme: Dublin, Ireland, 2021. [Google Scholar]
- The European Committee on Organ Transplantation (CD-P-TO). Guide to the Quality and Safety of Tissues and Cells for Human Application, 5th ed.; Conseil de l’Europe: Strasbourg, France, 2022; Available online: https://freepub.edqm.eu/publications/AUTOPUB_17/detail (accessed on 19 January 2023).
- Chimenti, I.; Gaetani, R.; Forte, E.; Angelini, F.; De Falco, E.; Zoccai, G.B.; Messina, E.; Frati, G.; Giacomello, A. Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture. J. Cell Mol. Med. 2014, 18, 624–634. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission directive 2003/94/EC. Off. J. Eur. Union 2003, 46, 22–26. [Google Scholar]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Petsoglou, C.; Wen, L.; Hoque, M.; Zhu, M.; Valtink, M.; Sutton, G.; You, J. Effects of human platelet lysate on the growth of cultured human corneal endothelial cells. Exp. Eye Res. 2021, 208, 108613. [Google Scholar] [CrossRef]
- Swiatkowska, M.; Szemraj, J.; Cierniewski, C.S. Induction of PAI-1 expression by tumor necrosis factor alpha in endothelial cells is mediated by its responsive element located in the 4G/5G site. FEBS J. 2005, 272, 5821–5831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sosne, G.; Kurpakus-Wheater, M. Plasminogen activator inhibitor-1 (PAI-1) stimulates human corneal epithelial cell adhesion and migration in vitro. Exp. Eye Res. 2005, 80, 1–8. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Xie, H.; Chen, L.; Zhou, Y.; Chen, W.; Liu, Z. Serine protease inhibitor A3K protects rabbit corneal endothelium from barrier function disruption induced by TNF-α. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5400–5407. [Google Scholar] [CrossRef]
- Wieben, E.D.; Baratz, K.H.; Aleff, R.A.; Kalari, K.R.; Tang, X.; Maguire, L.J.; Patel, S.V.; Fautsch, M.P. Gene Expression and Missplicing in the Corneal Endothelium of Patients With a TCF4 Trinucleotide Repeat Expansion Without Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3636–3643. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Suyeoka, G.; Papa, S.; Franzoso, G.; Neufeld, A.H. Growth arrest and DNA damage protein 45b (Gadd45b) protects retinal ganglion cells from injuries. Neurobiol. Dis. 2009, 33, 104–110. [Google Scholar] [CrossRef]
- Pinto, F.; Santos-Ferreira, L.; Pinto, M.T.; Gomes, C.; Reis, C.A. The Extracellular Small Leucine-Rich Proteoglycan Biglycan Is a Key Player in Gastric Cancer Aggressiveness. Cancers 2021, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
- Koulikovska, M.; Fagerholm, P. Effect of Biglycan on Interleukin1-Induced Apoptosis of Transformed Keratocytes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1495. [Google Scholar]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Benichou, G.; Yamada, Y.; Yun, S.H.; Lin, C.; Fray, M.; Tocco, G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy 2011, 3, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, H.; Wang, Z.; Xu, W.; Zhong, L.; Cao, J.; Yang, J.; Tian, Y.; Yu, D.; Ji, J.; et al. The Role of FABP5 in Radiation-Induced Human Skin Fibrosis. Radiat. Res. 2018, 189, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Yu, Z.; Li, H.; Cheng, J.; Wang, Y. Fatty acid-binding protein 5 aggravates pulmonary artery fibrosis in pulmonary hypertension secondary to left heart disease via activating wnt/β-catenin pathway. J. Adv. Res. 2022, 40, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chung, Y.W.; Jung, M.K.; Lee, J.H.; Ko, K.Y.; Jang, J.K.; Ham, M.; Kang, H.; Pack, C.G.; Mihara, H.; et al. Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes. Cell Mol. Life Sci. 2020, 77, 2367–2386. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Takahashi, K.; Hirashima, M.; Kawakita, T.; Tsubota, K. Selenoprotein P controls oxidative stress in cornea. PLoS ONE 2010, 5, e9911. [Google Scholar] [CrossRef]
- Toris, C.; Gulati, V. The biology, pathology and therapeutic use of prostaglandins in the eye. Clin. Lipidol. 2011, 6, 577–591. [Google Scholar] [CrossRef]
- Rubio-Ramos, A.; Labat-de-Hoz, L.; Correas, I.; Alonso, M.A. The MAL Protein, an Integral Component of Specialized Membranes, in Normal Cells and Cancer. Cells 2021, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Onodera, A.; Hosokawa, H.; Watanabe, Y.; Horiuchi, S.; Yamashita, J.; Tanaka, H.; Ogawa, Y.; Suzuki, Y.; Nakayama, T. Genome-Wide Gene Expression Profiling Revealed a Critical Role for GATA3 in the Maintenance of the Th2 Cell Identity. PLoS ONE 2013, 8, e66468. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Bouchard, M.; Musil, L.S.; Cvekl, A. Identification of Novel Gata3 Distal Enhancers Active in Mouse Embryonic Lens. Dev. Dyn. 2018, 247, 1186–1198. [Google Scholar] [CrossRef]
- Fuest, M.; Yam, G.H.; Peh, G.S.; Mehta, J.S. Advances in corneal cell therapy. Regen. Med. 2016, 11, 601–615. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, Cd012097. [Google Scholar] [CrossRef]
- Lohmann, T.; Baumgarten, S.; Plange, N.; Walter, P.; Fuest, M. Effects of uncomplicated Descemet membrane endothelial keratoplasty on the central retinal thickness. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2731–2741. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Fuest, M.; Yam, G.H.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. [Google Scholar] [CrossRef]
- Yam, G.H.; Teo, E.P.; Setiawan, M.; Lovatt, M.J.; Yusoff, N.; Fuest, M.; Goh, B.T.; Mehta, J.S. Postnatal periodontal ligament as a novel adult stem cell source for regenerative corneal cell therapy. J. Cell Mol. Med. 2018, 22, 3119–3132. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Fuest, M.; Yusoff, N.; Goh, T.W.; Bandeira, F.; Setiawan, M.; Seah, X.Y.; Lwin, N.C.; Stanzel, T.P.; Ong, H.S.; et al. Safety and Feasibility of Intrastromal Injection of Cultivated Human Corneal Stromal Keratocytes as Cell-Based Therapy for Corneal Opacities. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3340–3354. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. J. AMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Tarau, I.S.; Rossi, A.; Leonhardt, S.; Schwarz, T.; Schuerlein, S.; Lotz, C.; Hansmann, J. In Vivo-Like Culture Conditions in a Bioreactor Facilitate Improved Tissue Quality in Corneal Storage. Biotechnol. J. 2018, 13, 1700344. [Google Scholar] [CrossRef] [PubMed]
- Garcin, T.; Gauthier, A.S.; Crouzet, E.; He, Z.; Herbepin, P.; Perrache, C.; Acquart, S.; Cognasse, F.; Forest, F.; Thuret, G.; et al. Innovative corneal active storage machine for long-term eye banking. Am. J. Transpl. 2019, 19, 1641–1651. [Google Scholar] [CrossRef]
- Schroeter, J.; Rieck, P. Endothelial evaluation in the cornea bank. Dev. Ophthalmol. 2009, 43, 47–62. [Google Scholar] [CrossRef]
- Schnitzler, A.C.; Salla, S.; Hamsley, N.; Flammersfeld, A.; Fuest, M.; Walter, P.; Hermel, M. Role of the Endothelial Layer in the Deswelling Process of Organ-Cultured Human Corneas Before Transplantation. Cornea 2016, 35, 1216–1221. [Google Scholar] [CrossRef]
- Pels, E.; Schuchard, Y. Organ-culture preservation of human corneas. Doc. Ophthalmol. 1983, 56, 147–153. [Google Scholar] [CrossRef]
- Means, T.L.; Geroski, D.H.; Hadley, A.; Lynn, M.J.; Edelhauser, H.F. Viability of Human Corneal Endothelium Following Optisol-GS Storage. Arch. Ophthalmol. 1995, 113, 805–809. [Google Scholar] [CrossRef]
- Frueh, B.E.; Böhnke, M. Prospective, randomized clinical evaluation of Optisol vs organ culture corneal storage media. Arch. Ophthalmol. 2000, 118, 757–760. [Google Scholar] [CrossRef]
- Wojcik, G.; Ferrari, S.; Romano, V.; Ponzin, D.; Ahmad, S.; Parekh, M. Corneal storage methods: Considerations and impact on surgical outcomes. Expert Rev. Ophthalmol. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Schaub, F.; Enders, P.; Adler, W.; Bachmann, B.O.; Cursiefen, C.; Heindl, L.M. Impact of donor graft quality on deep anterior lamellar Keratoplasty (DALK). BMC Ophthalmol. 2017, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Schrittenlocher, S.; Siebelmann, S.; Le, V.N.H.; Matthaei, M.; Franklin, J.; Bachmann, B.; Cursiefen, C. Risk factors for endothelial cell loss after Descemet membrane endothelial keratoplasty (DMEK). Sci. Rep. 2020, 10, 11086. [Google Scholar] [CrossRef]
- Thieme, D.; Reuland, L.; Lindl, T.; Kruse, F.; Fuchsluger, T. Optimized human platelet lysate as novel basis for a serum-, xeno-, and additive-free corneal endothelial cell and tissue culture. J. Tissue Eng. Regen. Med. 2018, 12, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Qazi, Y.; Hamrah, P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J. Clin. Cell Immunol. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, R.; Song, Z.; Ye, S.; Sun, R.; Xue, Q.; Zhang, Z. Gene expression profiles during activation of cultured rat hepatic stellate cells by tumoral hepatocytes and fetal bovine serum. J. Cancer Res. Clin. Oncol. 2009, 136, 309. [Google Scholar] [CrossRef]
- Yang, Y.R.; Bu, F.T.; Yang, Y.; Li, H.; Huang, C.; Meng, X.M.; Zhang, L.; Lv, X.W.; Li, J. LEFTY2 alleviates hepatic stellate cell activation and liver fibrosis by regulating the TGF-β1/Smad3 pathway. Mol. Immunol. 2020, 126, 31–39. [Google Scholar] [CrossRef]
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694. [Google Scholar] [CrossRef]
- Ito, Y.; Oike, Y.; Yasunaga, K.; Hamada, K.; Miyata, K.; Matsumoto, S.; Sugano, S.; Tanihara, H.; Masuho, Y.; Suda, T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003, 63, 6651–6657. [Google Scholar]
- Teo, Z.; Chan, J.S.K.; Chong, H.C.; Sng, M.K.; Choo, C.C.; Phua, G.Z.M.; Teo, D.J.R.; Zhu, P.; Choong, C.; Wong, M.T.C.; et al. Angiopoietin-like 4 induces a β-catenin-mediated upregulation of ID3 in fibroblasts to reduce scar collagen expression. Sci. Rep. 2017, 7, 6303. [Google Scholar] [CrossRef]
- Chen, H.; Lui, Y.S.; Tan, Z.W.; Lee, J.Y.H.; Tan, N.S.; Tan, L.P. Migration and Phenotype Control of Human Dermal Fibroblasts by Electrospun Fibrous Substrates. Adv. Health Mater. 2019, 8, e1801378. [Google Scholar] [CrossRef] [PubMed]
- Kurth, I.; Willimann, K.; Schaerli, P.; Hunziker, T.; Clark-Lewis, I.; Moser, B. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J. Exp. Med. 2001, 194, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Hermel, M.; Salla, S.; Hamsley, N.; Steinfeld, A.; Walter, P. Detection of contamination during organ culture of the human cornea. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 117–126. [Google Scholar] [CrossRef]
- Gundersen, H.J.G. Notes on the estimation of the numerical density of arbitrary profiles: The edge effect. J. Microsc. 1977, 111, 219–223. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Merkel, D. Docker: Lightweight linux containers for consistent development and deployment. Linux J. 2014, 2014, 2. [Google Scholar]
- Krueger, F.; James, F.; Ewels, P.; Afyounian, E.; Schuster-Boeckler, B. FelixKrueger/TrimGalore: v0. 6.7-DOI via Zenodo. 2021. Available online: https://zenodo.org/record/5127899#.Y9iyC61BxPZ (accessed on 9 January 2023).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chuang, E.Y.; Yoon, K.C.; de Paiva, C.S.; Shine, H.D.; Jones, D.B.; Pflugfelder, S.C.; Li, D.Q. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol. Vis. 2007, 13, 1934–1941. [Google Scholar] [PubMed]
- Gavrilova, N.A.; Borzenok, S.A.; Revishchin, A.V.; Tishchenko, O.E.; Ostrovkiy, D.S.; Bobrova, M.M.; Safonova, L.A.; Efimov, A.E.; Agapova, O.I.; Agammedov, M.B.; et al. The effect of biodegradable silk fibroin-based scaffolds containing glial cell line-derived neurotrophic factor (GDNF) on the corneal regeneration process. Int. J. Biol. Macromol. 2021, 185, 264–276. [Google Scholar] [CrossRef]
- De Roo, A.-K.; Foets, B.; van den Oord, J.J. Superficial Conjunctival Epithelium as the Main Producer of Protective Tear Component Cystatin SN. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1846. [Google Scholar]
- Theriault, M.; Parent, N.; Gendron, S.; Brunette, I.; Rochette, P.J.; Proulx, S. Secreted protease imbalance in Fuchs Corneal Endothelial Dystrophy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1358. [Google Scholar]
- Garcia, J.G.; Liu, F.; Fau-Verin, A.D.; Verin Ad Fau-Birukova, A.; Birukova, A. Fau-Dechert, M.A.; Dechert Ma Fau-Gerthoffer, W.T.; Gerthoffer Wt Fau-Bamberg, J.R.; Bamberg Fau-English, D., Jr.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef]
- Kowtharapu, B.S.; Prakasam, R.K.; Murín, R.; Koczan, D.; Stahnke, T.; Wree, A.; Jünemann, A.G.M.; Stachs, O. Role of Bone Morphogenetic Protein 7 (BMP7) in the Modulation of Corneal Stromal and Epithelial Cell Functions. Int. J. Mol. Sci. 2018, 19, 1415. [Google Scholar] [CrossRef]
- Albert, R.; Veréb, Z.; Fau-Csomós, K.; Csomós, K.; Fau-Moe, M.C.; Moe Mc Fau-Johnsen, E.O.; Johnsen Eo Fau-Olstad, O.K.; Olstad Ok Fau-Nicolaissen, B.; Nicolaissen, B.; Fau-Rajnavölgyi, E.; et al. Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium. PLoS ONE 2012, 7, e47187. [Google Scholar] [CrossRef]
- Anderegg, U.; Breitschwerdt, K.; Köhler, M.J.; Sticherling, M.; Haustein, U.F.; Simon, J.C.; Saalbach, A. MEL4B3, a novel mRNA is induced in skin tumors and regulated by TGF-beta and pro-inflammatory cytokines. Exp. Dermatol. 2005, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kabza, M.; Karolak, J.A.; Rydzanicz, M.; Szcześniak, M.W.; Nowak, D.M.; Ginter-Matuszewska, B.; Polakowski, P.; Ploski, R.; Szaflik, J.P.; Gajecka, M. Collagen synthesis disruption and downregulation of core elements of TGF-β, Hippo, and Wnt pathways in keratoconus corneas. Eur. J. Hum. Genet. 2017, 25, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.Q. Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5503–5511. [Google Scholar] [CrossRef]
- Jalkanen, R.; Mäntyjärvi, M.; Tobias, R.; Isosomppi, J.; Sankila, E.M.; Alitalo, T.; Bech-Hansen, N.T. X linked cone-rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J. Med. Genet. 2006, 43, 699–704. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, P.; Wang, Z.C.; Zou, L.; Santiago, S.; Garbitt, V.; Gjoerup, O.V.; Iglehart, J.D.; Miron, A.; Richardson, A.L.; et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci. Signal. 2009, 2, ra35. [Google Scholar] [CrossRef]
- Consortium, T.A.o.G.R. Alliance of Genome Resources Portal: Unified model organism research platform. Nucleic Acids Res. 2019, 48, D650–D658. [Google Scholar] [CrossRef]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318 Pt 1, 1–14. [Google Scholar] [CrossRef]
- Hardcastle, A.J.; Liskova, P.; Bykhovskaya, Y.; McComish, B.J.; Davidson, A.E.; Inglehearn, C.F.; Li, X.; Choquet, H.; Habeeb, M.; Lucas, S.E.M.; et al. A multi-ethnic genome-wide association study implicates collagen matrix integrity and cell differentiation pathways in keratoconus. Commun. Biol. 2021, 4, 266. [Google Scholar] [CrossRef] [PubMed]
- Beaman, E.-M.; Carter, D.R.F.; Brooks, S.A. GALNTs: Master regulators of metastasis-associated epithelial-mesenchymal transition (EMT)? Glycobiology 2022, 32, 556–579. [Google Scholar] [CrossRef]
- Frausto, R.F.; Le, D.J.; Aldave, A.J. Transcriptomic Analysis of Cultured Corneal Endothelial Cells as a Validation for Their Use in Cell Replacement Therapy. Cell Transplant. 2016, 25, 1159–1176. [Google Scholar] [CrossRef] [PubMed]
- Meijers, R.; Smock, R.G.; Zhang, Y.; Wang, J.H. Netrin Synergizes Signaling and Adhesion through DCC. Trends Biochem. Sci. 2020, 45, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, R.J.; Cesar, Q.; Chédotal, A.; Nguyen-Ba-Charvet, K.T. Revisiting the role of Dcc in visual system development with a novel eye clearing method. Elife 2020, 9, 51275. [Google Scholar] [CrossRef]
- Han, Y.; Shao, Y.; Lin, Z.; Qu, Y.-L.; Wang, H.; Zhou, Y.; Chen, W.; Chen, Y.; Chen, W.-L.; Hu, F.-R.; et al. Netrin-1 Simultaneously Suppresses Corneal Inflammation and Neovascularization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Andrysik, Z.; Bernstein, W.Z.; Deng, L.; Myer, D.L.; Li, Y.-Q.; Tischfield, J.A.; Stambrook, P.J.; Bahassi, E.M. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 2010, 38, 2931–2943. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef]
- Jeon, K.I.; Kulkarni, A.; Woeller, C.F.; Phipps, R.P.; Sime, P.J.; Hindman, H.B.; Huxlin, K.R. Inhibitory effects of PPARγ ligands on TGF-β1-induced corneal myofibroblast transformation. Am. J. Pathol. 2014, 184, 1429–1445. [Google Scholar] [CrossRef]
- Du, Y.; Sundarraj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utsumi, J.; Matsui, M.; Sudo, T.; Nakamura, N.; Mutoh, M.; Kajita, A.; Sone, S.; Kigasawa, K.; Shibuya, M.; et al. Purification, molecular cloning, and expression of a novel growth-promoting factor for retinal pigment epithelial cells, REF-1/TFPI-2. Investig. Ophthalmol. Vis. Sci. 2004, 45, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Alles, V.V.; Bottazzi, B.; Peri, G.; Golay, J.; Introna, M.; Mantovani, A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 1994, 84, 3483–3493. [Google Scholar] [CrossRef]
- Jiang, Y.; Xing, X.; Niu, T.; Wang, H.; Wang, C.; Shi, X.; Liu, K.; Su, L. Protective effect of pentraxin 3 on pathological retinal angiogenesis in an in vitro model of diabetic retinopathy. Arch. Biochem. Biophys. 2022, 725, 109283. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Li, F.; Zhang, Y.; Chen, S.Y.; Tighe, S.; Lin, S.Y.; Tseng, S.C.G. HC-HA/PTX3 Purified From Human Amniotic Membrane Reverts Human Corneal Fibroblasts and Myofibroblasts to Keratocytes by Activating BMP Signaling. Investig. Ophthalmol. Vis. Sci. 2020, 61, 62. [Google Scholar] [CrossRef]
- Doni, A.; Mantovani, A.; Bottazzi, B.; Russo, R.C. PTX3 Regulation of Inflammation, Hemostatic Response, Tissue Repair, and Resolution of Fibrosis Favors a Role in Limiting Idiopathic Pulmonary Fibrosis. Front. Immunol. 2021, 12, 676702. [Google Scholar] [CrossRef]
- Peh, G.S.; Chng, Z.; Ang, H.P.; Cheng, T.Y.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Alpha, K.M.; Xu, W.; Turner, C.E. Paxillin family of focal adhesion adaptor proteins and regulation of cancer cell invasion. Int. Rev. Cell. Mol. Biol. 2020, 355, 1–52. [Google Scholar] [CrossRef]
- Rush, J.S.; Boeving, M.A.; Berry, W.L.; Ceresa, B.P. Antagonizing c-Cbl Enhances EGFR-Dependent Corneal Epithelial Homeostasis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Rosani, U.; Tarricone, E.; Venier, P.; Brun, P.; Deligianni, V.; Zuin, M.; Martines, E.; Leonardi, A.; Brun, P. Atmospheric-Pressure Cold Plasma Induces Transcriptional Changes in Ex Vivo Human Corneas. PLoS ONE 2015, 10, e0133173. [Google Scholar] [CrossRef] [PubMed]
- Sablina, A.A.; Hector, M.; Colpaert, N.; Hahn, W.C. Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res. 2010, 70, 10474–10484. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Ohlmeyer, M. Protein phosphatase 2A as a therapeutic target in inflammation and neurodegeneration. Pharmacol. Ther. 2019, 201, 181–201. [Google Scholar] [CrossRef]
- Liu, W.B.; Li, Y.; Zhang, L.; Chen, H.G.; Sun, S.; Liu, J.P.; Liu, Y.; Li, D.W. Differential expression of the catalytic subunits for PP-1 and PP-2A and the regulatory subunits for PP-2A in mouse eye. Mol. Vis. 2008, 14, 762–773. [Google Scholar]
- Campbell, G.; Swamynathan, S.; Tiwari, A.; Swamynathan, S.K. The secreted Ly-6/uPAR related protein-1 (SLURP1) stabilizes epithelial cell junctions and suppresses TNF-α-induced cytokine production. Biochem. Biophys. Res. Commun. 2019, 517, 729–734. [Google Scholar] [CrossRef]
- Laux-Fenton, W.T.; Donaldson, P.J.; Kistler, J.; Green, C.R. Connexin expression patterns in the rat cornea: Molecular evidence for communication compartments. Cornea 2003, 22, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Belinky, F.; Nativ, N.; Stelzer, G.; Zimmerman, S.; Iny Stein, T.; Safran, M.; Lancet, D. PathCards: Multi-source consolidation of human biological pathways. Database 2015, 2015, bav006. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Bojic, S.; Dorgau, B.; Moyse, N.; Molina, M.M.; Yang, C.; Dey, S.; Reynolds, G.; et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul. Surf. 2021, 21, 279–298. [Google Scholar] [CrossRef]
- Ligocki, A.J.; Fury, W.; Gutierrez, C.; Adler, C.; Yang, T.; Ni, M.; Bai, Y.; Wei, Y.; Lehmann, G.L.; Romano, C. Molecular characteristics and spatial distribution of adult human corneal cell subtypes. Sci. Rep. 2021, 11, 16323. [Google Scholar] [CrossRef]
- VanderWall, K.B.; Lu, B.; Alfaro, J.S.; Allsop, A.R.; Carr, A.S.; Wang, S.; Meyer, J.S. Differential susceptibility of retinal ganglion cell subtypes in acute and chronic models of injury and disease. Sci. Rep. 2020, 10, 17359. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Williams, G.P.; Setiawan, M.; Yusoff, N.Z.; Lee, X.W.; Htoon, H.M.; Zhou, L.; Fuest, M.; Mehta, J.S. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci. Rep. 2017, 7, 45396. [Google Scholar] [CrossRef]
- Yue, X.; Ai, J.; Xu, Y.; Chen, Y.; Huang, M.; Yang, X.; Hu, B.; Zhang, H.; He, C.; Yang, X.; et al. Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma. Hepatology 2017, 65, 1948–1962. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Chen, X.; Cao, Y.; Yang, Z. A Bioinformatic Analysis of Correlations between Polymeric Immunoglobulin Receptor (PIGR) and Liver Fibrosis Progression. BioMed Res. Int. 2021, 2021, 5541780. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.O.; Fernandez, M.P. Expression profile and structural divergence of novel human annexin 31. FEBS Lett. 1998, 434, 300–304. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, C.; Wang, T.; Tao, L.; Zhang, Z.; Yao, J. High Expression of Annexin A9 Promotes Cell Proliferation and Migration in Gastric Cancer via the TGF-β Signaling Pathway. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Draeger, A. The annexins: Spatial and temporal coordination of signaling events during cellular stress. Cell. Mol. Life Sci. 2009, 66, 2623–2642. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, S.; Schumacher, M.; Jun, A.; Chakravarti, S. Differential gene expression patterns of the developing and adult mouse cornea compared to the lens and tendon. Exp. Eye Res. 2008, 87, 214–225. [Google Scholar] [CrossRef] [PubMed]



| Donor | Gender | Age (Years) | Death to Cornea Collection Time (Hours) | Cause of Death |
|---|---|---|---|---|
| 1 | f | 79 | 61.17 | hemorrhagic shock |
| 2 | m | 59 | 59.18 | hemorrhagic shock; thoracoabdominal aneurysm of Aorta |
| 3 | m | 61 | 38.17 | multiple organ failure; liver cirrhosis |
| 4 | f | 81 | 14.25 | multiple organ failure; hepatic failure |
| 5 | m | 64 | 71.87 | multiple organ failure; liver cirrhosis |
| 6 | f | 85 | 25.30 | renal failure |
| 7 | f | 84 | 37.48 | respiratory failure; fall |
| 8 | f | 95 | 63.33 | basilar artery occlusion, ischemic stroke |
| 9 | f | 31 | 23.65 | pancreatic carcinoma |
| 10 | m | 62 | 39.85 | hemorrhagic shock; perforated coronary bypass |
| 11 | f | 54 | 32.17 | septic shock; acute myeloid leukemia |
| 12 | f | 57 | 30.17 | pancreatic carcinoma |
| 13 | m | 73 | 41.68 | cerebral hemorrhage |
| 14 | m | 67 | 32.13 | septic shock; carcinoma of colon |
| 15 | m | 72 | 17.07 | hepatic failure; bladder carcinoma |
| 16 | f | 84 | 28.58 | rectal carcinoma |
| Mean | 69.25 | 38.50 | ||
| SD | 15.72 | 17.08 |
| Donor | Culture | ECD TP1 2%HPL Cells/mm2 | ECD TP2 2%HPL Cells/mm2 | ECD Change Cells/mm2 | ECD Change% | Culture | ECD TP1 2%FBS Cells/mm2 | ECD TP2 2%FBS Cells/mm2 | ECD Change Cells/mm2 | ECD Change % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HPL | 1935 | 1995 | 60 | 3.10 | FBS | 1905 | 2040 | 135 | 7.09 |
| 2 | HPL | 2050 | 2055 | 5 | 0.24 | FBS | 2035 | 1950 | −85 | −4.18 |
| 3 | HPL | 2600 | 2560 | −40 | −1.54 | FBS | 2375 | 2295 | −80 | −3.37 |
| 4 | HPL | 2630 | 2590 | −40 | −1.52 | FBS | 2535 | 2515 | −20 | −0.79 |
| 5 | HPL | 1970 | 1940 | −30 | −1.52 | FBS | 1855 | 1840 | −15 | −0.81 |
| 6 | HPL | 2485 | 2440 | −45 | −1.81 | FBS | 2520 | 2465 | −55 | −2.18 |
| 7 | HPL | 1610 | 1570 | −40 | −2.48 | FBS | 1350 | 1310 | −40 | −2.96 |
| 8 | HPL | 1885 | 1870 | −15 | −0.80 | FBS | 1825 | 1750 | −75 | −4.11 |
| 9 | HPL | 2094 | 2088 | −6 | −0.29 | FBS | 2340 | 2125 | −215 | −9.19 |
| 10 | HPL | 2610 | 2525 | −85 | −3.26 | FBS | 2720 | 2560 | −160 | −5.88 |
| 11 | HPL | 2315 | 2400 | 85 | 3.67 | FBS | 2445 | 2255 | −190 | −7.77 |
| 12 | HPL | 2220 | 2125 | −95 | −4.28 | FBS | 2300 | 2230 | −70 | −3.04 |
| 13 | HPL | 2095 | 2105 | 10 | 0.48 | FBS | 2350 | 2200 | −150 | −6.38 |
| 14 | HPL | 2375 | 2325 | −50 | −2.11 | FBS | 2490 | 2285 | −205 | −8.23 |
| 15 | HPL | 2290 | 2335 | 45 | 1.97 | FBS | 2255 | 2255 | 0 | 0.00 |
| 16 | HPL | 1945 | 1935 | −10 | −0.51 | FBS | 1910 | 1725 | −185 | −9.69 |
| MEAN | 2194.31 | 2178.63 | −15.69 | −0.67 | 2200.63 | 2112.50 | −88.13 | −3.84 | ||
| SD | 297.35 | 288.14 | 48.90 | 2.17 | 355.47 | 330.99 | 93.11 | 4.21 |
| Time Point | Polymegethism | Pleomorphism | Granulation | Vacuolization | Segmentation of Cell Membranes | Endothelial Cell-Free Areas | Descemet Folds | Trypan Blue-Positive Cells | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 2% HPL | 1 | Mean | 1.44 | 1.19 | 0.00 | 0.13 | 0.94 | 0.13 | 1.88 | 0.00 |
| SD | 0.73 | 0.40 | 0.00 | 0.34 | 0.25 | 0.50 | 0.50 | 0.00 | ||
| 2% HPL | 2 | Mean | 1.38 | 1.25 | 0.00 | 0.06 | 1.00 | 0.00 | 2.13 | 0.00 |
| SD | 0.62 | 0.45 | 0.00 | 0.25 | 0.00 | 0.00 | 0.50 | 0.00 | ||
| 2% FBS | 1 | Mean | 1.38 | 1.44 | 0.00 | 0.06 | 1.00 | 0.00 | 1.94 | 0.00 |
| SD | 0.72 | 0.63 | 0.00 | 0.25 | 0.00 | 0.00 | 0.57 | 0.00 | ||
| 2% FBS | 2 | Mean | 1.50 | 1.31 | 0.00 | 0.13 | 0.94 | 0.00 | 2.06 | 0.00 |
| SD | 0.63 | 0.60 | 0.00 | 0.34 | 0.25 | 0.00 | 0.44 | 0.00 |
| Gene | Full Name | Molecular Function | Mean | log2FC | ||
|---|---|---|---|---|---|---|
| E N D O U P | 1 | GFRA1 | GDNF family receptor alpha 1 | glial cell-derived neurotrophic factor receptor activity | 10.67 | 4.46 |
| 2 | GFRA2 | GDNF family receptor alpha 2 | glial cell-derived neurotrophic factor receptor activity | 9.80 | 4.07 | |
| 3 | CST1 | cystatin SN | cysteine-type endopeptidase inhibitor activity | 50.55 | 3.97 | |
| 4 | S1PR5 | sphingosine-1-phosphate receptor 5 | G protein-coupled receptor activity | 23.90 | 3.89 | |
| 5 | C5orf46 | chromosome 5 open reading frame 46 | unclear | 46.64 | 3.86 | |
| 6 | DCX | doublecortin | microtubule binding, protein kinase binding | 11.45 | 3.81 | |
| 7 | LEFTY2 | left-right determination factor 2 | cytokine activity | 50.76 | 3.74 | |
| 8 | FNDC1 | fibronectin type III domain containing 1 | activator of G protein signaling | 159.43 | 3.67 | |
| 9 | NEURL1B | neuralized E3 ubiquitin protein ligase 1B | ubiquitin protein ligase activity | 28.72 | 3.45 | |
| 10 | HMOX1 | heme oxygenase 1 | protein homodimerization activity, oxidoreductase activity | 849.53 | 3.44 | |
| E N D O D O W N | 1 | CACNA1F | calcium voltage-gated channel subunit alpha1 F | ion channel activity, high voltage-gated calcium channel activity | 5.11 | −2.90 |
| 2 | SIK1B | salt inducible kinase 1B | transferase activity, transferring phosphorus-containing groups, protein tyrosine kinase activity | 8.18 | −2.89 | |
| 3 | TTC23L | tetratricopeptide repeat domain 23 like | unclear | 8.26 | −2.76 | |
| 4 | CCDC201 | coiled-coil domain containing 201 | unclear | 5.13 | −2.70 | |
| 5 | LCN12 | lipocalin 12 | transporter activity, retinoic acid binding | 12.63 | −2.54 | |
| 6 | GALNT16 | polypeptide N-acetylgalactosaminyltransferase 16 | carbohydrate binding, polypeptide N-acetylgalactosaminyltransferase activity | 5.55 | −2.52 | |
| 7 | RANBP3L | RAN binding protein 3 like | SMAD binding activity | 118.52 | −2.49 | |
| 8 | B3GALT1 | Beta-1,3-galactosyltransferase 1 | galactosyltransferase activity, UDP-galactose:beta-N-acetylglucosamine beta-1,3-galactosyltransferase activity | 7.00 | −2.48 | |
| 9 | DCC | DCC netrin 1 receptor | transmembrane signaling receptor activity, axon guidance | 89.05 | −2.45 | |
| 10 | ETNPPL | polo like kinase 5 | regulatory kinase of cell cycle, apoptosis | 567.33 | −2.45 | |
| S T R O M A U P | 1 | MKX | mohawk homeobox | sequence-specific DNA binding, DNA-binding transcription activator activity, RNA polymerase II-specific | 7.36 | 1.83 |
| 2 | ANGPTL4 | angiopoietin like 4 | enzyme inhibitor activity (inactivation of the lipoprotein lipase LPL) | 156.66 | 1.68 | |
| 3 | HMOX1 | heme oxygenase 1 | protein homodimerization activity, oxidoreductase activity | 428.25 | 1.35 | |
| 4 | PLIN2 | perilipin 2 | lipid globule surface membrane material | 2202.47 | 1.29 | |
| 5 | PDK4 | pyruvate dehydrogenase kinase 4 | protein kinase activity | 106.98 | 1.26 | |
| 6 | TFPI2 | tissue factor pathway inhibitor 2 | serine-type endopeptidase inhibitor activity, peptidase inhibitor activity | 78.66 | 1.25 | |
| 7 | PTX3 | pentraxin 3 | virion binding, (1->3)-beta-D-glucan binding | 705.56 | 1.24 | |
| 8 | ESM1 | endothelial cell specific molecule 1 | integrin binding, hepatocyte growth factor receptor binding | 50.20 | 1.12 | |
| 9 | LPXN | leupaxin | focal adhesion protein, cell type-specific signaling | 58.77 | 1.07 | |
| 10 | CBL | Cbl proto-oncogene | cell signaling and protein ubiquitination | 239.57 | 1.06 | |
| S T R O M A D O W N | 1 | ANXA8L1 | annexin A8 like 1 | calcium ion binding, calcium-dependent phospholipid binding | 48.27 | −8.37 |
| 2 | PPP2R3B | protein phosphatase 2 regulatory subunit B’’beta | calcium ion binding, protein serine/threonine phosphatase activity | 19.56 | −4.37 | |
| 3 | SLURP1 | secreted LY6/PLAUR domain containing 1 | cytokine activity | 31.10 | −3.93 | |
| 4 | GJA4 | gap junction protein alpha 4 | gap junction channel activity | 11.78 | −3.42 | |
| 5 | LYPD2 | LY6/PLAUR domain containing 2 | post-translational modification: synthesis of GPI-anchored proteins | 15.07 | −3.33 | |
| 6 | CEACAM7 | CEA cell adhesion molecule 7 | Post-translational modification: synthesis of GPI-anchored proteins | 8.64 | −3.17 | |
| 7 | FSTL4 | follistatin like 4 | calcium ion binding | 7.43 | −2.90 | |
| 8 | PIGR | polymeric immunoglobulin receptor | immunoglobulin receptor activity (innate immune system) | 27.42 | −2.81 | |
| 9 | ANXA9 | annexin A9 | calcium ion binding, phospholipid binding | 15.02 | −2.80 | |
| 10 | PCDH19 | protocadherin 19 | calcium-dependent cell-adhesion protein | 42.01 | −2.51 |
| Gene_Name | baseMean | log2FoldChange | padj | sig |
|---|---|---|---|---|
| Stroma | ||||
| LUM | 7339.60 | −0.04 | 0.97 | Non-sig. |
| ALDH3A1 | 5061.16 | −0.20 | 0.81 | Non-sig. |
| ALDH1A1 | 360.23 | −0.04 | 0.96 | Non-sig. |
| COL3A1 | 322.41 | −0.63 | 0.62 | Non-sig. |
| KERA | 247.90 | −0.43 | 0.56 | Non-sig. |
| THY1 | 119.26 | −0.06 | 0.96 | Non-sig. |
| ACTA2 | 92.73 | 0.40 | 0.64 | Non-sig. |
| TNC | 52.40 | 0.16 | 0.91 | Non-sig. |
| Endothelium | ||||
| PTGDS | 7862.98 | −1.27 | 0.0000 | Sig. genes |
| AQP1 | 4592.68 | −0.26 | 0.3667 | Non-sig. |
| ATP1A1 | 2506.61 | −0.69 | 0.0000 | Sig. genes |
| SLC4A11 | 2153.22 | −1.24 | 0.0000 | Sig. genes |
| COL8A2 | 1929.74 | −0.05 | 0.8879 | Non-sig. |
| CDH2 | 1667.72 | 0.69 | 0.0000 | Sig. genes |
| SLC4A4 | 1644.20 | −0.47 | 0.0406 | Sig. genes |
| TJP1 | 579.02 | −0.27 | 0.3754 | Non-sig. |
| ENO2 | 578.72 | 0.10 | 0.7347 | Non-sig. |
| VDAC2 | 532.68 | −0.14 | 0.1763 | Non-sig. |
| VDAC3 | 522.33 | −0.25 | 0.0629 | Non-sig. |
| CLCN3 | 475.47 | 0.16 | 0.5404 | Non-sig. |
| ACTA2 | 178.71 | 0.90 | 0.1877 | Non-sig. |
| F11R | 100.05 | −0.19 | 0.6834 | Non-sig. |
| CLCN2 | 9.39 | −0.29 | 0.7173 | Non-sig. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talpan, D.; Salla, S.; Meusel, L.; Walter, P.; Kuo, C.-C.; Franzen, J.; Fuest, M. Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas. Int. J. Mol. Sci. 2023, 24, 2882. https://doi.org/10.3390/ijms24032882
Talpan D, Salla S, Meusel L, Walter P, Kuo C-C, Franzen J, Fuest M. Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas. International Journal of Molecular Sciences. 2023; 24(3):2882. https://doi.org/10.3390/ijms24032882
Chicago/Turabian StyleTalpan, Delia, Sabine Salla, Linus Meusel, Peter Walter, Chao-Chung Kuo, Julia Franzen, and Matthias Fuest. 2023. "Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas" International Journal of Molecular Sciences 24, no. 3: 2882. https://doi.org/10.3390/ijms24032882
APA StyleTalpan, D., Salla, S., Meusel, L., Walter, P., Kuo, C.-C., Franzen, J., & Fuest, M. (2023). Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas. International Journal of Molecular Sciences, 24(3), 2882. https://doi.org/10.3390/ijms24032882






