Abstract
Despite advances in supportive care and antimicrobial treatment, bacterial meningitis remains the most serious infection of the central nervous system (CNS) that poses a serious risk to life. This clinical dilemma is largely due to our insufficient knowledge of the pathology behind this disease. By controlling the entry of molecules into the CNS microenvironment, the blood–brain barrier (BBB), a highly selective cellular monolayer that is specific to the CNS’s microvasculature, regulates communication between the CNS and the rest of the body. A defining feature of the pathogenesis of bacterial meningitis is the increase in BBB permeability. So far, several contributing factors for BBB disruption have been reported, including direct cellular damage brought on by bacterial virulence factors, as well as host-specific proteins or inflammatory pathways being activated. Recent studies have demonstrated that targeting pathological factors contributing to enhanced BBB permeability is an effective therapeutic complement to antimicrobial therapy for treating bacterial meningitis. Hence, understanding how these meningitis-causing pathogens affect the BBB permeability will provide novel perspectives for investigating bacterial meningitis’s pathogenesis, prevention, and therapies. Here, we summarized the recent research progress on meningitis-causing pathogens disrupting the barrier function of BBB. This review provides handy information on BBB disruption by meningitis-causing pathogens, and helps design future research as well as develop potential combination therapies.
1. Introduction
Bacterial meningitis is an inflammation of the meninges, including the dura mater, arachnoid mater, and pia mater, in response to bacterial infection [1]. It remains an important cause of the high mortality rate and incidence of neurological sequelae [2]. Case mortality rates have ranged between 5% and 40%, while between 25% and 50% of survivors have neurological conditions such as cerebral palsy, mental retardation, blindness, deafness, and seizures [3,4]. The most frequent causes of meningitis in infants and adults worldwide are Escherichia coli (E. coli), Group B Streptococcus (GBS), Listeria monocytogenes (L. monocytogenes), Mycobacterium tuberculosis (M. tuberculosis), Streptococcus pneumoniae (S. pneumoniae), Neisseria meningitidis (N. meningitidis), and Haemophilus influenzae type b (Hib) [5,6]. A significant new zoonotic pathogen that can also cause meningitis in humans is Streptococcus suis (S. suis) [7]. Generally, the pathological process of bacterial meningitis includes the following stages, namely mucosal colonization, microbial translocation of mucous membrane and invasion into the intravascular space, followed by intravascular survival and multiplication, reaching a high degree of bacteremia, translocation over the blood–brain barrier (BBB), and invasion of the meninges and central nervous system (CNS) [8]. In addition to the BBB, there are also a blood–cerebrospinal fluid barrier at the choroid plexus and a meningeal barrier at the subarachnoidal space between the CNS and the rest of the body. Bacteria can also enter the CNS by crossing these barriers [9]. Bacteria can then increase the BBB permeability and induce pleocytosis, which causes edema, an increase in intracranial pressure, and the release of inflammatory factors from white blood cells and other host cells that have been infiltrated [10].
The CNS is a shielded environment protected by the meninges, the vertebral column, and the skull [11]. The meninges are a membranous envelope in connective tissue whose primary function is to protect the spinal cord and brain from trauma [12]. The BBB provides a selective filter tightly regulating the exchange of water, ions, oxygen, nutrients, and other compounds between the CNS and the bloodstream [13]. In addition, it protects the CNS from invasive pathogens [14]. The basement membrane, astrocytes, microglial cells, pericytes, and the microvasculature support the brain microvascular endothelial cells (BMECs), which create the wall of the blood capillaries [15,16,17]. BMECs form many tight junctions (TJs) and some adherens junctions with adjacent cells, resulting in poorer paracellular permeability [18]. TJs, which are composed of Zonula Occludens proteins (especially ZO-1, -2, and -3), Occludins, Claudins (especially Claudin-5 and -12), and junction adhesion molecules, help to create high trans-endothelial electrical resistance (TEER) and control polarity to the BBB endothelium (Figure 1) [19,20,21]. Therefore, any deterioration or separation of these proteins from their counterparts may lead to an increase in barrier permeability [22]. In addition, astrocytes and pericytes maintain the barrier property of BMECs, but their effects on microbial entry into the CNS across the BBB appear to be limited [23].
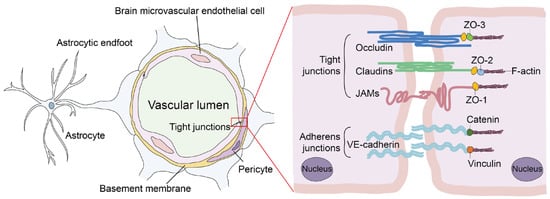
Figure 1.
Schematic architecture of cellular components and molecules involved in the regulation of BBB integrity.
Meningeal pathogens can penetrate the BBB and damage the BBB integrity, but such ability is lacking for non-meningitis-causing microorganisms [24]. With the availability of in vitro monolayer BMECs and in vivo mouse models, tremendous progress has been achieved up to this point in understanding the molecular interactions between meningeal infections and the BBB [25]. Meningeal pathogens have been shown to penetrate the host BBB by transcytosis (intracellular trafficking through the endothelial cells), paracytosis (through the intercellular junctional spaces), or Trojan horse mechanism (utilizing infected phagocytes as vehicles) [26]. Apart from these strategies, severe CNS inflammatory responses following microbial invasion and replication in the brain and the cytotoxicity of microbial toxins lead to the enhancement of BBB permeability, which also facilitates pathogens crossing the BBB [12]. Our limited knowledge of their etiology, particularly how pathogenic microorganisms increase BBB permeability, is a crucial factor contributing to such high mortality and morbidity. This paper provides an overview of the processes through which meningitis-causing bacteria increase BBB permeability. In order to maintain BBB integrity in CNS infectious disorders, it will be important to understand the molecular causes of increased BBB permeability. This will help create safer and more efficient therapeutic strategies.
2. Enhancement of BBB Permeability by Bacterial Virulence Factors
2.1. Bacterial Surface Structure
Lipopolysaccharide (LPS) is located on the outer leaflet of the outer membrane. It serves as the main surface component of the cell envelope of Gram-negative bacteria, responsible for activating the host’s innate immune system [27]. It was reported that LPS treatment reduced expression of TJs in BMECs and altered BBB structural integrity in vitro. Exposure to LPS decreased the expression of hydroxycarboxylic acid receptor 1 (HCAR1) and monocarboxylate transporters-1 in BMECs, and meanwhile induced interleukin (IL)-1β overproduction and a dose-dependent increase in lactate concentrations in the extracellular space, which led to the increase of BBB permeability [28]. Moreover, E. coli LPS affects the BBB via the crosstalk between protein kinase C (PKC) and RhoA signals, independent of the phosphatidylinositol 3-kinase (PI3K) and tyrosine kinase pathways [29]. Most recently, sulfasalazine was reported to improve the maintenance of BBB integrity and relieve E. coli LPS-induced inflammatory apoptosis [30]. In a rat model of Hib meningitis, intracisternal inoculation of Hib LPS (2 pg to 20 ng) resulted in dose-dependent increases in BBB permeability [31]. Outer membrane vesicles (OMV) are nano-sized spherical vesicles released by many Gram-negative bacteria, with a lipid bilayer structure that ranges in size from 20 to 250 nm. These vesicles include elements resembling those seen in bacteria, such as phospholipids, deoxyribonucleic acid, β-barrel proteins, lipoproteins, LPS, etc., because they are formed by the cell envelop of bacteria [32]. According to previous study, OMV may include a significant amount of Hib LPS, which could alter its activity. Intracisternal inoculation of Hib OMV in adult rats led to dose- and time-dependent increases in BBB permeability, much like inoculation of purified Hib LPS [33]. In addition, the capsule and peptidoglycan of Hib are also critical virulence factors to destroy host BBB integrity [34,35].
Bacillus anthracis (B. anthracis) is a sporulating Gram-positive rod that enters the human body mostly through the skin, and hemorrhagic meningitis is one of the deadly consequences of anthrax [36]. It has been recently shown that proteolytic breakdown of the monolayer hBMECs by B. anthracis is associated with the degradation of ZO-1. This procedure necessitates bacterial attachment to BMECs via the S-layer adhesin BslA [37].
The S. suis surface structure, including muramidase-released protein (MRP), factor H-binding protein (Fhb), and S. suis protein endopeptidase O (SsPepO), has been widely reported to destroy the integrity of BBB. MRP is a vital virulence marker of S. suis serotype 2 (SS2), which can bind to human fibrinogen [38]. It was discovered that the human fibrinogen-mediated adhesion and traversal ability of SS2 across BMECs are both considerably impaired by SS2 deletion of MRP. Meanwhile, measurement of the permeability to Evans blue extravasation in vivo and Lucifer yellow in vitro show that the MRP-human fibrinogen interaction dramatically enhances the BBB permeability via destroying the cell adherens junction protein p120-catenin of BMECs [39]. Another study showed that the Fhb contributed to S. suis-induced meningitis by interaction with globotriaosylceramide (Gb3). Gb3, also known as CD77, is restricted in expression to certain cell types, including some epithelial and endothelial cells [40]. Through Rho/Rho-associated protein kinase (ROCK) signaling, Gb3 may have an impact on the activation of myosin light chain 2 brought on by S. suis infection in hBMECs. Gb3 deficiency protected mice from severe brain inflammation or damage [41]. Furthermore, SsPepO also contributed to the pathogenesis of S. suis meningitis, which was identified as a predicted metalloendopeptidase that shares homology with the M13 peptidase family [42]. SsPepO was found to bind to human fibronectin to promote adherence and traversal ability of S. suis across monolayer hBMECs. Meanwhile, the SsPepO-human fibronectin-integrin interaction significantly increased the permeability of the BBB [43].
SS2 enolase was initially identified as a key glycolytic enzyme. Subsequently, enolase was identified to be expressed on the surface of S. suis [44]. Enolase can help pathogens infect host cells by interacting with the plasminogen receptor on the surfaces of various cell types [45]. The SS2 enolase has been shown to be important in disrupting BBB integrity by causing the release of IL-8 [46]. The extracellular adenosine of SS2 was also a contributor to promoting BBB permeability. The adenosine could activate the A1 adenosine receptor signaling pathway in BMECs and attendant cytoskeleton remodeling to damage BBB integrity. The study also found that adenosine orthologs from other bacterial species promote their translocation across BBB [47].
In addition, Streptococcus equi subsp. zooepidemicus (SEZ), which belongs to Group C streptococcal species, is another important animal pathogen and can cause meningitis in humans [48]. BifA is a recently identified virulence factor that facilitates SEZ adhesion to host tissue and immune evasion. BifA encodes a protein with an N-terminal RhuM domain and a C-terminal Fic domain. Fic (filamentation induced by cyclic AMP) domain-containing proteins are found in many animal and plant pathogens. It was reported that SEZ BifA’s Fic domain enables its binding and activation of cytoskeletal regulatory protein moesin. The phosphorylation of moesin could activate the downstream RhoA signaling pathway and thus destroy the integrity of BBB [49]. In summary, the surface structure of bacteria is considered the most critical factor in mediating the increase in permeability of the BBB (Figure 2).
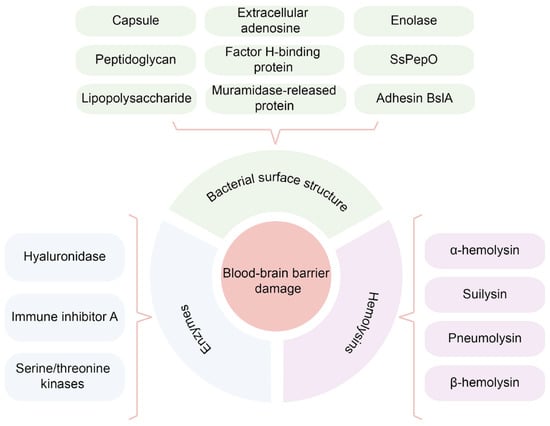
Figure 2.
A brief summary of bacterial virulence factors that affecting the BBB permeability.
2.2. Hemolysins
Hemolysin is an extracellular toxic protein produced by many Gram-negative and Gram-positive bacteria. Meningitis-causing pathogens such as E. coli, L. monocytogenes, S. pneumoniae, GBS, and S. suis can produce hemolysin [50]. As its name suggests, hemolysin is cytolytic. It binds to the host cell membrane, causing a pre-pore to form, then pierces the cell membrane and causes conformational changes within the host cell to form a mature ply pore. The mature ply pore in host cells drives protein influx and imbalances in signal transduction [51].
E. coli α-hemolysin is the best-studied repeat-in-toxin protein (repeat-in-toxin proteins are widespread among Gram-negative bacteria) released by the type I secretion system. It is an important virulence factor due to its cytolytic and cytotoxic activity against a diverse range of mammalian cell types (e.g., erythrocytes, monocytes, granulocytes, and endothelial cells) [52]. The α-hemolysin in meningitic E. coli K1 strain has been shown to reduce the TGFβ1 receptor TGFBRII and the critical transcription factor Gli2 of hedgehog signaling in BMECs, eventually leading to the BBB breakdown [53].
Another hemolysin, “suilysin”, is involved in modulating S. suis interactions with host cells [54]. Suilysin was discovered to cause a significant release of IL-6 and IL-8 by swine BMECs, destroying the integrity of the BBB [55]. In another study, suilysin was demonstrated to increase the paracellular permeability of BBB via the activation of group III secretory phospholipase A2 (PLA2G3) in vivo and in vitro [56].
Listeriolysin O, a pore-forming toxin generated by L. monocytogenes, is a particularly important virulence factor that performs many roles in guaranteeing the pathogen’s intracellular survival in hosts [57]. A result of L. monocytogenes infection in the CNS showed that listeriolysin O-mediated cytotoxicity against BMECs enables L. monocytogenes to effectively penetrate the BBB [58].
Pneumolysin is another widely studied hemolysin and a major virulence factor produced by S. pneumoniae [59]. When hBMECs were infected with S. pneumoniae, the cells rounded and detached, and the TEER of the monolayer hBMECs decreased significantly. An S. pneumoniae mutant deficient in pneumolysin did not affect the integrity of the hBMECs monolayer. However, purified pneumolysin-caused hBMECs monolayer damage was comparable to that caused by S. pneumoniae [60]. In another study, it was shown that pneumolysin causes a high expression of CREB-binding protein, which can result in the release of tumor necrosis factor-α (TNF-α) and then accelerate apoptosis of cells, which is a crucial factor contributing to BBB permeabilization [61]. In addition, pneumolysin-induced pore formation affects glial cells, altering astrocyte structure and increasing overall BBB permeability [62].
The hemolysin encoded by GBS was called β-hemolysin. It has been reported that GBS can repress the transcription of β-hemolysin under the regulation of the two-component system CovR/CovS. Moreover, the serine/threonine kinase Stk1 can phosphorylate CovR at threonine 65 to relieve the repression of β-hemolysin. Due to more β-hemolysin produced, CovR deficient GBS were more proficient in the induction of permeability and pro-inflammatory signaling pathways in BMECs [63]. In conclusion, hemolysin is one crucial virulence factor for meningitis-causing pathogens that damage the host BBB (Figure 2).
2.3. Enzymes
Bacteria produce a wide variety of secreted enzymes, including streptokinase, esterase, DNases, hyaluronidases, superoxide dismutase, and immunoglobulin degrading enzymes, many of which are considered virulence factors. Accumulating studies have shown that these enzymes are involved in the degradation of extracellular matrix between BMECs by pathogenic bacteria.
GBS produces a specific exotic enzyme, hyaluronidase (HylB). It was recently determined that HylB degrades hyaluronic acid into disaccharide fragments, which blocks toll-like receptor 2 (TLR2) and TLR4, preventing GBS ligands from activating the pro-inflammatory signaling pathway [64]. In GBS meningitis, it was found that the inactivation of HylB resulted in significantly decreased BBB permeability and the intravenous administration of purified HylB protein resulted in dose-dependent BBB opening [65]. In addition, research showed that type II CRISPR RNA-guided endonuclease Cas9 (Cas9) plays a vital role in GBS meningitis pathogenesis by repressing the regR regulator, further elevating HylB secretion that results in BBB integrity damage [66].
The metalloprotease immune inhibitor A (InhA), secreted by B. anthracis, can degrade matrix proteins. Purified InhA treatment of hBMECs resulted in a time-dependent decrease in TEER followed by ZO-1 degradation. Meanwhile, mice given purified InhA intravenously demonstrated a time-dependent Evans blue dye extravasation, leukocyte infiltration, and InhA distribution in the brain parenchyma, indicating BBB leakage and cerebral hemorrhage [22].
Eukaryotic-type serine/threonine kinases (STK) are expressed in many prokaryotes, including GBS, M. tuberculosis, and S. pneumoniae. STK was reported to regulate bacteria stress response, biofilm formation, cell wall biosynthesis, development, metabolism, antibiotic resistance, and virulence [67]. According to a recent study, the SS2 wild-type strain crossed the BBB model more easily than the STK mutant strain. STK may modulate the expression of E3 ubiquitin ligase HECTD1, increasing the degradation of Claudin-5 and allowing SS2 to cross the BBB [68]. Current studies have confirmed that bacterial enzymes can directly or indirectly affect the integrity of BBB. However, more in-depth research is still required to determine their specific contributions because of the diversity of mechanisms and functions of enzymes (Figure 2).
3. Host Signaling Mediators That Regulate BBB Permeability
3.1. Cytokines
There have been several studies on cytokines and chemokines in patients with bacterial meningitis, including the different stages of the infection process [69]. The most important factor in triggering BBB dysfunction is the formation of the CNS cytokine storm, which is caused by the excessive production of these pro-inflammatory molecules [70].
The production of various cytokines, including IL-1β, IL-6, TNF-α, and IL-8, is principally responsible for BBB breakdown during neuroinflammation [71]. TJs function alteration and BBB permeability increase are closely related to the production of cytokines (IL-1β, IL-6, and TNFα) in the brain [72]. For example, IL-6 and IL-1β were increased in the hippocampus in GBS-infected neonate rats, which increased the paracellular permeability of BMECs by decreasing TJs [73]. In addition, interferon-gamma (IFNγ), a pro-inflammatory cytokine, has been demonstrated to be a key player in the pathogenesis of experimental pneumococcal meningitis. The integrity of the BBB is impacted by IFN-modulated nitric oxide synthase 2 (NOS2), one of the many factors leading to pneumococcal meningitis pathogenesis [74]. In CNS infection with M. tuberculosis, it was shown that mycobacterium can induce granuloma formation on the monolayer BMECs, which led to cluster-associated destruction of the BMECs monolayer defined by mitochondrial stress, disruption of ZO-1 and Claudin-5, and enhanced transmigration of bacteria-infected cells across the BBB.
On the other hand, inhibition of TNF-α decreases the formation of clusters on BMECs and lessens damage from clusters [75]. It was found that the increase in BBB permeability induced by either meningitic E. coli or S. pneumoniae could be inhibited by anti-TNF-α antibodies [76]. Moreover, our study has shown that macrophage migration inhibitory factor (MIF) was significantly upregulated in meningitic E. coli infected-hBMECs. The recombinant MIF decreased the TEER values of the hBMECs monolayer dose-dependently and led to decreased expression of TJ proteins such as ZO-1 and Occludin [77].
L. monocytogenes cross the BBB in the form of “Trojan Horse”; therefore, macrophages’ migration and crossing the BBB is very important for L. monocytogenes to induce meningitis [78]. Recently, RhoA was reported to increase macrophage migration and trigger the production of IL-1β, IL-6, and TNF-α. In turn, the expression of IL-1β, IL-6, and TNF-α may facilitate the macrophage migration and adhesion across the BBB [79]. Taken together, it is clear that a variety of cytokines can increase BBB permeability by suppressing TJs, but more molecular mechanisms need to be further analyzed (Figure 3).
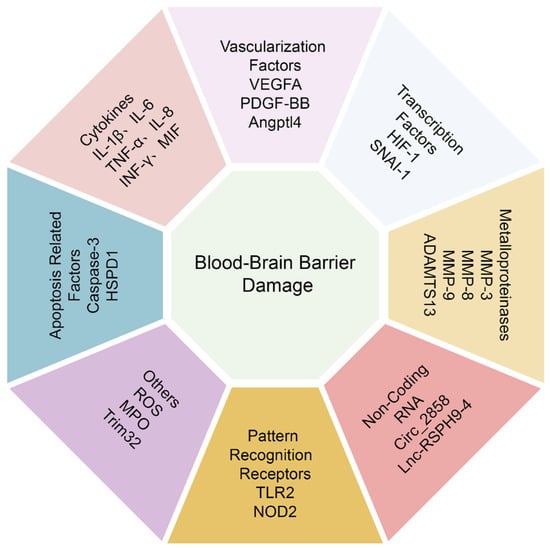
Figure 3.
A brief summary of host signaling mediators that regulating the BBB permeability.
3.2. Vascularization Factors
The mammalian vascular endothelial growth factor (VEGF) family has five members: VEGF-A, -B, -C, -D, and placental growth factor (PGF). Among them, VEGF-A has gotten the most attention. VEGF-A can promote angiogenesis and neuroprotection, and induce neurogenesis [80]. It has been known to be a potent activator of vascular permeability by activating multiple signaling pathways downstream of VEGFR2 [81]. Moreover, VEGF-A has been shown to alter the expression and distribution of TJs, resulting in BBB hyperpermeability in E. coli meningitis [82]. Most recently, resveratrol treatment was found to maintain BBB permeability by suppressing the activation of extracellular signal-regulated protein kinases1/2 (ERK1/2)-VEGFA signaling cascade [83]. Astrocytes and pericytes seem to be the primary sources of VEGF-A in pathological conditions in CNS diseases [84]. In a Haemophilus influenzae type a (HiA) study, the infection activated adenosine receptors A2A and A2B in hBMECs and pericytes, causing the pericytes to release large amount of VEGF-A. The high level of VEGF-A may cause pericytes detachment and hBMECs proliferation, thereby causing BBB damage [85]. In addition, VEGF-A also participates in the breakdown of the BBB in M. tuberculosis meningitis, further exacerbating the disease [86,87].
Platelet-derived growth factor (PDGF)-BB possesses chemotactic, differentiating, mitogenic, and angiogenic properties and is concerned with wound healing [88]. Furthermore, it is understood that PDGF-BB regulates BBB homeostasis and is essential for preserving CNS stability [89]. However, in addition to its neuroprotective effects, studies have shown that cocaine-mediated PDGF-BB induction in hBMECs resulted in BBB damage by decreasing ZO-1 expression [90]. In our work, we were the first to document how meningitic E. coli caused human and mouse BMECs to experience a time-dependent elevation of PDGF-BB, which led to TJ disarrangement [91].
Angiopoietin-like protein-4 (Angptl4) is a secreted glycoprotein with a physiological role in lipid metabolism. Angptl4 was reported to involve vascular permeability, angiogenesis, and inflammatory responses in different tissues [92]. In the field of cancer research, there are contradictory reports about the relevance of Angptl4 in regulating vascular permeability [93]. Our recent study has demonstrated that Angptl4 was markedly elevated in meningitic E. coli infection of hBMECs as well as in a mice model, and the induction of Angptl4 contributes to BBB disruption via ARHGAP5/RhoA/MYL5 signaling cascade [94]. Understanding the characterization of these vascularization targets involved in CNS infectious diseases, such as VEGF-A, PDGF-BB, and ANGPTL4 will open new opportunities for using these as potential therapeutic targets for bacterial meningitis (Figure 3).
3.3. Apoptosis Related Factors
A key cellular response called apoptosis is crucial for development and stability. Genetic studies have shown that the loss of pro-apoptotic genes leads to abnormalities in the CNS. In addition, the removal of dysfunctional cells is vital to the stability of the internal tissues and organs environment. However, when improperly controlled, apoptosis can potentially advance or even exacerbate disease processes [95]. By activating the extrinsic route, pro-inflammatory cytokines such as TNF-α, CD40/CD40 ligand, CD47, and its ligand thrombospondin-1 cause the apoptosis of BMECs [96]. Meningitis-causing pathogens, including S. pneumoniae, Haemophilus parasuis (H. parasuis), and S. suis, can induce BMECs apoptosis via several mechanisms. For instance, two different mitochondrial pathways are activated by S. pneumoniae to initiate apoptosis: a caspase-3-dependent pathway that is triggered by the physical contact between the bacteria and the BMECs, and a caspase-3-independent pathway that is triggered by pro-inflammatory components of the bacterial cell wall, or by the release of toxins like pneumolysin and H2O2 [97]. BMECs apoptosis was also detected during H. parasuis infection. In a time- and dose-dependent manner, H. parasuis induces caspase-3-mediated apoptosis of porcine BMECs [98].
It has been determined that the virulence factor SS2 Enolase affects BBB integrity. According to a recent study, SS2 Enolase binds to the 40S ribosomal protein SA on the surface of porcine BMECs. This causes the intracellular p38/ERK-eIF4E signaling pathway to be activated, which encourages the expression of the heat-shock protein family D member 1 (HSPD1) and starts the apoptosis process in the BMECs. This increases the BBB permeability and, in turn, facilitates bacterial invasion [99].
In addition, the type VI secretion system (T6SS) has recently been identified and characterized in several Gram-negative pathogens. It represents a complex secretion machinery that contributes to pathogenicity in many bacteria [100]. The Hcp1 was secreted in a T6SS-dependent manner in meningitic E. coli [101]. It was reported that Hcp1 could induce cytokines release, cytoskeleton rearrangement, and apoptosis in BMECs to damage BBB integrity [102]. BMECs apoptosis is a complex process involving distinct intracellular signaling pathways. At present, there are few studies on the apoptosis of BMECs in the field of bacterial meningitis, and further investigation is still needed (Figure 3).
3.4. Transcription Factors
HIF-1, a transcriptional factor, is linked to a variety of cerebral vascular pathological disorders. HIF-1 is a heterodimeric complex, which is mainly comprised of O2-sensitive α subunits (HIF-1α) and shared β subunits (HIF-1β), the latter being constitutively expressed under hypoxia [103]. HIF-1 could regulate transcriptional activation of several genes responsive to the lack of oxygen, such as glucose transporters, VEGF, glycolytic enzymes, and erythropoietin [104]. One of the most popular HIF-1 target genes in vascular biology is VEGF. It was reported that the upregulation of HIF-1α/VEGF pathway during S. pneumoniae infection is associated with BBB opening. In S. pneumoniae-infected mice, therapeutic rescue with the HIF-1 inhibitor echinomycin increased survival and enhanced BBB performance [105].
A zinc-finger transcription inhibitor known as Snail family transcriptional repressor 1 (SNAI1) is involved in a wide range of physiological and pathological processes, such as healthy embryonic development, epithelial injury healing, and cancer spread [106]. A growing number of studies have confirmed the role of SNAI1 in cell junctions. Overexpression of SNAI1 has been shown to destroy the top complex of vascular endothelial cells [107]. Moreover, SNAI1 can negatively regulate the expression of Claudin-5 and Occludin [108,109]. Most recently, GBS-infected hBMECs were found to increase the expression of SNAI1 that mediated the degradation of ZO-1, Occludin, and Claudin-5, and disrupted endothelial barrier integrity in cultured hBMECs [110]. In our investigation, we similarly showed that meningitic E. coli induces SNAI1 and that SNAI1 negatively regulates the junctional proteins ZO-1, Occludin, Claudin-5, and β-catenin. Although Snail-1 knocking-down did not fully restore the decreased expression of TJs, this reflected a negative effect of Snail-1 on the TJs to a certain degree [82]. The studies mentioned above show that transcription factors are important endogenous regulators in charge of controlling BBB permeability. However, the precise regulatory mechanisms involving other essential transcription factors in BBB damage still require more experimental endeavors (Figure 3).
3.5. Metalloproteinases
A class of zinc-dependent endopeptidases known as matrix metalloproteinases (MMPs) has been found to be an important regulator of the BBB integrity during bacterial meningitis [111]. Studies on CNS infectious diseases have concentrated on MMP-8 (collagenase-2) and MMP-9 (gelatinase B), as they both have the capacity to degrade basement membranes due to their substrate specificity for type IV collagen, laminin, and fibronectin, the main components of basal lamina surrounding cerebral vessels [112]. In vitro and in vivo, MMP-9 can break down Claudin-5, Occludin, and ZO-1, contributing to the breakdown of TJs [113]. Experimental evidence suggests that MMP-9, which is implicated in the breakdown of the BBB, is primarily produced by BMECs and infiltrating neutrophils [114]. For example, M. tuberculosis causes the breakdown of type IV collagen and decreases expression of TJs to increase the BBB permeability [115], which is driven by M. tuberculosis-dependent secretion of MMP-9 [116]. Except for causing direct degradation of the basement membrane and TJs, MMP-9 affects expression of TJs by inhibiting the Sonic hedgehog (Shh) signaling pathway in BMECs as well [117]. In addition, MMP-9 was found to contribute to brain damage associated with N. meningitides meningitis significantly, and inhibition of MMP-9 reduced intracranial complications in mice suffering from N. meningitides meningitis [118]. In another study, the infection of BMECs with N. meningitides could enhance permeability, which was accompanied by an increase in MMP-8 activity in supernatants taken from infected cells. MMP-8 was involved in the proteolytic cleavage of the TJs Occludin, causing it to vanish from the cell periphery. Moreover, MMP-8 affected cell adherence to the underlying matrix [119]. In a study of S. suis meningitis, dexamethasone was reported to significantly prevent S. suis-induced protein and morphological TJs alterations via attenuating MMP-3 expression [120].
In addition, a disintegrin and metalloprotease with thrombospondin type I repeats-13 (ADAMTS13) was reported to cleave a large polymeric adhesion protein von Willebrand factor, which was synthesized in vascular endothelial cells, maintaining the CNS function [121]. In L. monocytogenes-infected lambs, significantly elevated levels of ADAMTS-13 may help to control and safeguard BBB integrity and CNS cells from listeric encephalitis. Furthermore, increased ADAMTS-13 expression may be essential for promoting the survival of glia and neurons [122]. Together, these studies established that metalloproteinase activity is crucial in disassembling or maintaining cell junction components during meningitis-causing bacteria infection (Figure 3).
3.6. Non-Coding RNA
It was previously thought that only proteins were responsible for controlling BBB permeability, but non-coding RNA (ncRNA) has lately come to light as a crucial regulatory component of this process [123,124]. Many species of ncRNAs, including microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular ncRNAs (circRNAs), can directly or indirectly influence BBB integrity, which may hold therapeutic potential nucleic acid targets in the context of bacterial meningitis [15]. For example, our team discovered that circ_2858 was upregulated in BMECs after exposure to meningitic E. coli and showed that this circ_2858 might promote VEGFA production by actively competing with miR-93-5p, disrupting the TJs and impairing the BBB [125]. In another work, we revealed that meningitic E. coli infection-induced lncRSPH9-4 exacerbated TJ disruption in BMECs, most likely via the miR-17-5p/MMP3 axis [126]. Although miRNAs have been extensively studied as potential therapeutic targets, at the time of writing, new regulatory ncRNAs such as lncRNAs and circRNAs have received more attention and are still in the early phases of research. A few investigations on the modulation of BBB permeability by lncRNAs and circRNAs described indirect pathways involving miRNAs. The lncRNAs and circRNAs provide stable molecular targets that act as the magnet for the miRNAs, impairing the regulatory functions of the specific miRNAs to which the lncRNAs or circRNAs bind [17]. As a result, more strategies for protecting BBB permeability through lncRNAs and circRNAs need to be investigated (Figure 3).
3.7. Pattern-Recognition Receptors
Pattern-recognition receptors are believed to induce the expression of inflammatory factors initiating the brain immune injury. One of the most significant immune defense lines against infectious illnesses, TLRs are crucial for host defense [127]. TLR2 is involved in cell activation by the cell wall and membrane components of Gram-positive bacteria, such as lipoproteins, lipoteichoic acid, and peptidoglycan [128]. In the mouse S. pneumoniae meningitis model, the bacterial infection caused the alteration of BBB permeability in both wild-type and TLR2 deficient mice and the higher Evans blue concentration in the brains of TLR2 deficient mice, compared with the control mice. This indicates that the activation of TLR2 helps increase the permeability of the BBB [129].
The nucleotide-binding and oligomerization domain 2 protein (NOD2) belongs to the NOD-like receptor (NLR) family [130]. Studies have found that S. pneumoniae enhancement of the BBB permeability is closely related to the upregulated expression of NOD2 [131]. Since pattern-recognition receptors play crucial roles in stimulating the secretion of pro-inflammatory cytokines and subsequently the development of pro-inflammatory responses, inhibiting the activation of such pattern-recognition receptors, like TLR2 and NOD2, can effectively reduce the neuroinflammatory response and maintain the stability of BBB (Figure 3).
3.8. Others
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen that are typical byproducts of aerobic metabolism [132]. ROS are produced by immune cells and are essential for innate host defense as effectors due to their toxic action against pathogens. However, unmanaged ROS regulation during infection may result in persistent inflammatory conditions and diseases [133]. Staphylococcus aureus (S. aureus) is a common opportunistic pathogen that can cause CNS infection and elevated paracellular permeability. S. aureus infection of hBMECs resulted in the dose-dependent release of cytokines/chemokines (TNF-α, IL-6, macrophage cationic peptide 1 (MCP-1), C-X-C motif chemokine ligand 10 (CXCL10), and thrombomodulin), as well as the reduction of expression of TJs (Claudin-5 and ZO-1). These events were linked to the induction of ROS within hBMECs by S. aureus [134].
A heme-containing peroxidase known as myeloperoxidase (MPO) is largely expressed in neutrophils and to a lesser extent in monocytes. In several inflammatory illnesses, MPO has been shown to act as a local mediator of tissue injury and the inflammation, and plays a vital role in microbial killing by neutrophils [135]. Patients with bacterial meningitis showed elevated systemic and local MPO, which was accentuated during the feverish episodes. Reacting with cell-matrix metalloproteinase, MPO may contribute to BBB dysfunction [136].
Members of the tripartite motif (TRIM) protein family have a role in a number of biological functions, such as apoptosis, oncogenesis, development, differentiation, and cell proliferation [137]. Because of its wide-ranging role in triggering innate immune responses, TRIM32, a member of the TRIM protein family, is a potential candidate for causing broad and unbalanced cytokine production [138]. Following S. suis infection, TRIM32 deficiency markedly decreased bacteremia and the production of pro-inflammatory cytokines, shielding the infected mice from the streptococcal toxic shock-like syndrome. Additionally, it was discovered that during the early stages of S. suis infection, TRIM32 loss increased the BBB’s permeability and the recruitment of inflammatory monocytes [139]. This indicates that TRIM32 both positively and negatively regulates S. suis meningitis. Therefore, the function of TRIM32 in S. suis infection still needs further study to answer (Figure 3).
4. Conclusions
In the past few decades, the treatment of bacterial meningitis has mainly focused on killing invasive bacteria by using antibiotics and reducing the CNS inflammatory response by using corticosteroids [140]. However, the mortality and morbidity due to bacterial meningitis remains unacceptably high. Importantly, because of the immature development of the host defense system and BBB in neonates, the complications caused by both factors released by multiplying bacteria and as a result of the inflammatory host response to bacterial components are severe [141]. Because of the traditional treatment’s shortcomings in terms of efficacy, some new treatment strategies for bacterial meningitis have been proposed in recent years. A multimodal treatment concept that targets different steps of the pathophysiologic cascade, such as using non-bacteriolytic but bactericidal antibiotics (e.g., rifampicin and daptomycin) to limit bacterial component release, decreasing neutrophil life-span to reduce leukocyte accumulation, blocking a central proinflammatory factor (e.g., IL-1β, TNF-α, and MCP-1), and maintaining BBB permeability (e.g., vascularization factors and MMPs), represents a promising approach in the successful bacterial meningitis treatment [142]. Despite the existence of above novel treatment strategies, future work on novel therapeutic targets is still required.
In order to maintain brain homeostasis under varied circumstances, BBB permeability is highly dynamic and responsive to many internal and external cues. Changes in BBB permeability are typically caused by blood-borne substances, such as bacterial metabolites, hormones, or cytokines, which either directly affect the brain endothelium or induce an inflammatory response that leads to BMECs dysfunction [143]. Damage to the integrity of the BBB accelerates the CNS infectious diseases because this dysfunction can promote the infiltration of the leukocyte and pathogens into the CNS and accelerate the development of the disease. Therefore, the pathological analysis of BBB disruption and exploring the potential molecular targets to maintain BBB permeability are indispensable and may be a potential strategy for managing bacterial meningitis. The emphasis on possible BBB alterations in pathological and pathogenic scenarios could help design novel therapeutic strategies and optimize clinical drug administration practices.
Author Contributions
Conceptualization, X.W.; manuscript writing, R.Y.; visualization, J.W., F.W. and H.Z.; supervision, H.C. and C.T.; funding acquisition, R.Y. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
The National Key Research and Development Program of China (2021YFD1800800), the National Natural Science Foundation of China (NSFC) (32122086 and 32102749), and the China Postdoctoral Science Foundation (2021T140242 and 2022M721277) provided funding for this project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coureuil, M.; Lecuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.; Thompson, H.; Thakur, K.T. Nervous system infections and the global traveler. Semin. Neurol. 2018, 38, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Investigating bacterial penetration of the blood-brain barrier for the pathogenesis, prevention, and therapy of bacterial meningitis. ACS. Infect. Dis. 2020, 6, 34–42. [Google Scholar] [CrossRef]
- Lucas, M.J.; Brouwer, M.C.; van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 2016, 73, 18–27. [Google Scholar] [CrossRef]
- van Sorge, N.M.; Doran, K.S. Defense at the border: The blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012, 7, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; van Crevel, R.; Mai, N.T.H.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R.; et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017, 13, 581–598. [Google Scholar] [CrossRef]
- Segura, M.; Fittipaldi, N.; Calzas, C.; Gottschalk, M. Critical streptococcus suis virulence factors: Are they all really critical? Trends Microbiol. 2017, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, L.; Coureuil, M.; Nassif, X.; Bourdoulous, S. Strategies used by bacterial pathogens to cross the blood-brain barrier. Cell. Microbiol. 2020, 22, e13132. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Mollgard, K.; Habgood, M.D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. 2018, 596, 5723–5756. [Google Scholar] [CrossRef]
- Kim, K.S. Acute bacterial meningitis in infants and children. Lancet Infect. Dis. 2010, 10, 32–42. [Google Scholar] [CrossRef]
- Bayir, E.; Sendemir, A. Role of intermediate filaments in blood-brain barrier in health and disease. Cells 2021, 10, 1400. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.; Banerjee, A. Pneumococcal encounter with the blood-brain barrier endothelium. Front. Cell. Infect. Microbiol. 2020, 10, 590682. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.D.; Salimi, H.; Diamond, M.S.; Klein, R.S. Mechanisms of pathogen invasion into the central nervous system. Neuron 2019, 103, 771–783. [Google Scholar] [CrossRef]
- Yang, R.C.; Huang, F.; Fu, J.Y.; Dou, B.B.; Xu, B.J.; Miao, L.; Liu, W.T.; Yang, X.P.; Tan, C.; Chen, H.C.; et al. Differential transcription profiles of long non-coding RNAs in primary human brain microvascular endothelial cells in response to meningitic Escherichia coli. Sci. Rep. 2016, 6, 38903. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.C.; Huang, K.; Zhang, H.P.; Li, L.; Tan, C.; Chen, H.C.; Jin, M.L.; Wang, X.R. Transcriptional landscape of human neuroblastoma cells in response to SARS-CoV-2. BMC. Neurosci. 2022, 23, 43. [Google Scholar] [CrossRef]
- Yang, R.C.; Xu, B.J.; Yang, B.; Fu, J.Y.; Chen, H.C.; Wang, X.R. Non-coding RNAs: The extensive and interactive regulators of the blood-brain barrier permeability. RNA. Biol. 2021, 18, 108–116. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Campbell, M. Tight junction modulation at the blood-brain barrier: Current and future perspectives. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183298. [Google Scholar] [CrossRef]
- Yang, R.C.; Huang, K.; Zhang, H.P.; Li, L.; Zhang, Y.F.; Tan, C.; Chen, H.C.; Jin, M.L.; Wang, X.R. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J. Neuroinflamm. 2022, 19, 149. [Google Scholar] [CrossRef]
- Castro Dias, M.; Coisne, C.; Lazarevic, I.; Baden, P.; Hata, M.; Iwamoto, N.; Francisco, D.M.F.; Vanlandewijck, M.; He, L.; Baier, F.A.; et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep. 2019, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.V.; Tonry, J.H.; Kim, K.S.; Ramarao, N.; Popova, T.G.; Bailey, C.; Popov, S.; Chung, M.C. Bacillus anthracis protease InhA increases blood-brain barrier permeability and contributes to cerebral hemorrhages. PloS ONE 2011, 6, e17921. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, V.; Kim, Y.V.; Liu, S.; Kim, K.S. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain. Res. 2007, 1147, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Human meningitis-associated Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Doran, K.S.; Banerjee, A.; Disson, O.; Lecuit, M. Concepts and mechanisms: Crossing host barrier. Cold Spring Harb. Perspect. Med. 2013, 3, a010090. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Anisimov, A.P.; Kislichkina, A.A.; Kondakova, A.N.; Bystrova, O.V.; Vagaiskaya, A.S.; Shatalin, K.Y.; Shashkov, A.S.; Dentovskaya, S.V. Lipopolysaccharide of the Yersinia pseudotuberculosis complex. Biomolecules 2021, 11, 1410. [Google Scholar] [CrossRef]
- Boitsova, E.B.; Morgun, A.V.; Osipova, E.D.; Pozhilenkova, E.A.; Martinova, G.P.; Frolova, O.V.; Olovannikova, R.Y.; Tohidpour, A.; Gorina, Y.V.; Panina, Y.A.; et al. The inhibitory effect of LPS on the expression of GPR81 lactate receptor in blood-brain barrier model in vitro. J. Neuroinflamm. 2018, 15, 196. [Google Scholar] [CrossRef]
- He, F.; Yin, F.; Omran, A.; Yang, L.F.; Xiang, Q.L.; Peng, J. PKC and RhoA signals cross-talk in Escherichia coli endotoxin induced alterations in brain endothelial permeability. Biochem. Biophys. Res. Commun. 2012, 425, 182–188. [Google Scholar] [CrossRef]
- Chang, S.; Cao, Y. Sulfasalazine maintains blood-brain barrier integrity and relieves lipopolysaccharide-induced inflammation in hCMEC/D3 cells. Neuroreport 2021, 32, 672–677. [Google Scholar] [CrossRef]
- Wispelwey, B.; Lesse, A.J.; Hansen, E.J.; Scheld, W.M. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Investig. 1988, 82, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nieh, M.P.; Chen, W.; Lei, Y. Outer membrane vesicles (OMVs) enabled bio-applications: A critical review. Biotechnol. Bioeng. 2021, 119, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wispelwey, B.; Hansen, E.J.; Scheld, W.M. Haemophilus influenzae outer membrane vesicle-induced blood-brain barrier permeability during experimental meningitis. Infect. Immun. 1989, 57, 2559–2562. [Google Scholar] [CrossRef] [PubMed]
- Lesse, A.J.; Moxon, E.R.; Zwahlen, A.; Scheld, W.M. Role of cerebrospinal fluid pleocytosis and Haemophilus influenzae type b capsule on blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Investig. 1988, 82, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Roord, J.J.; Apicella, M.; Scheld, W.M. The induction of meningeal inflammation and blood-brain barrier permeability by Haemophilus influenzae type b peptidoglycan. J. Infect. Dis. 1994, 170, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.P.; Zaharia, M.; Nica, M.; Stanciu, D.; Moroti, R.; Benea, S.; Melinte, V.; Vasile, T.; Ceausu, E.; Ruta, S.; et al. Anthrax meningoencephalitis complicated with brain abscess—A case report. Int. J. Infect. Dis. 2021, 108, 217–219. [Google Scholar] [CrossRef]
- Ebrahimi, C.M.; Kern, J.W.; Sheen, T.R.; Ebrahimi-Fardooee, M.A.; van Sorge, N.M.; Schneewind, O.; Doran, K.S. Penetration of the blood-brain barrier by Bacillus anthracis requires the pXO1-encoded BslA protein. J. Bacteriol. 2009, 191, 7165–7173. [Google Scholar] [CrossRef]
- Pian, Y.; Wang, P.; Liu, P.; Zheng, Y.; Zhu, L.; Wang, H.; Xu, B.; Yuan, Y.; Jiang, Y. Proteomics identification of novel fibrinogen-binding proteins of Streptococcus suis contributing to antiphagocytosis. Front. Cell. Infect. Microbiol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Wang, J.; Kong, D.; Zhang, S.; Jiang, H.; Zheng, Y.; Zang, Y.; Hao, H.; Jiang, Y. Interaction of fibrinogen and muramidase-released protein promotes the development of Streptococcus suis meningitis. Front. Microbiol. 2015, 6, 1001. [Google Scholar] [CrossRef]
- Porubsky, S.; Federico, G.; Muthing, J.; Jennemann, R.; Gretz, N.; Buttner, S.; Obermuller, N.; Jung, O.; Hauser, I.A.; Grone, E.; et al. Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure. J. Pathol. 2014, 234, 120–133. [Google Scholar] [CrossRef]
- Kong, D.C.; Chen, Z.; Wang, J.P.; Lv, Q.Y.; Jiang, H.; Zheng, Y.L.; Xu, M.K.; Zhou, X.Y.; Hao, H.J.; Jiang, Y.Q. Interaction of factor H-binding protein of Streptococcus suis with globotriaosylceramide promotes the development of meningitis. Virulence 2017, 8, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Xia, J.; Tan, C.; Zhou, Y.; Wang, Y.; Zheng, C.K.; Chen, H.C.; Bei, W.C. Evaluation of the immunogenicity and the protective efficacy of a novel identified immunogenic protein, SsPepO, of Streptococcus suis serotype 2. Vaccine 2011, 29, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.Q.; Yan, K.; Li, H.; Sun, C.F.; Zhang, S.; Yuan, F.Y.; Wang, X.R.; Tan, C.; Chen, H.C.; et al. Binding of fibronectin to SsPepO facilitates the development of Streptococcus suis meningitis. J. Infect. Dis. 2018, 217, 973–982. [Google Scholar] [CrossRef]
- Esgleas, M.; Li, Y.; Hancock, M.A.; Harel, J.; Dubreuil, J.D.; Gottschalk, M. Isolation and characterization of alpha-enolase, a novel fibronectin-binding protein from Streptococcus suis. Microbiology 2008, 154 Pt 9, 2668–2679. [Google Scholar] [CrossRef]
- Lopez-Alemany, R.; Longstaff, C.; Hawley, S.; Mirshahi, M.; Fabregas, P.; Jardi, M.; Merton, E.; Miles, L.A.; Felez, J. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am. J. Hematol. 2003, 72, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, N.; Zhang, J.; Liu, H.; Liu, J.; Xia, X.; Sun, C.; Feng, X.; Gu, J.; Du, C.; et al. Enolase of Streptococcus Suis serotype 2 enhances blood-brain barrier permeability by inducing IL-8 release. Inflammation 2016, 39, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shang, X.; Chen, Y.; Zheng, Y.; Huang, W.; Jiang, H.; Lv, Q.; Kong, D.; Jiang, Y.; Liu, P. Bacteria elevate extracellular adenosine to exploit host signaling for blood-brain barrier disruption. Virulence 2020, 11, 980–994. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, J.; Zhang, H.; Xu, B.; Pei, X.M.; Lin, H.X.; Lu, C.P.; Fan, H.J. SILAC and LC-MS/MS identification of Streptococcus equi ssp zooepidemicus proteins that contribute to mouse brain microvascular endothelial cell infection. Appl. Microbiol. Biotechnol. 2016, 100, 7125–7136. [Google Scholar]
- Ma, Z.; Peng, J.; Yu, D.D.; Park, J.S.; Lin, H.X.; Xu, B.; Lu, C.P.; Fan, H.J.; Waldor, M.K. A streptococcal Fic domain-containing protein disrupts blood-brain barrier integrity by activating moesin in endothelial cells. PLoS Pathog. 2019, 15, e1007737. [Google Scholar] [CrossRef]
- Price, K.E.; Greene, N.G.; Camilli, A. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 2012, 194, 3651–3660. [Google Scholar] [CrossRef]
- van Pee, K.; Mulvihill, E.; Muller, D.J.; Yildiz, O. Unraveling the pore-forming steps of pneumolysin from Streptococcus pneumoniae. Nano Lett. 2016, 16, 7915–7924. [Google Scholar] [CrossRef]
- Gentschev, I.; Dietrich, G.; Goebel, W. The E. coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 2002, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Li, L.; Huo, D.; Yang, R.C.; Yang, B.; Xu, B.J.; Yang, X.P.; Dai, M.H.; Tan, C.; Chen, H.C.; et al. Meningitic Escherichia coli alpha-hemolysin aggravates blood-brain barrier disruption via targeting TGFβ1-triggered hedgehog signaling. Mol. Brain 2021, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Vanier, G.; Segura, M.; Friedl, P.; Lacouture, S.; Gottschalk, M. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun. 2004, 72, 1441–1449. [Google Scholar] [CrossRef]
- Vanier, G.; Segura, M.; Lecours, M.P.; Grenier, D.; Gottschalk, M. Porcine brain microvascular endothelial cell-derived interleukin-8 is first induced and then degraded by Streptococcus suis. Microb. Pathog. 2009, 46, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Chen, Y.; Lv, Q.; Zheng, Y.; Kong, D.; Jiang, H.; Huang, W.; Ren, Y.; Liu, P.; Jiang, Y. Suilyin disrupts the blood-brain barrier by activating group III secretory phospholipase A2. Life 2022, 12, 919. [Google Scholar] [CrossRef]
- Cossart, P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef]
- Zhang, T.; Bae, D.; Wang, C. Listeriolysin O mediates cytotoxicity against human brain microvascular endothelial cells. FEMS Microbiol. Lett. 2015, 362, fnv084. [Google Scholar] [CrossRef]
- Yau, B.; Hunt, N.H.; Mitchell, A.J.; Too, L.K. Blood-brain barrier pathology and CNS outcomes in Streptococcus pneumoniae meningitis. Int. J. Mol. Sci. 2018, 19, 3555. [Google Scholar] [CrossRef] [PubMed]
- Zysk, G.; Schneider-Wald, B.K.; Hwang, J.H.; Bejo, L.; Kim, K.S.; Mitchell, T.J.; Hakenbeck, R.; Heinz, H.P. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 2001, 69, 845–852. [Google Scholar] [CrossRef]
- Chen, J.Q.; Li, N.N.; Wang, B.W.; Liu, X.F.; Liu, J.L.; Chang, Q. Upregulation of CBP by PLY can cause permeability of blood-brain barrier to increase meningitis. J. Biochem. Mol. Toxicol. 2019, 33, e22333. [Google Scholar] [CrossRef]
- Hupp, S.; Heimeroth, V.; Wippel, C.; Fortsch, C.; Ma, J.T.; Mitchell, T.J.; Iliev, A.I. Astrocytic tissue remodeling by the meningitis neurotoxin pneumolysin facilitates pathogen tissue penetration and produces interstitial brain edema. Glia 2012, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Gurney, M.A.; Burnside, K.; Banerjee, A.; de los Reyes, M.; Connelly, J.E.; Lin, W.J.; Jewell, K.A.; Vo, A.; Renken, C.W.; et al. Regulation of CovR expression in Group B Streptococcus impacts blood-brain barrier penetration. Mol. Microbiol. 2010, 77, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Vornhagen, J.; Quach, P.; Boldenow, E.; Merillat, S.; Whidbey, C.; Ngo, L.Y.; Adams Waldorf, K.M.; Rajagopal, L. Bacterial hyaluronidase promotes ascending GBS infection and preterm birth. mBio 2016, 7, e00781-16. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Cao, Q.; Ma, K.; Wang, Z.; Liu, G.; Lu, C.; Liu, Y. Quantitative assessment of the blood-brain barrier opening caused by Streptococcus agalactiae hyaluronidase in a BALB/c mouse model. Sci. Rep. 2017, 7, 13529. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Cao, Q.; Luo, S.; Wang, Z.; Liu, G.; Lu, C.; Liu, Y. Cas9 enhances bacterial virulence by repressing the regR transcriptional regulator in Streptococcus agalactiae. Infect. Immun. 2018, 86, e00552-17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.F.; Goss, L.; Dworkin, J. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 192–212. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Weiyi, L.; Yu, M.; Hong, Z.; Jiao, Y.; Zhe, M.; Hongjie, F. The serine/threonine protein kinase of Streptococcus suis serotype 2 affects the ability of the pathogen to penetrate the blood-brain barrier. Cell. Microbiol. 2018, 20, e12862. [Google Scholar]
- Barichello, T.; Pereira, J.S.; Savi, G.D.; Generoso, J.S.; Cipriano, A.L.; Silvestre, C.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C.; Teixeira, A.L. A kinetic study of the cytokine/chemokines levels and disruption of blood-brain barrier in infant rats after pneumococcal meningitis. J. Neuroimmunol. 2011, 233, 12–17. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Bruggen, M.C.; O’Mahony, L.; Gao, Y.D.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- de Vries, H.E.; Blom-Roosemalen, M.C.; van Oosten, M.; de Boer, A.G.; van Berkel, T.J.; Breimer, D.D.; Kuiper, J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 1996, 64, 37–43. [Google Scholar] [CrossRef]
- Tanabe, S.; Gottschalk, M.; Grenier, D. Hemoglobin and Streptococcus suis cell wall act in synergy to potentiate the inflammatory response of monocyte-derived macrophages. Innate Immun. 2008, 14, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Lemos, J.C.; Generoso, J.S.; Cipriano, A.L.; Milioli, G.L.; Marcelino, D.M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C.; et al. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae. Neurochem. Res. 2011, 36, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Yau, B.; Mitchell, A.J.; Too, L.K.; Ball, H.J.; Hunt, N.H. Interferon-γ-induced nitric oxide synthase-2 contributes to blood/brain barrier dysfunction and acute mortality in experimental Streptococcus pneumoniae meningitis. J. Interferon Cytokine Res. 2016, 36, 86–99. [Google Scholar] [CrossRef]
- Gilpin, T.E.; Walter, F.R.; Herbath, M.; Wigand, K.; Sandor, M.; Fabry, Z. Mycobacterium bovis bacillus calmette-guérin-infected dendritic cells induce TNF-α-dependent cell cluster formation that damage brain endothelial cells in an in vitro model of the blood brain barrier. J. Immunol. 2020, 204, 1065–1077. [Google Scholar] [CrossRef]
- Tsao, N.; Hsu, H.P.; Wu, C.M.; Liu, C.C.; Lei, H.Y. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 2001, 50, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Lv, Y.J.; Yang, R.C.; Fu, J.Y.; Liu, L.; Wang, H.; Cao, Q.; Tan, C.; Chen, H.C.; Wang, X.R. New insights into meningitic Escherichia coli infection of brain microvascular endothelial cells from quantitative proteomics analysis. J. Neuroinflamm. 2018, 15, 291. [Google Scholar] [CrossRef] [PubMed]
- Ireton, K.; Mortuza, R.; Gyanwali, G.C.; Gianfelice, A.; Hussain, M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407–1419. [Google Scholar] [CrossRef]
- Shahid, A.D.; Lu, Y.; Iqbal, M.A.; Lin, L.; Huang, S.; Jiang, X.G.; Chen, S.X. Listeria monocytogenes crosses blood brain barrier through Rho GTPases induced migration of macrophages and inflammatory interleukin expression. Microb. Pathog. 2021, 159, 105143. [Google Scholar] [CrossRef]
- Ma, Y.; Zechariah, A.; Qu, Y.; Hermann, D.M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 2012, 90, 1873–1882. [Google Scholar] [CrossRef]
- Dzietko, M.; Derugin, N.; Wendland, M.F.; Vexler, Z.S.; Ferriero, D.M. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl. Stroke Res. 2013, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.C.; Liu, W.T.; Miao, L.; Yang, X.P.; Fu, J.Y.; Dou, B.B.; Cai, A.L.; Zong, X.; Tan, C.; Chen, H.C.; et al. Induction of VEGFA and Snail-1 by meningitic Escherichia coli mediates disruption of the blood-brain barrier. Oncotarget 2016, 7, 63839–63855. [Google Scholar] [CrossRef]
- Yang, R.C.; Lv, Y.J.; Miao, L.; Zhang, H.P.; Qu, X.Y.; Chen, J.Q.; Xu, B.J.; Yang, B.; Fu, J.Y.; Tan, C.; et al. Resveratrol attenuates meningitic Escherichia coli-mediated blood-brain barrier disruption. ACS. Infect. Dis. 2021, 7, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Pan, R.; Qin, X.J.; Yang, W.L.; Qi, Z.F.; Liu, W.L.; Liu, K.J. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J. Neurochem. 2014, 129, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Caporarello, N.; Olivieri, M.; Cristaldi, M.; Scalia, M.; Toscano, M.A.; Genovese, C.; Addamo, A.; Salmeri, M.; Lupo, G.; Anfuso, C.D. Blood-brain barrier in a Haemophilus influenzae type a in vitro infection: Role of adenosine receptors A2A and A2B. Mol. Neurobiol. 2018, 55, 5321–5336. [Google Scholar] [CrossRef]
- Zucchi, F.C.R.; Tsanaclis, A.M.C.; Moura-Dias, Q.; Silva, C.L.; Pelegrini-da-Silva, A.; Neder, L.; Takayanagui, O.M. Modulation of angiogenic factor VEGF by DNA-hsp65 vaccination in a murine CNS tuberculosis model. Tuberculosis 2013, 93, 373–380. [Google Scholar] [CrossRef]
- van der Flier, M.; Hoppenreijs, S.; van Rensburg, A.J.; Nurs, D.; Ruyken, M.; Kolk, A.H.J.; Springer, P.; Hoepelman, A.I.M.; Geelen, S.P.M.; Kimpen, J.L.L.; et al. Vascular endothelial growth factor and blood-brain barrier disruption in tuberculous meningitis. Pediatr. Infect. Dis. J. 2004, 23, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V.; Hackler, L.; Pan, W.H. Different mechanisms influencing permeation of PDGF-AA and PDGF-BB across the blood-brain barrier. J. Neurochem. 2003, 87, 7–12. [Google Scholar] [CrossRef]
- Yao, H.H.; Duan, M.; Buch, S. Cocaine-mediated induction of platelet-derived growth factor: Implication for increased vascular permeability. Blood 2011, 117, 2538–2547. [Google Scholar] [CrossRef]
- Yang, R.C.; Qu, X.Y.; Xiao, S.Y.; Li, L.; Xu, B.J.; Fu, J.Y.; Lv, Y.J.; Amjad, N.; Tan, C.; Kim, K.S.; et al. Meningitic Escherichia coli-induced upregulation of PDGF-B and ICAM-1 aggravates blood-brain barrier disruption and neuroinflammatory response. J. Neuroinflamm. 2019, 16, 101. [Google Scholar] [CrossRef]
- Fernandez-Hernando, C.; Suarez, Y. ANGPTL4: A multifunctional protein involved in metabolism and vascular homeostasis. Curr. Opin. Hematol. 2020, 27, 206–213. [Google Scholar] [CrossRef]
- Garcia-Roman, J.; Zentella-Dehesa, A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013, 335, 259–269. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.X.; Huo, D.; Peng, Z.; Yang, R.C.; Fu, J.Y.; Xu, B.J.; Yang, B.; Chen, H.C.; Wang, X.R. Meningitic Escherichia coli induction of ANGPTL4 in brain microvascular endothelial cells contributes to blood-brain barrier disruption via ARHGAP5/RhoA/MYL5 signaling cascade. Pathogens 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.T.; Leaver, H.A. Brain endothelial cell death: Modes, signaling pathways, and relevance to neural development, homeostasis, and disease. Mol. Neurobiol. 2010, 42, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C422–C432. [Google Scholar] [CrossRef]
- Bermpohl, D.; Halle, A.; Freyer, D.; Dagand, E.; Braun, J.S.; Bechmann, I.; Schroder, N.W.J.; Weber, J.R. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J. Clin. Investig. 2005, 115, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, B.; Vanier, G.; Jacques, M.; Gottschalk, M. Interactions of Haemophilus parasuis and its LOS with porcine brain microvascular endothelial cells. Vet. Res. 2008, 39, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lei, S.; Jia, L.; Xia, X.; Sun, Y.; Jiang, H.; Zhu, R.; Li, S.; Qu, G.; Gu, J.; et al. Streptococcus suis serotype 2 enolase interaction with host brain microvascular endothelial cells and RPSA-induced apoptosis lead to loss of BBB integrity. Vet. Res. 2021, 52, 30. [Google Scholar] [CrossRef] [PubMed]
- Jurenas, D.; Journet, L. Activity, delivery, and diversity of Type VI secretion effectors. Mol. Microbiol. 2021, 115, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, X.; Shou, J.; Zong, B.; Zhang, Y.; Tan, J.; Chen, J.; Hu, L.; Zhu, Y.; Chen, H.; et al. Roles of Hcp family proteins in the pathogenesis of the porcine extraintestinal pathogenic Escherichia coli type VI secretion system. Sci. Rep. 2016, 6, 26816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, J.; Yu, H.; Ni, J.; Zeng, L.; Teng, Q.; Kim, K.S.; Zhao, G.P.; Guo, X.; Yao, Y. Hcp family proteins secreted via the type VI secretion system coordinately regulate Escherichia coli K1 interaction with human brain microvascular endothelial cells. Infect. Immun. 2012, 80, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Bruick, R.K.; McKnight, S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001, 294, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Agani, F.H.; Pichiule, P.; Chavez, J.C.; LaManna, J.C. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J. Biol. Chem. 2000, 275, 35863–35867. [Google Scholar] [CrossRef] [PubMed]
- Devraj, G.; Guerit, S.; Seele, J.; Spitzer, D.; Macas, J.; Khel, M.I.; Heidemann, R.; Braczynskiz, A.K.; Ballhorn, W.; Gunther, S.; et al. HIF-1 alpha is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis. Acta Neuropathol. 2020, 140, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Arzumanyan, A.; Friedman, T.; Kotei, E.; Ng, I.O.L.; Lian, Z.; Feitelson, M.A. Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene 2012, 31, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, E.L.; Liu, C.J.; Fearon, E.R.; Margolis, B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 2008, 27, 3875–3879. [Google Scholar] [CrossRef]
- Carrozzino, F.; Soulie, P.; Huber, D.; Mensi, N.; Orci, L.; Cano, A.; Feraille, E.; Montesano, R. Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am. J. Physiol. Cell Physiol. 2005, 289, C1002–C1014. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, T.; Ozawa, M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 2004, 117, 1675–1685. [Google Scholar] [CrossRef]
- Kim, B.J.; Hancock, B.M.; Bermudez, A.; Del Cid, N.; Reyes, E.; van Sorge, N.M.; Lauth, X.; Smurthwaite, C.A.; Hilton, B.J.; Stotland, A.; et al. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J. Clin. Investig. 2015, 125, 2473–2483. [Google Scholar] [CrossRef]
- Rohlwink, U.K.; Walker, N.F.; Ordonez, A.A.; Li, Y.J.; Tucker, E.W.; Elkington, P.T.; Wilkinson, R.J.; Wilkinson, K.A. Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis-A Review. Int. J. Mol. Sci. 2019, 20, 1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.L.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Meli, D.N.; Christen, S.; Leib, S.L. Matrix metalloproteinase-9 in pneumococcal meningitis: Activation via an oxidative pathway. J. Infect. Dis. 2003, 187, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Pabisiak, P.J.; Brilha, S.; Singh, P.; Roncaroli, F.; Elkington, P.T.; Friedland, J.S. Complex regulation of neutrophil-derived MMP-9 secretion in central nervous system tuberculosis. J. Neuroinflamm. 2017, 14, 31. [Google Scholar] [CrossRef]
- Mailankody, S.; Dangeti, G.V.; Soundravally, R.; Joseph, N.M.; Mandal, J.; Dutta, T.K.; Kadhiravan, T. Cerebrospinal fluid matrix metalloproteinase 9 levels, blood-brain barrier permeability, and treatment outcome in tuberculous meningitis. PLoS ONE 2017, 12, e0181262. [Google Scholar] [CrossRef]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef]
- Ricci, S.; Grandgirard, D.; Wenzel, M.; Braccini, T.; Salvatore, P.; Oggioni, M.R.; Leib, S.L.; Koedel, U. Inhibition of matrix metalloproteinases attenuates brain damage in experimental meningococcal meningitis. BMC. Infect. Dis. 2014, 14, 726. [Google Scholar] [CrossRef]
- Schubert-Unkmeir, A.; Konrad, C.; Slanina, H.; Czapek, F.; Hebling, S.; Frosch, M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: A role for MMP-8. PLoS Pathog. 2010, 6, e1000874. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Matalon, D.; Adam, R.; Seibt, A.; Wewer, C.; Schwerk, C.; Galla, H.J.; Schroten, H. Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Res. 2008, 1229, 1–17. [Google Scholar] [CrossRef]
- Levi, M.; Scully, M.; Singer, M. The role of ADAMTS-13 in the coagulopathy of sepsis. J. Thromb. Haemost. 2018, 16, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Karayigit, M.O.; Dincel, G.C. Role of ADAMTS-13 and nNOS expression in neuropathogenesis of listeric encephalitis of small ruminants. Biotech. Histochem. 2020, 95, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, Z.; Shi, Z.S. Non-coding RNA in acute ischemic stroke: Mechanisms, biomarkers and therapeutic targets. Cell Transplant. 2018, 27, 1763–1777. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, B.; Yang, B.; Fu, J.; Liu, L.; Amjad, N.; Cai, A.; Tan, C.; Chen, H.; Wang, X. Circular RNA transcriptomic analysis of primary human brain microvascular endothelial cells infected with meningitic Escherichia coli. Mol. Ther. Nucleic Acids 2018, 13, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.; Xu, B.; Yang, B.; Fu, J.; Xiao, S.; Tan, C.; Chen, H.; Wang, X. Circ_2858 helps blood-brain barrier disruption by increasing VEGFA via sponging miR-93-5p during Escherichia coli meningitis. Mol. Ther. Nucleic Acids 2020, 22, 708–721. [Google Scholar] [CrossRef]
- Xu, B.; Yang, R.; Fu, J.; Yang, B.; Chen, J.; Tan, C.; Chen, H.; Wang, X. LncRSPH9-4 facilitates meningitic Escherichia coli-caused blood-brain barrier disruption via miR-17-5p/MMP3 axis. Int. J. Mol. Sci. 2021, 22, 6364. [Google Scholar] [CrossRef]
- Zheng, K.; He, F.L.B.; Liu, H.S.; He, Q.S. Genetic variations of toll-like receptors: Impact on susceptibility, severity and prognosis of bacterial meningitis. Infect. Genet. Evol. 2021, 93, 104984. [Google Scholar] [CrossRef]
- Brightbill, H.D.; Libraty, D.H.; Krutzik, S.R.; Yang, R.B.; Belisle, J.T.; Bleharski, J.R.; Maitland, M.; Norgard, M.V.; Plevy, S.E.; Smale, S.T.; et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999, 285, 732–736. [Google Scholar] [CrossRef]
- Echchannaoui, H.; Frei, K.; Schnell, C.; Leib, S.L.; Zimmerli, W.; Landmann, R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 2002, 186, 798–806. [Google Scholar] [CrossRef]
- Kim, H.J. Role of nucleotide-binding and oligomerization domain 2 protein (NOD2) in the development of atherosclerosis. Korean J. Physiol. Pharmacol. 2015, 19, 479–484. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Liu, Q. NOD2 expression in Streptococcus pneumoniae meningitis and its influence on the blood-brain barrier. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7292084. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.B.; Zhou, J. Role of reactive oxygen species and iron in host defense against infection. Front. Biosci. 2020, 25, 1600–1616. [Google Scholar]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.K. Mitochondrial reactive oxygen species: Double-edged weapon in host defense and pathological inflammation during infection. Front. Immunol. 2020, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, A.; Rochfort, K.D.; McDonnell, C.J.; Kerrigan, S.W.; Cummins, P.M. Staphylococcus aureus-mediated blood-brain barrier injury: An in vitro human brain microvascular endothelial cell model. Cell. Microbiol. 2017, 19, e12664. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Miric, D.; Katanic, R.; Kisic, B.; Zoric, L.; Miric, B.; Mitic, R.; Dragojevic, I. Oxidative stress and myeloperoxidase activity during bacterial meningitis: Effects of febrile episodes and the BBB permeability. Clin. Biochem. 2010, 43, 246–252. [Google Scholar] [CrossRef]
- Nisole, S.; Stoye, J.P.; Saib, A. TRIM family proteins: Retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005, 3, 799–808. [Google Scholar] [CrossRef]
- Albor, A.; El-Hizawi, S.; Horn, E.J.; Laederich, M.; Frosk, P.; Wrogemann, K.; Kulesz-Martin, M. The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkappaB. J. Biol. Chem. 2006, 281, 25850–25866. [Google Scholar] [CrossRef]
- OuYang, X.; Guo, J.; Lv, Q.; Jiang, H.; Zheng, Y.; Liu, P.; Zhao, T.; Kong, D.; Hao, H.; Jiang, Y. TRIM32 drives pathogenesis in Streptococcal toxic shock-like syndrome and Streptococcus suis meningitis by regulating innate immune responses. Infect. Immun. 2020, 88, e00957-19. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Martinez-Martin, P.; Kennedy, P.G.; Andrew Seaton, R.; Portegies, P.; Bojar, M.; Steiner, I.; Force, E.T. EFNS guideline on the management of community-acquired bacterial meningitis: Report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur. J. Neurol. 2008, 15, 649–659. [Google Scholar] [CrossRef]
- Xie, Y.; Kim, K.J.; Kim, K.S. Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS. Immunol. Med. Microbiol. 2004, 42, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Woehrl, B.; Klein, M.; Grandgirard, D.; Koedel, U.; Leib, S. Bacterial meningitis: Current therapy and possible future treatment options. Expert Rev. Anti-Infect. Ther. 2011, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).