Pulsed Electric Field Ablation of Esophageal Malignancies and Mitigating Damage to Smooth Muscle: An In Vitro Study
Abstract
1. Introduction
2. Results
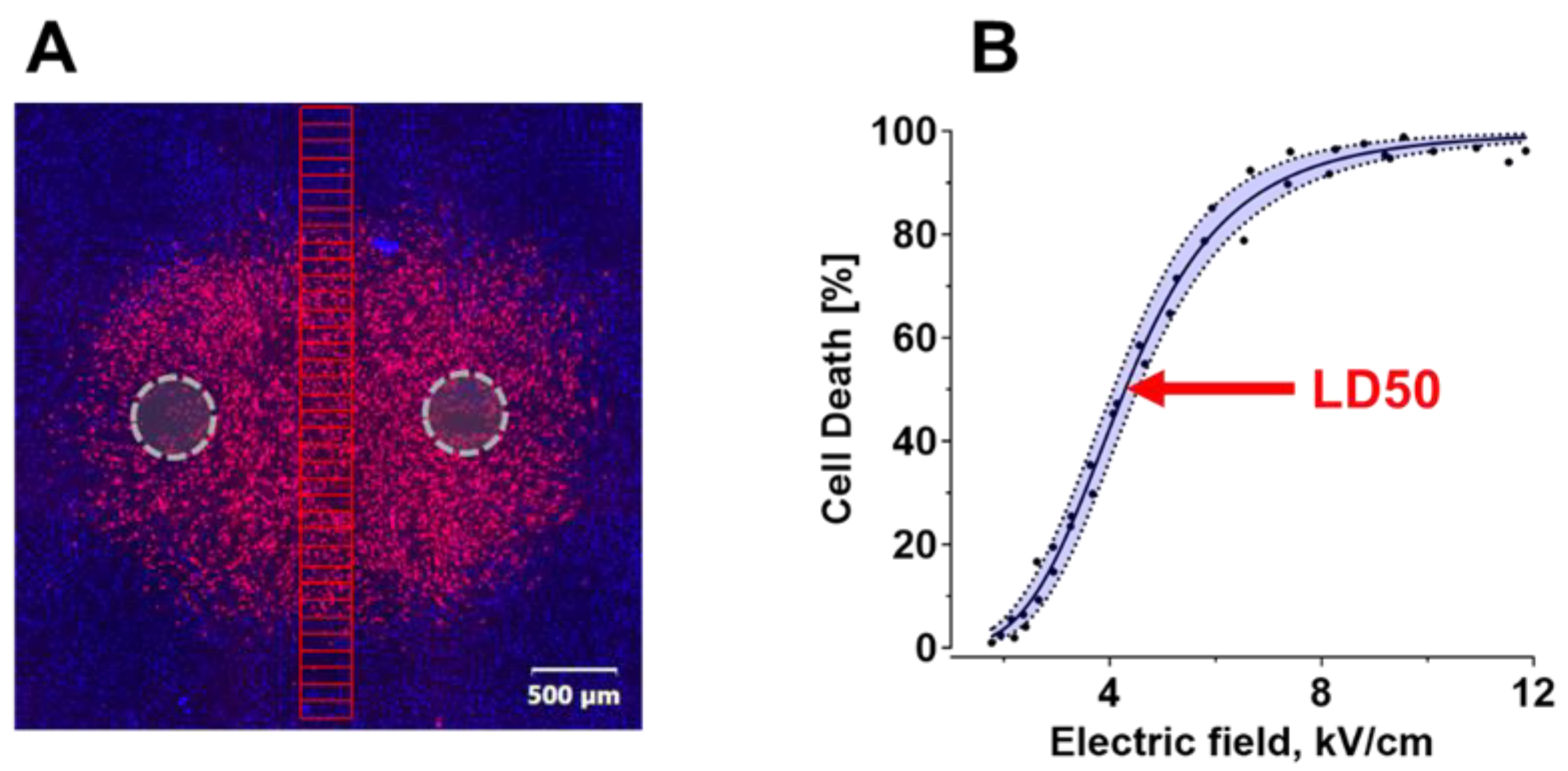
2.1. Electric Field LD50 for PEF Ablation with Uni- and Bi-Polar Pulses
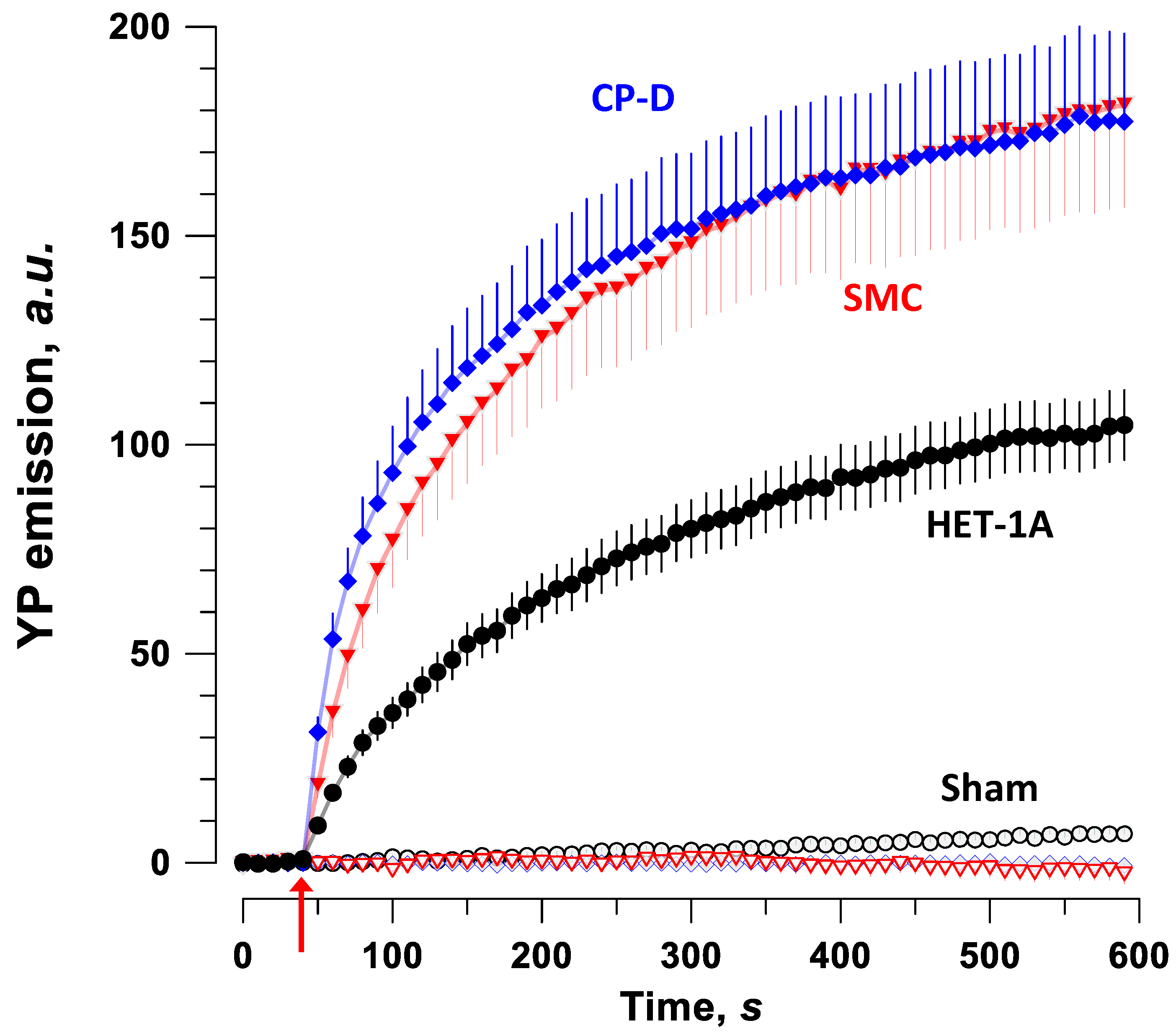
2.2. Permeabilization with PEF in Different Cell Types
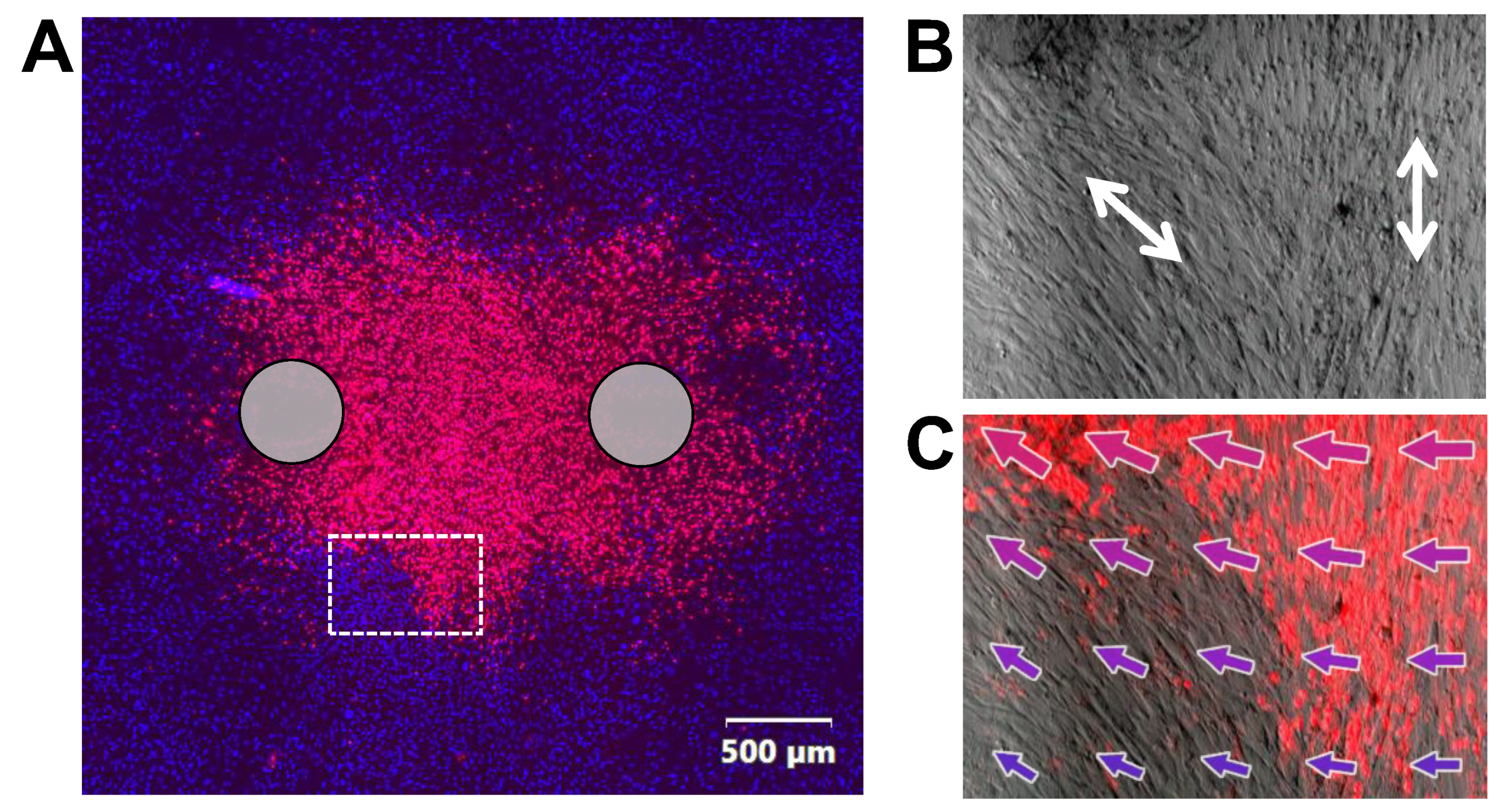
2.3. Permeabilization of SMC by Nanosecond Pulses Is Minimized by Aligning Cells with the Electric Field
2.4. Cell Alignment Impacts Membrane Charging and Relaxation of the Induced Membrane Potential
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Maintenance
4.2. PEF Generators
4.3. PEF Efficiency for Cell Killing in Monolayers
4.4. Image Analysis of PEF Lesions in a Cell Monolayer
4.5. Electroporative Dye Uptake in Individual Cells
4.6. Ca2+ Transients in Smooth Muscle Cells
4.7. Kinetics of Membrane Charging and Discharging in SMC Exposed to Nanosecond PEF
4.8. Electric Field Simulations
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Nowicki-Osuch, K.; Zhuang, L.; Jammula, S.; Bleaney, C.W.; Mahbubani, K.T.; Devonshire, G.; Katz-Summercorn, A.; Eling, N.; Wilbrey-Clark, A.; Madissoon, E. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science 2021, 373, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Early, D.S.; Fukami, N.; Ben-Menachem, T.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Fanelli, R.D.; Fisher, D.A.; Foley, K.Q. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest. Endosc. 2012, 76, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J. Barrett’s esophagus. Princ. Deglutition 2013, 315, 723–738. [Google Scholar]
- Ertan, A.; Zaheer, I.; Correa, A.M.; Thosani, N.; Blackmon, S.H. Photodynamic therapy vs radiofrequency ablation for Barrett’s dysplasia: Efficacy, safety and cost-comparison. World J. Gastroenterol. WJG 2013, 19, 7106. [Google Scholar] [CrossRef]
- Pellegatta, G.; Dal Buono, A.; Repici, A. Novel endoscopic therapies in Barrett’s esophagus: Narrative review. Ann. Esophagus 2020, 4, 29. [Google Scholar] [CrossRef]
- Lahat, E.; Eshkenazy, R.; Zendel, A.; Zakai, B.B.; Maor, M.; Dreznik, Y.; Ariche, A. Complications after percutaneous ablation of liver tumors: A systematic review. Hepatobiliary Surg. Nutr. 2014, 3, 317. [Google Scholar]
- Li, J.; Gao, M.; Zhang, M.; Liu, D.; Li, Z.; Du, J.; Hou, Y. Treatment of atrial fibrillation: A comprehensive review and practice guide. Cardiovasc. J. Afr. 2020, 31, 153–158. [Google Scholar] [CrossRef]
- Teissié, J.; Eynard, N.; Gabriel, B.; Rols, M.-P. Electropermeabilization of cell membranes. Adv. Drug Deliv. Rev. 1999, 35, 3–19. [Google Scholar] [CrossRef]
- Zimmermann, U.; Neil, G.A. Electromanipulation of Cells; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Neumann, E.; Sowers, A.E.; Jordan, C.A. Electroporation and Electrofusion in Cell Biology; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible electroporation: A new ablation modality-clinical implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef]
- Neal, R.E., 2nd; Millar, J.L.; Kavnoudias, H.; Royce, P.; Rosenfeldt, F.; Pham, A.; Smith, R.; Davalos, R.V.; Thomson, K.R. In vivo characterization and numerical simulation of prostate properties for non-thermal irreversible electroporation ablation. Prostate 2014, 74, 458–468. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: A systematic review of safety and efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011, quiz 1011. [Google Scholar] [CrossRef]
- Rubinsky, B. Irreversible Electroporation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D. High-voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Aycock, K.N.; Davalos, R.V. Irreversible electroporation: Background, theory, and review of recent developments in clinical oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef]
- Skeate, J.G.; Da Silva, D.M.; Chavez-Juan, E.; Anand, S.; Nuccitelli, R.; Kast, W.M. Nano-Pulse Stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE 2018, 13, e0191311. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Munavalli, G.S.; Zelickson, B.D.; Selim, M.M.; Kilmer, S.L.; Rohrer, T.E.; Newman, J.; Jauregui, L.; Knape, W.A.; Ebbers, E.; Uecker, D.; et al. Safety and Efficacy of Nanosecond Pulsed Electric Field Treatment of Sebaceous Gland Hyperplasia. Derm. Surg. 2020, 46, 803–809. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lu, D.S.; Osuagwu, F.; Lassman, C. Irreversible electroporation in porcine liver: Short-and long-term effect on the hepatic veins and adjacent tissue by CT with pathological correlation. Investig. Radiol. 2012, 47, 671–675. [Google Scholar] [CrossRef]
- Wendler, J.J.; Pech, M.; Blaschke, S.; Porsch, M.; Janitzky, A.; Ulrich, M.; Dudeck, O.; Ricke, J.; Liehr, U.-B. Angiography in the isolated perfused kidney: Radiological evaluation of vascular protection in tissue ablation by nonthermal irreversible electroporation. Cardiovasc. Interv. Radiol. 2012, 35, 383–390. [Google Scholar] [CrossRef]
- Joe, W.B.; Zarzour, J.G.; Gunn, A.J. Renal Cell Carcinoma Ablation: Preprocedural, Intraprocedural, and Postprocedural Imaging. Radiol. Imaging Cancer 2019, 1, e190002. [Google Scholar] [CrossRef]
- Gudvangen, E.; Kim, V.; Novickij, V.; Battista, F.; Pakhomov, A.G. Electroporation and cell killing by milli- to nanosecond pulses and avoiding neuromuscular stimulation in cancer ablation. Sci. Rep. 2022, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, B.; Arena, C.B.; Davalos, R.V.; Ivorra, A. Avoiding nerve stimulation in irreversible electroporation: A numerical modeling study. Phys. Med. Biol. 2017, 62, 8060–8079. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Di Bernardo, E.; D’Alessio, V.; Salati, S.; Cadossi, M. Reduction of muscle contraction and pain in electroporation-based treatments: An overview. World J. Clin. Oncol. 2021, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Kirks, R.C.; Latouche, E.L.; DeWitt, M.R.; Swet, J.H.; Baker, E.H.; Vrochides, D.; Iannitti, D.A.; Davalos, R.V.; McKillop, I.H. High-Frequency Irreversible Electroporation: Safety and Efficacy of Next-Generation Irreversible Electroporation Adjacent to Critical Hepatic Structures. Surg. Innov. 2017, 24, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H., Jr.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 2011, 10, 102. [Google Scholar] [CrossRef]
- Sano, M.B.; Arena, C.B.; Bittleman, K.R.; DeWitt, M.R.; Cho, H.J.; Szot, C.S.; Saur, D.; Cissell, J.M.; Robertson, J.; Lee, Y.W.; et al. Bursts of Bipolar Microsecond Pulses Inhibit Tumor Growth. Sci. Rep. 2015, 5, 14999. [Google Scholar] [CrossRef]
- Dong, S.L.; Yao, C.G.; Zhao, Y.J.; Lv, Y.P.; Liu, H.M. Parameters Optimization of Bipolar High Frequency Pulses on Tissue Ablation and Inhibiting Muscle Contraction. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 207–216. [Google Scholar] [CrossRef]
- Sano, M.B.; Fan, R.E.; Cheng, K.; Saenz, Y.; Sonn, G.A.; Hwang, G.L.; Xing, L. Reduction of Muscle Contractions during Irreversible Electroporation Therapy Using High-Frequency Bursts of Alternating Polarity Pulses: A Laboratory Investigation in an Ex Vivo Swine Model. J. Vasc. Interv. Radiol. JVIR 2018, 29, 893–898.e4. [Google Scholar] [CrossRef]
- Kim, V.; Gudvangen, E.; Kondratiev, O.; Redondo, L.; Xiao, S.; Pakhomov, A.G. Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. Int. J. Mol. Sci 2021, 22, 7051. [Google Scholar] [CrossRef]
- Reilly, J.P. Neuroelectric mechanisms applied to low frequency electric and magnetic field exposure guidelines—Part I: Sinusoidal waveforms. Health Phys. 2002, 83, 341–355. [Google Scholar] [CrossRef]
- Ibey, B.L.; Roth, C.C.; Pakhomov, A.G.; Bernhard, J.A.; Wilmink, G.J.; Pakhomova, O.N. Dose-dependent thresholds of 10-ns electric pulse induced plasma membrane disruption and cytotoxicity in multiple cell lines. PLoS ONE 2011, 6, e15642. [Google Scholar] [CrossRef]
- Ibey, B.L.; Pakhomov, A.G.; Gregory, B.W.; Khorokhorina, V.A.; Roth, C.C.; Rassokhin, M.A.; Bernhard, J.A.; Wilmink, G.J.; Pakhomova, O.N. Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim. Biophys. Acta 2010, 1800, 1210–1219. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Y.; Yin, D.; Koeffler, H.; Sawcer, D.; Vernier, P.; Gundersen, M. Differential sensitivities of malignant and normal skin cells to nanosecond pulsed electric fields. Technol. Cancer Res. Treat. 2011, 10, 281–286. [Google Scholar] [CrossRef]
- Yin, D.; Yang, W.G.; Weissberg, J.; Goff, C.B.; Chen, W.; Kuwayama, Y.; Leiter, A.; Xing, H.; Meixel, A.; Gaut, D. Cutaneous papilloma and squamous cell carcinoma therapy utilizing nanosecond pulsed electric fields (nsPEF). PLoS ONE 2012, 7, e43891. [Google Scholar] [CrossRef]
- Garon, E.B.; Sawcer, D.; Vernier, P.T.; Tang, T.; Sun, Y.; Marcu, L.; Gundersen, M.A.; Koeffler, H.P. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int. J. Cancer 2007, 121, 675–682. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell Mol. Life Sci. 2017, 74, 1741–1754. [Google Scholar] [CrossRef]
- Xie, F.; Varghese, F.; Pakhomov, A.G.; Semenov, I.; Xiao, S.; Philpott, J.; Zemlin, C. Ablation of myocardial tissue with nanosecond pulsed electric fields. PLoS ONE 2015, 10, e0144833. [Google Scholar] [CrossRef]
- Maor, E.; Sugrue, A.; Witt, C.; Vaidya, V.R.; DeSimone, C.V.; Asirvatham, S.J.; Kapa, S. Pulsed electric fields for cardiac ablation and beyond: A state-of-the-art review. Heart Rhythm 2019, 16, 1112–1120. [Google Scholar] [CrossRef]
- Dermol-Černe, J.; Batista Napotnik, T.; Reberšek, M.; Miklavčič, D. Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field. Sci. Rep. 2020, 10, 9149. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Lee, J.; Jiang, C.; Xiao, S.; Ibey, B.L.; Pakhomov, A.G. Electroporation of mammalian cells by nanosecond electric field oscillations and its inhibition by the electric field reversal. Sci. Rep. 2015, 5, 13818. [Google Scholar] [CrossRef]
- Bo, W.; Silkunas, M.; Mangalanathan, U.; Novickij, V.; Casciola, M.; Semenov, I.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Probing Nanoelectroporation and Resealing of the Cell Membrane by the Entry of Ca2+ and Ba2+ Ions. Int. J. Mol. Sci. 2020, 21, 3386. [Google Scholar] [CrossRef] [PubMed]
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Kiester, A.S.; Ibey, B.L.; Coker, Z.N.; Pakhomov, A.G.; Bixler, J.N. Strobe photography mapping of cell membrane potential with nanosecond resolution. Bioelectrochemistry 2021, 142, 107929. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Semenov, I.; Kiester, A.S.; Keppler, M.A.; Ibey, B.L.; Bixler, J.N.; Pakhomov, A.G. Action spectra and mechanisms of (in)efficiency of bipolar electric pulses at electroporation. Bioelectrochemistry 2022, 149, 108319. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Semenov, I.; Casciola, M.; Xiao, S. Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye. Biochim. Biophys. Acta 2017, 1859, 1273–1281. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Pakhomova, O.N. Nanopores: A distinct transmembrane passageway in electroporated cells. In Advanced Electroporation Techniques in Biology in Medicine; Pakhomov, A.G., Miklavcic, D., Markov, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 178–194. [Google Scholar]
- Bhattacharya, S.; Silkunas, M.; Gudvangen, E.; Mangalanathan, U.; Pakhomova, O.N.; Pakhomov, A.G. Ca2+ dependence and kinetics of cell membrane repair after electropermeabilization. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183823. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.; Semenov, I.; Pakhomov, A.G. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim. Biophys. Acta 2014, 1838, 2547–2554. [Google Scholar] [CrossRef]
- De Oliveira, P.X.; Bassani, R.A.; Bassani, J.W. Lethal effect of electric fields on isolated ventricular myocytes. IEEE Trans. Biomed. Eng. 2008, 55, 2635–2642. [Google Scholar] [CrossRef]
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and injury of adult ventricular cardiomyocytes by nano- to millisecond electric shocks. Sci. Rep. 2018, 8, 8233. [Google Scholar] [CrossRef]
- Tekle, E.; Oubrahim, H.; Dzekunov, S.M.; Kolb, J.F.; Schoenbach, K.H.; Chock, P. Selective field effects on intracellular vacuoles and vesicle membranes with nanosecond electric pulses. Biophys. J. 2005, 89, 274–284. [Google Scholar] [CrossRef]
- Kolb, J.F.; Kono, S.; Schoenbach, K.H. Nanosecond pulsed electric field generators for the study of subcellular effects. Bioelectromagnetics 2006, 27, 172–187. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Beebe, S.J.; Buescher, E.S. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics 2001, 22, 440–448. [Google Scholar] [CrossRef]
- Kandratsyeu, A.; Sabaleuski, U.; Redondo, L.; Pakhomov, A.G. Four Channel 6.5 kV, 65 A, 100 ns-100 micros Generator with Advanced Control of Pulse and Burst Protocols for Biomedical and Biotechnological Applications. Appl. Sci. 2021, 11, 11782. [Google Scholar] [CrossRef]
- Hristov, K.; Mangalanathan, U.; Casciola, M.; Pakhomova, O.N.; Pakhomov, A.G. Expression of voltage-gated calcium channels augments cell susceptibility to membrane disruption by nanosecond pulsed electric field. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 2175–2183. [Google Scholar] [CrossRef]
- Sukhorukov, V.L.; Reuss, R.; Zimmermann, D.; Held, C.; Müller, K.J.; Kiesel, M.; Geßner, P.; Steinbach, A.; Schenk, W.A.; Bamberg, E. Surviving high-intensity field pulses: Strategies for improving robustness and performance of electrotransfection and electrofusion. J. Membr. Biol. 2005, 206, 187–201. [Google Scholar] [CrossRef]
- Kiełbik, A.; Sowa, P.W.; Pakhomov, A.G.; Gudvangen, E.; Mangalanathan, U.; Kulbacka, J.; Pakhomova, O.N. Urine protects urothelial cells against killing with nanosecond pulsed electric fields. Bioelectrochemistry 2023, 149, 108289. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose-response curves. J. Pharm. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef]
- Muratori, C.; Silkuniene, G.; Mollica, P.A.; Pakhomov, A.G.; Pakhomova, O.N. The role of ESCRT-III and Annexin V in the repair of cell membrane permeabilization by the nanosecond pulsed electric field. Bioelectrochemistry 2021, 140, 107837. [Google Scholar] [CrossRef]
- Sozer, E.B.; Vernier, P.T. Modulation of biological responses to 2ns electrical stimuli by field reversal. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1228–1239. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gudvangen, E.; Xiao, S.; Semenov, I. Interference targeting of bipolar nanosecond electric pulses for spatially focused electroporation, electrostimulation, and tissue ablation. Bioelectrochemistry 2021, 141, 107876. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudvangen, E.; Mangalanathan, U.; Semenov, I.; Kiester, A.S.; Keppler, M.A.; Ibey, B.L.; Bixler, J.N.; Pakhomov, A.G. Pulsed Electric Field Ablation of Esophageal Malignancies and Mitigating Damage to Smooth Muscle: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 2854. https://doi.org/10.3390/ijms24032854
Gudvangen E, Mangalanathan U, Semenov I, Kiester AS, Keppler MA, Ibey BL, Bixler JN, Pakhomov AG. Pulsed Electric Field Ablation of Esophageal Malignancies and Mitigating Damage to Smooth Muscle: An In Vitro Study. International Journal of Molecular Sciences. 2023; 24(3):2854. https://doi.org/10.3390/ijms24032854
Chicago/Turabian StyleGudvangen, Emily, Uma Mangalanathan, Iurii Semenov, Allen S. Kiester, Mark A. Keppler, Bennett L. Ibey, Joel N. Bixler, and Andrei G. Pakhomov. 2023. "Pulsed Electric Field Ablation of Esophageal Malignancies and Mitigating Damage to Smooth Muscle: An In Vitro Study" International Journal of Molecular Sciences 24, no. 3: 2854. https://doi.org/10.3390/ijms24032854
APA StyleGudvangen, E., Mangalanathan, U., Semenov, I., Kiester, A. S., Keppler, M. A., Ibey, B. L., Bixler, J. N., & Pakhomov, A. G. (2023). Pulsed Electric Field Ablation of Esophageal Malignancies and Mitigating Damage to Smooth Muscle: An In Vitro Study. International Journal of Molecular Sciences, 24(3), 2854. https://doi.org/10.3390/ijms24032854






