1H-NMR Karplus Analysis of Molecular Conformations of Glycerol under Different Solvent Conditions: A Consistent Rotational Isomerism in the Backbone Governed by Glycerol/Water Interactions
Abstract
1. Introduction
2. Materials and Methods
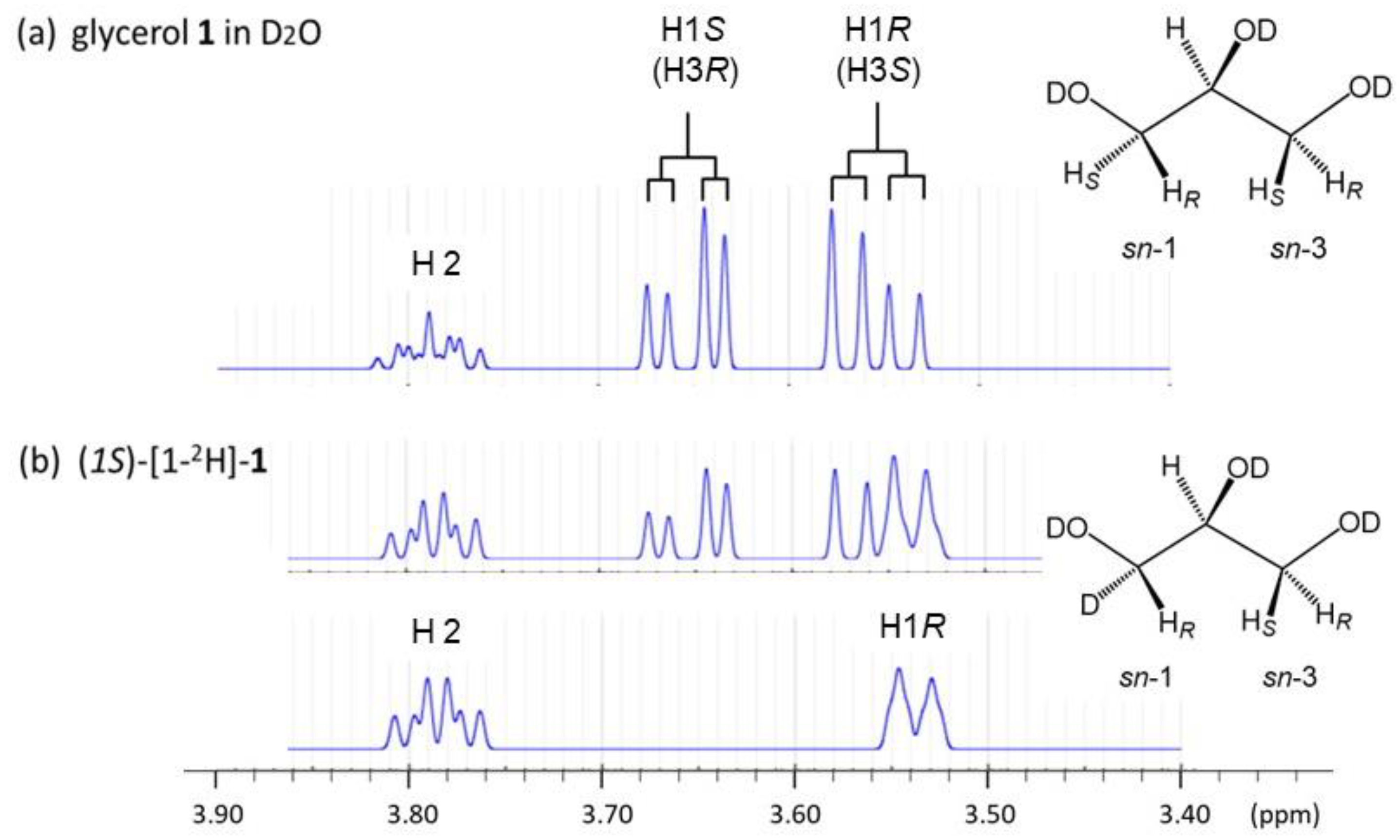
2.1. Glycerol in D2O Solutions for 1H-NMR Karplus Analysis
2.2. 1H-NMR Spectroscopic Measurement and Spectral Data Acquisition
2.3. 1H-NMR Karplus Analysis of Glycerol in the Dilute Aqueous Phase
- (a)
- A general Karplus equation [25]:where Ri = Δχi {0.87 − 2.4cos2(ζΦ + 19.9∣Δχi∣)}3JH1,H2 (Hz) = 13.22cos2Φ − 0.99cosΦ + ∑ Ri (O1, C2, O2),
- (b)
- Equation (1) (+60, −60, 180 in degrees) = eq 1 [16]:
- (c)
- Equation (2) (+65, −65, 180 in degrees) = eq 2 [16]:
System of Equations
3. Results and Discussion
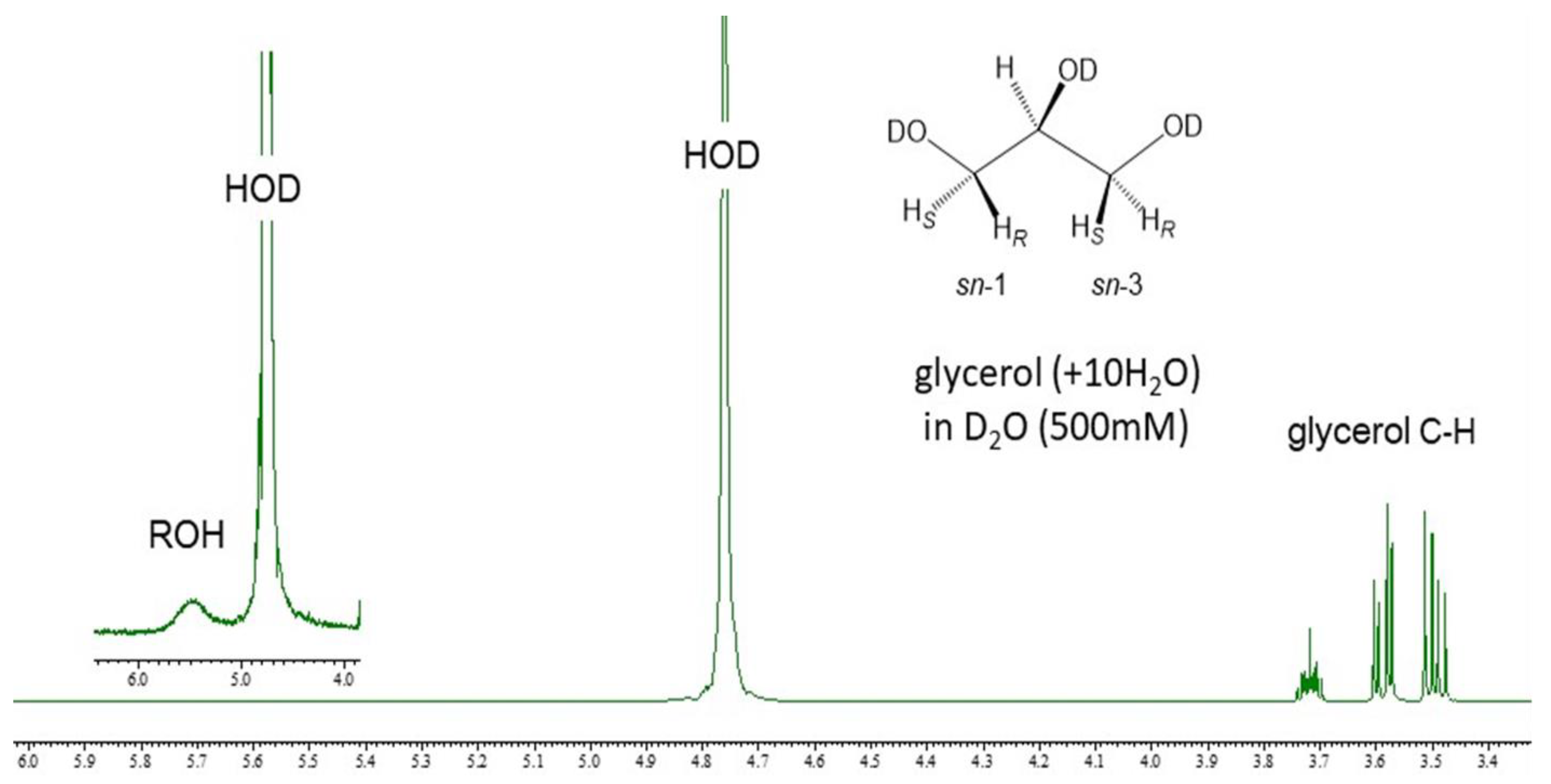
3.1. 1H-NMR Spectroscopic Profile of Glycerol in D2O Solutions without DSS
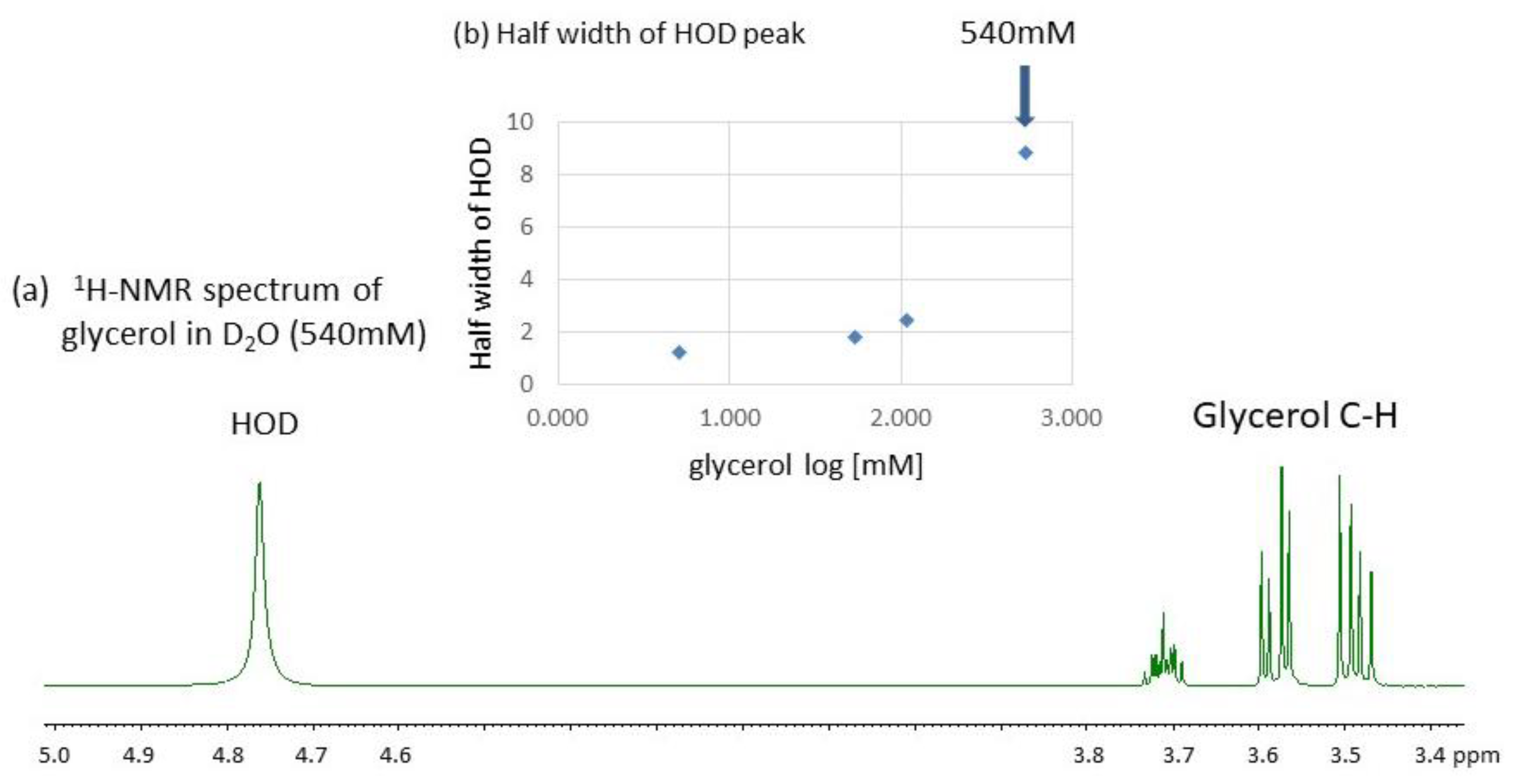
3.2. Effect of Concentrations on the Conformational Behavior of Glycerol in Water (D2O)
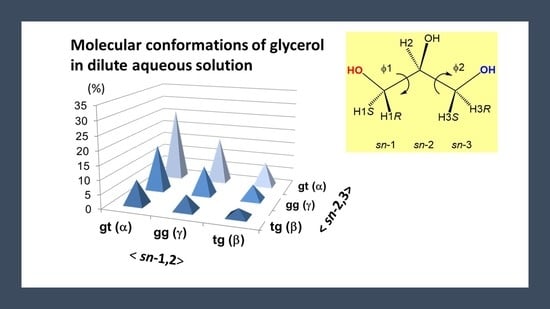
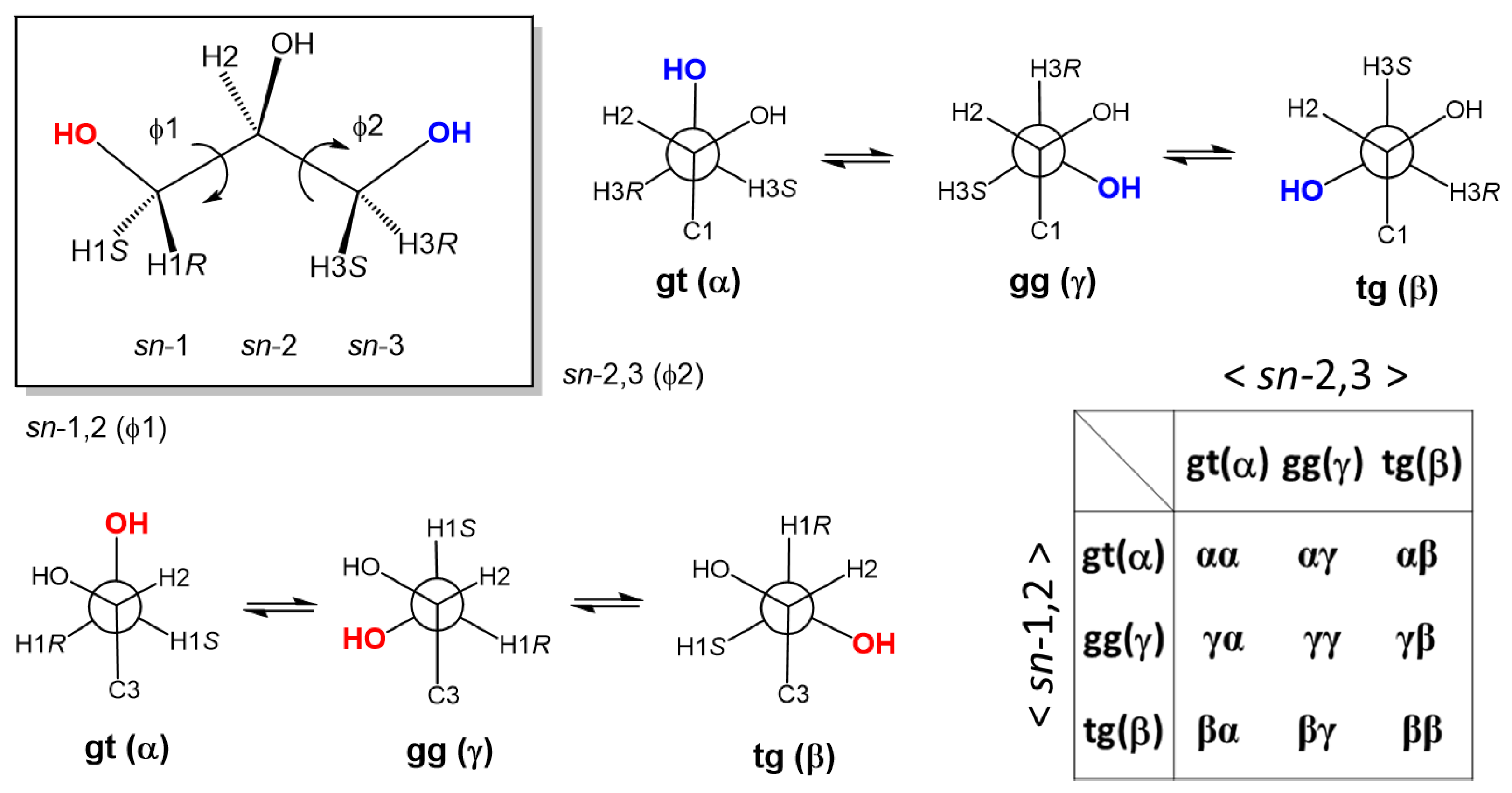
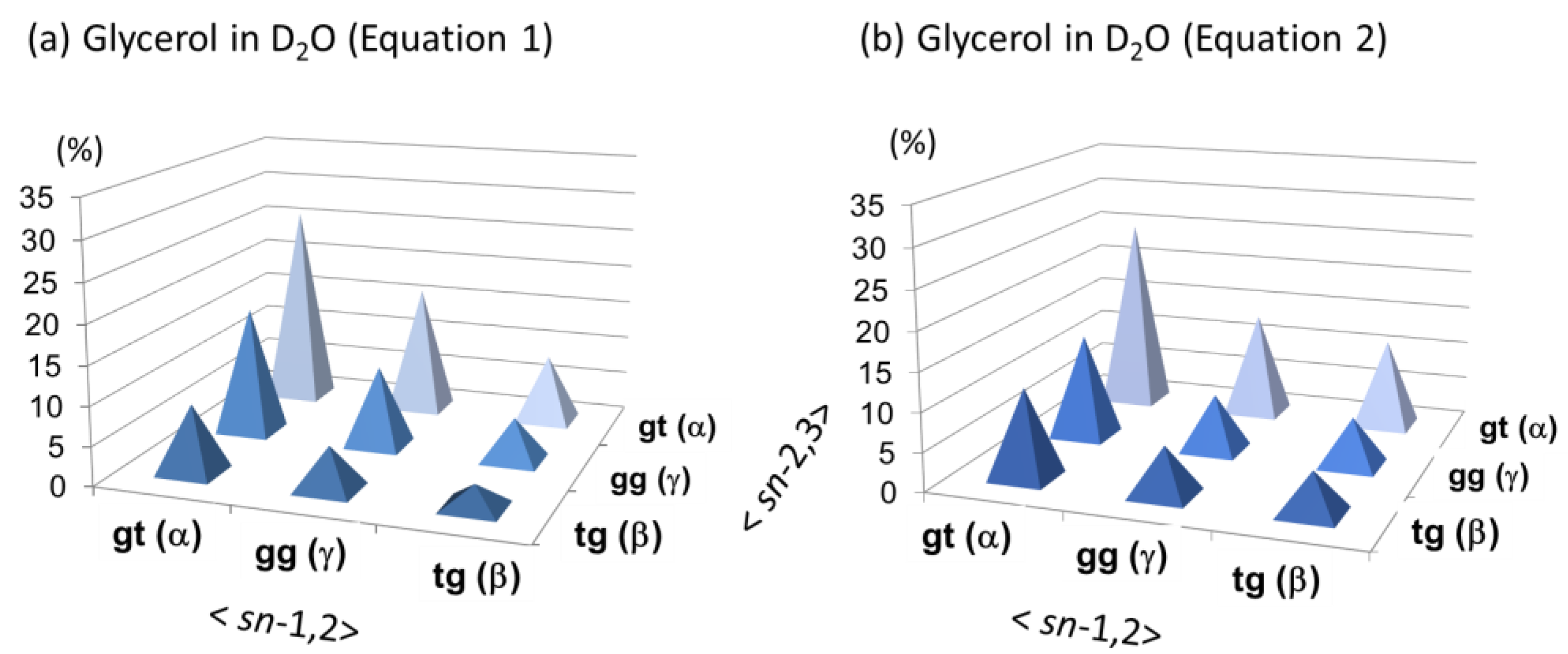
3.3. The Backbone Rotational Isomerism of Glycerol in the Dilute Aqueous Phase Generating the Nine or Six Different Conformers
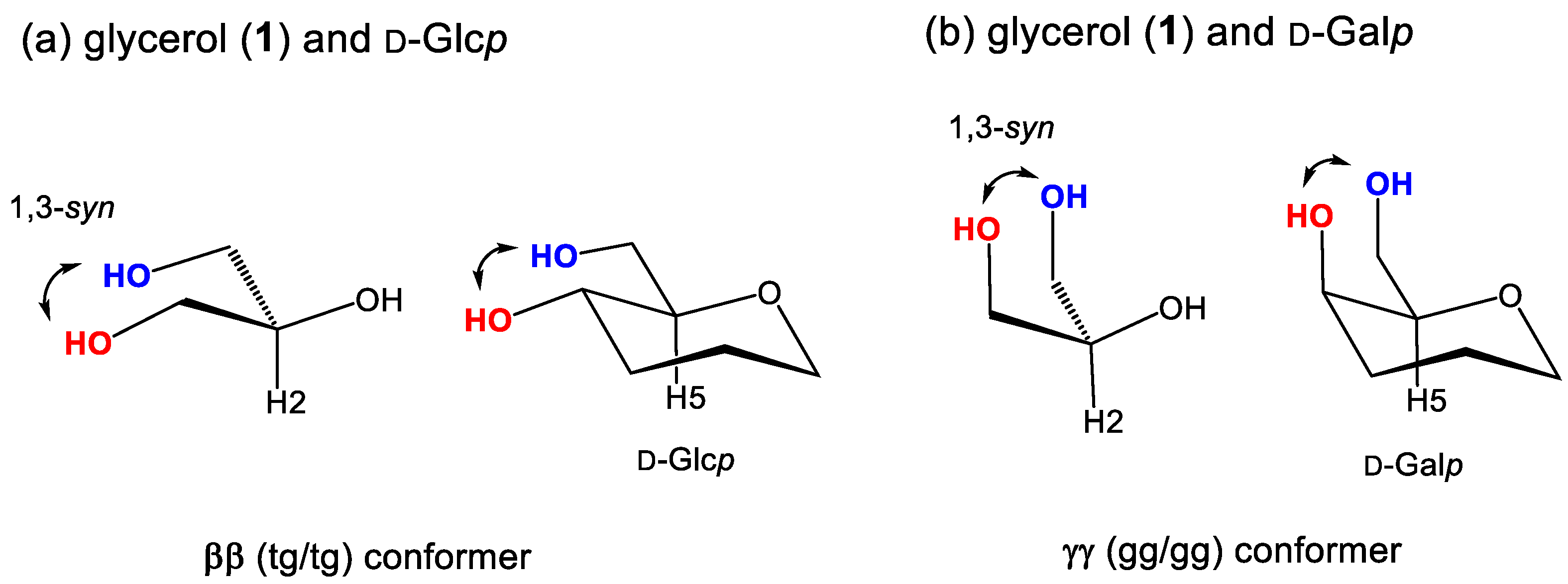
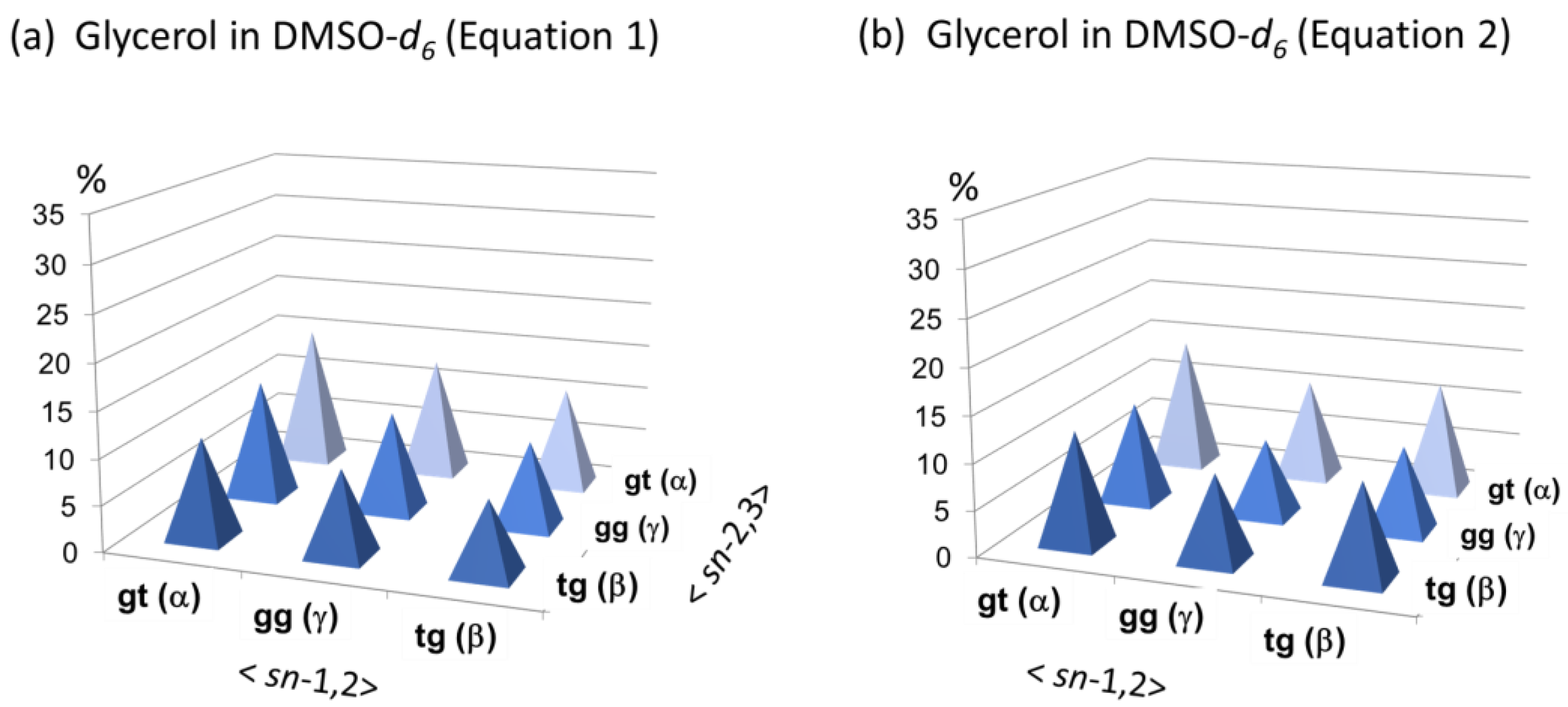
3.4. Conformational Behaviors of Glycerol under Different Solvent Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastiansen, O. Intra-molecular hydrogen bonds in ethylene glycol, glycerol, and ethylene chlorohydrin. Acta Chem. Scand. 1949, 3, 415. [Google Scholar] [CrossRef]
- Koningsveld, H. The crystal structure of glycerol and its conformation. Recl. Trav. Chim. Pays-Bas. 1968, 87, 243. [Google Scholar] [CrossRef]
- Koningsveld, H. A conformational study on glycerol in a D2O solution by means of 220 Mc PMR data. Recl. Trav. Chim. Pays-Bas. 1970, 89, 801. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Della Valle, R.G.; Califano, S. Glycerol condensed phases Part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1999, 1, 871–877. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Califano, S. Glycerol condensed phases Part II. A molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1999, 1, 879–885. [Google Scholar] [CrossRef]
- Pagot, G.; Tonello, S.; Vezzù, K.; Di Noto, V.A. A new glass-forming electrolyte based on lithium glycerolate. Batteries 2018, 4, 41. [Google Scholar] [CrossRef]
- Kusukawa, T.; Niwa, G.; Sasaki, T.; Oosawa, R.; Himeno, W.; Kato, M. Observation of a hydrogen-bonded 3D structure of crystalline glycerol. Bull. Chem. Soc. Jpn. 2013, 86, 351–353. [Google Scholar] [CrossRef]
- Egorov, A.V.; Lyubartsev, A.P.; Laaksonen, A. Molecular dynamics simulation study of glycerol–water liquid mixtures. J. Phys. Chem. B 2011, 115, 14572–14581. [Google Scholar] [CrossRef]
- Towery, J.J.; Soper, A.K.; Dougan, L. The structure of glycerol in the liquid state: A neutron diffraction study. Phys. Chem. Chem. Phys. 2011, 13, 9397–9406. [Google Scholar] [CrossRef]
- Towery, J.J.; Soper, A.K.; Dougan, L. What happens to the structure of water in cryoprotectant solutions? Faraday Discuss. 2013, 167, 159. [Google Scholar] [CrossRef]
- Chen, C.; Li, W.-Z. Molecular dynamics simulation of hydrogen bonding characteristics in aqueous glycerol solutions. Acta Phys. Chim. Sin. 2009, 25, 507–512. [Google Scholar]
- Chen, C.; Li, W.Z.; Song, Y.S.; Yang, J. Hydrogen bonding analysis of glycerol aqueous solutions: A molecular dynamics simulation study. J. Mol. Liq. 2009, 146, 23. [Google Scholar] [CrossRef]
- Callam, C.S.; Singer, S.J.; Lowary, T.L.; Hadad, C.M. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous Phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 2001, 123, 11743–11754. [Google Scholar] [CrossRef]
- Yongye, A.B.; Foley, B.L.; Woods, R.J. On achieving experimental accuracy from molecular dynamics simulations of flexible molecules: Aqueous glycerol. J. Phys. Chem. A 2008, 112, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.–H.; Byung, B.J.; Kang, Y.K. Conformational preferences of glycerol in the gas phase and in water. Bull. Korean Chem. Soc. 2012, 33, 917–924. [Google Scholar] [CrossRef]
- Yuan, M.; Fukuda, K.; Dohi, H.; Uzawa, H.; Nishida, Y. Comparative analyses of helical properties in asymmetric 1,2-diacyl-sn-glycerols by means of circular dichroism and proton NMR spectroscopies: Notable effects of substituting groups at sn-3 position. Tetrahedron Asym. 2015, 26, 1138–1144. [Google Scholar] [CrossRef]
- Nishida, Y.; Yuan, M.; Fukuda, K.; Fujisawa, K.; Dohi, H.; Uzawa, H. Remarkable functions of sn-3 hydroxy and phosphocholine groups in 1,2-diacyl-sn-glycerolipids to induce clockwise (+)-helicity around the 1,2-diacyl moiety: Evidence from conformation analysis by 1H NMR spectroscopy. Beilstein J. Org. Chem. 2017, 13, 1999–2009. [Google Scholar] [CrossRef]
- Nishida, Y.; Yuan, M.; Fujisawa, K.; Kitagawa, S.; Dohi, H.; Uzawa, H. Verification study for an empirical rule in diverse helical conformational behaviors of asymmetric 1,2-diacyl-sn-glycerols in the solution state. Tetrahedron Asym. 2017, 28, 1435–1443. [Google Scholar] [CrossRef]
- Uzawa, H.; Nishida, Y.; Hanada, S.; Ohrui, H.; Meguro, H. Syntheses and characterizations of chirally deuteriated glycerols. Chem. Commun. 1989, 862–863. [Google Scholar] [CrossRef]
- Nishida, Y.; Uzawa, H.; Hanada, S.; Ohrui, H.; Meguro, H. Synthesis and characterization of chirally deuteriated sn-glycerols. Agric. Biol. Chem. 1989, 53, 2319–2326. [Google Scholar] [CrossRef]
- Uzawa, H.; Nishida, Y.; Ohrui, H.; Meguro, H. Application of the dibenzoate chirality method to determine the absolute configuration of glycerols and related acyclic alcohols. J. Org. Chem. 1990, 55, 116–122. [Google Scholar] [CrossRef]
- Uzawa, H.; Nishida, Y.; Ohrui, H.; Meguro, H. A new approach to determine the stereospecificity in lipase catalysed hydrolysis using circular dichroism (CD): Lipases produce optically active diglycerides from achiral triglycerides. Biochem. Biophys. Res. Comm. 1990, 168, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Tamakoshi, H.; Kitagawa, Y.; Kobayashi, K.; Thiem, J. A novelbovine β-1,4-galactosyltransferase reaction to yield β-D-galactopyranosyl-(1-3)-linked disaccharides from L-sugars. Angew. Chem. Int. Ed. 2000, 39, 2000–2003. [Google Scholar] [CrossRef]
- Nishida, Y.; Tamakoshi, H.; Kobayashi, K.; Thiem, J. The first bovine β1,4-galactosyltransferase reaction with an acyclic acceptor substrate, 3-acetamido-1,2-propanediol, to yield a 3-O-β-D-galactopyranosyl-sn-glycerol skeleton. Org. Lett. 2001, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, C.A.G.; de Leeuw, F.A.A.M.; Altona, C. The relationship between proton-proton NMR coupling constants and substituent electronegativities—I: An empirical generalization of the karplus equation. Tetrahedron 1980, 36, 2783–2790. [Google Scholar] [CrossRef]
- HMDB Database. Available online: https://hmdb.ca/spectra/nmr_one_d/1101 (accessed on 10 June 2022).
- Nowick, J.S.; Khakshoor, O.; Hashemzadeh, M.; Brower, J.O. DSA: A New Internal Standard for NMR Studies in Aqueous Solution. Org. Lett. 2003, 5, 3511–3513. [Google Scholar] [CrossRef]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and accurate algorithm for the simulation of NMR spectra of large spin systems. J. Magn. Reson. 2011, 209, 123–130. [Google Scholar] [CrossRef]
- Nishida, Y.; Hori, H.; Ohrui, H.; Meguro, H. 1H NMR Analyses of Rotameric Distribution of C5-C6 bonds of D-Glucopyranoses in Solution. J. Carbohydr. Chem. 1988, 7, 239–250. [Google Scholar] [CrossRef]
- Matteson, D.S.; Kandil, A.A. Soundarrajan, R.S. Synthesis of Asymmetrically Deuterated Glycerol and Dibenzylglyceraldehyde via Boronic Esters. J. Am. Chem. Soc. 1990, 112, 3964–3969. [Google Scholar] [CrossRef]
- Morita, M.; Matsumura, F.; Shikata, T.; Ogawa, Y.; Kondo, N.; Shiraga, K. Hydrogen-Bond Configurations of Hydration Water around Glycerol Investigated by HOH Bending and OH Stretching Analysis. J. Phys. Chem. B 2022, 126, 9871–9880. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular Basis of Water Activity in Glycerol–Water Mixtures. Front. Chem. 2019, 7, 731. [Google Scholar] [CrossRef] [PubMed]
- Akinkunmi, F.O.; Jahn, D.A.; Giovambattista, N. Effects of Temperature on the Thermodynamic and Dynamical Properties of Glycerol–Water Mixtures: A Computer Simulation Study of Three Different Force Fields. J. Phys. Chem. B 2015, 119, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Dashnau, J.L.; Nucci, N.V.; Sharp, K.A.; Vanderkooi, J.M. Hydrogen Bonding and the Cryoprotective Properties of Glycerol/Water Mixtures. J. Phys. Chem. B 2006, 110, 13670–13677. [Google Scholar] [CrossRef]
- Mizuno, A.; Mitsuiki, M.; Toba, S.; Motoki, M. Antifreeze Activities of Various Food Components. J. Agric. Food Chem. 1997, 45, 14–18. [Google Scholar] [CrossRef]
- Sato, Y.; Miyawaki, O. Relationship between proton NMR relaxation time and viscosity of saccharide solutions. Food Sci. Technol. Res. 2000, 6, 136–139. [Google Scholar] [CrossRef]
- Wexler, A.D.; Woisetschlaeger, J.; Reiter, U.; Reiter, G.; Fuchsjaeger, M.; Fuchs, E.C.; Brecker, L. Nuclear Magnetic Relaxation Mapping of Spin Relaxation in Electrically Stressed Glycerol. ACS Omega 2020, 5, 22057–22070. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hori, H.; Ohrui, H.; Meguro, H.; Uzawa, J.; Reimer, D.; Sinnwell, V.; Paulsen, H. Conformational analyses of 04,06-branching tri-D-glucopyranosides: Influence of 04 linked residues on solution conformations about C5-C6 bonds at (1-6)-linkages. Tetrahedron Lett. 1988, 29, 4461–4464. [Google Scholar] [CrossRef]
- Hori, H.; Nishida, Y.; Ohrui, H.; Meguro, H.; Uzawa, J. Conformational analyses of α and β (1–6) mannodisaccharides by deuterium substitution effect on relaxation rate and NOE. Tetrahedron Lett. 1988, 29, 4457–4460. [Google Scholar] [CrossRef]
- Ohrui, H.; Nishida, Y.; Itoh, H.; Meguro, H. Preferred conformation about the C5-C6 bond of N-acetylneuraminyl(2-6)-D-galacto- and -D-glucopyranosides in solution. J. Org. Chem. 1991, 56, 1726–1729. [Google Scholar] [CrossRef]
- Meulendijks, G.H.W.M.; Van Es, W.; de Haan, J.W.; Buck, H.M. Conformational transmission in the glycerol backbone of phospholipid model compounds, induced by a P(4-coordinated) into trigonal bipyramidal P(5-coord) transition. Eur. J. Biochem. 1986, 157, 421. [Google Scholar] [CrossRef]
- Kosugi, Y.; Matsubara, K.J. Conformational analysis of triacylglycerols by means of nuclear magnetic resonance and molecular mechanics. Jpn. Oil Chem. Soc. 1989, 38, 415–420. [Google Scholar] [CrossRef]
- Woods, R.J.; Kirschner, K. Solvent interactions determine carbohydrate conformation. Proc. Natl. Acad. Sci. USA 2001, 98, 10541–10545. [Google Scholar]
- Ohrui, H.; Nishida, Y.; Watanabe, M.; Hori, H.; Meguro, H. 1H-NMR Studies on (6R)- and (6S)-deuterated (1–6)-linked disaccharides: Assignment of the preferred rotamers about C5–C6 bond of (1–6)-disaccharides in solution. Tetrahedron Lett. 1985, 26, 3251–3254. [Google Scholar] [CrossRef]
- Ashraf Kharaz, Y.; Zamboulis, D.; Sanders, K.; Comerford, E.; Clegg, P.; Peffers, M. Comparison between chaotropic and detergent-based sample preparation workflow in tendon for mass spectrometry analysis. Proteomics 2017, 17, 1700018. [Google Scholar] [CrossRef] [PubMed]
- Nollert, P.; Harries, W.E.C.; Fu, D.; Miercke, L.J.W.; Stroud, R.M. Atomic structure of a glycerol channel and implications for substrate permeation in aqua(glycero)porins. FEBS Lett. 2001, 504, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a Glycerol-Conducting Channel and the Basis for Its Selectivity. Science 2000, 290, 481–486. [Google Scholar] [CrossRef]
- Savage, D.F.; O’Connell, J.D., III; Miercke, L.J.; Finer-Moore, J.; Stroud, R.M. Structural context shapes the aquaporin selectivity filter. Proc. Natl. Acad. Sci. USA 2010, 107, 17164–17169. [Google Scholar] [CrossRef]
- Galleyway, F.B.; Hawkes, J.E.; Haycock, P.; Lewis, D.J. 1H NMR spectra and conformations of propane-1,2-diol, meso- and racemic butane-2,3-diols, and some alditols in non-aqueous media. Chem. Soc. Perkin Trans. 1990, 2, 1979–1985. [Google Scholar] [CrossRef]
- Petterson, K.A.; Stein, R.S.; Drake, M.D.; Roberts, J.D. An NMR investigation of the importance of intramolecular hydrogen bonding in determining the conformational equilibrium of ethylene glycol in solution. Magn. Reson. Chem. 2005, 43, 225–230. [Google Scholar] [CrossRef]
- John, S. Lomas, Cooperativity in alkane-1,2- and 1,3-polyols: NMR, QTAIM, and IQA study of O─H…OH and C─H…OH bonding interactions. Magn. Reson. Chem. 2020, 58, 666–684. [Google Scholar]
- Wolfe, S. Gauche effect. Stereochemical consequences of adjacent electron pairs and polar bonds. Acc. Chem. Res. 1972, 5, 102. [Google Scholar] [CrossRef]
- Thiehoff, C.; Rey, Y.P.; Gilmour, R. The Fluorine Gauche Effect: A Brief History. Isr. J. Chem. 2017, 57, 92–100. [Google Scholar] [CrossRef]
- Tvaroska, I.; Taravel, F.R.; Utille, J.P.; Carver, J.P. Quantum mechanical and NMR spectroscopy studies on theconformations of the hydroxymethyl and methoxymethyl groups in aldohexosides. Carbohydr. Res. 2002, 337, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Swick, R.W.; Nakao, A. The biological asymmetry of glycerol. J. Biol. Chem. 1956, 206, 883–886. [Google Scholar] [CrossRef]
- Crans, D.C. Whitesides, Glycerol Kinase: Synthesis of Dihydroxyacetone Phosphate, sn-Glycerol-3-Phosphate, and Chiral Analogues. J. Am. Chem. Soc. 1985, 107, 7019–7027. [Google Scholar] [CrossRef]
- Habe, H.; Fukuoka, T.; Kitamoto, D.; Sakaki, K. Biotechnological production of D-glyceric acid and its application. Appl. Microbiol. Biotechnol. 2008, 86, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Vustin, M.M. The Biological Role of Glycerol in Yeast Cells. Yeast as Glycerol Producers. Appl. Biochem Microbiol. 2021, 57, 907–916. [Google Scholar] [CrossRef]
- Gull, M.; Pasek, M.A. The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts 2021, 11, 86. [Google Scholar] [CrossRef]
- Mathew, T.; Esteves, P.M.; Prakash, G.K.S. Methanol in the RNA world: An astrochemical perspective. Front. Astron. Space Sci. 2022, 9, 809928. [Google Scholar] [CrossRef]
- Fedoseev, G.; Chuang, K.-J.; Ioppolo, S.; Qasim, D.; van Dishoeck, E.F.; Linnartz, H. Formation of glycerol through hydrogenation of CO ice under prestellar core conditions. Astrophys. J. 2017, 842, 842–852. [Google Scholar] [CrossRef]

| Entry | Solvent | 1H NMR Data 1 d ppm/2J and 3J (Hz) | Rotamers (%) 2 | ||||
|---|---|---|---|---|---|---|---|
| H2 | H1S/H3R | H1R/H3S | gt (α) | gg (γ) | tg (β) | ||
| 1 | D2O (100 mM) | 3.78 | 3.652 | 3.557 | 52 | 33 | 15 |
| m | 4.2, 11.7 | 6.6, 11.7 | |||||
| 2 | D2O | 3.775 | 3.644 | 3.551 | 50 | 32 | 18 |
| (90 mM) | m | 4.4, 11.7 | 6.5, 11.7 | ||||
| 3 | D2O (540 mM) | 3.772 | 3.642 | 3.551 | 47 | 34 | 19 |
| m | 4.4, 11.8 | 6.3, 11.8 | |||||
| Entry | Solvent (mM) | 1H NMR Spectral Data d ppm/J (Hz) | Chemical Shift Difference (D ppm) (Entry X − Entry 1) | Rotamers (%) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| by Equation (1) | ||||||||||
| /Equation (2) | ||||||||||
| H2 | H1S/H3R | H1R/H3S | DH2 | DH1S | DH1R | gt (α) | gg (γ) | tg (β) | ||
| 1 | D2O (5 mM) | 3.727 | 3.595 | 3.505 | 0 | 0 | 0 | 51 | 32 | 17 |
| m | 4.3 | 6.6, 11.7 | (50 | 27 | 23) | |||||
| 2 | D2O (54 mM) | 3.721 | 3.589 | 3.499 | −0.006 | −0.006 | −0.006 | 51 (50 | 32 27 | 17 23) |
| m | 4.3 | 6.6, 11.7 | ||||||||
| 3 | D2O (108 mM) | 3.719 | 3.587 | 3.497 | −0.008 | −0.008 | −0.008 | |||
| m | 4.3 | 6.6, 11.7 | ||||||||
| 4 | D2O (540 mM) | 3.711 | 3.58 | 3.489 | −0.016 | −0.015 | −0.016 | |||
| m | 4.3 | 6.6, 11.7 | ||||||||
| 5 | D2O (+H2O) (500 mM) 1 | 3.719 | 3.587 | 3.497 | −0.008 | −0.008 | −0.008 | 51 | 32 | 17 |
| m | 4.3 | 6.6, 11.7 | (50 | 27 | 23) | |||||
| Conformers | 1H-NMR Spectroscopic Studies 1 | Simulation Studies (B, C, and D) 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| eq 1 | eq 2 | eq-1 (A) | eq-2 (A) | A | B | C | D | |
| aa | 26 | 25 | 22 | 21 | 18~21 | 18 | 17 | 24.4 |
| ag+ga | 33 | 27 | 32 | 28 | 28~30 | 27 | 33 | 31.8 |
| ab+ba | 17 | 23 | 18 | 22 | 20~21 | 23 | 31 | 20 |
| bb | 3 | 5 | 4 | 6 | 5 | 3 | 2 | 1.2 |
| bg+gb | 11 | 12 | 13 | 14 | 15~17 | 24 | 14 | 13.4 |
| gg | 10 | 7 | 12 | 9 | 10~12 | 4 | 3 | 9.2 |
| Entry | Solvent (mM) | 1H NMR Spectroscopic Data 1 d ppm/J (Hz) | Chemical Shift Difference (D ppm) (Entry X − Entry 1) | Three Rotamers (%) by Equation (1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| /Equation (2) | ||||||||||
| H2 | H1S/H3R | H1R/H3S | DH2 | DH1S | DH1R | gt (α) | gg (γ) | tg (β) | ||
| 1 | D2O (54 mM) | 3.721 | 3.589 | 3.499 | 0 | 0 | 0 | 51 | 32 | 17 |
| m | 4.3 | 6.6, 11.7 | (50 | 27 | 23) | |||||
| 2 | D2O (+Gnd:HCl) 2 (50 mM) | 3.648 | 3.51 | 3.425 | −0.073 | −0.079 | −0.074 | 51 | 32 | 17 |
| m | 4.3 | 6.6, 11.7 | (50 | 27 | 23) | |||||
| 3 | DMSO-d6 (+10% D2O) (50 mM) | 3.46 | 3.382 | 3.308 | −0.261 | −0.207 | −0.191 | 39 | 33 | 28 |
| m | 5.1 | 5.9, 11.0 | (38 | 29 | 33) | |||||
| 4 | DMF-d7 (+10% D2O) (50 mM) | 3.652 | 3.563 | 3.498 | −0.069 | −0.026 | −0.001 | 39 | 33 | 28 |
| m | 5.1 | 5.9, 11.0 | (38 | 29 | 33) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Aono, R.; Dohi, H.; Ding, W.; Uzawa, H. 1H-NMR Karplus Analysis of Molecular Conformations of Glycerol under Different Solvent Conditions: A Consistent Rotational Isomerism in the Backbone Governed by Glycerol/Water Interactions. Int. J. Mol. Sci. 2023, 24, 2766. https://doi.org/10.3390/ijms24032766
Nishida Y, Aono R, Dohi H, Ding W, Uzawa H. 1H-NMR Karplus Analysis of Molecular Conformations of Glycerol under Different Solvent Conditions: A Consistent Rotational Isomerism in the Backbone Governed by Glycerol/Water Interactions. International Journal of Molecular Sciences. 2023; 24(3):2766. https://doi.org/10.3390/ijms24032766
Chicago/Turabian StyleNishida, Yoshihiro, Reina Aono, Hirofumi Dohi, Wuxiao Ding, and Hirotaka Uzawa. 2023. "1H-NMR Karplus Analysis of Molecular Conformations of Glycerol under Different Solvent Conditions: A Consistent Rotational Isomerism in the Backbone Governed by Glycerol/Water Interactions" International Journal of Molecular Sciences 24, no. 3: 2766. https://doi.org/10.3390/ijms24032766
APA StyleNishida, Y., Aono, R., Dohi, H., Ding, W., & Uzawa, H. (2023). 1H-NMR Karplus Analysis of Molecular Conformations of Glycerol under Different Solvent Conditions: A Consistent Rotational Isomerism in the Backbone Governed by Glycerol/Water Interactions. International Journal of Molecular Sciences, 24(3), 2766. https://doi.org/10.3390/ijms24032766