Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury–The EPITARGET Cohort
Abstract
1. Introduction
2. Results
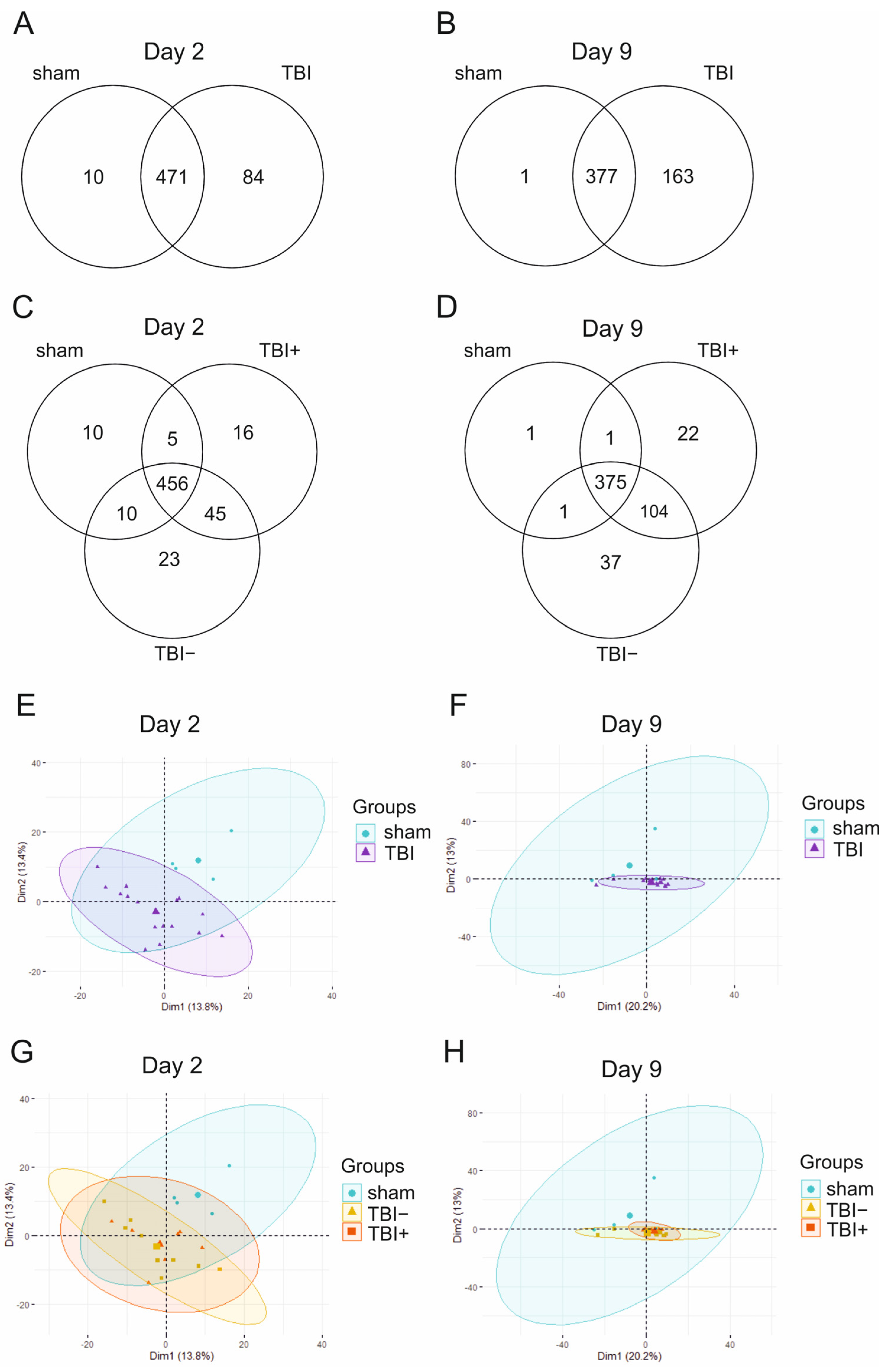
2.1. MiRNA Sequencing
2.1.1. MiRNA Quantification
2.1.2. Differential Expression Analysis
2.1.3. Expression Pattern Differences from Machine Learning Analysis
2.2. Technical Validation with RT-qPCR–Discovery Cohort
2.2.1. Selection of Differentially Expressed miRNAs for PCR Validation in D2 Samples
2.2.2. Quantitative Reverse Transcription PCR
2.3. D2 Plasma Levels of miRNAs in the Validation Cohort
2.3.1. Selection of miRNAs for ddPCR Analysis
2.3.2. Sample Quality
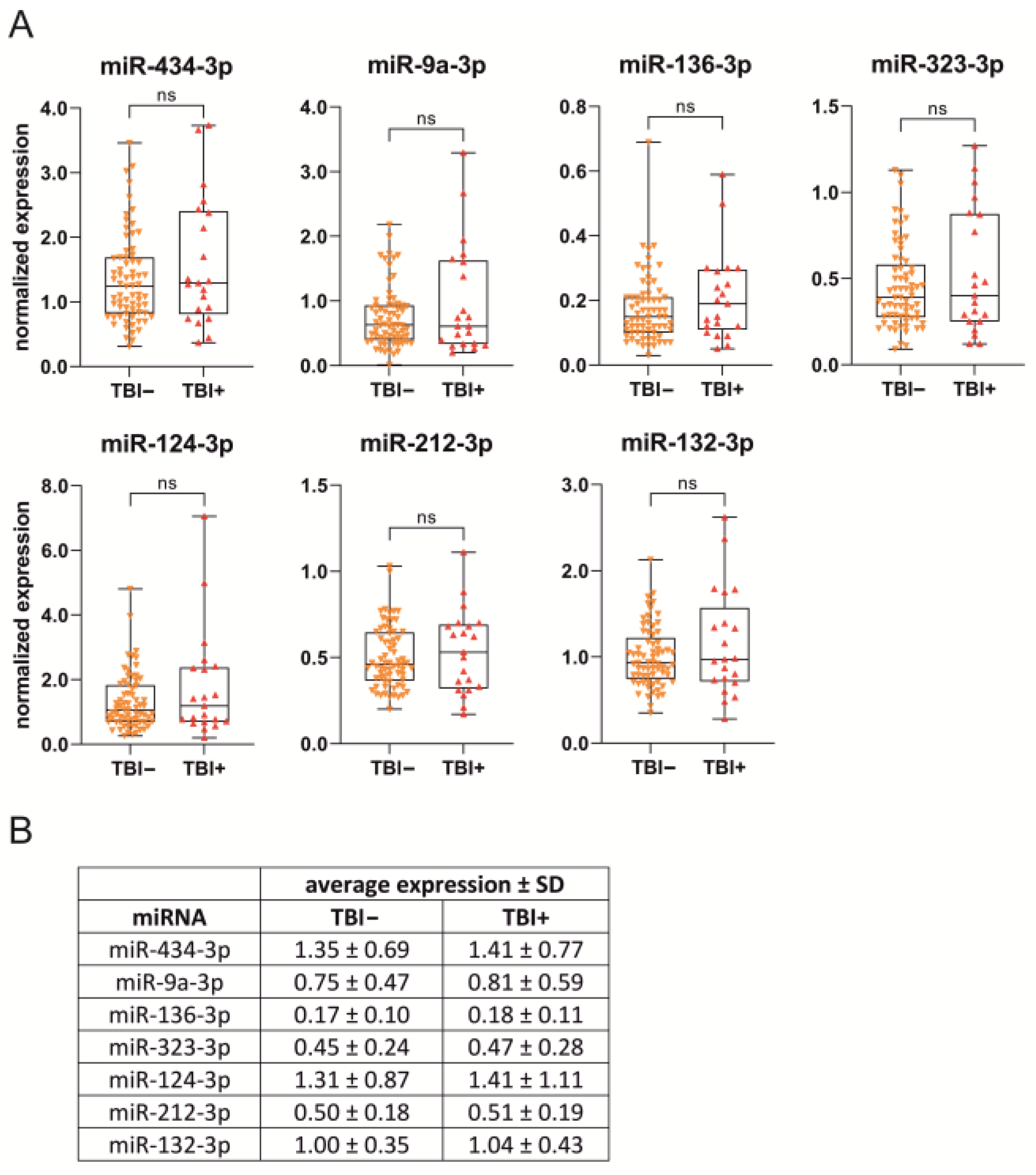
2.4. D2 Plasma miRNA Levels in Different Treatment Groups
2.4.1. Naïve vs. Sham vs. TBI
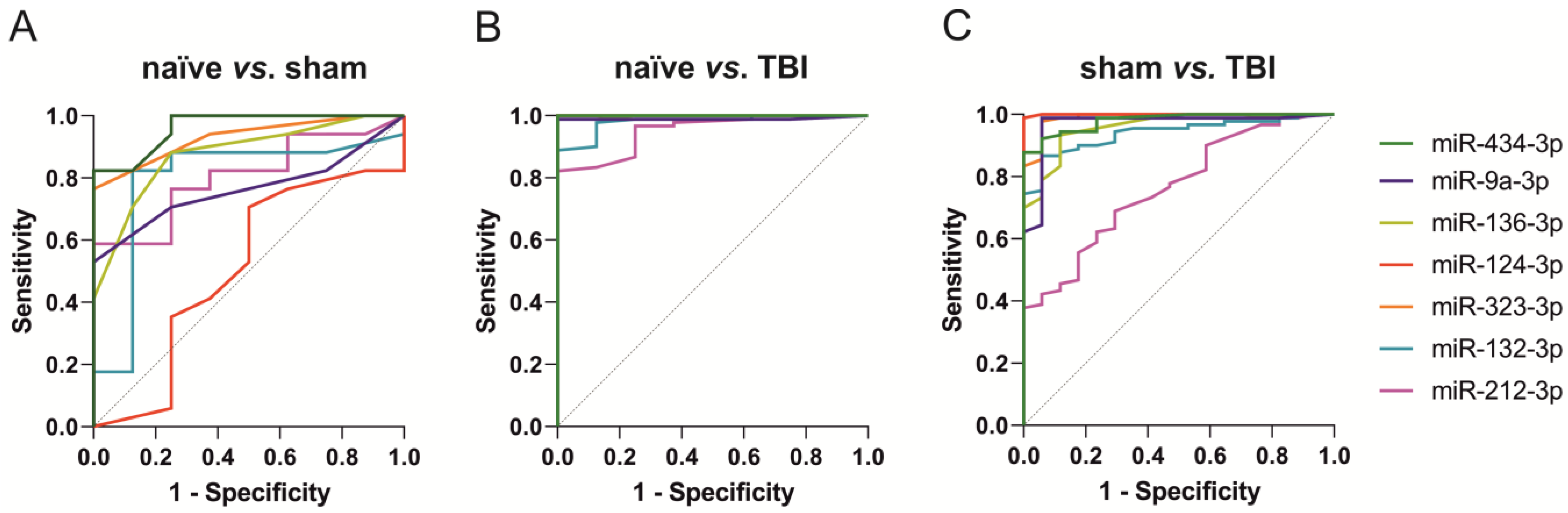
2.4.2. ROC Analysis
2.5. Epilepsy Outcome
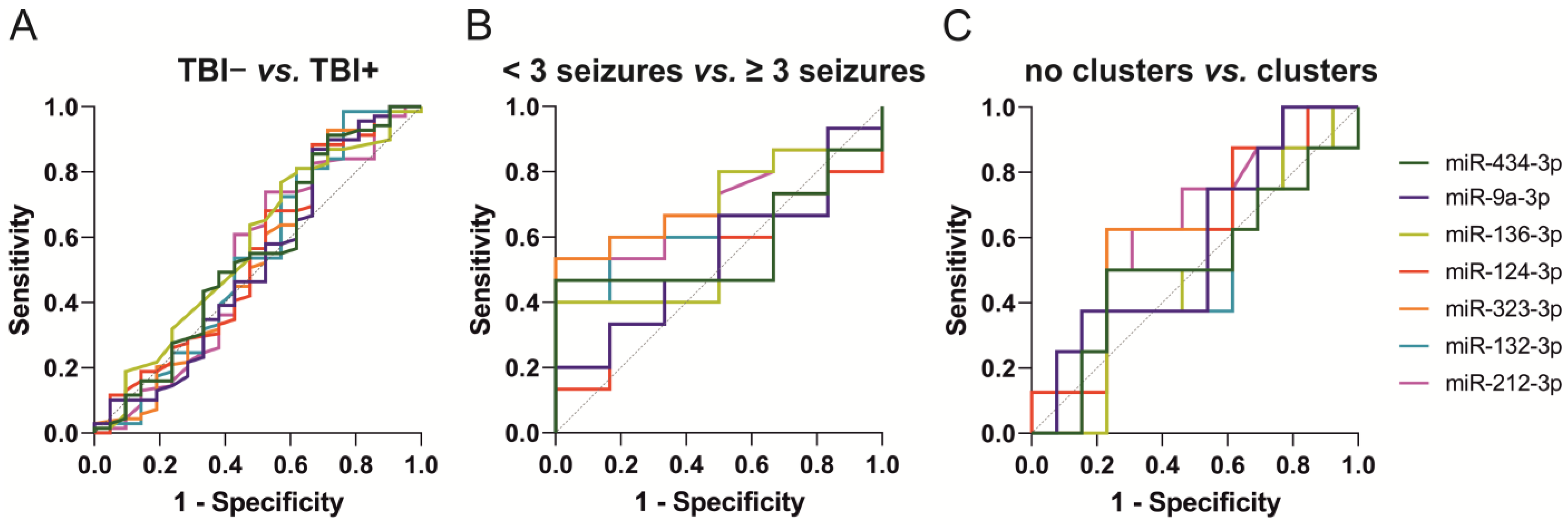
2.5.1. D2 Plasma miRNA Levels
2.5.2. ROC Analysis
2.5.3. Glmnet Analysis
2.6. Correlation of Acute Post-Injury Plasma miRNA Levels with Structural Outcome
2.6.1. Correlation of miRNA Levels with Cortical Lesion Volume in MRI
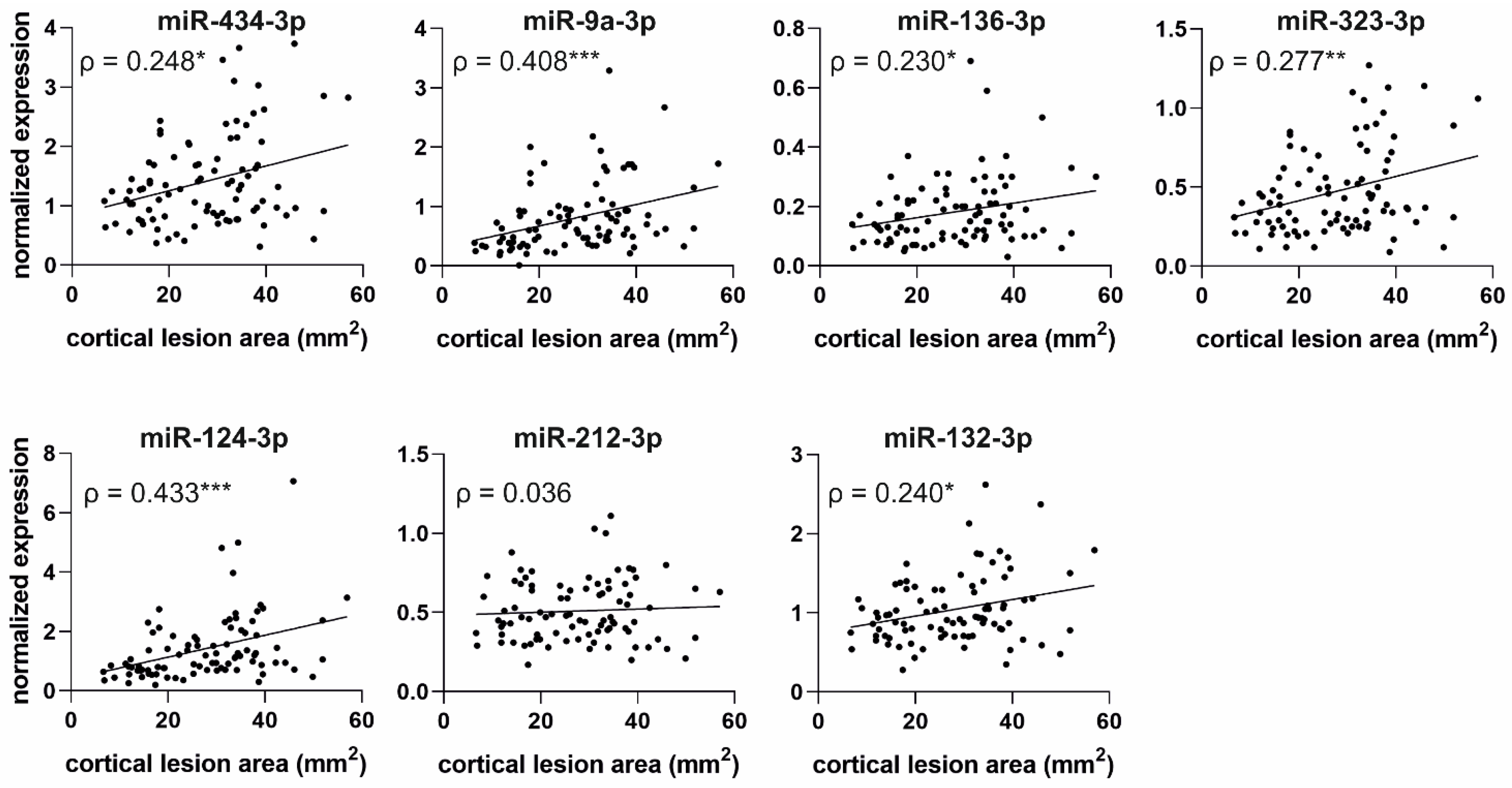
2.6.2. Correlation of miRNA Levels with Cortical Lesion Area in Unfolded Maps
2.7. MiRNA Target Analysis
3. Discussion
3.1. Acute Post-Injury Regulation of Circulating Neuron-Enriched miRNAs Is Clear but Short-Lasting
3.2. Plasma miRNA Levels Detect Mild Injury Caused by Craniotomy during Sham Operation
3.3. Functions of the Analyzed miRNAs
3.4. Acute Elevation of Plasma miRNA Levels Does Not Predict the Development of PTE
3.5. Increased Plasma miRNA Levels at an Acute Post-TBI Time-Point Correlate with a Larger Cortical Lesion Area at Acute, Subacute, and Chronic Time-Points
4. Materials and Methods
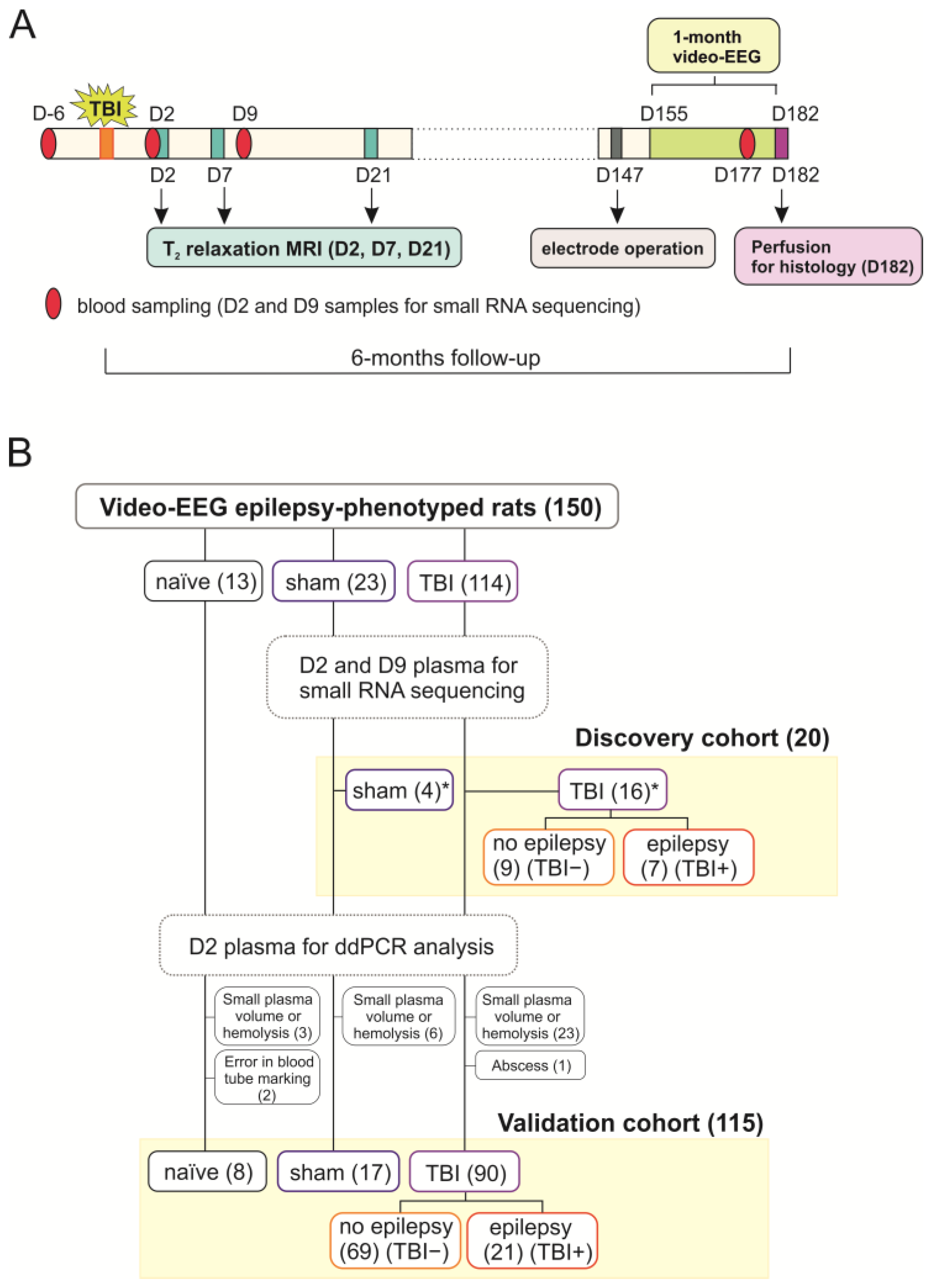
4.1. Animals
4.2. Induction of TBI by LFPI
4.3. Blood Collection
4.4. Video-EEG Monitoring
4.5. Analysis of Structural Outcome
4.5.1. Magnetic Resonance Imaging and Lesion Analysis
4.5.2. Histology and Preparation of Unfolded Maps
4.6. Small RNA Sequencing from Plasma
4.6.1. Library Preparation and Sequencing
4.6.2. Quantification of miRNAs and Differential Expression Analysis
4.6.3. Identification of Expression Pattern Differences with Machine Learning
4.6.4. Visualization of Sequencing Data
4.7. Technical Validation of miRNA-Sequencing Data by RT-qPCR
4.7.1. MiRNA Extraction from Plasma
4.7.2. Reverse Transcription
4.7.3. RT-qPCR
4.8. DdPCR Analysis
4.8.1. Plasma Quality Control
4.8.2. MiRNA Extraction from Plasma
4.8.3. DdPCR Validation of miRNAs
4.9. DdPCR Validation of Downregulated miRNAs
4.10. Glmnet Logistic Regression Analysis
4.11. Ingenuity Pathway Analysis
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitkänen, A.; Immonen, R. Epilepsy Related to Traumatic Brain Injury. Neurotherapeutics 2014, 11, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Annegers, J.F.; Hauser, W.A.; Coan, S.P.; Rocca, W.A. A Population-Based Study of Seizures after Traumatic Brain Injuries. N. Engl. J. Med. 1998, 338, 20–24. [Google Scholar] [CrossRef]
- Klein, P.; Tyrlikova, I. No Prevention or Cure of Epilepsy as Yet. Neuropharmacology 2020, 168, 107762. [Google Scholar] [CrossRef] [PubMed]
- Dulla, C.G.; Pitkänen, A. Novel Approaches to Prevent Epileptogenesis After Traumatic Brain Injury. Neurotherapeutics 2021, 18, 1582–1601. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Pitkänen, A.; Loeb, J.A.; Dudek, F.E.; Bertram, E.H.; Cole, A.J.; Moshé, S.L.; Wiebe, S.; Jensen, F.E.; Mody, I.; et al. Epilepsy Biomarkers. Epilepsia 2013, 54, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian MicroRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of MiRNA Expression across Human Tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Dogini, D.B.; Avansini, S.H.; Vieira, A.S.; Lopes-Cendes, I. MicroRNA Regulation and Dysregulation in Epilepsy. Front. Cell Neurosci. 2013, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in Epilepsy: Pathophysiology and Clinical Utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Pitkänen, A.; Paananen, T.; Kyyriäinen, J.; Das Gupta, S.; Heiskanen, M.; Vuokila, N.; Bañuelos-Cabrera, I.; Lapinlampi, N.; Kajevu, N.; Andrade, P.; et al. Biomarkers for Posttraumatic Epilepsy. Epilepsy Behav. 2021, 121, 107080. [Google Scholar] [CrossRef]
- Toffolo, K.; Osei, J.; Kelly, W.; Poulsen, A.; Donahue, K.; Wang, J.; Hunter, M.; Bard, J.; Wang, J.; Poulsen, D. Circulating MicroRNAs as Biomarkers in Traumatic Brain Injury. Neuropharmacology 2019, 145, 199–208. [Google Scholar] [CrossRef]
- Lapinlampi, N.; Andrade, P.; Paananen, T.; Hämäläinen, E.; Ekolle Ndode-Ekane, X.; Puhakka, N.; Pitkänen, A. Postinjury Weight Rather than Cognitive or Behavioral Impairment Predicts Development of Posttraumatic Epilepsy after Lateral Fluid-Percussion Injury in Rats. Epilepsia 2020, 61, 2035–2052. [Google Scholar] [CrossRef]
- Das Gupta, S.; Ciszek, R.; Heiskanen, M.; Lapinlampi, N.; Kukkonen, J.; Leinonen, V.; Puhakka, N.; Pitkänen, A. Plasma MiR-9-3p and MiR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 1563. [Google Scholar] [CrossRef]
- Bushel, P.R.; Caiment, F.; Wu, H.; O’Lone, R.; Day, F.; Calley, J.; Smith, A.; Li, J. RATEmiRs: The Rat Atlas of Tissue-Specific and Enriched MiRNAs Database. BMC Genomics 2018, 19, 825. [Google Scholar] [CrossRef]
- Manninen, E.; Chary, K.; Lapinlampi, N.; Andrade, P.; Paananen, T.; Sierra, A.; Tohka, J.; Gröhn, O.; Pitkänen, A. Early Increase in Cortical T(2) Relaxation Is a Prognostic Biomarker for the Evolution of Severe Cortical Damage, but Not for Epileptogenesis, after Experimental Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2580–2594. [Google Scholar] [CrossRef]
- Miska, E.A.; Alvarez-Saavedra, E.; Townsend, M.; Yoshii, A.; Sestan, N.; Rakic, P.; Constantine-Paton, M.; Horvitz, H.R. Microarray Analysis of MicroRNA Expression in the Developing Mammalian Brain. Genome Biol. 2004, 5, R68. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression Profiling of Mammalian MicroRNAs Uncovers a Subset of Brain-Expressed MicroRNAs with Possible Roles in Murine and Human Neuronal Differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; Sonntag, K.-C.; Isacson, O.; Kosik, K.S. Specific MicroRNAs Modulate Embryonic Stem Cell-Derived Neurogenesis. Stem Cells 2006, 24, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Makeyev, E.V.; Zhang, J.; Carrasco, M.A.; Maniatis, T. The MicroRNA MiR-124 Promotes Neuronal Differentiation by Triggering Brain-Specific Alternative Pre-MRNA Splicing. Mol. Cell 2007, 27, 435–448. [Google Scholar] [CrossRef]
- Mishima, T.; Mizuguchi, Y.; Kawahigashi, Y.; Takizawa, T.; Takizawa, T. RT-PCR-Based Analysis of MicroRNA (MiR-1 and -124) Expression in Mouse CNS. Brain Res. 2007, 1131, 37–43. [Google Scholar] [CrossRef]
- Olsen, L.; Klausen, M.; Helboe, L.; Nielsen, F.C.; Werge, T. MicroRNAs Show Mutually Exclusive Expression Patterns in the Brain of Adult Male Rats. PLoS ONE 2009, 4, e7225. [Google Scholar] [CrossRef]
- Juhila, J.; Sipilä, T.; Icay, K.; Nicorici, D.; Ellonen, P.; Kallio, A.; Korpelainen, E.; Greco, D.; Hovatta, I. MicroRNA Expression Profiling Reveals MiRNA Families Regulating Specific Biological Pathways in Mouse Frontal Cortex and Hippocampus. PLoS ONE 2011, 6, e21495. [Google Scholar] [CrossRef]
- Jovičić, A.; Roshan, R.; Moisoi, N.; Pradervand, S.; Moser, R.; Pillai, B.; Luthi-Carter, R. Comprehensive Expression Analyses of Neural Cell-Type-Specific MiRNAs Identify New Determinants of the Specification and Maintenance of Neuronal Phenotypes. J. Neurosci. 2013, 33, 5127–5137. [Google Scholar] [CrossRef]
- Vuokila, N.; Das Gupta, S.; Huusko, R.; Tohka, J.; Puhakka, N.; Pitkänen, A. Elevated Acute Plasma MiR-124-3p Level Relates to Evolution of Larger Cortical Lesion Area after Traumatic Brain Injury. Neuroscience 2020, 433, 21–35. [Google Scholar] [CrossRef]
- Wang, P.; Ma, H.; Zhang, Y.; Zeng, R.; Yu, J.; Liu, R.; Jin, X.; Zhao, Y. Plasma Exosome-Derived MicroRNAs as Novel Biomarkers of Traumatic Brain Injury in Rats. Int. J. Med. Sci. 2020, 17, 437–448. [Google Scholar] [CrossRef]
- O’Connell, G.C.; Smothers, C.G.; Winkelman, C. Bioinformatic Analysis of Brain-Specific MiRNAs for Identification of Candidate Traumatic Brain Injury Blood Biomarkers. Brain Inj. 2020, 34, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-Q.; Xu, X.-X.; He, Z.-Z.; Li, X.-H.; Luo, J.-M. Dynamic Changes in Peripheral Blood-Targeted MiRNA Expression Profiles in Patients with Severe Traumatic Brain Injury at High Altitude. Mil. Med. Res. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.R.; Woschek, M.; Vollrath, J.T.; Kontradowitz, K.; Lustenberger, T.; Störmann, P.; Marzi, I.; Henrich, D. MiR-142-3p Expression Is Predictive for Severe Traumatic Brain Injury (TBI) in Trauma Patients. Int. J. Mol. Sci. 2020, 21, 5381. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Yarnell, A.; Kean, W.S.; Gold, E.; Lewis, B.; Ren, M.; McMullen, D.C.; Jacobowitz, D.M.; Pollard, H.B.; O’Neill, J.T.; et al. Craniotomy: True Sham for Traumatic Brain Injury, or a Sham of a Sham? J. Neurotrauma 2011, 28, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, M.; Jääskeläinen, O.; Manninen, E.; Das Gupta, S.; Andrade, P.; Ciszek, R.; Gröhn, O.; Herukka, S.-K.; Puhakka, N.; Pitkänen, A. Plasma Neurofilament Light Chain (NF-L) Is a Prognostic Biomarker for Cortical Damage Evolution but Not for Cognitive Impairment or Epileptogenesis Following Experimental TBI. Int. J. Mol. Sci. 2022, 23, 5208. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, Y.; Wang, D.; He, Y.; Cao, D.; Zhang, F.; Dou, K. Altered Expression Levels of MiRNAs in Serum as Sensitive Biomarkers for Early Diagnosis of Traumatic Injury. J. Cell Biochem. 2011, 112, 2435–2442. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. MiR-9: A Versatile Regulator of Neurogenesis. Front. Cell Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Sim, S.-E.; Lim, C.-S.; Kim, J.-I.; Seo, D.; Chun, H.; Yu, N.-K.; Lee, J.; Kang, S.J.; Ko, H.-G.; Choi, J.-H.; et al. The Brain-Enriched MicroRNA MiR-9-3p Regulates Synaptic Plasticity and Memory. J. Neurosci. 2016, 36, 8641–8652. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Sørensen, S.S.; Nygaard, A.-B.; Carlsen, A.L.; Heegaard, N.H.H.; Bak, M.; Christensen, T. Elevation of Brain-Enriched MiRNAs in Cerebrospinal Fluid of Patients with Acute Ischemic Stroke. Biomark Res. 2017, 5, 24. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, C.; Chao, D.; Chen, Z.; Li, S.; Shi, M.; Pei, Y.; Dai, Y.; Ji, J.; Ji, Y.; et al. Acute Cerebral Ischemia Increases a Set of Brain-Specific MiRNAs in Serum Small Extracellular Vesicles. Front. Mol. Neurosci. 2022, 15, 874903. [Google Scholar] [CrossRef] [PubMed]
- Beske, R.P.; Bache, S.; Abild Stengaard Meyer, M.; Kjærgaard, J.; Bro-Jeppesen, J.; Obling, L.; Olsen, M.H.; Rossing, M.; Nielsen, F.C.; Møller, K.; et al. MicroRNA-9-3p: A Novel Predictor of Neurological Outcome after Cardiac Arrest. Eur. Heart J. Acute Cardiovasc. Care. 2022, 11, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Chan, C.P.Y.; Leung, Y.K.; Jiang, H.L.; Abrigo, J.M.; Wang, D.F.; Chung, J.S.H.; Rainer, T.H.; Graham, C.A. Comparison of MiR-124-3p and MiR-16 for Early Diagnosis of Hemorrhagic and Ischemic Stroke. Clin. Chim. Acta 2014, 433, 139–144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Han, R.; Liu, H.; Sun, D.; Liu, X. Downregulation of Serum Brain Specific MicroRNA Is Associated with Inflammation and Infarct Volume in Acute Ischemic Stroke. J. Clin. Neurosci. 2015, 22, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sheinerman, K.S.; Tsivinsky, V.G.; Abdullah, L.; Crawford, F.; Umansky, S.R. Plasma MicroRNA Biomarkers for Detection of Mild Cognitive Impairment: Biomarker Validation Study. Aging 2013, 5, 925–938. [Google Scholar] [CrossRef]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Maniati, M.; Koros, C.; Simitsi, A.-M.; Bozi, M.; Pachi, I.; Stamelou, M.; Paraskevas, G.P.; et al. Circulating Brain-Enriched MicroRNAs for Detection and Discrimination of Idiopathic and Genetic Parkinson’s Disease. Mov. Disord. 2020, 35, 457–467. [Google Scholar] [CrossRef]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Koros, C.; Simitsi, A.M.; Pachi, I.; Breza, M.; Stefanis, L.; Doxakis, E. Validation of Differentially Expressed Brain-Enriched MicroRNAs in the Plasma of PD Patients. Ann. Clin. Transl. Neurol. 2020, 7, 1594–1607. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Zhu, D.; Liu, P.; Yin, J.; Liu, D.; Zheng, M.; Gao, J.; Zhang, C.; Gao, Y. MiR-136-3p Targets PTEN to Regulate Vascularization and Bone Formation and Ameliorates Alcohol-Induced Osteopenia. FASEB J. 2020, 34, 5348–5362. [Google Scholar] [CrossRef]
- Kitahara, Y.; Nakamura, K.; Kogure, K.; Minegishi, T. Role of MicroRNA-136-3p on the Expression of Luteinizing Hormone-Human Chorionic Gonadotropin Receptor MRNA in Rat Ovaries. Biol. Reprod. 2013, 89, 114. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered MicroRNA Profiles in Cerebrospinal Fluid Exosome in Parkinson Disease and Alzheimer Disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef]
- Shang, F.-F.; Xia, Q.-J.; Liu, W.; Xia, L.; Qian, B.-J.; You, L.; He, M.; Yang, J.-L.; Wang, T.-H. MiR-434-3p and DNA Hypomethylation Co-Regulate EIF5A1 to Increase AChRs and to Improve Plasticity in SCT Rat Skeletal Muscle. Sci. Rep. 2016, 6, 22884. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, K.-P.; Milholland, B.; Shin, Y.J.; Kang, J.S.; Kwon, K.-S.; Suh, Y. Comprehensive MiRNA Profiling of Skeletal Muscle and Serum in Induced and Normal Mouse Muscle Atrophy During Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1483–1491. [Google Scholar] [CrossRef]
- Pardo, P.S.; Hajira, A.; Boriek, A.M.; Mohamed, J.S. MicroRNA-434-3p Regulates Age-Related Apoptosis through EIF5A1 in the Skeletal Muscle. Aging 2017, 9, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Manakov, S.A.; Morton, A.; Enright, A.J.; Grant, S.G.N. A Neuronal Transcriptome Response Involving Stress Pathways Is Buffered by Neuronal MicroRNAs. Front. Neurosci. 2012, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chandran, R.; Barry, E.S.; Bhomia, M.; Hutchison, M.A.; Balakathiresan, N.S.; Grunberg, N.E.; Maheshwari, R.K. Identification of Serum MicroRNA Signatures for Diagnosis of Mild Traumatic Brain Injury in a Closed Head Injury Model. PLoS ONE 2014, 9, e112019. [Google Scholar] [CrossRef] [PubMed]
- Wanet, A.; Tacheny, A.; Arnould, T.; Renard, P. MiR-212/132 Expression and Functions: Within and beyond the Neuronal Compartment. Nucleic Acids Res. 2012, 40, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. A CAMP-Response Element Binding Protein-Induced MicroRNA Regulates Neuronal Morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar] [CrossRef]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated MiR-146a Expression in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef]
- Mehta, A.; Mann, M.; Zhao, J.L.; Marinov, G.K.; Majumdar, D.; Garcia-Flores, Y.; Du, X.; Erikci, E.; Chowdhury, K.; Baltimore, D. The MicroRNA-212/132 Cluster Regulates B Cell Development by Targeting Sox4. J. Exp. Med. 2015, 212, 1679–1692. [Google Scholar] [CrossRef]
- Jin, W.; Reddy, M.A.; Chen, Z.; Putta, S.; Lanting, L.; Kato, M.; Park, J.T.; Chandra, M.; Wang, C.; Tangirala, R.K.; et al. Small RNA Sequencing Reveals MicroRNAs That Modulate Angiotensin II Effects in Vascular Smooth Muscle Cells. J. Biol. Chem. 2012, 287, 15672–15683. [Google Scholar] [CrossRef]
- Malm, H.A.; Mollet, I.G.; Berggreen, C.; Orho-Melander, M.; Esguerra, J.L.S.; Göransson, O.; Eliasson, L. Transcriptional Regulation of the MiR-212/MiR-132 Cluster in Insulin-Secreting β-Cells by CAMP-Regulated Transcriptional Co-Activator 1 and Salt-Inducible Kinases. Mol. Cell Endocrinol. 2016, 424, 23–33. [Google Scholar] [CrossRef]
- Korotkov, A.; Broekaart, D.W.M.; Banchaewa, L.; Pustjens, B.; van Scheppingen, J.; Anink, J.J.; Baayen, J.C.; Idema, S.; Gorter, J.A.; van Vliet, E.A.; et al. MicroRNA-132 Is Overexpressed in Glia in Temporal Lobe Epilepsy and Reduces the Expression of pro-Epileptogenic Factors in Human Cultured Astrocytes. Glia 2020, 68, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Davare, M.; Ando, H.; Fortin, D.; Varlamova, O.; Cheng, H.-Y.M.; Marks, D.; Obrietan, K.; Soderling, T.R.; Goodman, R.H.; et al. An Activity-Regulated MicroRNA Controls Dendritic Plasticity by down-Regulating P250GAP. Proc. Natl. Acad. Sci. USA 2008, 105, 9093–9098. [Google Scholar] [CrossRef]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. MicroRNA-132 Regulates Dendritic Growth and Arborization of Newborn Neurons in the Adult Hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Luikart, B.W.; Bensen, A.L.; Washburn, E.K.; Perederiy, J.V.; Su, K.G.; Li, Y.; Kernie, S.G.; Parada, L.F.; Westbrook, G.L. MiR-132 Mediates the Integration of Newborn Neurons into the Adult Dentate Gyrus. PLoS ONE 2011, 6, e19077. [Google Scholar] [CrossRef] [PubMed]
- Remenyi, J.; van den Bosch, M.W.M.; Palygin, O.; Mistry, R.B.; McKenzie, C.; Macdonald, A.; Hutvagner, G.; Arthur, J.S.C.; Frenguelli, B.G.; Pankratov, Y. MiR-132/212 Knockout Mice Reveal Roles for These MiRNAs in Regulating Cortical Synaptic Transmission and Plasticity. PLoS ONE 2013, 8, e62509. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rapp, J.; Smith, P.Y.; Filali, M.; Goupil, C.; Planel, E.; Magill, S.T.; Goodman, R.H.; Hébert, S.S. Memory Formation and Retention Are Affected in Adult MiR-132/212 Knockout Mice. Behav. Brain Res. 2015, 287, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.F.; Sakamoto, K.; Aten, S.; Price, K.H.; Loeser, J.; Hesse, A.M.; Page, C.E.; Pelz, C.; Arthur, J.S.C.; Impey, S.; et al. Targeted Deletion of MiR-132/-212 Impairs Memory and Alters the Hippocampal Transcriptome. Learn. Mem. 2016, 23, 61–71. [Google Scholar] [CrossRef]
- Cheng, H.-Y.M.; Papp, J.W.; Varlamova, O.; Dziema, H.; Russell, B.; Curfman, J.P.; Nakazawa, T.; Shimizu, K.; Okamura, H.; Impey, S.; et al. MicroRNA Modulation of Circadian-Clock Period and Entrainment. Neuron 2007, 54, 813–829. [Google Scholar] [CrossRef]
- Pichler, S.; Gu, W.; Hartl, D.; Gasparoni, G.; Leidinger, P.; Keller, A.; Meese, E.; Mayhaus, M.; Hampel, H.; Riemenschneider, M. The MiRNome of Alzheimer’s Disease: Consistent Downregulation of the MiR-132/212 Cluster. Neurobiol. Aging 2017, 50, e1–e167. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. MiR-212 and MiR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [PubMed]
- Enright, N.; Simonato, M.; Henshall, D.C. Discovery and Validation of Blood MicroRNAs as Molecular Biomarkers of Epilepsy: Ways to Close Current Knowledge Gaps. Epilepsia Open 2018, 3, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Agoston, D.V.; Brooks-Kayal, A.; Dulla, C.; Fureman, B.; Henshall, D.C.; Pitkänen, A.; Theodore, W.H.; Twyman, R.E.; Kobeissy, F.H.; et al. Identification of Clinically Relevant Biomarkers of Epileptogenesis—A Strategic Roadmap. Nat. Rev. Neurol. 2021, 17, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, A.S.; DiRocco, D.P.; Lambert, T.J.; Garelick, M.G.; Le, J.; Nathanson, N.M.; Storm, D.R. Neuronal Activity Rapidly Induces Transcription of the CREB-Regulated MicroRNA-132, In Vivo. Hippocampus 2010, 20, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.M.; Dębski, K.J.; Lukasiuk, K. Alterations in MiRNA Levels in the Dentate Gyrus in Epileptic Rats. PLoS ONE 2013, 8, e76051. [Google Scholar] [CrossRef]
- Gorter, J.A.; Iyer, A.; White, I.; Colzi, A.; van Vliet, E.A.; Sisodiya, S.; Aronica, E. Hippocampal Subregion-Specific MicroRNA Expression during Epileptogenesis in Experimental Temporal Lobe Epilepsy. Neurobiol. Dis. 2014, 62, 508–520. [Google Scholar] [CrossRef]
- Guo, J.; Wang, H.; Wang, Q.; Chen, Y.; Chen, S. Expression of P-CREB and Activity-Dependent MiR-132 in Temporal Lobe Epilepsy. Int. J. Clin. Exp. Med. 2014, 7, 1297–1306. [Google Scholar]
- Bencurova, P.; Baloun, J.; Hynst, J.; Oppelt, J.; Kubova, H.; Pospisilova, S.; Brazdil, M. Dynamic MiRNA Changes during the Process of Epileptogenesis in an Infantile and Adult-Onset Model. Sci. Rep. 2021, 11, 9649. [Google Scholar] [CrossRef]
- Venø, M.T.; Reschke, C.R.; Morris, G.; Connolly, N.M.C.; Su, J.; Yan, Y.; Engel, T.; Jimenez-Mateos, E.M.; Harder, L.M.; Pultz, D.; et al. A Systems Approach Delivers a Functional MicroRNA Catalog and Expanded Targets for Seizure Suppression in Temporal Lobe Epilepsy. Proc. Natl. Acad. Sci. USA 2020, 117, 15977–15988. [Google Scholar] [CrossRef]
- Jimenez-Mateos, E.M.; Bray, I.; Sanz-Rodriguez, A.; Engel, T.; McKiernan, R.C.; Mouri, G.; Tanaka, K.; Sano, T.; Saugstad, J.A.; Simon, R.P.; et al. MiRNA Expression Profile after Status Epilepticus and Hippocampal Neuroprotection by Targeting MiR-132. Am. J. Pathol. 2011, 179, 2519–2532. [Google Scholar] [CrossRef]
- Peng, J.; Omran, A.; Ashhab, M.U.; Kong, H.; Gan, N.; He, F.; Yin, F. Expression Patterns of MiR-124, MiR-134, MiR-132, and MiR-21 in an Immature Rat Model and Children with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2013, 50, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, G.; Tang, H.; Kong, L.; Wang, X.; Cui, C.; Xiao, Q.; Ji, H. Relationships between Plasma Expression Levels of MicroRNA-146a and MicroRNA-132 in Epileptic Patients and Their Cognitive, Mental and Psychological Disorders. Bioengineered 2022, 13, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Zhao, Y.; Chhibber, A.; Kaiboriboon, K.; Do, L.V.; Vogelgesang, S.; Barbaro, N.M.; Alldredge, B.K.; Lowenstein, D.H.; Cascorbi, I.; et al. SOX11 Identified by Target Gene Evaluation of MiRNAs Differentially Expressed in Focal and Non-Focal Brain Tissue of Therapy-Resistant Epilepsy Patients. Neurobiol. Dis. 2015, 77, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Long, L.; Zeng, C.; Ni, G.; Meng, Y.; Guo, Q.; Chen, Z.; Li, Z. LncRNA ILF3-AS1 Mediated the Occurrence of Epilepsy through Suppressing Hippocampal MiR-212 Expression. Aging 2020, 12, 8413–8422. [Google Scholar] [CrossRef]
- Vuokila, N.; Lukasiuk, K.; Bot, A.M.; van Vliet, E.A.; Aronica, E.; Pitkänen, A.; Puhakka, N. MiR-124-3p Is a Chronic Regulator of Gene Expression after Brain Injury. Cell Mol. Life Sci. 2018, 75, 4557–4581. [Google Scholar] [CrossRef]
- Ambrogini, P.; Albertini, M.C.; Betti, M.; Galati, C.; Lattanzi, D.; Savelli, D.; di Palma, M.; Saccomanno, S.; Bartolini, D.; Torquato, P.; et al. Neurobiological Correlates of Alpha-Tocopherol Antiepileptogenic Effects and MicroRNA Expression Modulation in a Rat Model of Kainate-Induced Seizures. Mol. Neurobiol. 2018, 55, 7822–7838. [Google Scholar] [CrossRef]
- Brennan, G.P.; Dey, D.; Chen, Y.; Patterson, K.P.; Magnetta, E.J.; Hall, A.M.; Dube, C.M.; Mei, Y.-T.; Baram, T.Z. Dual and Opposing Roles of MicroRNA-124 in Epilepsy Are Mediated through Inflammatory and NRSF-Dependent Gene Networks. Cell Rep. 2016, 14, 2402–2412. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, C.; Long, L.; Long, X.; Feng, L.; Li, Y.; Xiao, B. Expression Profile of MicroRNAs in Rat Hippocampus Following Lithium-Pilocarpine-Induced Status Epilepticus. Neurosci. Lett. 2011, 488, 252–257. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Q.-Y.; Edson, J.; Zhang, Z.H.; Li, X.; Wei, W.; Bredy, T.; Reutens, D.C. Genome-Wide MicroRNA Profiling in Brain and Blood Samples in a Mouse Model of Epileptogenesis. Epilepsy Res. 2020, 166, 106400. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, W. The Neuroprotective Effect of MiR-136 on Pilocarpine-Induced Temporal Lobe Epilepsy Rats by Inhibiting Wnt/β-Catenin Signaling Pathway. Comput. Math Methods Med. 2022, 2022, 1938205. [Google Scholar] [CrossRef]
- Rainer, T.H.; Leung, L.Y.; Chan, C.P.Y.; Leung, Y.K.; Abrigo, J.M.; Wang, D.; Graham, C.A. Plasma MiR-124-3p and MiR-16 Concentrations as Prognostic Markers in Acute Stroke. Clin. Biochem. 2016, 49, 663–668. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Puhakka, N.; Mills, J.D.; Srivastava, P.K.; Johnson, M.R.; Roncon, P.; Das Gupta, S.; Karttunen, J.; Simonato, M.; Lukasiuk, K.; et al. Standardization Procedure for Plasma Biomarker Analysis in Rat Models of Epileptogenesis: Focus on Circulating MicroRNAs. Epilepsia 2017, 58, 2013–2024. [Google Scholar] [CrossRef]
- Kharatishvili, I.; Nissinen, J.P.; McIntosh, T.K.; Pitkänen, A. A Model of Posttraumatic Epilepsy Induced by Lateral Fluid-Percussion Brain Injury in Rats. Neuroscience 2006, 140, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Ekolle Ndode-Ekane, X.; Kharatishvili, I.; Pitkänen, A. Unfolded Maps for Quantitative Analysis of Cortical Lesion Location and Extent after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Ciszek, R.; Pitkänen, A.; Ndode-Ekane, X.E. A Web-Based Application for Generating 2D-Unfolded Cortical Maps to Analyze the Location and Extent of Cortical Lesions Following Traumatic Brain Injury in Adult Rats. J. Neurosci. Methods 2018, 308, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating High Confidence MicroRNAs Using Deep Sequencing Data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Tohka, J.; Moradi, E.; Huttunen, H.; Initiative, A.D.N. Comparison of Feature Selection Techniques in Machine Learning for Anatomical Brain MRI in Dementia. Neuroinformatics 2016, 14, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Ambroise, C.; McLachlan, G.J. Selection Bias in Gene Extraction on the Basis of Microarray Gene-Expression Data. Proc. Natl. Acad. Sci. USA 2002, 99, 6562–6566. [Google Scholar] [CrossRef] [PubMed]
- Tohka, J.; van Gils, M. Evaluation of Machine Learning Algorithms for Health and Wellness Applications: A Tutorial. Comput. Biol. Med. 2021, 132, 104324. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Evans, A.C.; Tohka, J. T1 White/Gray Contrast as a Predictor of Chronological Age, and an Index of Cognitive Performance. Neuroimage 2018, 173, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.P. The Use of the Area under the ROC Curve in the Evaluation of Machine Learning Algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiskanen, M.; Das Gupta, S.; Mills, J.D.; van Vliet, E.A.; Manninen, E.; Ciszek, R.; Andrade, P.; Puhakka, N.; Aronica, E.; Pitkänen, A. Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury–The EPITARGET Cohort. Int. J. Mol. Sci. 2023, 24, 2823. https://doi.org/10.3390/ijms24032823
Heiskanen M, Das Gupta S, Mills JD, van Vliet EA, Manninen E, Ciszek R, Andrade P, Puhakka N, Aronica E, Pitkänen A. Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury–The EPITARGET Cohort. International Journal of Molecular Sciences. 2023; 24(3):2823. https://doi.org/10.3390/ijms24032823
Chicago/Turabian StyleHeiskanen, Mette, Shalini Das Gupta, James D. Mills, Erwin A. van Vliet, Eppu Manninen, Robert Ciszek, Pedro Andrade, Noora Puhakka, Eleonora Aronica, and Asla Pitkänen. 2023. "Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury–The EPITARGET Cohort" International Journal of Molecular Sciences 24, no. 3: 2823. https://doi.org/10.3390/ijms24032823
APA StyleHeiskanen, M., Das Gupta, S., Mills, J. D., van Vliet, E. A., Manninen, E., Ciszek, R., Andrade, P., Puhakka, N., Aronica, E., & Pitkänen, A. (2023). Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury–The EPITARGET Cohort. International Journal of Molecular Sciences, 24(3), 2823. https://doi.org/10.3390/ijms24032823




