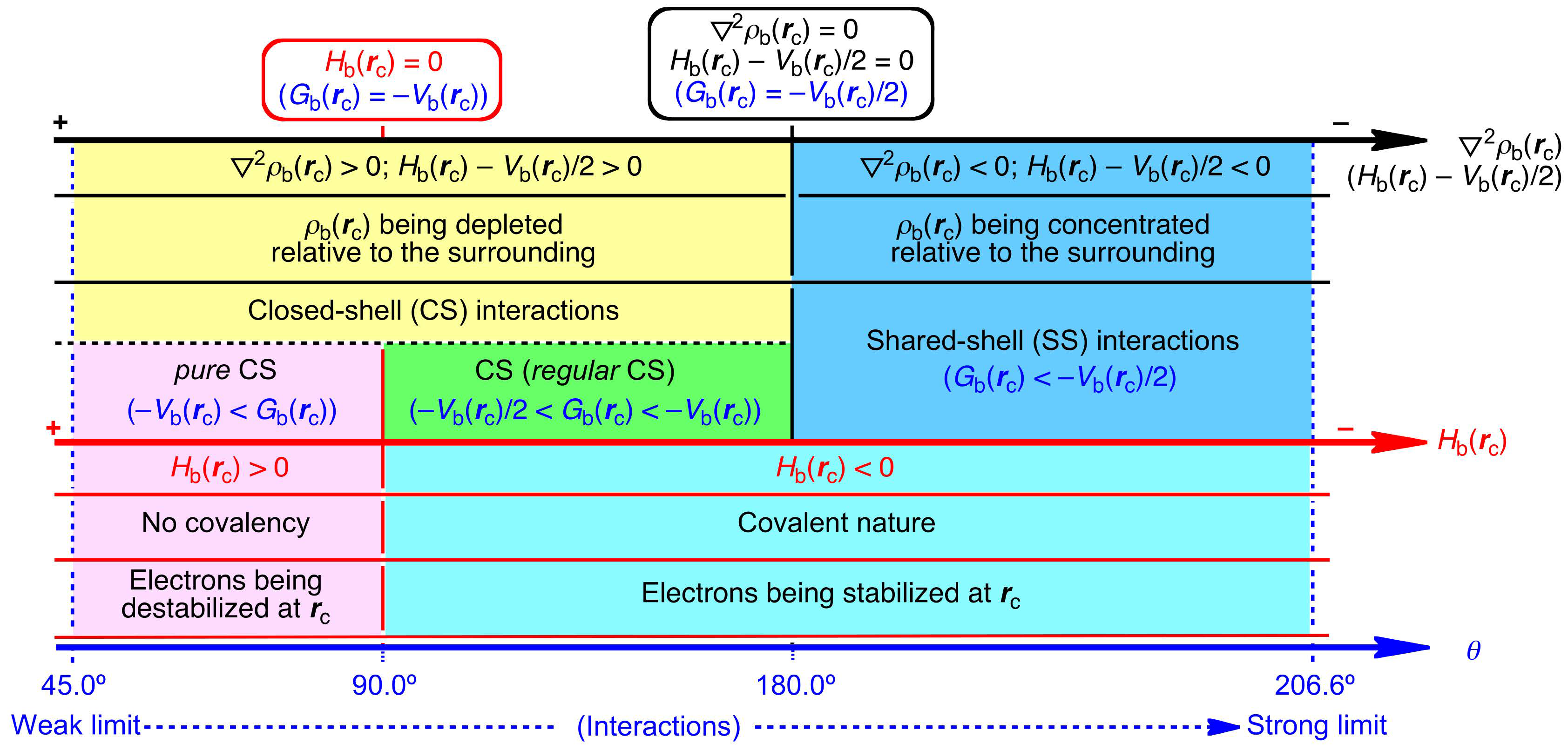
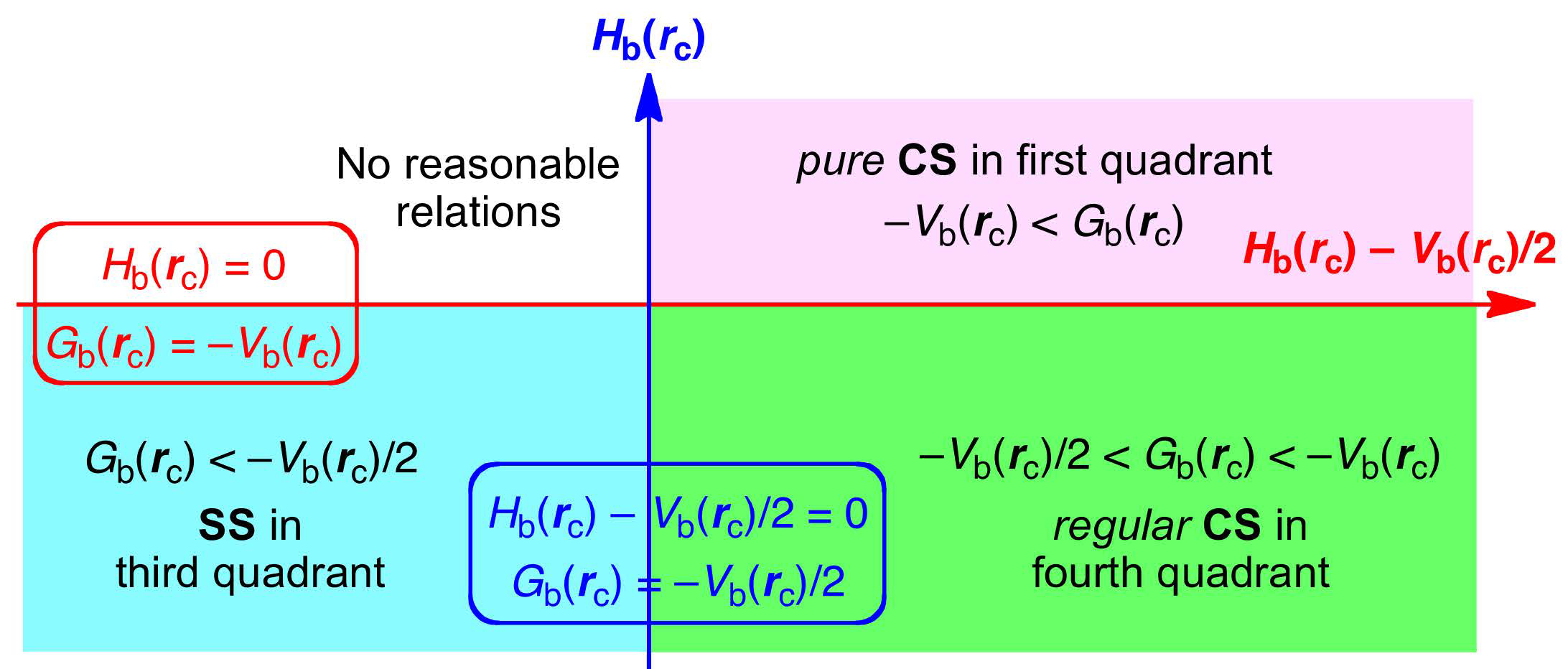
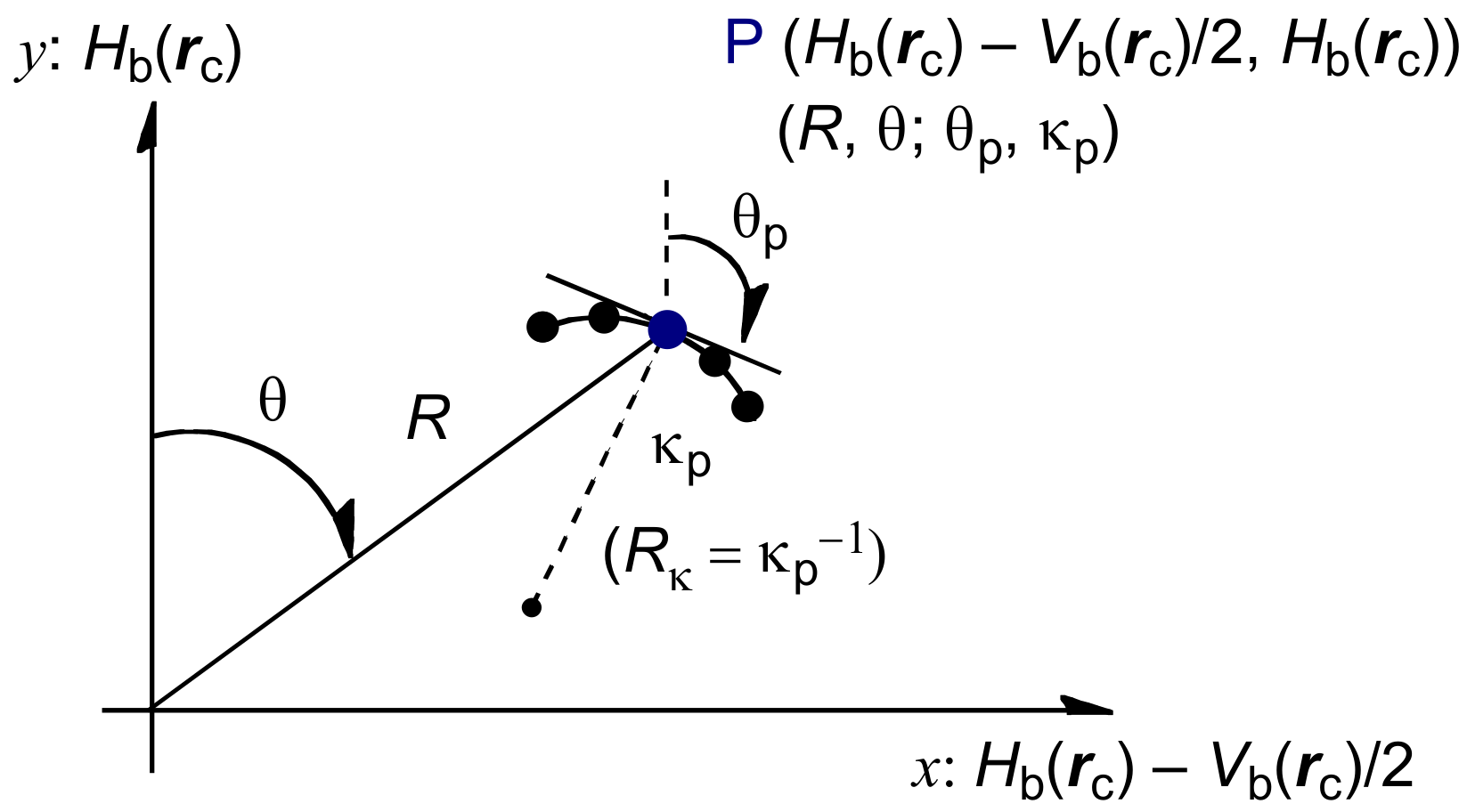
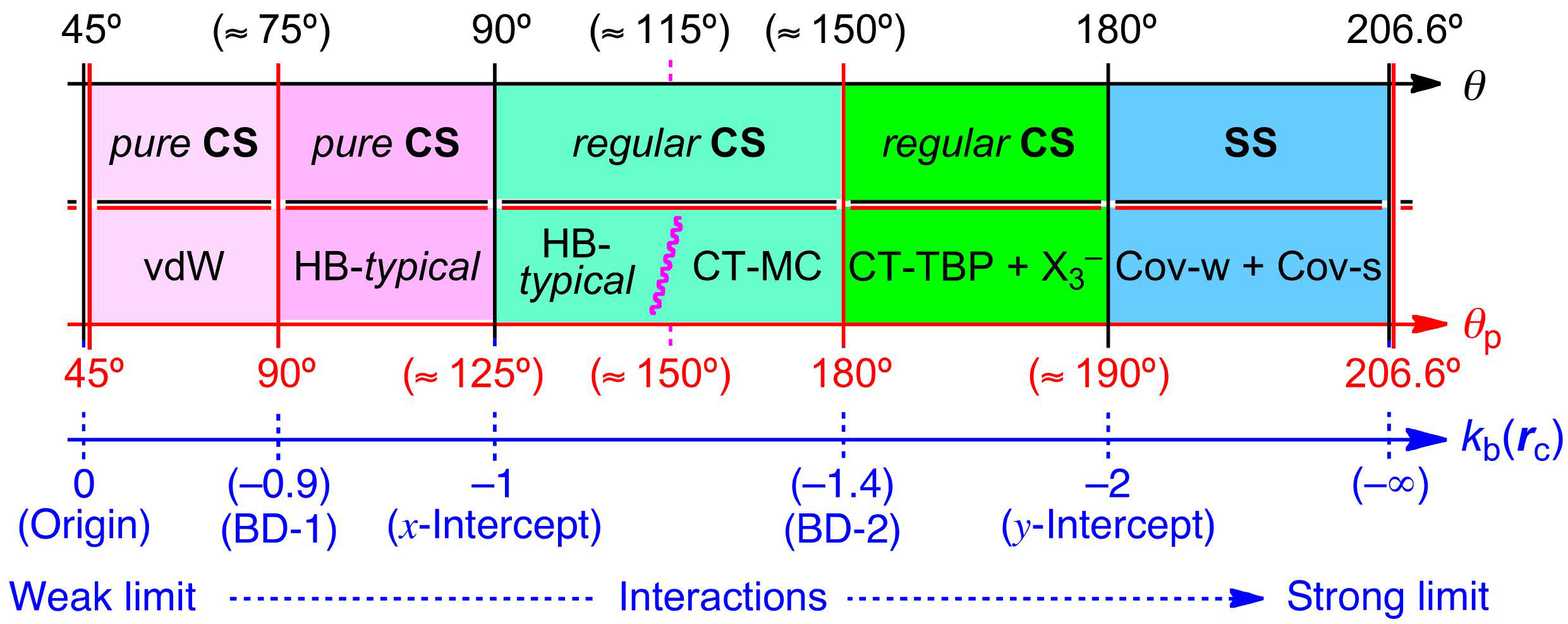
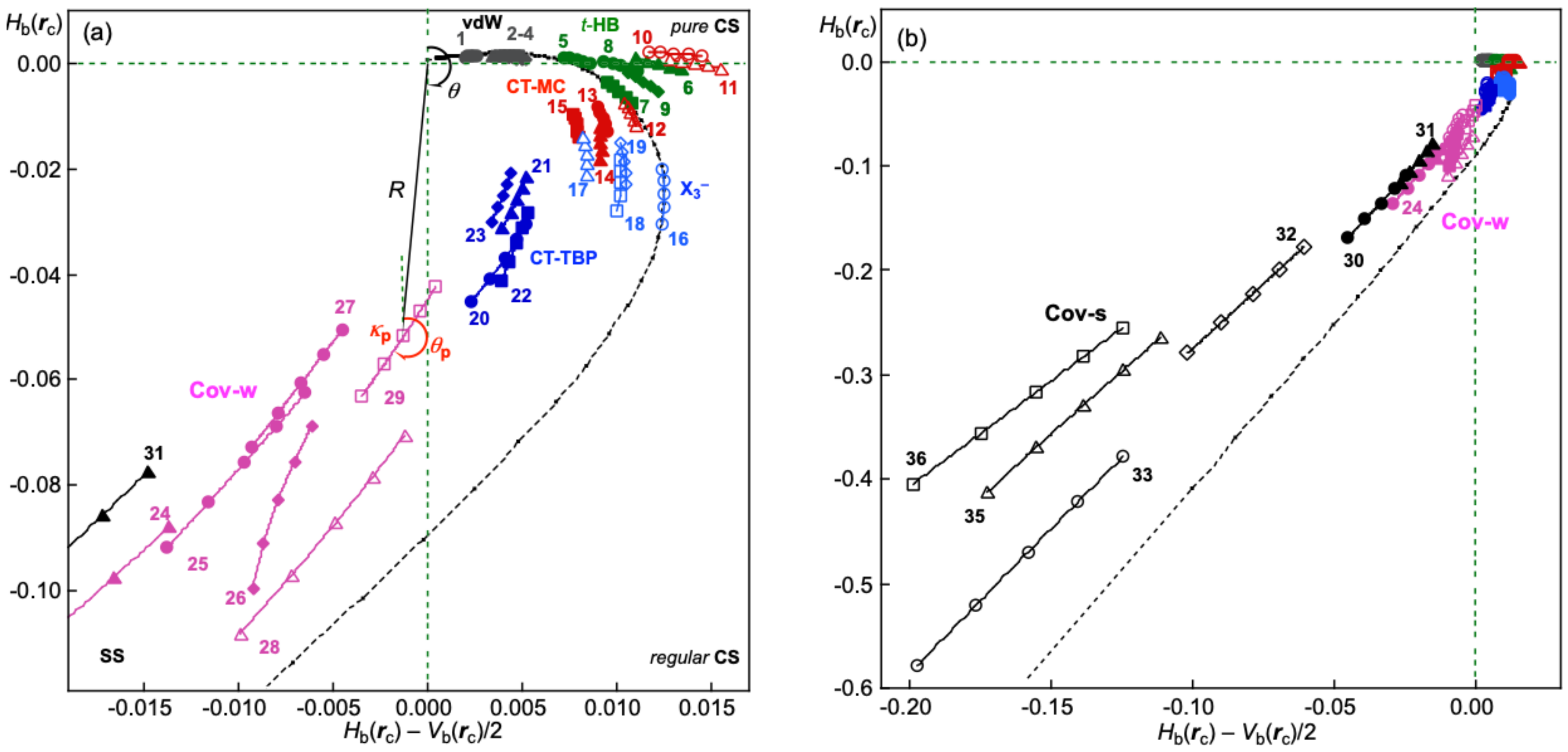
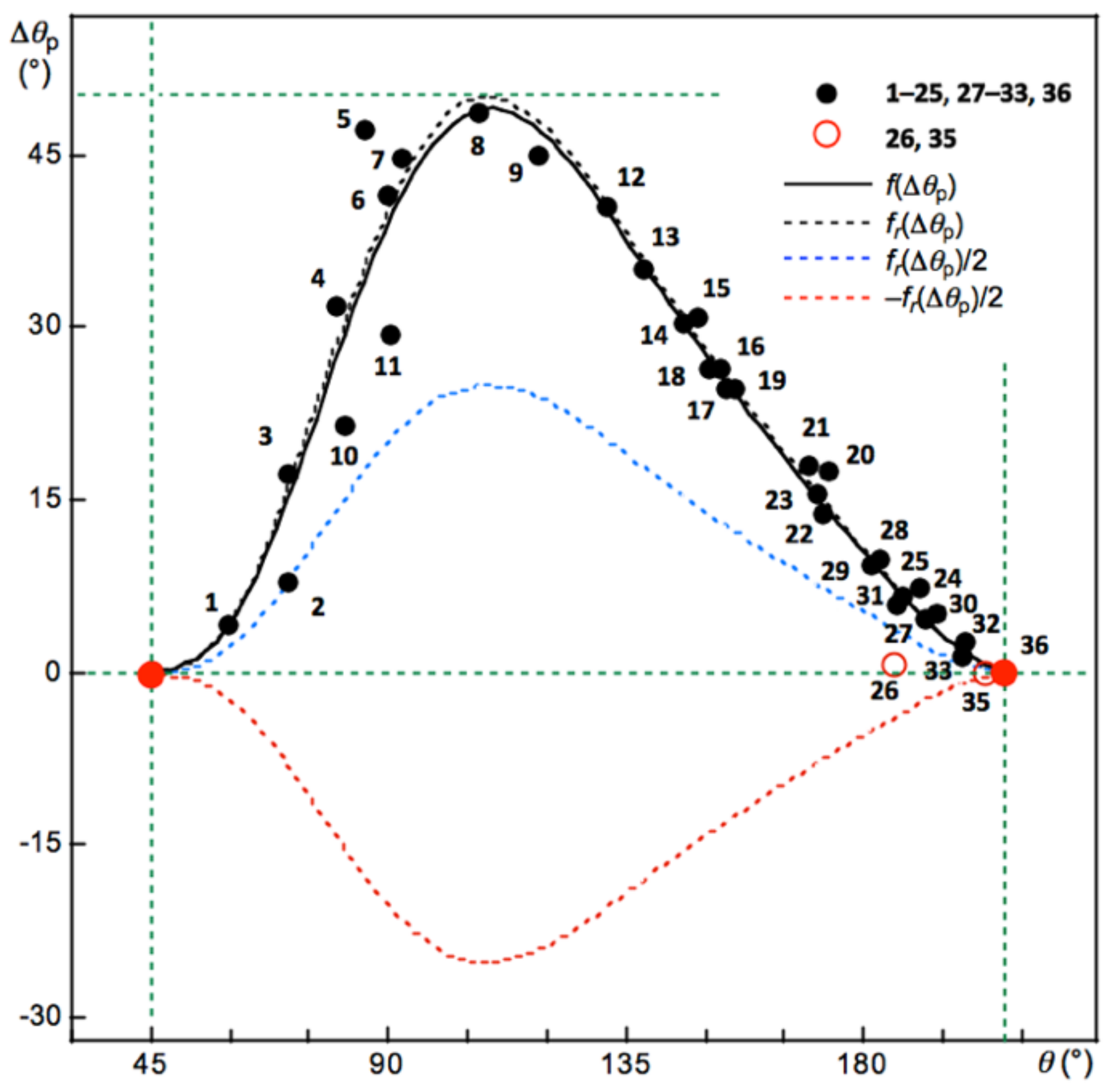
3.1. Basic Trend in the Δθp Values
The
Hb(
rc) −
Vb(
rc)/2 and
Hb(
rc) values of the QTAIM functions and the QTAIM-DFA parameters of (
R,
θ) and (
θp,
κp) [
1,
2] for
1–
36 (
Scheme 1) calculated under MP2/BSS-A are collected in
Table S3 of the Supplementary Materials, together with the Δ
θp and
Cii values and the predicted nature. The Δ
θp values are positive for all standard interactions of
1–
36, except for C-∗-H of H
3C-∗-H (
35), for which (
θ, Δ
θp) = (202.8°, –0.2°). It is reasonable to assume that an interaction shows normal behavior if Δ
θp > 0, whereas it does the inverse behavior when Δ
θp < 0, which we propose in this work. Namely, all standard interactions in
1–
36 behave normally, except for
35, which shows inverse behavior, although the magnitude of Δ
θp is very small. To elucidate the nature of the interactions based on the Δ
θp values, investigations are started by examining the basic trend in the standard interactions of
1–
36, employing the Δ
θp values.
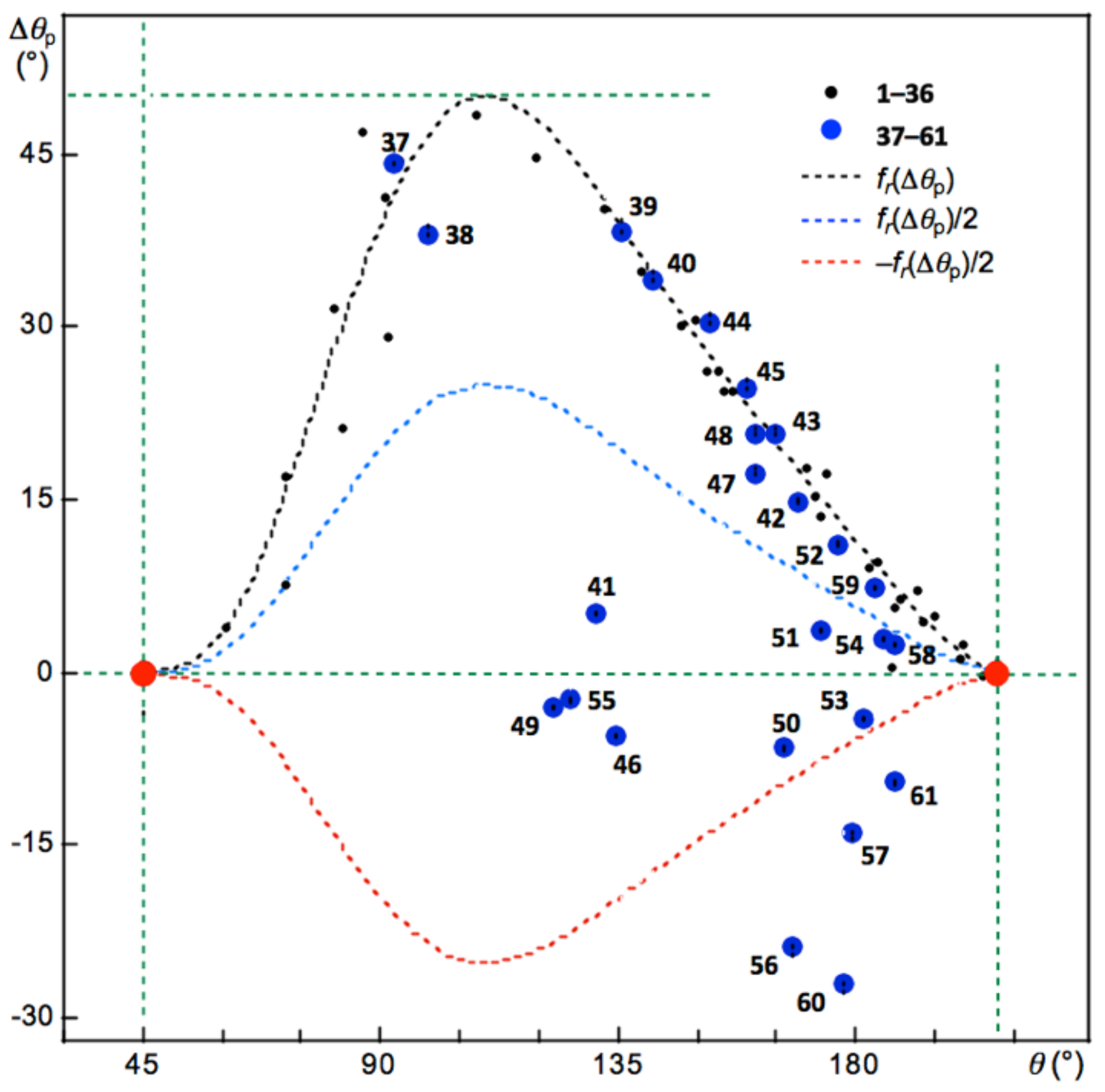
Figure 2 shows the plot of Δ
θp versus
θ for
1–
36 (
Scheme 1). The plot is analyzed using a regression curve of the eighth-order function after the addition of some fictional points, such as (
θ, Δ
θp) = (45°, 0°) and (206.6°, 0°), and omitting the data for
35 and Me
2Se
+-∗-Cl (
26). Fortunately, a smooth regression curve, described by
f(Δ
θp), was obtained, and the curve passes very close to the points of (45°, 0°) and (206.6°, 0°). The regression curve is shown by a black solid line in
Figure 2. The maximum point of the regression curve, shown by the solid line, was (
θ, Δ
θp) = (109.7°, 48.9°). The regression curve was revised to a new curve by amplifying the maximum value of (109.7°, 48.9°) to (109.7°, 50.0°). The new curve is described by
fr(Δ
θp) and shown by the dotted line. The treatment helps the discussion to be simpler, because the maximum value of 48.9° should be tentative and change depending on the employed species. Two more curves of
fr(Δ
θp)/2 and −
fr(Δ
θp)/2 are added in
Figure 2, which are shown by the blue and red dotted lines, respectively, for a better explanation of the behavior.
The data for the normal behavior of the interactions with Δ
θp > 0 appear on the upper side of the
x-axis in
Figure 2. Interactions show inverse behavior if the (
θ, Δ
θp) points appear downside of the
x-axis. The typical data for the normal behavior of interactions should appear around the black dotted line of
fr(Δ
θp), whereas data for the inverse behavior of interactions appear around the red dotted line of −
fr(Δ
θp)/2. An interaction is called to show weak normal behavior if the point appears between
fr(Δ
θp)/2 and the
x-axis. The interaction in Ne-∗-HF (
2) seems to show borderline to weak normal behavior (
Figure 2). While the interactions in
26 are borderline between weak normal and inverse behavior, that in
35 show inverse behavior, close to weak normal behavior.
Very similar results were obtained in the plot of Δ
θp versus
θ for
1–
36 when calculated with BSS-B (see
Table S4 and Figure S1 of the Supplementary Materials for the data calculated with BSS-B). The Δ
θp values are similarly plotted versus
θ for the wide range of data of [Cl–Cl-∗-Cl]
− (
16), calculated under aug-cc-pVTZ, where the perturbed structures of
16 are generated with POM. The plot is shown in
Figure S2 of the Supplementary Materials, together with the data for
1–
36.
Figure A1 is essentially the same as
Figure 2 (see
Figure 1 for the QTAIM-DFA plot of
16, shown by the dotted line). A detailed comparison of the two figures revealed that the plots are very close to each other when the Cl-∗-Cl distances in question are longer than the optimized values, whereas the Δ
θp values for
16 are evaluated to be somewhat larger than those expected based on the data for
1–
36 when the Cl-∗-Cl distances in question become shorter than the optimized values of 2.300 Å. When the interaction distance becomes shorter than the optimized value, the energy curve sharply tightens. The calculation conditions for POM under the interaction distances are widely shorter than the optimized values, where the shortened distance are 0.5 Å for 20 data points. The very severe conditions would be responsible for the results.
The close similarity between the two figures can be explained as follows: Starting from interatomic distances that are long enough, chemical bonds or interactions form as the distances shorten. The processes for the interactions are similar to each other, if the interactions of the normal behavior are compared. The processes can be understood based on the Hb(rc) and Hb(rc) − Vb(rc)/2 values or the plot of Hb(rc) versus Hb(rc) − Vb(rc)/2. In these cases, the (θ, θp) values are very close to each other, if they are calculated at the points of substantially the same positions on the plots. Namely, starting from interaction distances that are far enough, stable interactions form as if they go along the similar plot of Hb(rc) versus Hb(rc) − Vb(rc)/2. The drive on the plot arrives at a point of the minimum energy of the interaction, where the (θ, θp) values are given for the interaction. Different (θ, θp) values are obtained because the minimum energy is mainly determined depending on the interacting atoms. There must also be some differences in the energy curves, depending on the characteristics of the interactions, which result in the somewhat different curves of the plots. This should be the reason that the data points of various interactions appear close to the curve for the plot of (θ, Δθp) of 16.
The next investigation is to search for the interactions with negative Δθp values after the establishment of the basic trend in the normal behavior of the standard interactions.
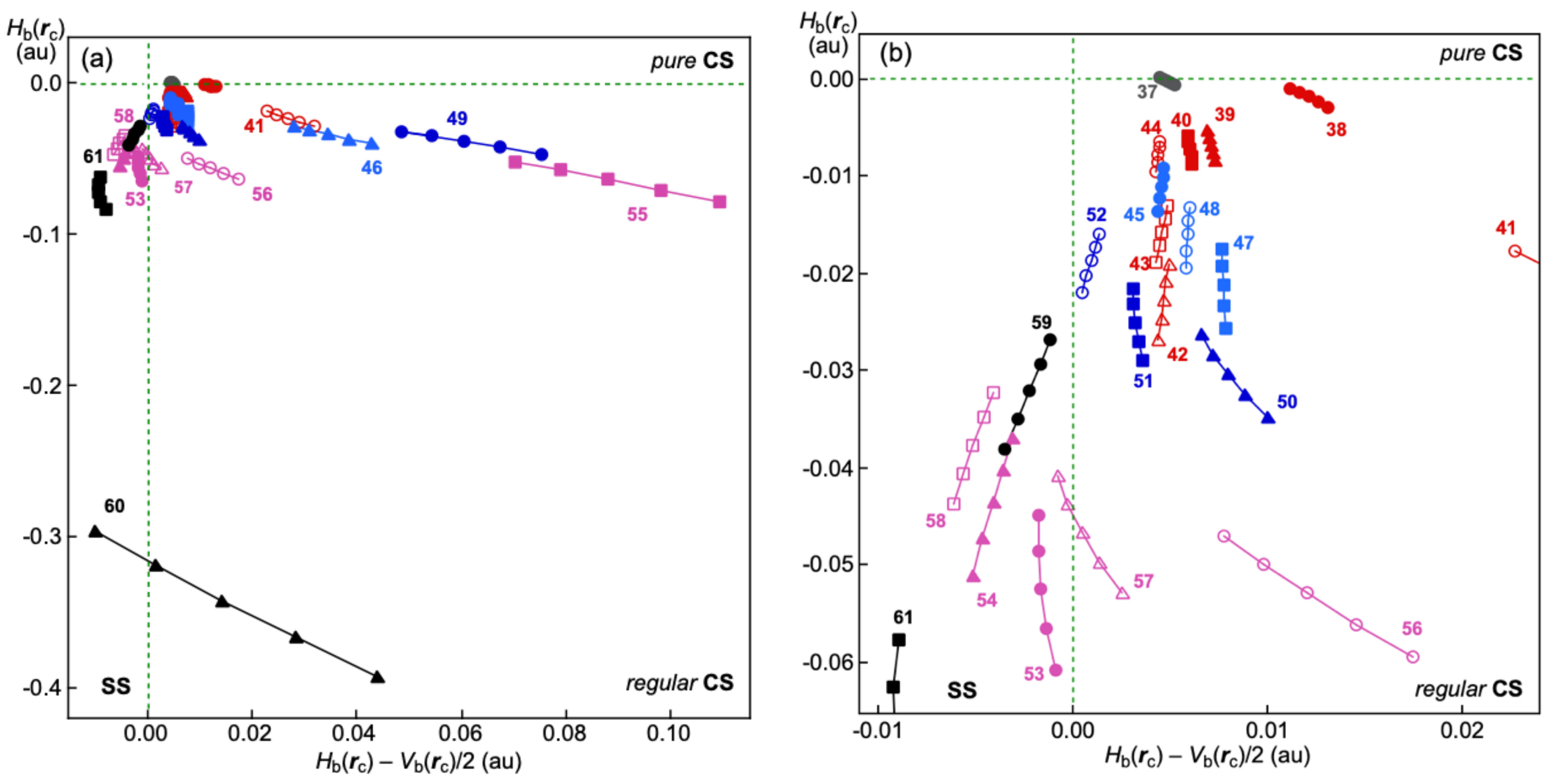
3.2. Behavior of Various Interactions Based on θ and Δθp
Table 1 collects the
Hb(
rc) −
Vb(
rc)/2 and
Hb(
rc) values for
37–
61, similarly calculated under MP2/BSS-A’.
Figure 3 shows the plots of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 for the data of
37–
61 shown in
Table 1, where the perturbed structures are generated with CIV. Contrary to the case of
Figure 1, some plots for the interactions in
Figure 3 show different streams from the main (averaged) stream, which must be a reflection of the different behaviors of the interactions. The (
R,
θ) and (
θp,
κp) values were similarly calculated for
37–
61 under MP2/BSS-A’.
Table 1 collects the values, together with the Δ
θp and
Cii values and the predicted natures. The Δ
θp values were plotted versus
θ for the data of
37–
61 shown in
Table 1.
Figure 4 shows the plots.
As shown in
Figure 4 (and
Table 1), the data of the (
θ, Δ
θp) values drop very near the
fr(Δ
θp) curve for most of
37–
61, which shows the normal behavior of the interactions, although some points appear much further below the
fr(Δ
θp) curve. The data of the (
θ, Δ
θp) values appear between the
fr(Δ
θp)/2 curve and the
x-axis for Me
2Te-∗-F
2 (
41; (
θ, Δ
θp) = (131.0°, 5.0°)), Me
2BrTe-∗-Br (
51; (173.1°, 3.5°)), Me
2Se
+-∗-I (
54; (185.0°, 2.9°)) and Me
2Te
+-∗-I (
58; (187.3°, 2.3°)). The interactions show weak normal behavior. The (
θ, Δ
θp) points appear between the
x-axis and the −
fr(Δ
θp)/2 curve for F-∗-IF
− (
46; (
θ, Δ
θp) = (134.5°, −5.5°)), Me
2FTe-∗-F (
49; (122.6°, −3.0°)), Me
2ClTe-∗-Cl (
50; (166.3°, −6.6°)), Me
2S
+-∗-I (
53; (181.7°, −4.0°)) and Me
2Te
+-∗-F (
55; (126.0°, −2.4°)), which show the inverse behavior of the interactions. In the case of Me
2Te
+-∗-Cl (
56;
θ, Δ
θp) = (168.0°, −23.9°)), Me
2Te
+-∗-Br (
57; (179.5°, −14.0°)), H
3C-∗-F (
60; (177.6°, −27.0°)) and H
3C-∗-I (
61; (187.2°, −9.5°)), the data appear below the −
fr(Δ
θp)/2 curve. Therefore, the interactions are recognized to show inverse behavior stronger than that supposed from the behavior of the standard interactions in
1–
36.
What are the differences in the nature of the interactions among the three groups? The (
θ, Δ
θp) values are examined in more detail for the interactions shown in
Table 1. The typical (
θ, Δ
θp) values for Me
2Te-∗-X
2 (
41–
44), Me
2(X)Te-∗-X (
49–
52), Me
2Te
+-∗-X (
55–
58) and H
3C-∗-X (
30,
31,
60, and
61) (X = F, Cl, Br, and I) are shown in Equations (12)–(15), respectively. The interactions in the equations are arranged in ascending order of Δ
θp.
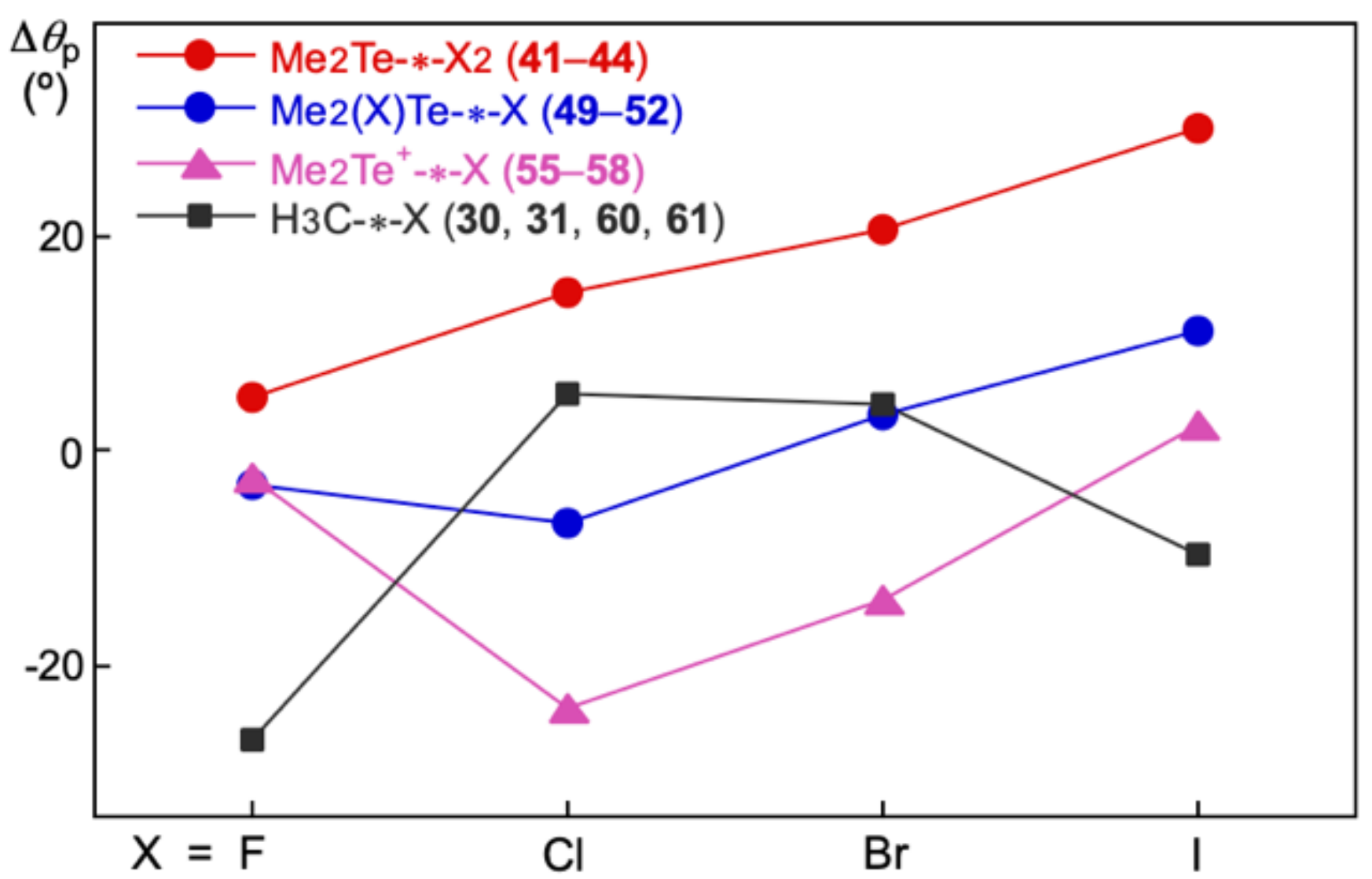
Figure 5 shows the plot of Δ
θp versus X (X = F, Cl, Br, and I) for
41–
44,
49–
52,
55–
58 and
30,
31,
60, and
61. The Δ
θp values for the interactions in
41–
44,
49–
52 and
55–
58 are in the order of Me
2Te-∗-X
2 > Me
2(X)Te-∗-X > Me
2Te
+-∗-X, as a whole, if those of the same X are compared. The strength of Te–X is in the order of Me
2Te-∗-X
2 < Me
2(X)Te-∗-X < Me
2Te
+-∗-X; therefore, the strength of Te–X in the species operates to decrease the Δ
θp values for the species.
The Δθp values for the interactions in 41–44 become more positive, as X goes from F to Cl, then Br, and then to I, where the character (or atomic number) of X becomes closer to Te in 41–44. The Δθp values in 49–52 and 55–58 show the trends, similar to that in 41–44, for X = Cl, Br and I. The strength of Te–X operates to decrease the Δθp values also for the species. However, the Δθp values in 49 and 55 become more negative, when X goes F to Cl, contrary to the case in 41. The differences in the strength of Te–F in 41, 49 and 55 would result in smaller differences in the plot. In the case of H3C-∗-X, the Δθp values become more negative in the order of X = Cl ≥ Br > I >> F. The behavior of the interactions should be controlled by the differences in the characters (or atomic numbers) of X. The character of C in H3C-∗-X should be closer to those of Cl and Br, relative to the case of I and F. The calculated results are well explained based on Equations (5) and (6).
What are the behavior of the E-∗-E’ bonds in the neutral, monoanionic, monocationic, and dicationic forms of [MeE-∗-E’Me]* (E, E’ = S, Se, and Te; * = null, −, +, and 2+)? The species are optimized, first. The optimized structures of the neutral form have
C2 symmetry for E = E’ and close to
C2 symmetry for E ≠ E’. Here, the structures are called the
C2 type. The structures of the
C2 and
trans types are optimized for the monoanionic form. The structures of the
C2 type are only discussed here because the Δ
θp values are larger than 20° for both forms (
Table 2). The structures of the
trans type are optimized for the mono- and dicationic forms.
Table 2 collects the (
θ,
θp, Δ
θp) values of the species calculated under MP2/BSS-C. Numbers for the species are shown in
Table 1. The calculated results, other than those in
Table 2, are collected in
Table S8 of the Supplementary Materials.
The behavior of the interactions is examined next, based on the (
θ, Δ
θp) values. Positive Δ
θp values of 28.2–32.3° are predicted for the monoanionic forms of [MeE-∗-E’Me]
− (E, E’ = S, Se and Te) shown in
Table 2. Positive Δ
θp values (2.6–6.8°) are predicted for S-∗-S, S-∗-Se, Se-∗-Se and Te-∗-Te of the neutral, mono- and dicationic forms of [MeE-∗-E’Me]* (E, E’ = S, Se, and Te; * = null, +, and 2+). However, negative Δ
θp values are predicted for the neutral forms of MeS-∗-TeMe (Δ
θp = −14.2°) and MeSe-∗-TeMe (−3.7°); the monocationic forms of [MeS-∗-TeMe]
+ (c−15.5°) and [MeSe-∗-TeMe]
+ (−3.9°); and the dicationic forms of [MeS-∗-TeMe]
2+ (−11.3°) and [MeSe-∗-TeMe]
2+ (−3.2°).
Equation (16) shows the common order for Δθp of E-∗-E’ in the neutral, mono- and dicationic forms of [MeE-∗-E’Me]* (E, E’ = S, Se, and Te), where the equation is arranged in the ascending order of Δθp. The order can also be understood under the guidance of the conclusions derived from Equations (5) and (6). That is, the Δθp values are more negative as the difference in the atomic numbers of A and B in A-∗-B becomes larger. However, it is not so clear that the Δθp values for the same A-∗-B become more negative if the interaction in question becomes stronger.
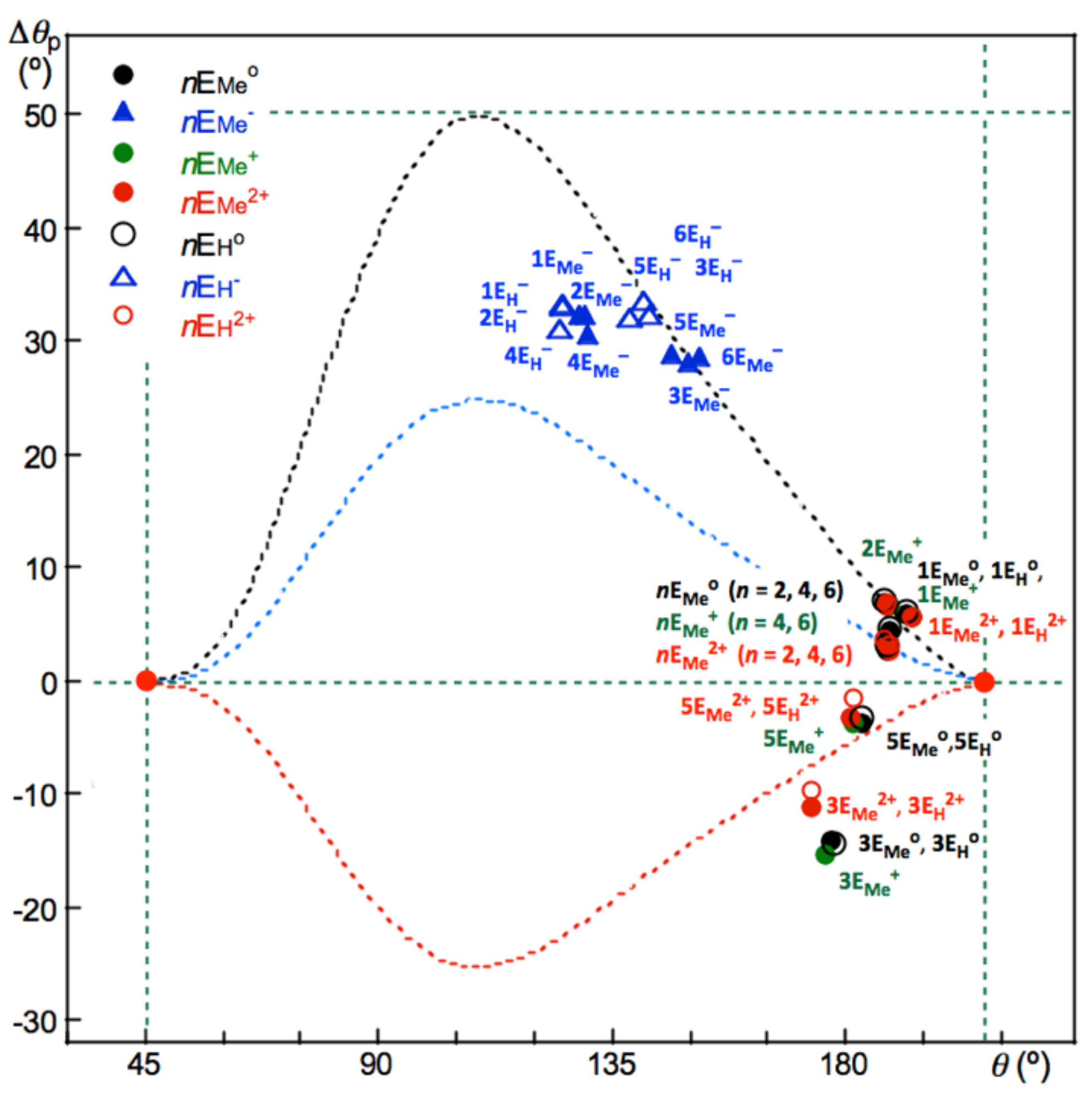
Figure 6 shows the plot of Δ
θp versus
θ for the E-∗-E’ interactions in the neutral, monoanionic, monocationic and dicationic forms of [MeEE’Me]* (E, E’ = S, Se and Te; * = null, −, + and 2+). (See
Table 2 for the values). The normal, weak normal and inverse behavior of interactions is clearly illustrated in the figure. A very similar trend is observed in the neutral, anionic and dicationic forms of [HE-∗-E’H]* (E, E’ = S, Se and Te; * = null, − and 2+). The calculated results are collected in
Table S8 of the Supplementary Materials, and the values are plotted in
Figure 6. Some (data) points of (Δ
θp,
θ) appear below the –
fr(Δ
θp)/2 curve for S-∗-Te in 3E
Me0, 3E
H0, 3E
Me+ and 3E
Me2+, and 3E
H2+ with Se-∗-Te in 5E
Me0 and 5E
H0. The results show that some S-∗-Te and Se-∗-Te interactions show stronger inverse behavior than that supposed from the behavior for the standard interactions in
1–
36.
The behavior of E-∗-E’ in [HE-∗-E’H]* (E, E’ = S, Se and Te; * = null, − and 2+) is very similar to the behavior in [MeE-∗-E’Me]* (E, E’ = S, Se and Te; * = null, −, + and 2+); therefore, the behavior of E-∗-E’ in [HE-∗-E’H]* (E, E’ = S, Se and Te; * = null, −, + and 2+) is not discussed in detail here. Nevertheless, the behavior of [HS-∗-TeH]2+ is discussed later.
The (
θ,
θp, Δ
θp) values for E-∗-C and E’-∗-C, together with E-∗-H and E’-∗-H, in [REE’R]* (R = Me and H: E, E’ = S, Se, and Te; * = null, −, +, and 2+) are collected in
Table S8 of the Supplementary Materials.
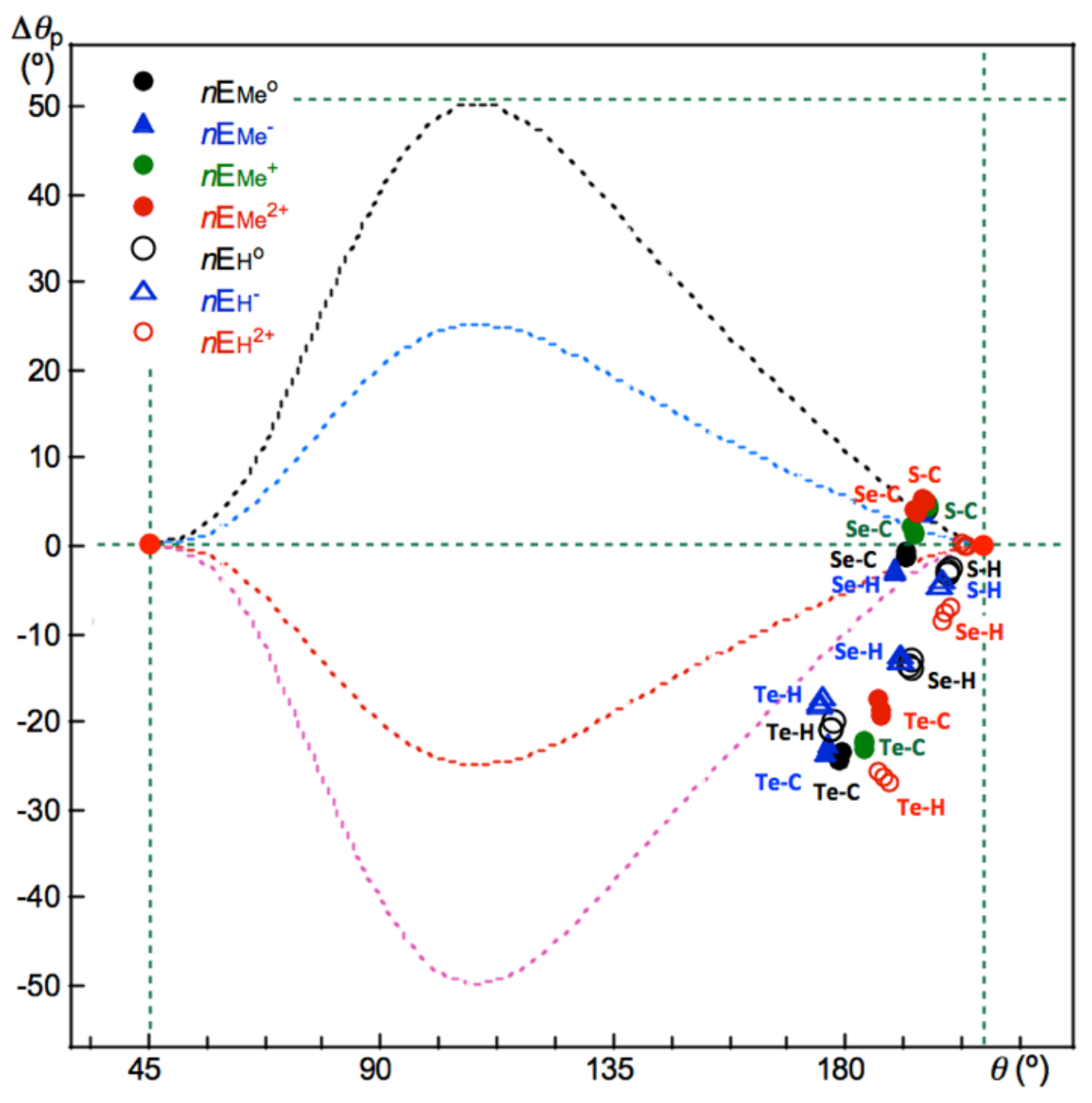
Figure 7 shows the plot of Δ
θp versus
θ, for the data in
Table S8. A lot of data are well visualized.
As shown in the table and the figure, the Δθp values are negative for Te-∗-C in MeETe-∗-Me (E = S, Se and Te: −24.5° ≤ Δθp ≤ −23.5°), [MeETe-∗-Me]– (E = S, Se and Te: −23.5° ≤ Δθp ≤ −22.5°), [MeETe-∗-Me]+ (E = S, Se and Te: −23.1° ≤ Δθp ≤ −22.3°), and [MeETe-∗-Me]2+ (E = S, Se and Te: −19.4° ≤ Δθp ≤ −17.3°), whereas the values are all positive for S-v-C of [Me-∗-SE’Me]* (E’ = S, Se and Te; * = null, –, + and 2+: 3.9° ≤ Δθp ≤ 5.4°). In the case of Se-∗-C, the Δθp values are negative for MeESe-∗-Me (E = S, Se and Te: −1.4° ≤ Δθp ≤ −0.7°) and [MeESe-∗-Me]− (E = S, Se and Te: −2.8° ≤ Δθp ≤ −2.5°), whereas the values are positive for [MeESe-∗-Me]+ (E = S, Se and Te: 1.4° ≤ Δθp ≤ 2.2°) and [MeESe-∗-Me]2+ (E = S, Se and Te: 3.7° ≤ Δθp ≤ 4.1°). The Δθp values of E-∗-C and E’-∗-C are plotted in [Me-∗-EE’-∗-Me]* (E, E’ = S, Se and Te; * = null, −, + and 2+), containing the values from the symmetric [Me-∗-EE-∗-Me]* (E = S, Se and Te; * = null, −, + and 2+). In the case of E-∗-H and E’-∗-H, the Δθp values are negative for all S-∗-H, Se-∗-H, and Te-∗-H shown in the table. The Δθp values are −4.5° ≤ Δθp ≤ −2.5° for HES-∗-H and [HES-∗-H]−, −13.9° ≤ Δθp ≤ −12.2° for HESe-∗-H and [HESe-∗-H]− and −20.9° ≤ Δθp ≤ −17.1° for HETe-∗-H and [HETe-∗-H]−, where E = S, Se and Te. Most of the (data) points of (Δθp, θ) for E-∗-H (E = S, Se, and Te) and Te-∗-H appear below the −fr(Δθp) curve, tentatively drawn in the plot. The interactions show much severer the inverse behavior is than that supposed from the behavior for the standard interactions of 1–36.
After recognizing the interactions with Δθp < 0, the next extension is to clarify the origin and mechanisms that cause the negative Δθp values.
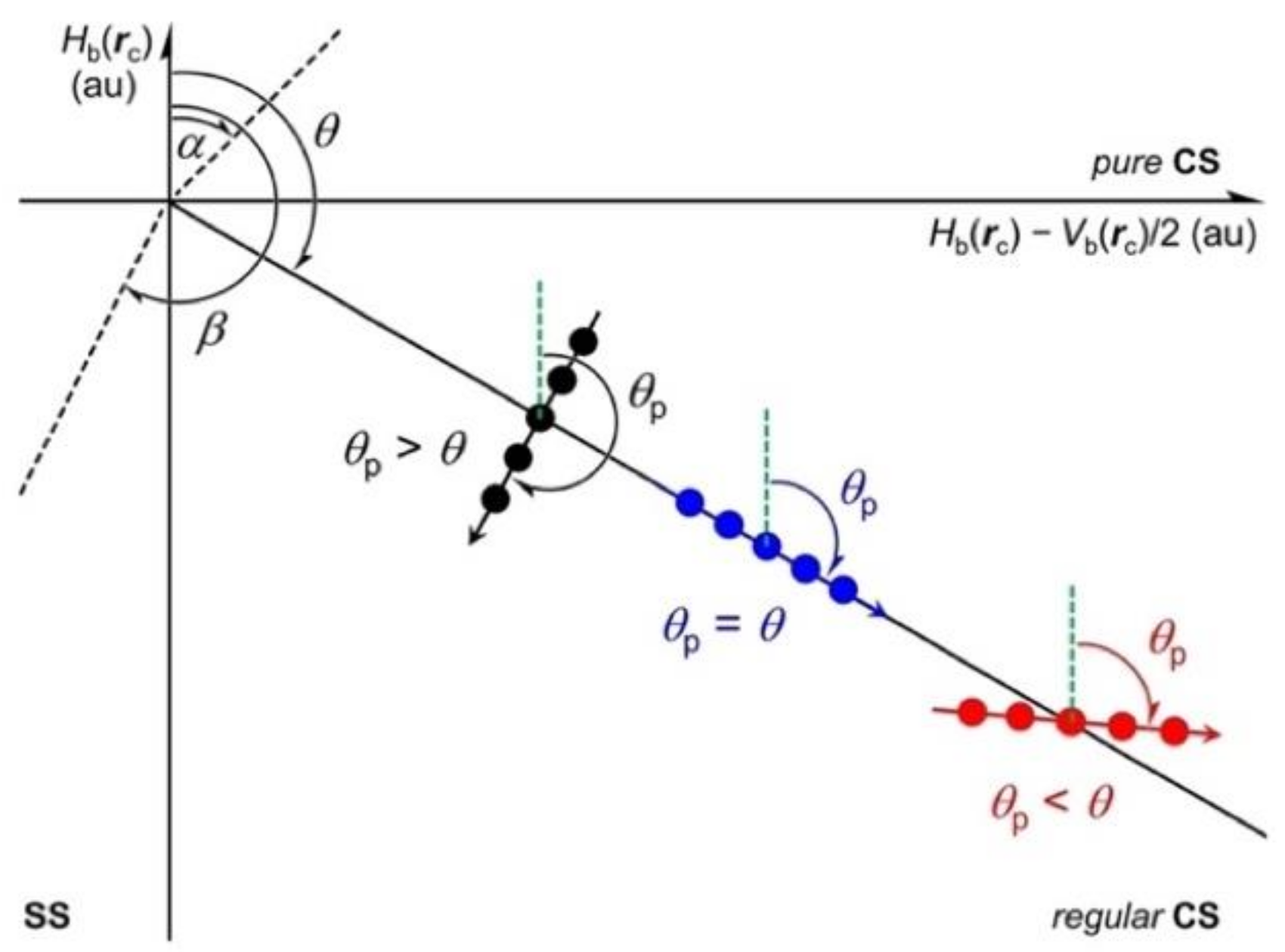
3.4. Processes to Arise the Negative Δθp Values around the Optimized Structures
The processes to give the negative Δθp values are examined for some interactions from the sufficiently distant states to around the optimized structures. Hb(rc) values are plotted versus Hb(rc) − Vb(rc)/2 for the reaction processes over wide ranges of the interaction distances in question. It is more suitable if the singlet state is retained throughout the process, which relieves us from the trouble of considering the effect of the change on the multiplicity in the process. The structural change is also expected to be limited to the minimum extent if the singlet state is retained throughout the processes. The analysis is much simpler under these conditions, together with the discussion. As a result, the mechanisms to give the negative Δθp values are more clearly understood as the reactions proceed if the singlet state is retained throughout the reaction processes. Such processes seem rather rare and satisfy the above conditions.
The negative Δ
θp value of −4.2° is calculated for [F–I-∗-F]
− (
46), as shown in
Table 1, where the reaction process is shown by Equation (17). The reaction shown by Equation (18) has been reported to proceed in the singlet state under metal-free conditions [
19,
20]. A negative value of −22.8° was also calculated for Tf–N-∗-IMe (Tf = SO
2CF
3) (
Table 3). The reaction processes for [F–I-∗-F]
– (
46) in Equation (17) and Tf–N-∗-IMe in Equation (18) could be good candidates to achieve this purpose. Therefore, the reaction processes to give the negative Δ
θp values are examined, exemplified by the reactions shown in Equations (17) and (18).
Figure 9 shows the QTAIM-DFA plot of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 for [F–I-∗-F]
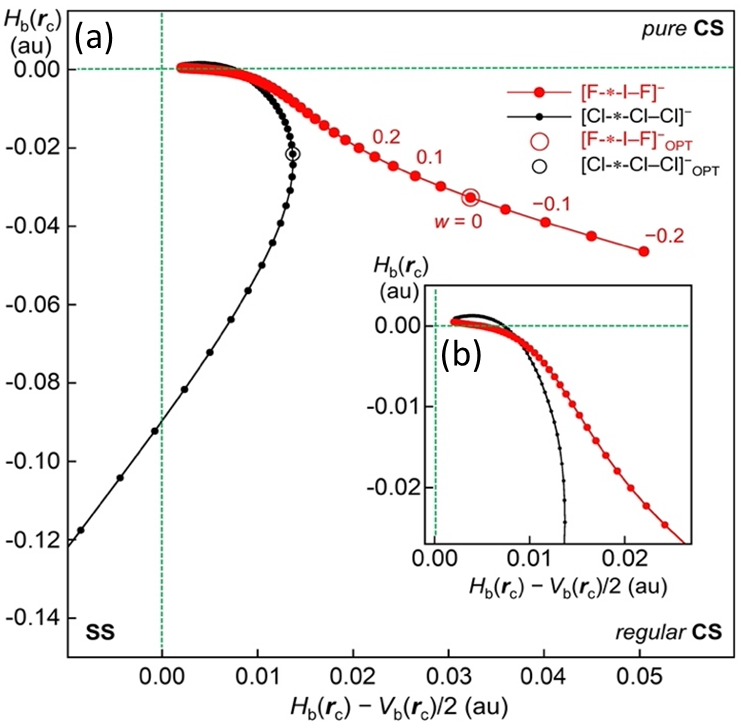
− for a wide range of the interaction distances in question. Fortunately, the perturbed structures were successfully (and easily) obtained for a wide range of interaction distances under MP2/BSS-C.
Figure 9 contains the plot for [Cl–Cl-∗-Cl]
−, similarly calculated, for comparison (see also
Table 1). The plot for [Cl–Cl-∗-Cl]
− shows a smooth and monotonic curve, starting from a point close to the origin. The plot for [Cl–Cl-∗-Cl]
− forms a spiral curve overall. As a result, the curve of the plot for [Cl–Cl-∗-Cl]
− satisfies the requirements for
θp >
θ (Δ
θp > 0) throughout the reaction process, as illustrated in
Figure 9. This is the reason that [Cl–Cl-∗-Cl]
− typically shows the normal behavior of interactions.
However, the plot for [F–I-∗-F]
− seems different from the plot for [Cl–Cl-∗-Cl]
− in some points. The plot does not show the spiral figure as a whole. The plot of [F–I-∗-F]
− seems similar to the plot of [Cl–Cl-∗-Cl]
− when
Hb(
rc) −
Vb(
rc)/2 < 0.01 au, as shown in the magnified
Figure 9b. However, the plots for the two interactions become much different if
Hb(
rc) −
Vb(
rc)/2 > 0.02 au. The
Hb(
rc) value seems to decrease almost linearly as the
Hb(
rc) −
Vb(
rc)/2 value becomes larger, especially for the range of
Hb(
rc) −
Vb(
rc)/2 > 0.02 au. The plot for [F–I-∗-F]
− seems to show a downwardly convex character for the range, which is very different from the plot for [Cl–Cl-∗-Cl]
−. The characteristic figure in the plot for [F–I-∗-F]
− prevents us from showing the spiral stream. The curve for the plot of [F–I-∗-F]
− would satisfy the requirements for
θp >
θ (Δ
θp > 0) in the range of
Hb(
rc) −
Vb(
rc)/2 < 0.02 au, as illustrated in
Figure 9. The curve gradually changes to show the downwardly convex character to satisfy the requirements for
θp =
θ (Δ
θp = 0). The optimized structure, shown by
w = 0.0 in
Figure 9, appears in the range satisfying the requirements for
θp <
θ (Δ
θp < 0), illustrated in
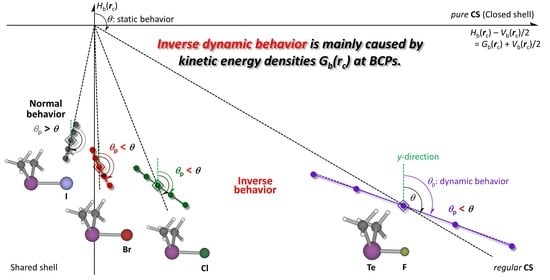
Figure 8. Indeed, the plot in
Figure 9 shows the outline to give the negative Δ
θp value (–5.5°) for [F–I-∗-F]
− (
46), but it is better if the requirement for Δ
θp < 0 is illustrated more clearly in the plot for a process. Next, this process was examined and exemplified by the ligand exchange reaction at N via TS [MeBr-∗-N(Tf)-∗-IMe].
Table 3 collects the (
θ,
θp, Δ
θp) values for the interactions of Tf–N-∗-BrMe, Tf–N-∗-IMe and TS [MeBr-∗-N(Tf)-∗-IMe], which have been calculated at the BCPs under MP2/BSS-C’.
Table 3 contains the values for TS [MeBr-∗-N(Tf)-∗-BrMe], similarly calculated, for reference. The perturbed structures were calculated in the processes shown in Equations (17) and (18) with the IRC method, starting from TS [MeBr-∗-N(Tf)-∗-IMe] and TS [MeBr-∗-N(Tf)-∗-BrMe]. The perturbed structures of the Br-∗-N and N-∗-I interaction distances of wide ranges could be successfully calculated with the IRC method under MP2/BSS-C’.
The reaction processes shown in Equation (19) were examined before investigations on those shown in Equation (18).
Hb(
rc) is plotted versus
Hb(
rc) −
Vb(
rc)/2 for both Br-∗-N bonds in the ligand exchange reaction via TS [MeBr-∗-N(Tf)-∗-BrMe]. The plot is shown in
Figure S3 of the Supplementary Materials, consisting of two plots, very close to each other, because TS [MeBr-∗-N(Tf)-∗-BrMe] has no symmetry due to the slightly different geometry around the Br-∗-N-∗-Br moiety. Therefore, the nature of Br-∗-N on both sides of the TS [MeBr-∗-N(Tf)-∗-BrMe] is slightly different. The Δ
θp values are calculated to be 50.3° and 49.1° for TS [MeBr-∗-N(Tf)-∗-BrMe], respectively.
The plots for the two N-∗-Br interactions via TS [MeBr-∗-N(Tf)-∗-BrMe] show a smooth, monotonic and spiral curve, very similar to the curve for [Cl–Cl-∗-Cl]
– (
Figure 1 and
Figure 9). Consequently, both Br-∗-N are recognized to show the typical normal behavior of interactions, which leads to positive Δ
θp values for the final products. The Δ
θp values of the final products of MeBr-∗-N–Tf and the isomer are 7.4° and 5.8°, respectively, depending on slight differences in the geometry. The similarity in the reaction processes between MeBr-∗-N–Tf and [Cl-∗-Cl-∗-Cl]
− is of significant interest. The processes proceed in both directions and give the final products from TS [MeBr-∗-N(Tf)-∗-BrMe] of the highest energy for the former but from the global minimum of the lowest energy for the latter.
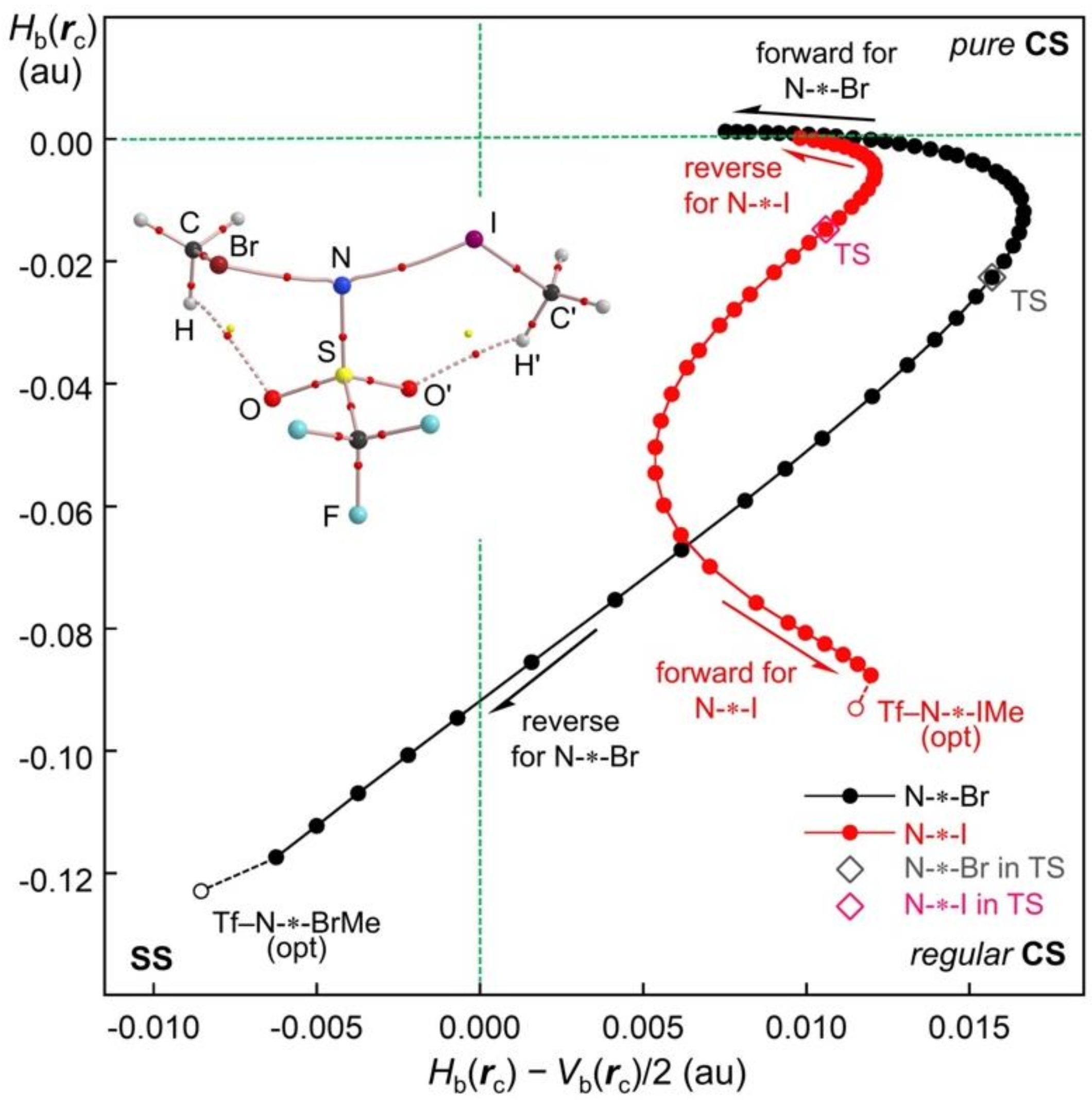
Figure 10 shows the QTAIM-DFA plots for the process shown by Equation (18).
Figure 10 is briefly explained to avoid misunderstanding. It is not a plot for the energy profile, but a QTAIM-DFA plot for both Br-∗-N and N-∗-I, so two plots appear corresponding to the two bonds, while a single transition state contributes to the reaction. (This is also the case for the plots in
Figure S3 of the Supplementary Materials.) The forward and reverse processes for the reaction shown by Equation (18) are clearly specified in
Figure 10.
In spite of the forward and reverse processes for the reaction, the plot for Br-∗-N is drawn by the black dots in
Figure 10, which corresponds to the reaction process of Equation (18). The process forms MeI + Tf–N-∗-BrMe, starting from Tf–N-∗-IMe + MeBr via TS [MeBr-∗-N(Tf)-∗-IMe]. The Δ
θp value of Br-∗-N changes from 7.9° for Tf–N-∗-BrMe to 42.6° for TS [MeBr-∗-N(Tf)-∗-IMe], then to 0.0°, with no interaction state, if the reaction proceeds in the direction shown in Equation (18).
The plot for Br-∗-N in
Figure 10 also shows a smooth, monotonic and spiral curve, which is very similar to the curve for [Cl–Cl-∗-Cl]
− (
Figure 1 and
Figure 9) and the curves for Br-∗-N illustrated in
Figure S3 of the Supplementary Materials (see Equation (19)). Consequently, the Br-∗-N bond in the ligand exchange reactions via TS [MeBr-∗-N(Tf)-∗-IMe] shows normal behavior similar to the case of TS [MeBr-∗-N(Tf)-∗-BrMe] and [Cl–Cl-∗-Cl]
−. However, the plot for I-∗-N in
Figure 10 is drawn by the red dots. The process forms MeBr + Tf–N-∗-IMe, starting from Tf–N-∗-BrMe + MeI via TS [MeBr-∗-N(Tf)-∗-IMe]. The Δ
θp value of I-∗-N goes from 0.0° for MeI to 48.4° for TS [MeBr-∗-N(Tf)-∗-IMe], then to −22.8° for Tf–N-∗-IMe if the reaction proceeds in the direction shown in Equation (18). The plot for I-∗-N is very different from the plot for N-∗-Br, as shown in
Figure 10. The difference between the plots clarifies the mechanism to give the negative Δ
θp value for Tf–N-∗-IMe.
The plot for N-∗-I in red shows a distorted Z shape. This is of great interest because the plot for N-∗-I seems to largely overlap the plot for N-∗-Br in the range of
Hb(
rc) > −0.02 au, if the plot for N-∗-I is moved to the right. The plot for N-∗-I curves to the left in the range of −0.05 <
Hb(
rc) < −0.006 au, then crosses the plot for N-∗-Br at
Hb(
rc) around −0.07 au. The plot for N-∗-I shows a downward-sloping linear shape in the range of
Hb(
rc) < –0.08 au. The mechanism to cause the negative Δ
θp value for Tf–N-∗-IMe, based on the characteristic figure in the plot of N-∗-I shown in
Figure 10, can be explained as follows: The Δ
θp value for N-∗-I in the reaction, shown in Equation (18), must be positive if
Hb(
rc) > −0.06 au for N-∗-I, starting from Tf–N-∗-BrMe + MeI (no interaction between N and I). The positive Δ
θp value decreases for the plot curving to the left in the range of −0.05 <
Hb(
rc) < −0.01 au, then approaches zero, and finally gives the negative Δ
θp value of −12.9° for Tf–N-∗-IMe in the range of
Hb(
rc) < −0.09 au.
In the plot for N-∗-I in
Figure 10,
Hb(
rc) decreases monotonically, whereas
Hb(
rc) –
Vb(
rc)/2 shows abnormal behavior when
Hb(
rc) < 0. Therefore, it is strongly suggested that the strange behavior of
Hb(
rc) –
Vb(
rc)/2 should be responsible for the negative Δ
θp value.
The process for N-∗-I to give the negative Δ
θp value is well clarified by the plot for N-∗-I in
Figure 10. However, it would be difficult to visually recognize the value of Δ
θp, positive or negative, from the plot. We searched for a typical example that can be recognized as a clear negative Δ
θp value visually from the plot. The (
θ,
θp, Δ
θp) values for [HS-∗-TeH]
2+ are given in
Table S8 of the Supplementary Materials, of which Δ
θp is a negative value of −9.7°. The process for [HS-∗-TeH]
2+ was calculated with POM by elongating the S-∗-Te distance, starting from the structure around the optimized one. The calculations were performed in the range where the rational structures were optimized at the singlet state.
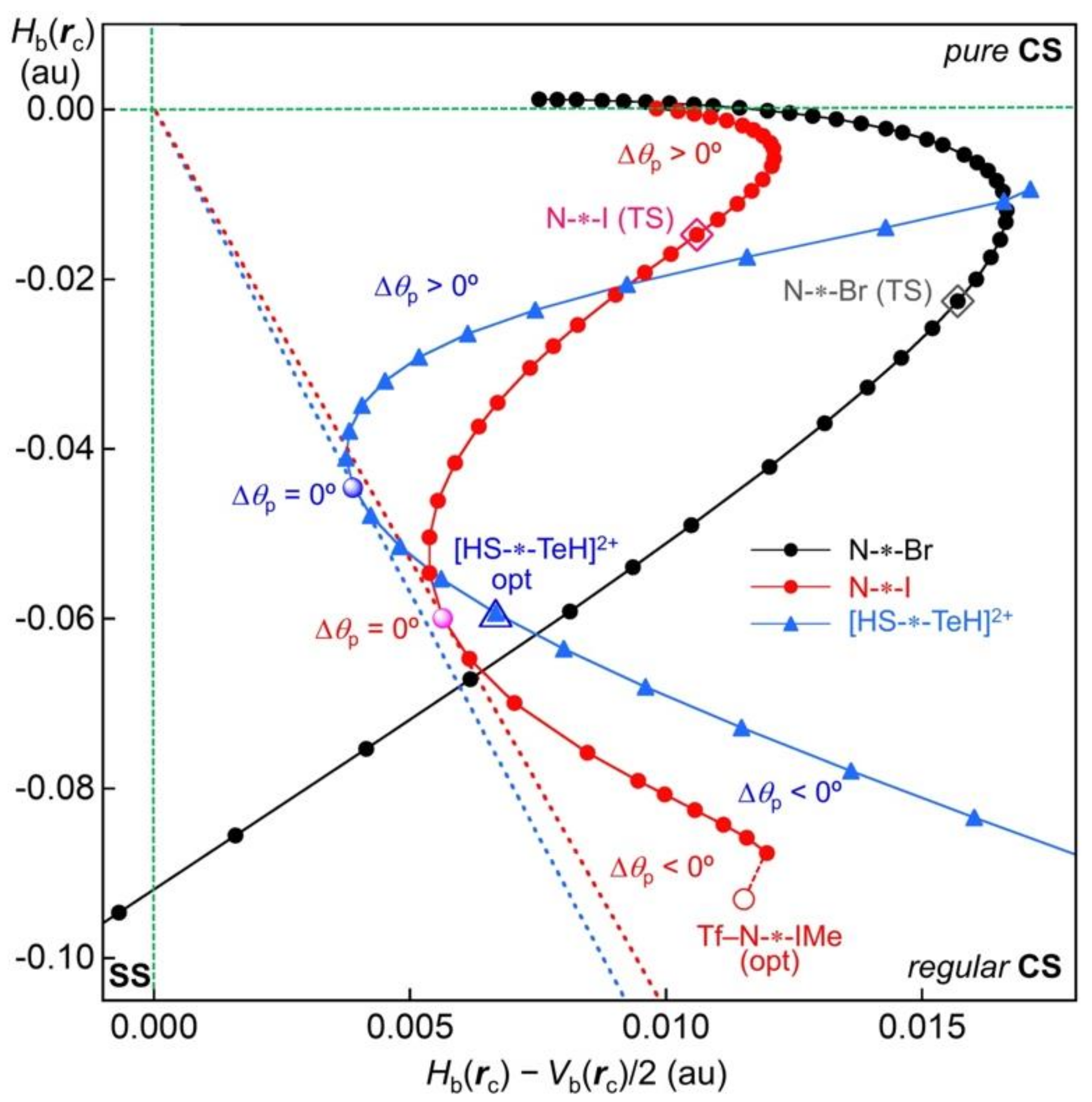
Figure 11 draws the reaction process for [HS-∗-TeH]
2+, although the interaction distance is limited to the distance around the optimized structure, where the perturbed structures are rationally optimized at the singlet state. The plots for N-∗-I and N-∗-Br in the reaction processes shown in Equation (18) are added to
Figure 11 for reference. The partial plot for [HS-∗-TeH]
2+, shown in
Figure 11, seems to correspond to the plot for N-∗-I in the range of
Hb(
rc) < −0.005 au. The lines of Δ
θp = 0° for the plots of N-∗-I and [HS-∗-TeH]
2+ are drawn by the red and blue dotted lines, respectively. The tangent directions from the origin to the curves correspond to the lines of Δ
θp = 0° (see also
Figure 8).
As discussed above, the plot for N-∗-Br satisfies the requirement for Δθp > 0° in the whole range of the reaction process, while the plot for N-∗-I should satisfy the requirement for Δθp < 0° around the optimized structure. However, it seems visually unclear from the plot, as mentioned above. In the case of the plot for [HS-∗-TeH]2+, there exists an area that clearly satisfies the requirements for Δθp < 0°, after the blue dotted line for Δθp = 0°. The area with Δθp < 0° is the area around the optimized structure in this case. The crossing point on the plot with the tangent line corresponds to Δθp = 0°; therefore, the perturbed structure of which the S-∗-Te distance is shorter than the value at Δθp = 0° should show positive Δθp values, namely the area of Δθp ≥ 0°.
After clarification of the origin for the positive and negative Δθp values through the plots, the next extension is to elucidate the mechanisms based on the behavior of Gb(rc) and Vb(rc).
3.5. Mechanisms for the Origin of the Negative Δθp Values
The reaction processes can be directly expressed using the interaction distances. However, the
ρb(
rc) values can also be used for the analyses, where the values decrease exponentially as the interaction distances increase. The distances are also expressed by
w in Equation (10). The relation between
ρb(
rc) and
w is confirmed by the plot of
ρb(
rc) versus
w for an interaction. Such a plot is shown in
Figure S6 of the Supplementary Materials, exemplified by [Cl–Cl-∗-Cl]
− and [F–I-∗-F]
−. As a result,
ρb(
rc) would be the better parameter to describe the characteristics of interactions around the optimizes structures, in more detail, relative to the case of
r and
w for the longer distances. The characteristic behavior of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 can be attributed to the behavior of
Gb(
rc) and
Vb(
rc), because
Hb(
rc) and
Hb(
rc) −
Vb(
rc)/2 are expressed by
Gb(
rc) and
Vb(
rc) (see Equations (1) and (2)). The reaction processes are investigated employing the plots of
Hb(
rc) −
Vb(
rc)/2,
Hb(
rc),
Gb(
rc) and
Vb(
rc) versus
ρb(
rc) and the related plots.
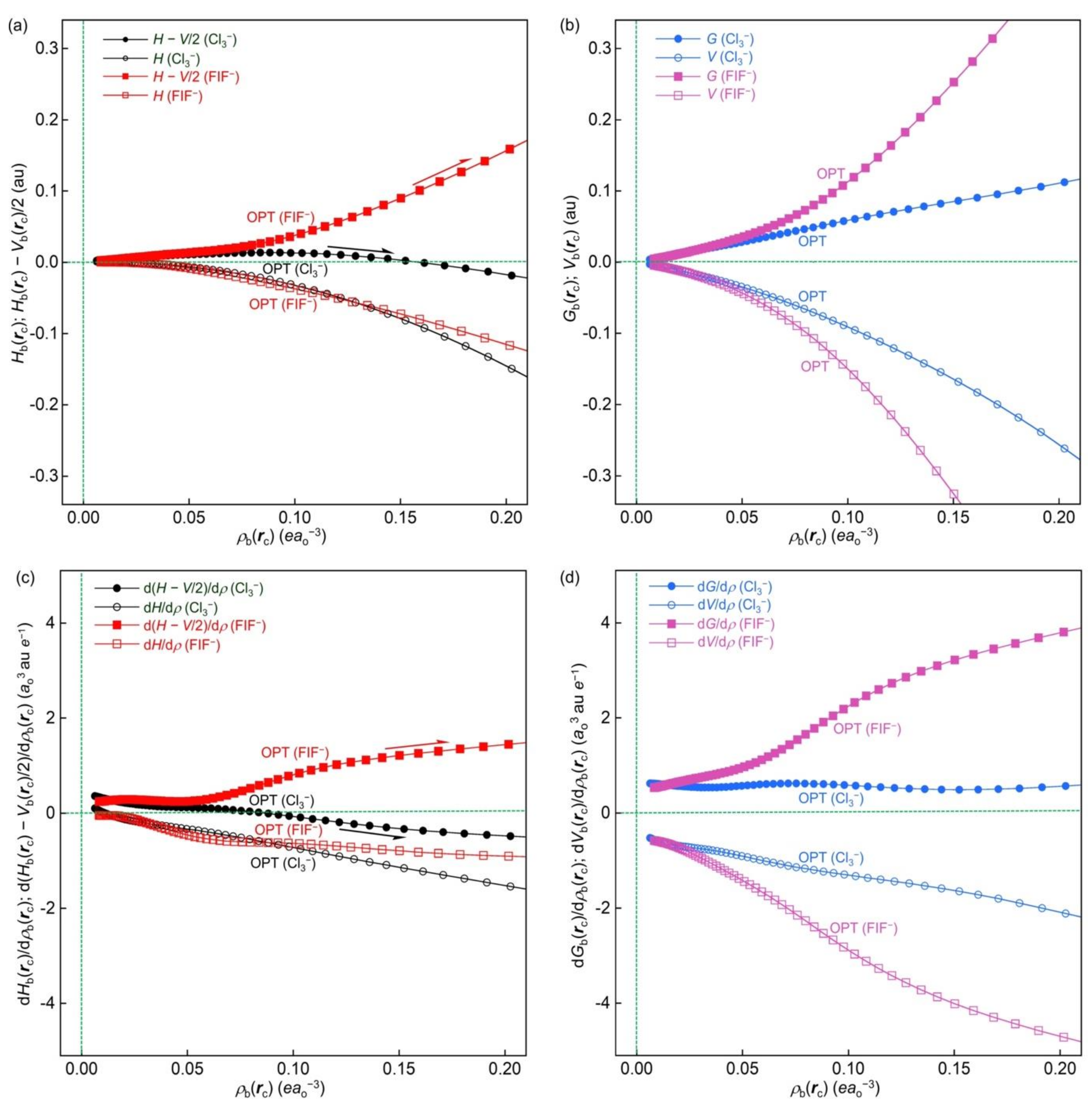
Figure 12a,b shows the plots of
Hb(
rc) −
Vb(
rc)/2,
Hb(
rc),
Gb(
rc) and
Vb(
rc) versus
ρb(
rc) for the reaction processes of [Cl–Cl-∗-Cl]
− and [F–I-∗-F]
−, where the perturbed structures are generated with POM over the wide range of
w. The plots of
Gb(
rc) versus
ρb(
rc) and
Vb(
rc) versus
ρb(
rc) for [Cl–Cl-∗-Cl]
− go upside and downside, respectively, very smoothly and monotonically, starting from a point close to the origin. The plot of the former goes upside almost linearly, while that of the latter goes downside, showing a slight convex upward shape. The magnitude of the former seems close to but less than two times the magnitude of the latter for the range of
ρb(
rc) < 0.16 (
e/
a03). The plots of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) and
Hb(
rc) versus
ρb(
rc) are also smooth and gentle for [Cl–Cl-∗-Cl]
−. The
Hb(
rc) −
Vb(
rc)/2 value is positive and then negative after
ρb(
rc) ≥ 0.16 (
e/
a03), while the positive
Hb(
rc) becomes negative when
ρb(
rc) reaches approximately 0.026 (
e/
a03), leading to the normal behavior of the [Cl–Cl-∗-Cl]
− interactions (Δ
θp > 0). The plot of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 for [Cl–Cl-∗-Cl]
− shown in
Figure 9 is well explained through the plots of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) and
Hb(
rc) versus
ρb(
rc) shown in
Figure 12a.
In the case of [F–I-∗-F]
−, the plots of
Gb(
rc) versus
ρb(
rc) and
Vb(
rc) versus
ρb(
rc) go upside and downside, respectively, smoothly and monotonically, starting from a point close to the origin. However, the plots show convex upward and downward shapes, respectively, and the magnitude of the former seems 0.8 times larger than the magnitude of the latter. As a result, the plot of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) is mainly controlled by the plot of
Gb(
rc) versus
ρb(
rc), whereas the plot of
Hb(
rc) versus
ρb(
rc) is almost controlled by the plot of
Vb(
rc) versus
ρb(
rc) (cf; Equations (1) and (2)).
Hb(
rc) −
Vb(
rc)/2 and
Hb(
rc) are positive and negative in the calculated whole range for [F–I-∗-F]
−, except for
Hb(
rc) of the substantially large range of
r where
ρb(
rc) is substantially very small. Therefore, the plot of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 is close to the linear relationship of the
y = −
ax (
a > 0) type, which essentially explains the plot for [F–I-∗-F]
−, as shown in
Figure 9. The character of
Gb(
rc) versus
ρb(
rc) remains in the character of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) for [F–I-∗-F]
−. The negative Δ
θp value of [F–I-∗-F]
− must arise from the characteristic plot of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2, which is close to the linear relationship of the
y = −a
x (
a > 0) type, which originates from the characteristic plot of
Hb(
rc) versus
Gb(
rc).
Figure 12c,d show the plots of d(
Hb(
rc) −
Vb(
rc)/2)/d
ρb(
rc), d
Hb(
rc)/d
ρb(
rc), d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc) for the reaction process of [Cl–C-∗-Cl]
− and [F–I-∗-F]
−. All plots seem almost linearly flat, except for the plots of d(
Hb(
rc) −
Vb(
rc)/2)/d
ρb(
rc), d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) for [F–I-∗-F]
−. The results for F–I-∗-F]
− expose the behavior in the plot of d(
Hb(
rc) −
Vb(
rc)/2)/d
ρb(
rc) versus
ρb(
rc). The behavior of the plot of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) is shown to be the main factor leading to the negative Δ
θp value for F–I-∗-F]
−, as expected.
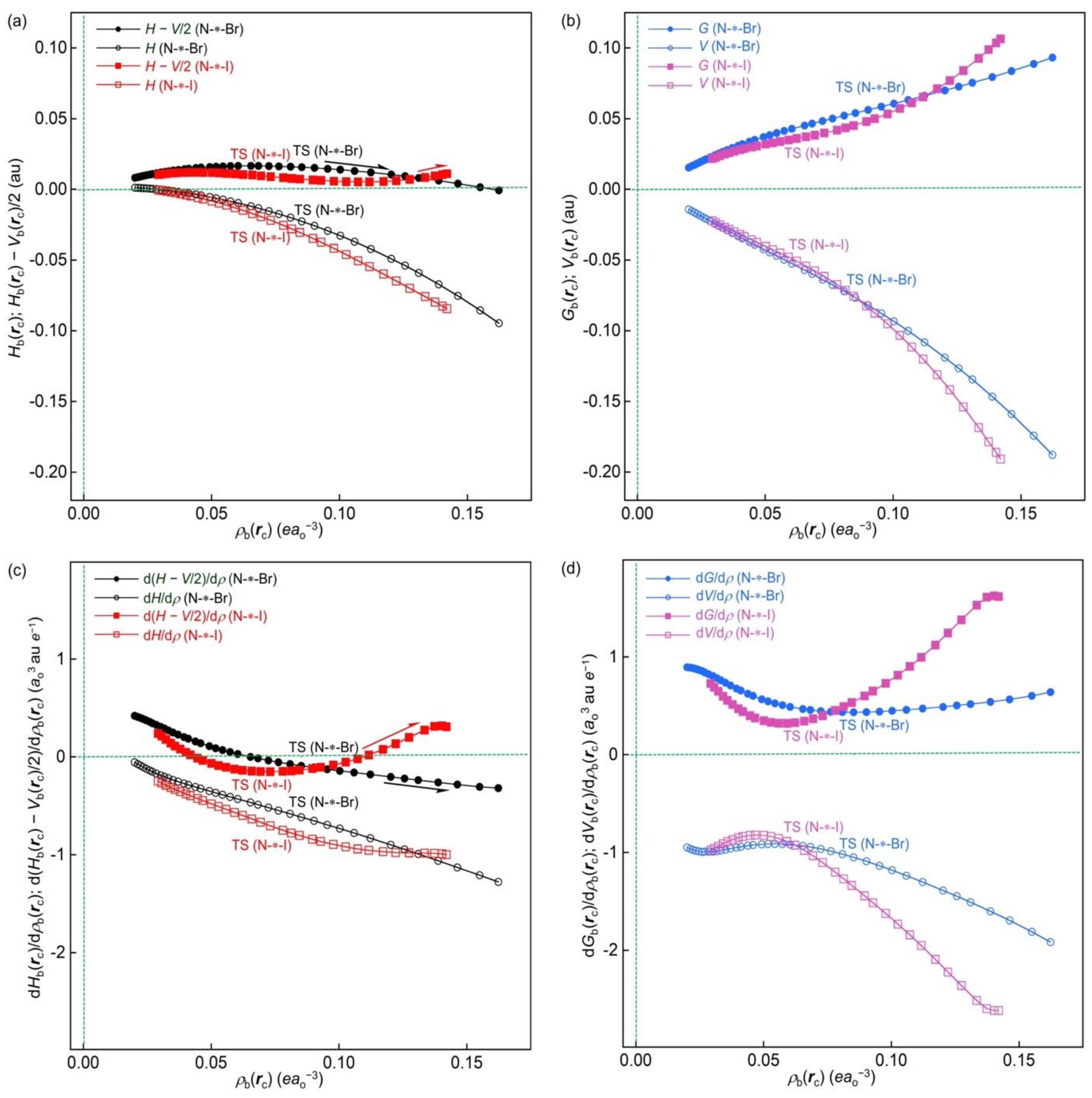
Figure 13a,b illustrate the plots of
Hb(
rc) −
Vb(
rc)/2,
Hb(
rc)
Gb(
rc) and
Vb(
rc) versus
ρb(
rc) for N-∗-Br and N-∗-I in the reaction processes from TS [MeBr-∗-N(Tf)-∗-IMe], where the perturbed structures are generated with the IRC method (see Equation (18)).
The plots of Hb(rc) − Vb(rc)/2, Hb(rc), Gb(rc), and Vb(rc) versus ρb(rc) for N-∗-Br are very similar to the corresponding plots for [Cl–C-∗-Cl]−, although the magnitudes of the plots are different. Consequently, the plot of Hb(rc) versus Hb(rc) − Vb(rc)/2 for N-∗-Br should be very similar to the plot for [Cl–C-∗-Cl]−. The plot for N-∗-Br is well explained based on the analyzed results discussed above.
However, the plots of Gb(rc) versus ρb(rc) and Vb(rc) versus ρb(rc) for N-∗-I go upside and downside, respectively, starting from a point close to the origin. The plot of Gb(rc) versus ρb(rc) for N-∗-I gives a distorted Z-shaped curve, while the plot of Vb(rc) versus ρb(rc) shows a slightly distorted Z-shaped character. The magnitude of the plot of Gb(rc) versus ρb(rc) seems to be 0.7 times the magnitude of Vb(rc) versus ρb(rc) for N-∗-I. Therefore, the plot of Hb(rc) − Vb(rc)/2 versus ρb(rc) is controlled by the plot of Gb(rc) versus ρb(rc), whereas the plot of Hb(rc) versus ρb(rc) is controlled by the plot of Vb(rc) versus ρb(rc). Hb(rc) − Vb(rc)/2 and Hb(rc) are positive and negative in the whole range calculated for N-∗-I, except for Hb(rc) of the substantially very small range ρb(rc). Therefore, the plot of Hb(rc) versus Hb(rc) − Vb(rc)/2 appears in the regular CS region, except for the region of Hb(rc) > 0, very close to the origin. The character of Gb(rc) versus ρb(rc) remains in the plot of Hb(rc) − Vb(rc)/2 versus ρb(rc) for N-∗-I, again. The positive value of Hb(rc) becomes negative at approximately 0.028 (e/a03) for ρb(rc), and then the value becomes more negative as ρb(rc) becomes larger.
In the case of
Hb(
rc) −
Vb(
rc)/2, the value is positive in the whole region calculated for N-∗-I (
Figure 13a). The plot of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc) for N-∗-I gives a rather clear distorted Z-shaped curve. The plot goes upside after the start of the plot at around the origin, then it goes downside and then goes upside. Consequently, the Z-shaped figure of
Hb(
rc) versus
Hb(
rc) −
Vb(
rc)/2 for N-∗-I shown in
Figure 10 is well analyzed based on the characteristic behavior of the plot of
Hb(
rc) −
Vb(
rc)/2 versus
ρb(
rc), together with the plot of
Hb(
rc) versus
ρb(
rc). The abnormal behavior of
Hb(
rc) −
Vb(
rc)/2, derived from the abnormal behavior of
Gb(
rc), must be responsible for the negative Δ
θp value of N-∗-I in CF
3SO
2N-∗-IMe.
Figure 13c,d show the plots of d(
Hb(
rc) −
Vb(
rc)/2)/d
ρb(
rc), d
Hb(
rc)/d
ρb(
rc), d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc) for the process for N-∗-Br and N-∗-I in TS [MeI–N(Tf)-∗-BrMe]. The characteristic behaviors in the plots of
Hb(
rc) −
Vb(
rc)/2,
Hb(
rc)
Gb(
rc) and
Vb(
rc) versus
ρb(
rc) is be emphasized by the plots of d(
Hb(
rc) −
Vb(
rc)/2)/d
ρb(
rc), d
Hb(
rc)/d
ρb(
rc), d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc). The plots of d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc) for N-∗-Br show concave and convex figures, respectively, while the plots for N-∗-I show apparent wave figures. The plots of d
Gb(
rc)/d
ρb(
rc) versus
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc) behave oppositely for the both cases of N-∗-Br and N-∗-I. The magnitudes of the changes in the plots of d
Gb(
rc)/d
ρb(
rc) and d
Vb(
rc)/d
ρb(
rc) versus
ρb(
rc) are larger for N-∗-I than for N-∗-Br.
The plot of dHb(rc)/dρb(rc) versus ρb(rc) for N-∗-I is very close to the plot for N-∗-Br, which decreases very smoothly and monotonically with increasing magnitude as ρb(rc) becomes larger due to the expected relation of dHb(rc)/dρb(rc) = dGb(rc)/dρb(rc) + dVb(rc)/dρb(rc). However, the plot of d(Hb(rc) − Vb(rc)/2)/dρb(rc) versus ρb(rc) for N-∗-I shows a concave then convex (wave) figure. The character in the plot of dGb(rc)/dρb(rc) versus ρb(rc) must remain in the plot of d(Hb(rc) − Vb(rc)/2)/dρb(rc) versus ρb(rc), mainly due to the expected relation of d(Hb(rc) − Vb(rc)/2)/dρb(rc) = dGb(rc)/dρb(rc) + dVb(rc)/2dρb(rc). The results support the following statements: The inverse behavior of the N-∗-I interaction must originate based on the convex then concave curve in the plot of Hb(rc) − Vb(rc)/2 versus ρb(rc). The loose Z-shaped plot of Hb(rc) versus Hb(rc) − Vb(rc)/2 for N-∗-I is observed, starting from a point close to the origin. The N-∗-Br and N-∗-I interactions are demonstrated through the above discussion, which show typical normal and inverse behavior, respectively.
Applications of the proposed methodology to wider range of bonds and interactions, such as a bird’s-eye view of the periodic table, are in progress. The results will be discussed elsewhere, together with the results, related to ours, reported so far by others.