Localisation of Intracellular Signals and Responses during Phagocytosis
Abstract
1. Introduction
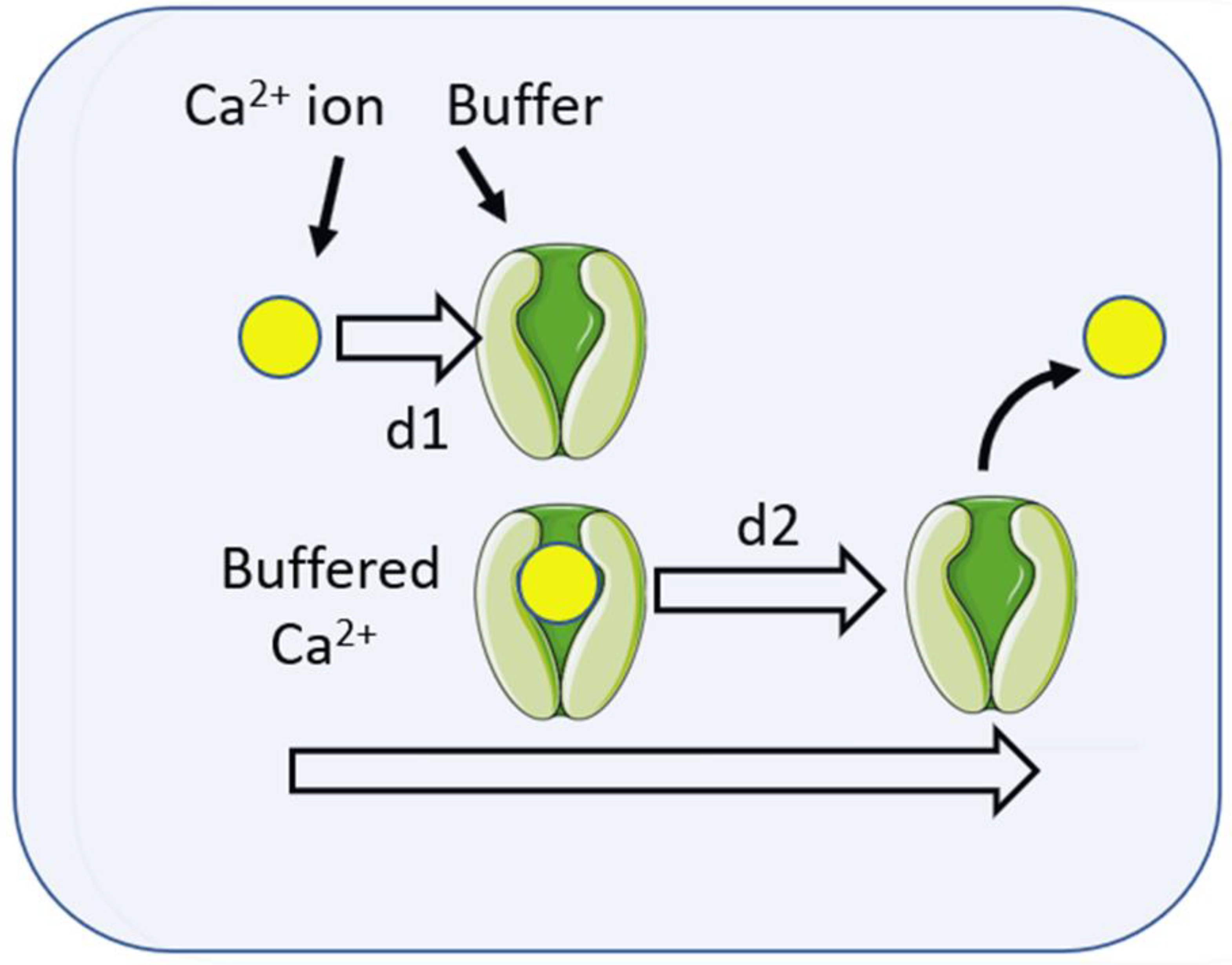
2. Diffusion of Chemical Signals
3. Theoretical Basis of Localisation of Ca2+ Signalling
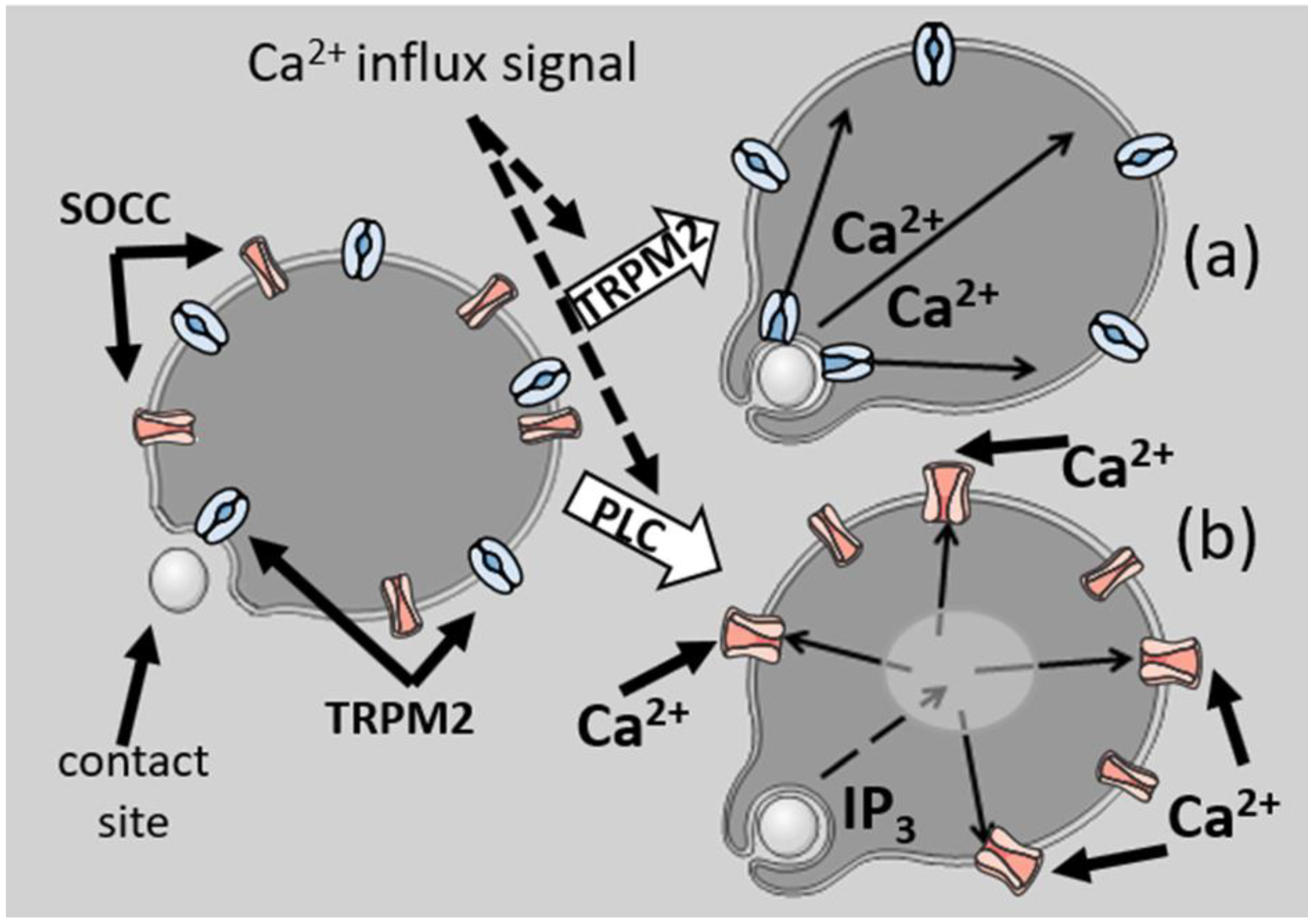
4. Localisation of Ca2+ Signalling of Phagocytosis
5. Localisation of Ca2+ Signals after Phagocytosis
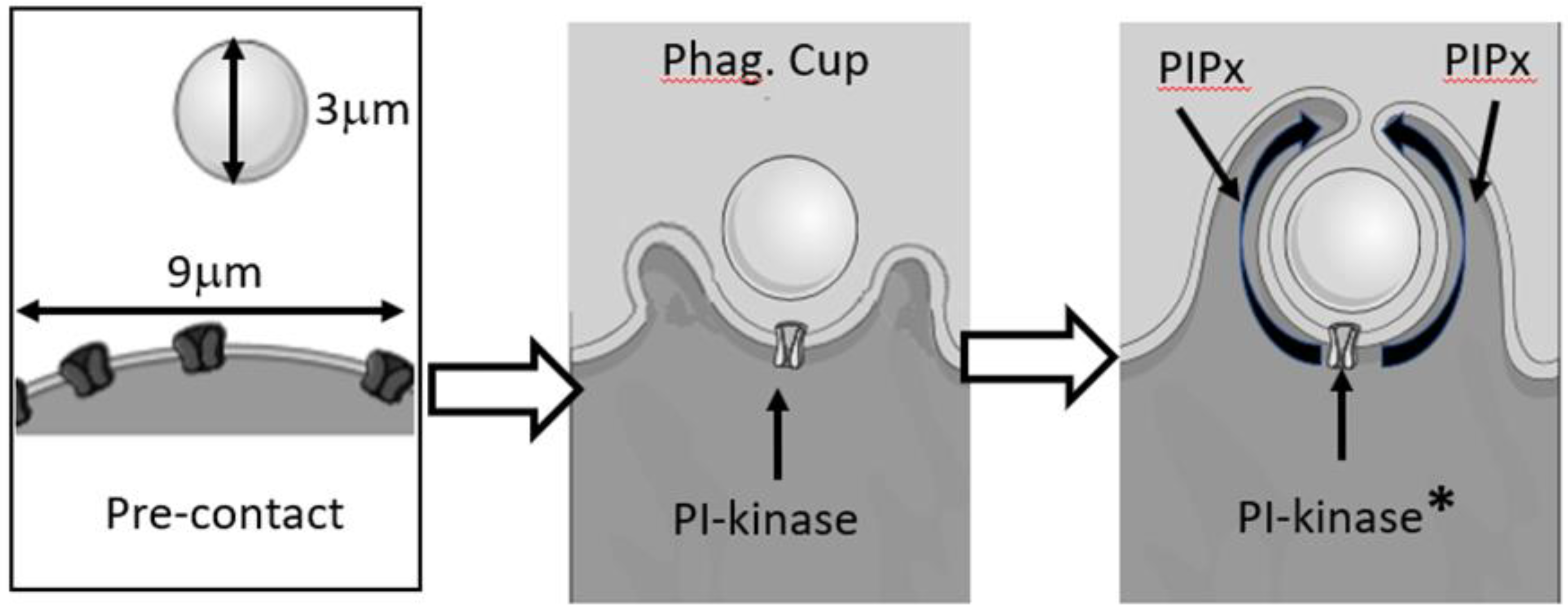
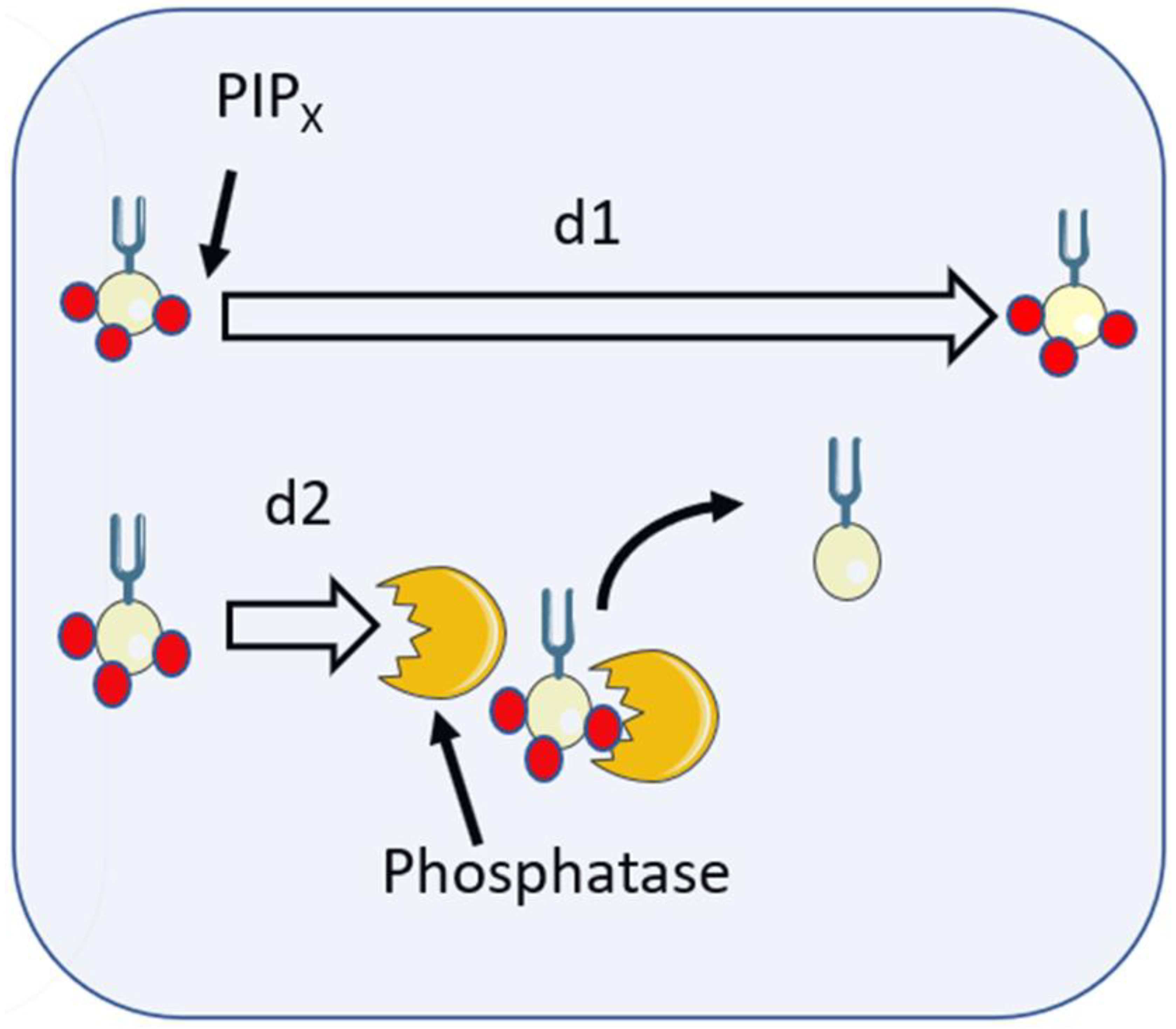
6. Membrane Lipid Signals
7. Localisation of Lipid Signalling of Phagocytosis
8. Localisation of Lipid Signals after Phagocytosis
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Underhill, D.M. Mechanisms of Phagocytosis in Macrophages. Ann. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Woo, Y.; Hahn, T.-W.; Jung, Y.M.; Jung, Y.-J. Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection. Microorganisms 2020, 8, 1298. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotry, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.M. Receptor Models of Phagocytosis: The Effect of Target Shape. Adv. Expt. Med. Biol. 2020, 1246, 55–70. [Google Scholar]
- Richards, D.M.; Endres, R.G. How cells engulf: A review of theoretical approaches to phagocytosis. Rep. Prog. Phys. 2017, 80, 126601. [Google Scholar] [CrossRef]
- Berg, H.C. Random Walks in Biology; Princeton University Press: Princeton, NJ, USA, 1993. [Google Scholar]
- Nunes-Hasler, P.; Kaba, M.; Demaurex, N. Molecular mechanisms of calcium signaling during phagocytosis. Adv. Expt. Med. Biol. 2020, 1246, 103–128. [Google Scholar]
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leuk. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef]
- Hamburger, H.J. Researches on phagocytosis. Nature 1915, 96, 19–23. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Meyer, B.C.; Greenberg, S. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J. Cell Biol. 1988, 106, 657–666. [Google Scholar] [CrossRef]
- Westman, J.; Grinstein, S.; Maxson, M. Revisiting the role of calcium in phagosome formation and maturation. J. Leuk. Biol. 2019, 106, 837–851. [Google Scholar] [CrossRef]
- Dewitt, S.; Hallett, M.B. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J. Cell Biol. 2002, 159, 181–189. [Google Scholar] [CrossRef]
- Francis, E.A.; Heinrich, V. Extension of chemotactic pseudopods by nonadherent human neutrophils does not require or cause calcium bursts. Sci. Signal. 2018, 11, eaal4289. [Google Scholar] [CrossRef]
- Kruskal, B.A.; Shak, S.; Maxfield, F.R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc. Natl. Acad. Sci. USA 1986, 83, 2919–2923. [Google Scholar] [CrossRef]
- Dewitt, S.; Francis, R.J.; Hallett, M.B. Ca2+ and calpain control membrane expansion during the rapid cell spreading of neutrophils. J. Cell Sci. 2013, 126, 4627–4635. [Google Scholar]
- Wang, J.H. Tracer-diffusion in liquids. IV. Self-diffusion of calcium and chloride ion in aqueous calcium chloride solutions. J. Am. Chem. Soc. 1952, 75, 1769–1770. [Google Scholar] [CrossRef]
- Donahue, B.S.; Abercrombie, R.F. Free diffusion coefficient of ionic calcium in cytoplasm. Cell Calcium 1987, 8, 437–448. [Google Scholar] [CrossRef]
- Allbritton, N.L.; Meyer, T.; Stryer, L. Range of Messenger Action of Calcium Ion and Inositol 1,4,5-Trisphosphate. Science 1992, 258, 1812–1815. [Google Scholar] [CrossRef]
- Villarruel, C.; Aguilar, P.S.; Dawson, S.P. High rates of calcium-free diffusion in the cytosol of living cells. Biophys. J. 2021, 120, 3960–3972. [Google Scholar] [CrossRef]
- Von Tscharner, V.; Deranleau, D.A.; Baggiolini, M. Calcium fluxes and calcium buffering in human neutrophils. J. Biol. Chem. 1986, 261, 10163–10168. [Google Scholar] [CrossRef]
- Al-Mohanna, F.A.; Hallett, M.B. The use of fura-2 to determine the relationship between cytoplasmic free Ca2+ and oxidase activation in rat neutrophils. Cell Calcium 1988, 9, 17–26. [Google Scholar] [CrossRef]
- Hillson, E.J.; Hallett, M.B. Localised and rapid Ca2+ micro-events in human neutrophils: Conventional Ca2+ puffs and global waves without peripheral-restriction or wave cycling. Cell Calcium 2007, 41, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Bill, C.A.; Vines, C.M. Phospholipsae C. Adv. Expt. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [CrossRef]
- Hellberg, C.; Molony, L.; Zheng, L.; Andersson, T. Ca2+ signalling mechanisms of the beta 2 integrin on neutrophils: Involvement of phospholipase C gamma 2 and Ins(1, 4, 5)P3. Biochem. J. 1996, 317, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W. Capacitative calcium entry: Sensing the calcium stores. J. Cell Biol. 2005, 169, 381–382. [Google Scholar] [CrossRef]
- Putney, J.W. Store-Operated Calcium Entry: An Historical Overview. Adv. Expt. Med. Biol. 2017, 981, 205–214. [Google Scholar]
- Lunz, V.; Romanin, C.; Frischauf, I. STIM1 activation of Orai1. Cell Calcium 2019, 77, 29–38. [Google Scholar] [CrossRef]
- Yeung, P.S.-W.; Yamashita, M.; Prakriya, M. Molecular basis of allosteric Orai1 channel activation by STIM1. J. Physiol. 2020, 598, 1707–1723. [Google Scholar] [CrossRef]
- Tedeschi, V.; La Russa, D.; Franco, C.; Vinciguerra, A.; Amantea, D.; Secondo, A. Plasma membrane and organellar targets of STIM1 for intracellular calcium handling. Cells 2021, 10, 2518. [Google Scholar] [CrossRef]
- Demaurex, N.; Nunes, P. The role of STIM and ORAI proteins in phagocytic immune cells. Am. J. Physiol. Cell Physiol. 2016, 310, C496–C508. [Google Scholar] [CrossRef]
- Nunes-Hasler, P.; Maschalidi, S.; Lippens, C.; Castelbou, C.; Bouvet, S.; Guido, D.; Bermont, F.; Bassoy, E.Y.; Page, N.; Merkler, D.; et al. STIM1 promotes migration, phagosomal maturation and antigen cross-presentation in dendritic cells. Nat. Commun. 2017, 8, 1852. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.; Cornut, D.; Bochet, V.; Demaurex, N. STIM1 juxtaposes ER to phagosomes, generating Ca2+ hotspots that boost phagocytosis. Curr. Biol. 2012, 22, 1990–1997. [Google Scholar] [CrossRef]
- Heiner, I.; Eisfeld, J.; Lückhoff, A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 2003, 33, 533–540. [Google Scholar] [CrossRef]
- Heiner, I.; Eisfeld, J.; Halaszovich, C.R.; Wehage, E.; Jüngling, E.; Zitt, C.; Lückhoff, A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: Evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem. J. 2003, 371, 1045–1053. [Google Scholar] [CrossRef]
- Najder, K.; Musset, B.; Lindemann, O.; Bulk, E.; Schwab, A.; Fels, B. The function of TRP channels in neutrophil granulocytes. Pflug. Arch. 2018, 470, 1017–1033. [Google Scholar] [CrossRef]
- Syed Mortadza, S.A.; Wang, L.; Li, D.; Jiang, L.H. TRPM2 Channel-Mediated ROS-Sensitive Ca(2+) Signaling mechanisms in Immune cells. Front. Immunol. 2015, 6, 407. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Ann. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Eisfeld, J.; Lückhoff, A. TRPM2. Transient Receptor Potential (TRP) Channels. In Handbook of Experimental Pharmacology; Flockerzi, V., Nilius, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 179. [Google Scholar]
- Knowles, H.; Heizer, J.W.; Li, Y.; Chapman, K.; Ogden, C.A.; Andreasen, K.; Shapland, E.; Kucera, G.; Mogan, J.; Humann, J.; et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 11578–11583. [Google Scholar] [CrossRef]
- Di, A.; Gao, X.P.; Qian, F.; Kawamura, T.; Han, J.; Hecquet, C.; Ye, R.D.; Vogel, S.M.; Malik, A.B. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 2011, 13, 29–34. [Google Scholar] [CrossRef]
- Sawyer, D.W.; Sullivan, J.A.; Mandell, G.L. Intracellular free calcium localization in neutrophils during phagocytosis. Science 1985, 230, 663–666. [Google Scholar] [CrossRef]
- Marks, P.W.; Maxfield, F.R. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium 1990, 11, 181–190. [Google Scholar] [CrossRef]
- Dewitt, S.; Darley, R.L.; Hallett, M.B. Translocation or just location? Pseudopodia affect fluorescent signals. J. Cell Biol. 2009, 184, 197–203. [Google Scholar] [PubMed]
- Dewitt, S.; Hallett, M.B. Ironing out the wrinkles of neutrophil phagocytosis. Trends Cell. Biol. 2007, 17, 209–214. [Google Scholar]
- Dewitt, S.; Hallett, M.B. Calpain activation by Ca2+ and its role in phagocytosis. Adv. Expt. Med. Biol. 2020, 1246, 129–151. [Google Scholar]
- Roberts, R.E.; Hallett, M.B. Neutrophil cell shape change: Mechanism and signalling during cell spreading and phagocytosis. Intl. J. Mol. Sci. 2019, 20, 1383. [Google Scholar] [CrossRef]
- Brückner, B.R.; Pietuch, A.; Nehls, S.; Rother, J.; Janshoff, A. Ezrin is a Major Regulator of Membrane Tension in Epithelial Cells. Sci. Rep. 2015, 5, 14700. [Google Scholar] [CrossRef]
- Roberts, R.E.; Dewitt, S.; Hallett, M.B. Membrane tension and the role of ezrin during phagocytosis. Adv. Expt. Med. Biol. 2020, 1246, 83–102. [Google Scholar]
- Herant, M.; Heinrich, V.; Dembo, M. Mechanics of neutrophil phagocytosis: Experiments and quantitative models. J. Cell. Sci. 2006, 119, 1903–1913. [Google Scholar] [CrossRef]
- Petty, H.R.; Hafeman, D.G.; McConnell, H.M. Disappearance of macrophage surface folds after antibody-dependent phagocytosis. J. Cell. Biol. 1981, 89, 223–229. [Google Scholar] [CrossRef]
- Jumaa, M.A.A.; Dewitt, S.; Hallett, M.B. Topographical interrogation of the living cell surface reveals its role in rapid cell shape changes during phagocytosis and spreading. Sci. Rep. 2017, 7, 9790. [Google Scholar] [CrossRef]
- Roberts, R.E.; Martin, M.; Marion, S.; Elumalai, G.L.; Lewis, K.; Hallett, M.B. Ca2+-activated cleavage of ezrin visualised dynamically in living myeloid cells during cell surface area expansion. J. Cell. Sci. 2020, 133, 236968. [Google Scholar] [CrossRef]
- Bisaria, A.; Hayer, A.; Garbett, D.; Cohen, D.; Meyer, T. Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science 2020, 12, 1205–1210. [Google Scholar] [CrossRef]
- Welf, E.S.; Miles, C.E.; Huh, J.; Sapoznik, E.; Chi, J.; Driscoll, M.K.; Isogai, T.; Noh, J.; Weems, A.D.; Pohlkamp, T.; et al. Actin-Membrane Release Initiates Cell Protrusions. Dev. Cell. 2020, 21, 723–736. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Brasen, J.C.; Olsen, L.F.; Hallett, M.B. Cell surface topology creates high Ca2+ signalling microdomains. Cell Calcium 2010, 47, 339–349. [Google Scholar] [CrossRef]
- Roberts, R.E.; Vervliet, T.; Bultynck, G.; Parys, J.B.; Hallett, M.B. EPIC3, a novel Ca2+ indicator located at the cell cortex and in microridges, detects high Ca2+ subdomains during Ca2+ influx and phagocytosis. Cell Calcium 2020, 92, 102291. [Google Scholar] [CrossRef]
- Kruskal, B.A.; Maxfield, F.R. Cytosolic free calcium increases before and oscillates during frustrated phagocytosis in macrophages. J. Cell. Biol. 1987, 105, 2685–2693. [Google Scholar] [CrossRef]
- Samie, M.; Wang, X.; Zhang, X.; Goschka, A.; Li, X.; Cheng, X.; Gregg, E.; Azar, M.; Zhuo, Y.; Garrity, A.G.; et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 2013, 26, 511–524. [Google Scholar] [CrossRef]
- Guido, D.; Demaurex, N.; Nunes, P. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell. Sci. 2015, 128, 4074–4082. [Google Scholar] [CrossRef]
- Di, A.; Kiya, T.; Gong, H.; Gao, X.; Malik, A.B. Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages. J. Cell. Sci. 2017, 130, 735–744. [Google Scholar] [CrossRef]
- Qian, X.; Numata, T.; Zhang, K.; Li, C.; Hou, J.; Mori, Y.; Fang, X. Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology 2014, 121, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Vorselen, D. Dynamics of phagocytosis mediated by phosphatidylserine. Biochem. Soc. Trans 2022, 50, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nature Rev. Mol. Cell. Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef]
- Hammond, G.R.; Hong, Y. Phosphoinositides and membrane targeting in cell polarity. Cold Spring Harb. Perspect. Biol. 2018, 10, a027938. [Google Scholar] [CrossRef] [PubMed]
- Katan, M. and Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar]
- Dickson, E.J.; Hille, B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019, 476, 1–23. [Google Scholar] [CrossRef]
- Hammond, G.R.V.; Burke, J.E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell. Biol. 2020, 63, 57–67. [Google Scholar] [CrossRef]
- Botelho, R.J.; Teruel, M.; Dierckman, R.; Anderson, R.; Wells, A.; York, J.D.; Meyer, T.; Grinstein, S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell. Biol. 2000, 151, 1353–1367. [Google Scholar] [CrossRef]
- Scott, C.C.; Dobson, W.; Botelho, R.J.; Coady-Osberg, N.; Chavrier, P.; Knecht, D.A.; Heath, C.; Stahl, P.; Grinstein, S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell. Biol. 2005, 169, 139–149. [Google Scholar] [CrossRef]
- Minakami, R.; Maehara, Y.; Kamakura, S.; Kumano, O.; Miyano, K.; Sumimoto, H. Membrane phospholipid metabolism during phagocytosis in human neutrophils. Genes Cells 2010, 15, 409–424. [Google Scholar] [CrossRef]
- Mu, L.; Tu, Z.; Miao, L.; Xia, T.; Shi, Y. A phosphatidylinositol 4,5-bisphosphate redistribution-based sensing mechanism initiates a phagocytosis programing. Nat. Commun. 2018, 9, 4259. [Google Scholar] [CrossRef]
- Panikerm, S.R.; Yago, T.; Shao, B.; McEver, R.P. Neutrophils lacking ERM proteins polarize and crawl directionally but have decreased adhesion strength. Blood Adv. 2020, 4, 3559–3571. [Google Scholar] [CrossRef]
- Marshall, J.G.; Booth, J.W.; Stambolic, V.; Mak, T.; Balla, T.; Schreiber, A.D.; Meyer, T.; Grinstein, S. Restricted Accumulation of Phosphatidylinositol 3-Kinase Products in a Plasmalemmal Subdomain during Fcγ Receptor-Mediated Phagocytosis. J. Cell. Biol. 2001, 153, 1369–1380. [Google Scholar] [CrossRef]
- Montaño-Rendón, F.; Walpole, G.F.W.; Krause, M.; Hammond, G.R.V.; Grinstein, S.; Fairn, G.D. PtdIns(3,4)P2, Lamellipodin, and VASP coordinate actin dynamics during phagocytosis in macrophages. J. Cell. Biol. 2022, 221, e202207042. [Google Scholar] [CrossRef]
- Goulden, B.D.; Pacheco, J.; Dull, A.; Zewe, J.P.; Deiters, A.; Hammond, G.R.V. A high-avidity biosensor reveals plasma membrane PI(3,4)P2 is predominantly a class I PI3K signaling product. J. Cell. Biol. 2019, 218, 10661079. [Google Scholar] [CrossRef]
- Naish, E.; Wood, A.J.T.; Stewart, A.P.; Routledge, M.; Morris, A.C.; Chilvers, E.R.; Lodge, K.M. The formation and function of the neutrophil phagosome. Immunol. Rev. 2023, in press. [Google Scholar] [CrossRef]
- Gullapalli, R.R.; Demirel, M.C.; Butler, P.J. Molecular dynamics simulations of DiI-C18(3) in a DPPC lipid bilayer. Phys. Chem. Chem. Phys. 2008, 10, 3548–3560. [Google Scholar] [CrossRef]
- Golebiewska, U.; Nyako, M.; Woturski, W.; Zaitseva, I.; McLaughlin, S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate (PIP2) in the plasma membrane of cells. Mol. Biol. Cell 2008, 19, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.D.; Lerner, M.G.; Marcano-Velázquez, J.G.; Pastor, R.W.; Falke, J.J. Single molecule diffusion of membrane-bound proteins: Window into lipid contacts and bilayer dynamics. Biophys. J. 2010, 99, 2879–2887. [Google Scholar] [CrossRef]
- Pacheco, J.; Cassidy, A.C.; Zewe, J.P.; Wills, J.C.; Hammond, G.R.V. PI(4,5)P2 diffuses freely in the plasma membrane even within high-density effector protein complexes. J. Cell. Biol. 2023, 222, e202204099. [Google Scholar] [CrossRef]
- Dewitt, S.; Tian, W.; Hallett, M.B. Localised PtdIns(3,4,5)P-3 or PtdIns(3,4)P-2 at the phagocytic cup is required for both phagosome closure and Ca2+ signalling in HL60 neutrophils. J. Cell. Sci. 2006, 119, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Fehon, R.; McClatchey, A.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell. Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, A.; Serrador, J.M. ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. Int. J. Mol. Sci. 2020, 21, 1502. [Google Scholar] [CrossRef]
- Gerisch, G.; Ecke, M.; Schroth-Diez, B.; Gerwig, S.; Engel, U.; Maddera, L.; Clarke, M. Self-organizing actin waves as planar phagocytic cup structures. Cell. Adh. Migr. 2009, 3, 373–382. [Google Scholar] [CrossRef]
- Gerhardt, M.; Ecke, M.; Walz, M.; Stengl, A.; Beta, C.; Gerisch, G. Actin and PIP3 waves in giant cells reveal the inherent length scale of an excited state. Cell. Sci. 2014, 127, 4507–4517. [Google Scholar] [CrossRef]
- Golebiewska, U.; Kay, J.G.; Masters, T.; Grinstein, S.; Im, W.; Pastor, R.W.; Scarlata, S.; McLaughlin, S. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell. 2011, 22, 3498–3507. [Google Scholar] [CrossRef]
- Woodward, X.; Stimpson, E.E.; Kelly, C.V. Single-lipid tracking on nanoscale membrane buds: The effects of curvature on lipid diffusion and sorting. Biochim. Biophys. Acta 2018, 1860, 2064–2075. [Google Scholar] [CrossRef]
- Myeong, J.; Park, C.-G.; Suh, B.-C.; Hille, B. Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquid-ordered/raft domains. Proc. Natl. Acad. Sci. USA 2021, 118, e2025343118. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Heberle Lipid rafts: Controversies resolved, mysteries remain. Trends Cell. Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Yip, S.C.; Eddy, R.J.; Branch, A.M.; Pang, H.; Wu, H.; Yan, Y.; Drees, B.E.; Neilsen, P.O.; Condeelis, J.; Backer, J.M. Quantification of PtdIns(3,4,5)P(3) dynamics in EGF-stimulated carcinoma cells: A comparison of PH-domain-mediated methods with immunological methods. Biochem. J. 2008, 411, 441–448. [Google Scholar] [CrossRef]
- Ellson, C.D.; Gobert-Gosse, S.; Anderson, K.E.; Chilvers, E.R.; Hawkins, P.T.; Stephens, L.R. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat. Cell. Biol. 2001, 3, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Bouchab, L.; Hudik, E.; Le Bars, R.; Nüsse, O.; Dupré-Crochet, S. Phosphoinositol 3-phosphate acts as a timer for reactive oxygen species production in the phagosome. J. Leukoc. Biol. 2017, 101, 1155–1168. [Google Scholar] [CrossRef]
- Kanai, F.; Liu, H.; Field, S.J.; Akbary, H.; Matsuo, T.; Brown, G.E.; Cantley, L.; Yaffe, M.B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell. Biol. 2001, 3, 675–678. [Google Scholar] [CrossRef]
- Karathanassis, D.; Stahelin, R.V.; Bravo, J.; Perisic, O.; Pacold, C.M.; Cho, W.; Williams, R. Binding of the PX domain of p47phox to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002, 21, 5057–5068. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallett, M.B. Localisation of Intracellular Signals and Responses during Phagocytosis. Int. J. Mol. Sci. 2023, 24, 2825. https://doi.org/10.3390/ijms24032825
Hallett MB. Localisation of Intracellular Signals and Responses during Phagocytosis. International Journal of Molecular Sciences. 2023; 24(3):2825. https://doi.org/10.3390/ijms24032825
Chicago/Turabian StyleHallett, Maurice B. 2023. "Localisation of Intracellular Signals and Responses during Phagocytosis" International Journal of Molecular Sciences 24, no. 3: 2825. https://doi.org/10.3390/ijms24032825
APA StyleHallett, M. B. (2023). Localisation of Intracellular Signals and Responses during Phagocytosis. International Journal of Molecular Sciences, 24(3), 2825. https://doi.org/10.3390/ijms24032825





