Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective
Abstract
1. Introduction
2. Blood–Brain Barrier (BBB): A Detailed Account
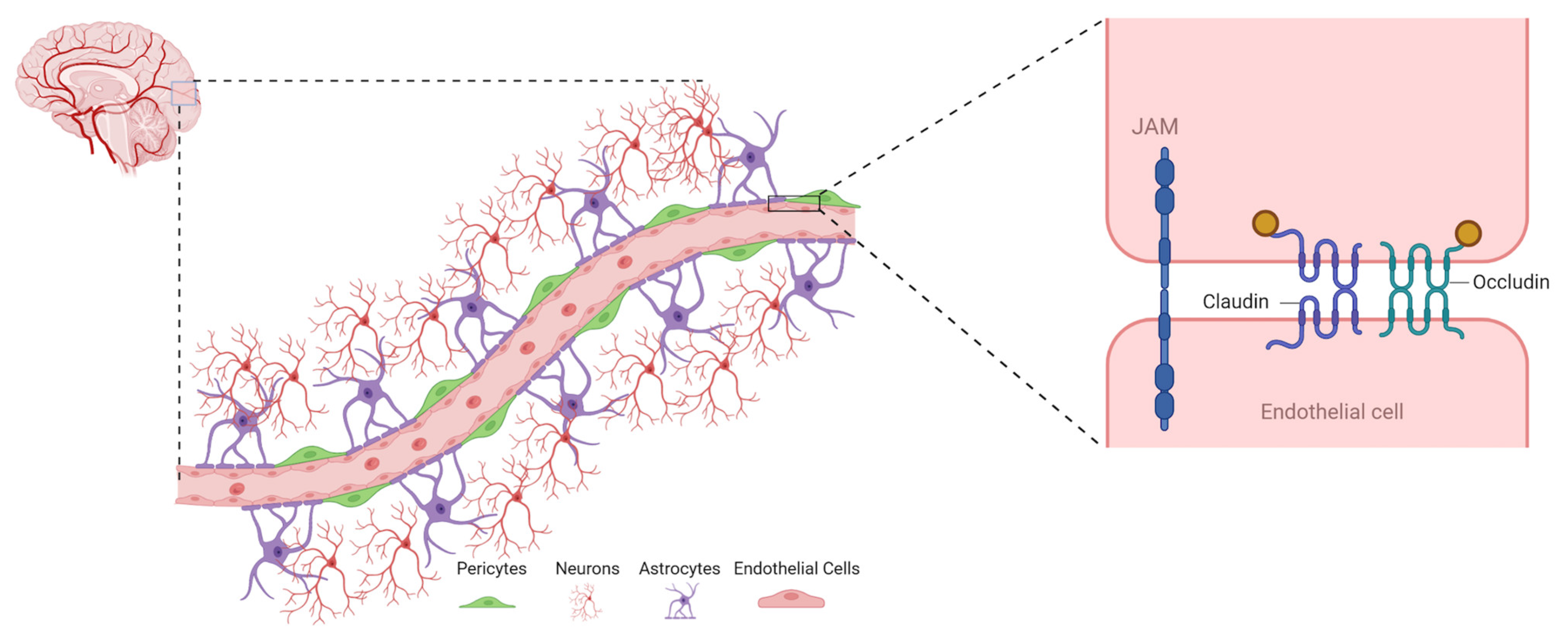
2.1. Components of BBB
2.1.1. Endothelial Cells (ECs)
2.1.2. Astrocytes
2.1.3. Pericytes
2.1.4. Basement Membrane
2.2. Functions of BBB
2.2.1. Protection against Neurotoxins
2.2.2. Prevention of Macromolecules Influx into the Brain
2.2.3. Regulation of Neurotransmitters
2.2.4. Maintenance of Ionic Homeostasis
2.2.5. Supplementation of Brain Nutrition
3. Transport across BBB
3.1. Passive Diffusion
3.2. Active Efflux
3.3. Carrier-Mediated Transport (CMT)/Influx by the Major Facilitator Superfamily
3.4. Receptor-Mediated Transport (RMT)
4. Embryonic Development of BBB
5. In Vitro Models of BBB
5.1. 2D Models of BBB
5.1.1. Cells Used in 2D Models
Endothelial Cells
Stem Cells
5.1.2. Monoculture BBB Model
| Cell Types | Origin | References |
|---|---|---|
| Immortalized Cells | ||
| bEnd.3 cells | Mouse | [98,99,100] |
| cEND | Mouse | [101] |
| BB19 | Human | [102] |
| hBMEC | Human | [102] |
| bEnd.5 | Mouse | [103,104] |
| cerebEND | Mouse | [101] |
| ECV304 | Human | [105] |
| CRL-2583 | Mouse | [106] |
| EaHy929 | Human | [107] |
| HCEC | Human | [108] |
| hCMEC/D3 | Human | [109,110,111,112,113] |
| TY10 | Human | [102] |
| TY08 | Human | [114] |
| TR-BBB | Rat | [114,115] |
| TM-BBB | Mouse | [116] |
| THBMEC | Human | [117] |
| HUVEC-304 | Human | [118] |
| Primary Cells | ||
| BMEC | Bovine | [119] |
| BCEC | Bovine | [120,121] |
| PBEC | Porcine | [122] |
| Brain capillary endothelial cells | Monkey | [84] |
| Stem Cells | ||
| Induced pluripotent cells | Human | [94,123,124,125] |
| Induced multipotent cells | Human | [125] |
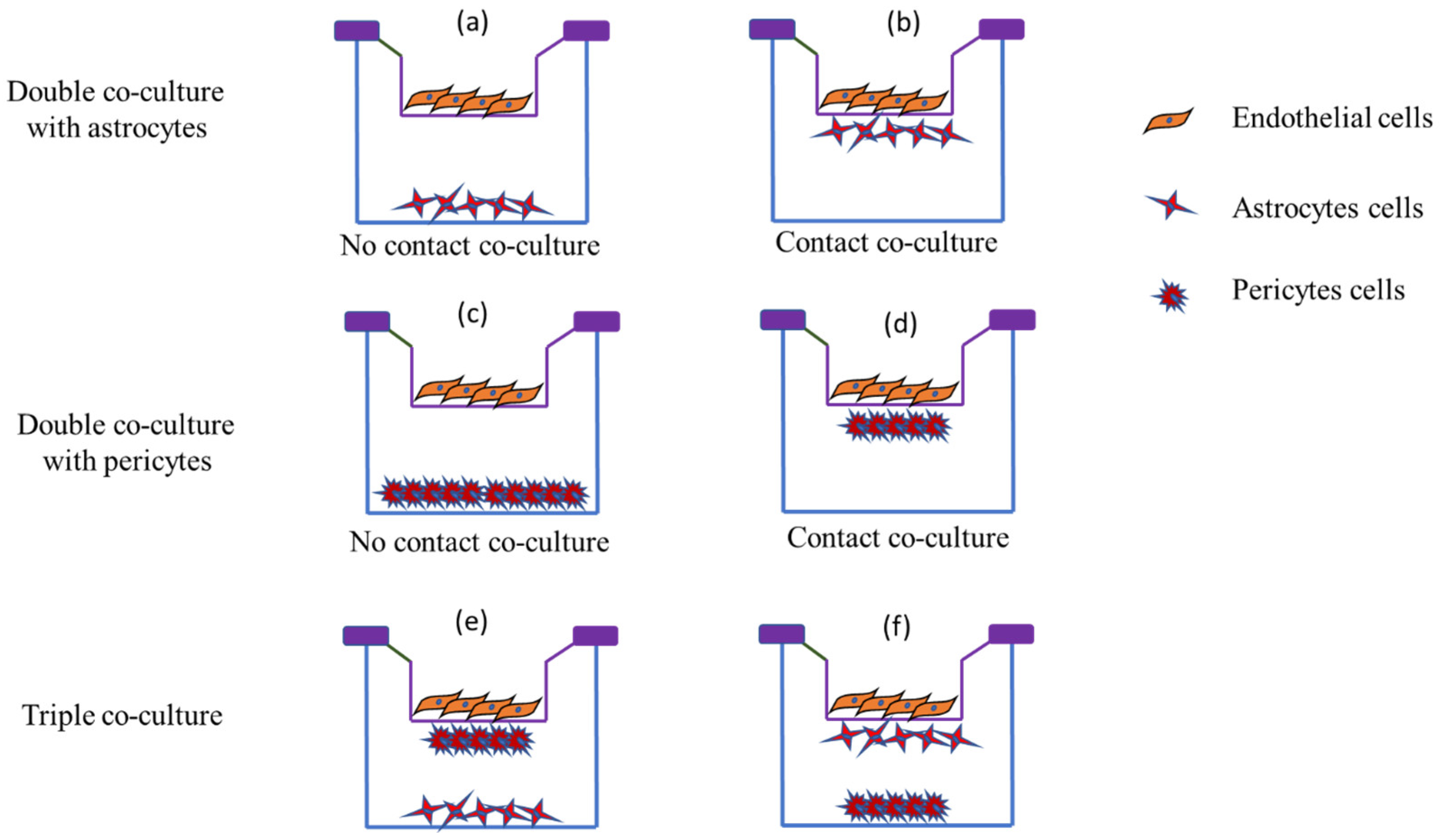
5.1.3. Co-Culture BBB Models
| Cells | Inference | Reference |
|---|---|---|
| Human induced pluripotent (hiPSC) and multipotent cells (neural stem cells) | High TEER (2500 Ωcm2); complex in vivo-like tight junction network | [125] |
| iPSC and GM25256 cells | High TEER (1560 Ωcm2 ± 230 Ωcm2); TJ protein and endothelial marker expression | [138] |
| Human hCMEC/D3 | Feasible alternative to primary ECs in in vitro BBB models | [139] |
| bEnd.3 and glial cells | Intact BBB model with 178.4 ± 10 Ωcm2 TEER | [133] |
| bEnd.3 and glial cells | TEER ~400 Ωcm2 | [134] |
| bEnd.3 and glial cells | TEER ~600 Ωcm2 | [63] |
| ECs and pericytes | Occludin gene expression | [95] |
| Human ECs and pericytes | TEER ~3500 Ωcm2 | [140] |
| Bovine ECs and rat astrocytes | TEER ~850 Ωcm2 | [119] |
| Porcine ECs and rat astrocytes | TEER ~1693 Ωcm2 | [122] |
| Monkey ECs and rat astrocytes, pericytes | TEER~150 Ωcm2 | [84] |
5.1.4. In Vitro Characterization of 2D BBB Models
In Vitro Permeability Measurement
- Papp = apparent permeability;
- dQ/dt = amount of permeability marker transported per min (μg/min);
- A = surface area of the transwell membrane (cm2);
- C0 = initial concentration of the permeability marker;
- 60 is the conversion factor.
TEER Measurement
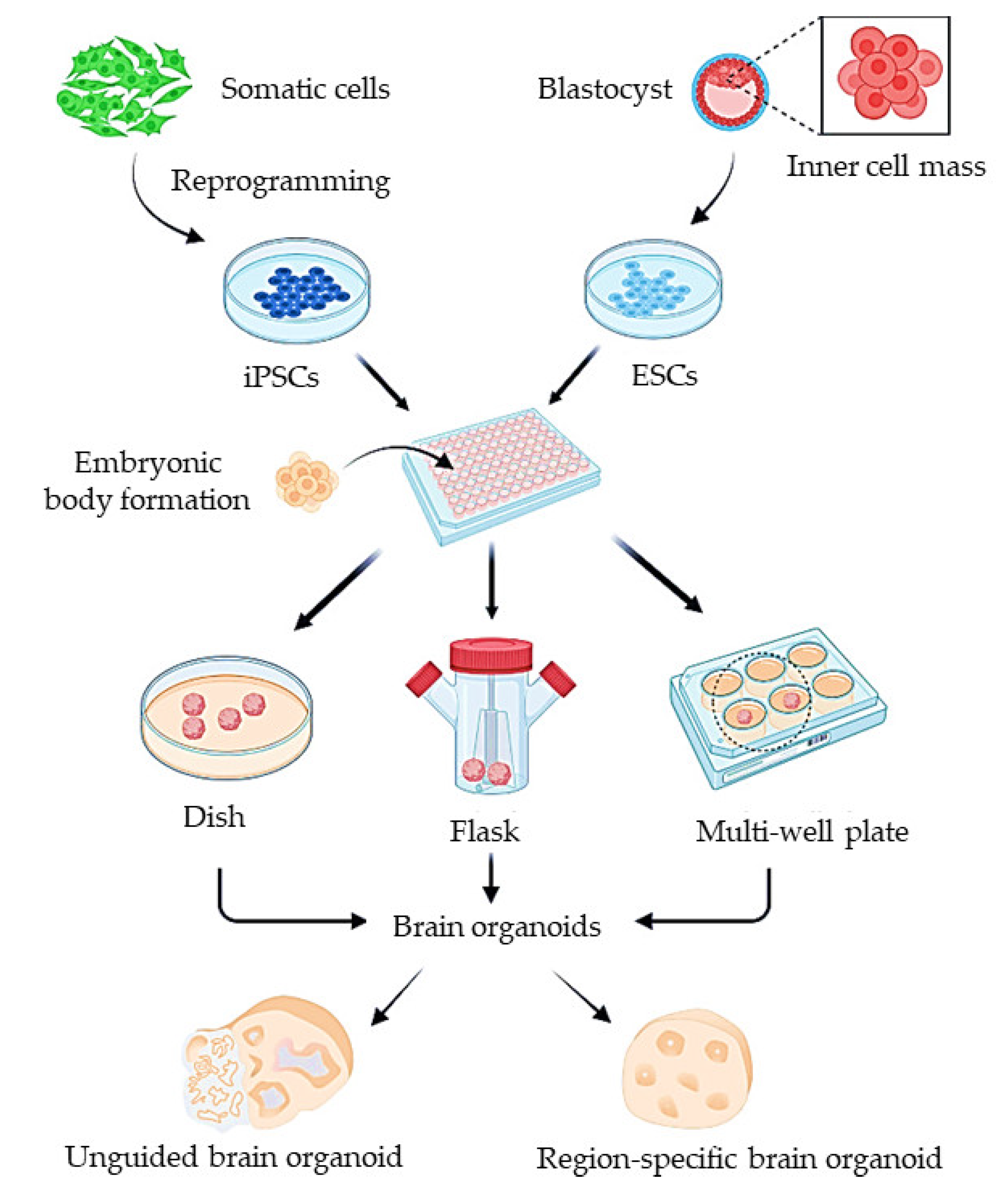
5.2. Organoid Models
5.3. Microfluidic Models for BBB
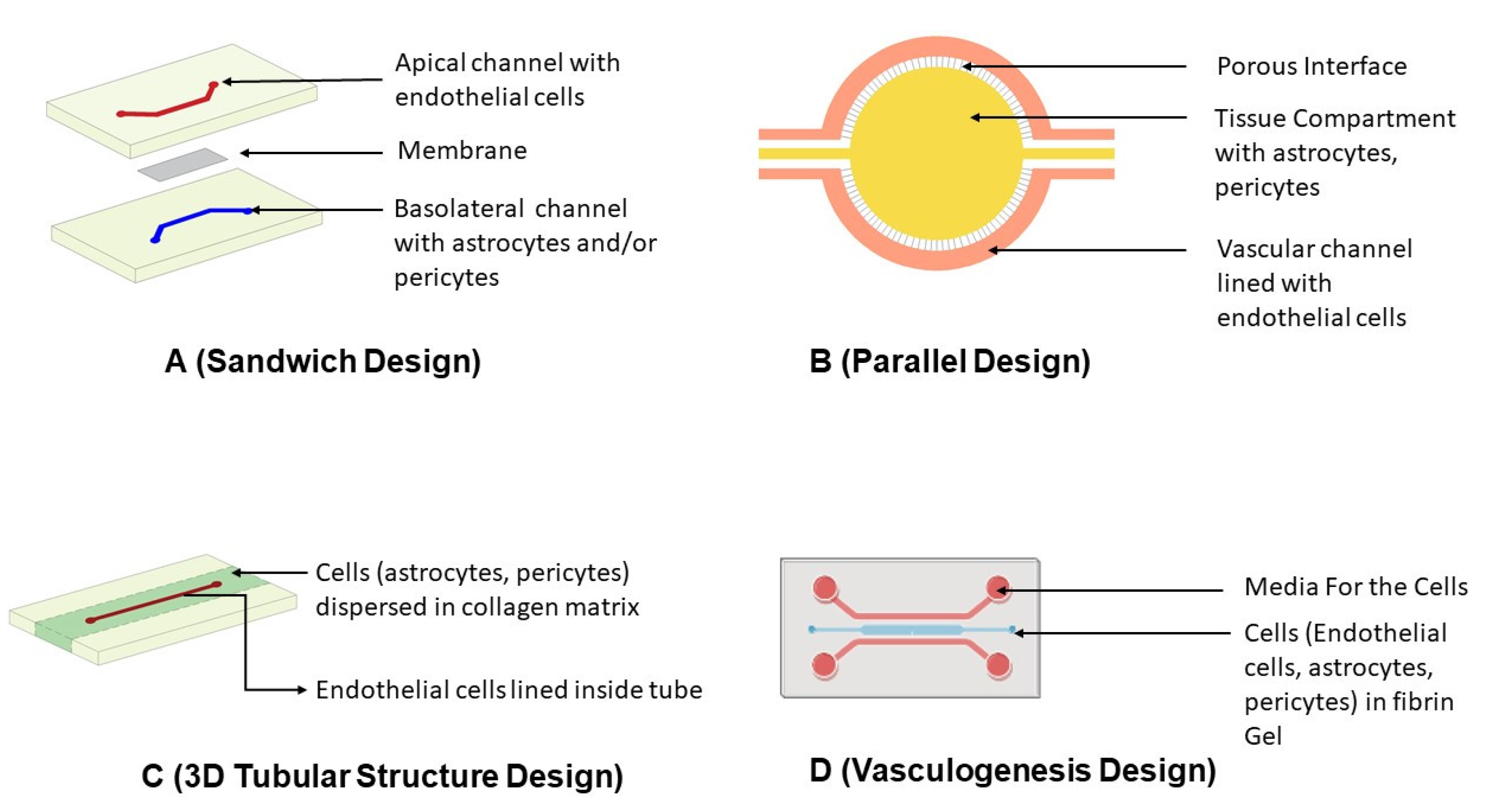
5.3.1. Designing of Microfluidic Devices
Sandwich Design
Parallel Design
3D Tubular Structure Design
Vasculogenesis Design
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Hwang, T.J.; Franklin, J.M. Two decades of new drug development for central nervous system disorders. Nat. Rev. Drug Discov. 2015, 14, 815–816. [Google Scholar] [CrossRef]
- Danon, J.J.; Reekie, T.A.; Kassiou, M. Challenges and Opportunities in Central Nervous System Drug Discovery. Trends Chem. 2019, 1, 612–624. [Google Scholar] [CrossRef]
- Ribeiro, M.; Castanho, M.; Serrano, I. In vitro blood-brain barrier models-latest advances and therapeutic applications in a chronological perspective. Mini Rev. Med. Chem. 2010, 10, 263–271. [Google Scholar] [CrossRef]
- Liddelow, S.A. Fluids and barriers of the CNS: A historical viewpoint. Fluids Barriers CNS 2011, 8, 2. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- van Der Helm, M.W.; Van Der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S. Preclinical research: Make mouse studies work. Nature 2014, 507, 423–425. [Google Scholar] [CrossRef]
- Begley, C.G.; Ellis, L.M. Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Aday, S.; Cecchelli, R.; Hallier-Vanuxeem, D.; Dehouck, M.; Ferreira, L. Stem cell-based human blood–brain barrier models for drug discovery and delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood–brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Huber, J.D.; Egleton, R.D.; Davis, T.P. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 2001, 24, 719–725. [Google Scholar] [CrossRef]
- Tam, S.J.; Watts, R.J. Connecting vascular and nervous system development: Angiogenesis and the blood-brain barrier. Annu. Rev. Neurosci. 2010, 33, 379–408. [Google Scholar] [CrossRef]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr. Med. Chem. 2007, 14, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Fraemohs, L.; Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 2007, 7, 467–477. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, P.F.; Nourshargh, S.; Aurrand-Lions, M.; Imhof, B.A. JAM family and related proteins in leukocyte migration (Vestweber series). Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Tao-Cheng, J.-H.; Brightman, M.W. Development of membrane interactions between brain endothelial cells and astrocytes in vitro. Int. J. Dev. Neurosci. 1988, 6, 25–37. [Google Scholar] [CrossRef]
- Gee, J.R.; Keller, J.N. Astrocytes: Regulation of brain homeostasis via apolipoprotein E. Int. J. Biochem. Cell Biol. 2005, 37, 1145–1150. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [PubMed]
- Somjen, G.G. Ions in the Brain: Normal Function, Seizures, and Stroke; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Gynther, M.; Laine, K.; Ropponen, J.; Leppänen, J.; Mannila, A.; Nevalainen, T.; Savolainen, J.; Järvinen, T.; Rautio, J. Large Neutral Amino Acid Transporter Enables Brain Drug Delivery via Prodrugs. J. Med. Chem. 2008, 51, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. How pathogens penetrate the blood-brain barrier. Microbe 2014, 9, 487–492. [Google Scholar] [CrossRef]
- Dwivedi, D.; Megha, K.; Mishra, R.; Mandal, P.K. Glutathione in brain: Overview of its conformations, functions, biochemical characteristics, quantitation and potential therapeutic role in brain disorders. Neurochem. Res. 2020, 45, 1461–1480. [Google Scholar] [CrossRef] [PubMed]
- Ooms, F.; Weber, P.; Carrupt, P.-A.; Testa, B. A simple model to predict blood–brain barrier permeation from 3D molecular fields. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2002, 1587, 118–125. [Google Scholar] [CrossRef]
- Kaznessis, Y.N.; Snow, M.E.; Blankley, C.J. Prediction of blood-brain partitioning using Monte Carlo simulations of molecules in water. J. Comput.-Aided Mol. Des. 2001, 15, 697–708. [Google Scholar] [CrossRef]
- Fischer, H.; Gottschlich, R.; Seelig, A. Blood-brain barrier permeation: Molecular parameters governing passive diffusion. J. Membr. Biol. 1998, 165, 201–211. [Google Scholar] [CrossRef]
- Lobell, M.; Molnár, L.; Keserü, G.M. Recent advances in the prediction of blood–brain partitioning from molecular structure. J. Pharm. Sci. 2003, 92, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Conradi, R.; Burton, P.; Borchardt, R. Physico-chemical and biological factors that influence a drug’s cellular permeability by passive diffusion. In Lipophilicity in Drug Action and Toxicology; Wiley Online Library: Hoboken, NJ, USA, 1996; pp. 233–252. [Google Scholar]
- Clark, D.E. In silico prediction of blood–brain barrier permeation. Drug Discov. Today 2003, 8, 927–933. [Google Scholar] [CrossRef]
- Marrink, S.; Jähnig, F.; Berendsen, H. Proton transport across transient single-file water pores in a lipid membrane studied by molecular dynamics simulations. Biophys. J. 1996, 71, 632–647. [Google Scholar] [CrossRef]
- Träuble, H. The movement of molecules across lipid membranes: A molecular theory. J. Membr. Biol. 1971, 4, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Sugiyama, Y. Active efflux across the blood-brain barrier: Role of the solute carrier family. NeuroRx 2005, 2, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.-F. Efflux transporters in blood-brain interfaces of the developing brain. Front. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Steiner, P. Brain fuel utilization in the developing brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Ligong, C. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian, J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Nałęcz, K.A. Solute carriers in the blood–brain barier: Safety in abundance. Neurochem. Res. 2017, 42, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [PubMed]
- Ashraf, T.; Kao, A.; Bendayan, R. Functional expression of drug transporters in glial cells: Potential role on drug delivery to the CNS. Adv. Pharmacol. 2014, 71, 45–111. [Google Scholar]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.E.; Abbott, N.J.; Begley, D.J. Transcytosis of macromolecules at the blood–brain barrier. Adv. Pharmacol. 2014, 71, 147–163. [Google Scholar]
- dos Santos Rodrigues, B.; Arora, S.; Kanekiyo, T.; Singh, J. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res. 2020, 1734, 146738. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Dual-modified liposome for targeted and enhanced gene delivery into mice brain. J. Pharmacol. Exp. Ther. 2020, 374, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, N.; Zeng, Z.; Huang, J.; Xiang, Z.; Guan, Y.-Q. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson’s disease models. J. Mater. Sci. Technol. 2020, 43, 197–207. [Google Scholar] [CrossRef]
- Zhou, Y.; Nathans, J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 2014, 31, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, M.; Abbott, N.J. All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia 2008, 56, 699–708. [Google Scholar] [CrossRef]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L. Brain and retinal pericytes: Origin, function and role. Front. Cell. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood–brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PloS ONE 2010, 5, e13741. [Google Scholar] [CrossRef] [PubMed]
- Rice, O.; Surian, A.; Chen, Y. Modeling the blood-brain barrier for treatment of central nervous system (CNS) diseases. J. Tissue Eng. 2022, 13, 20417314221095997. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Wilson, M.L.; Banks, W.A. In vitro modeling of blood–brain barrier and interface functions in neuroimmune communication. Fluids Barriers CNS 2020, 17, 26. [Google Scholar] [CrossRef]
- Banerjee, J.; Shi, Y.; Azevedo, H.S. In vitro blood–brain barrier models for drug research: State-of-the-art and new perspectives on reconstituting these models on artificial basement membrane platforms. Drug Discov. Today 2016, 21, 1367–1386. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef]
- Marroni, M.; Kight, K.M.; Hossain, M.; Cucullo, L.; Desai, S.Y.; Janigro, D. Dynamic in vitro model of the blood-brain barrier. In The Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 2003; pp. 419–434. [Google Scholar]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Voorwinden, L.H.; Nielsen, J.L.; Ivanov, A.; Atsumi, R.; Engman, H.; Ringbom, C.; de Boer, A.G.; Breimer, D.D. Establishment and functional characterization of an in vitro model of the blood–brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur. J. Pharm. Sci. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Muok, L.; Zeng, C.; Li, Y. Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases. Cells 2021, 10, 3183. [Google Scholar] [CrossRef] [PubMed]
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A novel dynamic neonatal blood-brain barrier on a chip. PloS ONE 2015, 10, e0142725. [Google Scholar] [CrossRef]
- Gomes, M.J.; Mendes, B.; Martins, S.; Sarmento, B. Cell-based in vitro models for studying blood–brain barrier (BBB) permeability. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 169–188. [Google Scholar]
- Wilhelm, I.; Krizbai, I.A. In vitro models of the blood–brain barrier for the study of drug delivery to the brain. Mol. Pharm. 2014, 11, 1949–1963. [Google Scholar] [CrossRef]
- Abbott, N.J.; Yusof, S.R.; Reichel, A.; Dolman, D.E.; Preston, J.E. In vitro models of CNS barriers. In Drug Delivery to the Brain; Springer: Berlin/Heidelberg, Germany, 2022; pp. 211–254. [Google Scholar]
- Rahman, N.A.; Rasil, A.N.A.H.M.; Meyding-Lamade, U.; Craemer, E.M.; Diah, S.; Tuah, A.A.; Muharram, S.H. Immortalized endothelial cell lines for in vitro blood–brain barrier models: A systematic review. Brain Res. 2016, 1642, 532–545. [Google Scholar] [CrossRef]
- Weksler, B.; Subileau, E.; Perriere, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood–brain barrier. J. Physiol. 2008, 586, 1937–1949. [Google Scholar] [CrossRef]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef]
- Yamanaka, S.; Blau, H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010, 465, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Dekmak, A.; Mantash, S.; Shaito, A.; Toutonji, A.; Ramadan, N.; Ghazale, H.; Kassem, N.; Darwish, H.; Zibara, K. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav. Brain Res. 2018, 340, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Bao, X.; Al-Ahmad, A.; Liu, J.; Wu, Y.; Dong, W.; Dunn, K.K.; Shusta, E.V.; Palecek, S.P. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014, 3, 804–816. [Google Scholar] [CrossRef]
- Hori, S.; Ohtsuki, S.; Hosoya, K.I.; Nakashima, E.; Terasaki, T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 2004, 89, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Wiley, M. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev. Biol. 1981, 84, 183–192. [Google Scholar] [CrossRef]
- Toimela, T.; Mäenpää, H.; Mannerström, M.; Tähti, H. Development of an in vitro blood–brain barrier model—Cytotoxicity of mercury and aluminum. Toxicol. Appl. Pharmacol. 2004, 195, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood–brain barrier model. Ann. Biomed. Eng. 2014, 42, 2379–2391. [Google Scholar] [CrossRef]
- Koto, T.; Takubo, K.; Ishida, S.; Shinoda, H.; Inoue, M.; Tsubota, K.; Okada, Y.; Ikeda, E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am. J. Pathol. 2007, 170, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Oh, S.; Ahn, J.-H.; Park, E.-M. Estrogen receptor-mediated resveratrol actions on blood-brain barrier of ovariectomized mice. Neurobiol. Aging 2015, 36, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Silwedel, C.; Förster, C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J. Neuroimmunol. 2006, 179, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood–brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Paolinelli, R.; Corada, M.; Ferrarini, L.; Devraj, K.; Artus, C.; Czupalla, C.J.; Rudini, N.; Maddaluno, L.; Papa, E.; Engelhardt, B. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PloS ONE 2013, 8, e70233. [Google Scholar] [CrossRef]
- Kraus, J.; Voigt, K.; Schuller, A.; Scholz, M.; Kim, K.; Schilling, M.; Schäbitz, W.; Oschmann, P.; Engelhardt, B. Interferon-β stabilizes barrier characteristics of the blood–brain barrier in four different species in vitro. Mult. Scler. J. 2008, 14, 843–852. [Google Scholar] [CrossRef]
- Dobbie, M.S.; Hurst, R.D.; Klein, N.J.; Surtees, R.A. Upregulation of intercellular adhesion molecule-1 expression on human endothelial cells by tumour necrosis factor-α in an in vitro model of the blood–brain barrier. Brain Res. 1999, 830, 330–336. [Google Scholar] [CrossRef]
- Zhang, Z.; McGoron, A.J.; Crumpler, E.T.; Li, C.-Z. Co-culture based blood-brain barrier in vitro model, a tissue engineering approach using immortalized cell lines for drug transport study. Appl. Biochem. Biotechnol. 2011, 163, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Dobbie, M.S.; Felix, R.A.; Barrand, M.A.; Hurst, R.D. A comparison of the induction of immortalized endothelial cell impermeability by astrocytes. Neuroreport 2001, 12, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Chakrabarti, R.; Tang, D.; Kim, K.; Kaplowitz, N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: Evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res. 2000, 852, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, A.; Favretto, M.E.; Ioannou, P.V.; Romero, I.A.; Couraud, P.-O.; Weksler, B.B.; Parker, T.L.; Kallinteri, P. Permeability of PEGylated immunoarsonoliposomes through in vitro blood brain barrier-medulloblastoma co-culture models for brain tumor therapy. Pharm. Res. 2015, 32, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.R.; Banerjee, A.; Banks, W.A.; Ercal, N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood–brain barrier endothelial cells. Free Radic. Biol. Med. 2011, 50, 801–810. [Google Scholar] [CrossRef]
- Ilina, P.; Partti, S.; Niklander, J.; Ruponen, M.; Lou, Y.-R.; Yliperttula, M. Effect of differentiation on endocytic profiles of endothelial and epithelial cell culture models. Exp. Cell Res. 2015, 332, 89–101. [Google Scholar] [CrossRef]
- Shimizu, F.; Sano, Y.; Tominaga, O.; Maeda, T.; Abe, M.-a.; Kanda, T. Advanced glycation end-products disrupt the blood–brain barrier by stimulating the release of transforming growth factor–β by pericytes and vascular endothelial growth factor and matrix metalloproteinase–2 by endothelial cells in vitro. Neurobiol. Aging 2013, 34, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. Influence of glioma cells on a new co-culture in vitro blood–brain barrier model for characterization and validation of permeability. Int. J. Pharm. 2015, 490, 94–101. [Google Scholar] [CrossRef]
- Sano, Y.; Shimizu, F.; Abe, M.; Maeda, T.; Kashiwamura, Y.; Ohtsuki, S.; Terasaki, T.; Obinata, M.; Kajiwara, K.; Fujii, M. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood–brain barrier function. J. Cell. Physiol. 2010, 225, 519–528. [Google Scholar] [CrossRef]
- Hosoya, K.-I.; Takashima, T.; Tetsuka, K.; Nagura, T.; Ohtsuki, S.; Takanaga, H.; Ueda, M.; Yanai, N.; Obinata, M.; Terasaki, T. mRNA expression and transport characterization of conditionally immortalized rat brain capillary endothelial cell lines; a new in vitro BBB model for drug targeting. J. Drug Target. 2000, 8, 357–370. [Google Scholar] [CrossRef]
- Pan, W.; Cain, C.; Yu, Y.; Kastin, A.J. Receptor-mediated transport of LIF across blood–spinal cord barrier is upregulated after spinal cord injury. J. Neuroimmunol. 2006, 174, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.; Häckel, S.; Lucka, L.; Danker, K. Interleukin-1β induces an inflammatory response and the breakdown of the endothelial cell layer in an improved human THBMEC-based in vitro blood–brain barrier model. J. Neurosci. Methods 2014, 228, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Fritz, I. Properties of an immortalised vascular endothelial/glioma cell co-culture model of the blood-brain barrier. J. Cell. Physiol. 1996, 167, 81–88. [Google Scholar] [CrossRef]
- Parran, D.K.; Magnin, G.; Li, W.; Jortner, B.S.; Ehrich, M. Chlorpyrifos alters functional integrity and structure of an in vitro BBB model: Co-cultures of bovine endothelial cells and neonatal rat astrocytes. Neurotoxicology 2005, 26, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Candela, P.; Boucau, M.-C.; Fenart, L.; Gosselet, F. Oxysterols decrease apical-to-basolateral transport of Aß peptides via an ABCB1-mediated process in an in vitro Blood-brain barrier model constituted of bovine brain capillary endothelial cells. Brain Res. 2013, 1517, 1–15. [Google Scholar] [CrossRef]
- Di Marco, A.; Vignone, D.; Gonzalez Paz, O.; Fini, I.; Battista, M.R.; Cellucci, A.; Bracacel, E.; Auciello, G.; Veneziano, M.; Khetarpal, V. Establishment of an in vitro human blood-brain barrier model derived from induced pluripotent stem cells and comparison to a porcine cell-based system. Cells 2020, 9, 994. [Google Scholar] [CrossRef]
- Cantrill, C.A.; Skinner, R.A.; Rothwell, N.J.; Penny, J.I. An immortalised astrocyte cell line maintains the in vivo phenotype of a primary porcine in vitro blood–brain barrier model. Brain Res. 2012, 1479, 17–30. [Google Scholar] [CrossRef]
- Patel, R.; Page, S.; Al-Ahmad, A.J. Isogenic blood–brain barrier models based on patient-derived stem cells display inter-individual differences in cell maturation and functionality. J. Neurochem. 2017, 142, 74–88. [Google Scholar] [CrossRef]
- DeStefano, J.G.; Xu, Z.S.; Williams, A.J.; Yimam, N.; Searson, P.C. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 2017, 14, 20. [Google Scholar] [CrossRef]
- Appelt-Menzel, A.; Cubukova, A.; Günther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stüber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri-and multipotent stem cells. Stem Cell Rep. 2017, 8, 894–906. [Google Scholar] [CrossRef]
- Kaisar, M.A.; Sajja, R.K.; Prasad, S.; Abhyankar, V.V.; Liles, T.; Cucullo, L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin. Drug Discov. 2017, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Shayan, G.; Choi, Y.S.; Shusta, E.V.; Shuler, M.L.; Lee, K.H. Murine in vitro model of the blood–brain barrier for evaluating drug transport. Eur. J. Pharm. Sci. 2011, 42, 148–155. [Google Scholar] [CrossRef]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the blood–brain barrier: Beyond the endothelial cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef]
- Abbott, N.J.; Dolman, D.E.; Drndarski, S.; Fredriksson, S.M. An improved in vitro blood–brain barrier model: Rat brain endothelial cells co-cultured with astrocytes. In Astrocytes; Springer: Berlin/Heidelberg, Germany, 2012; pp. 415–430. [Google Scholar]
- Wolburg, H.; Neuhaus, J.; Kniesel, U.; Krauß, B.; Schmid, E.-M.; Ocalan, M.; Farrell, C.; Risau, W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J. Cell Sci. 1994, 107, 1347–1357. [Google Scholar] [CrossRef]
- Coisne, C.; Dehouck, L.; Faveeuw, C.; Delplace, Y.; Miller, F.; Landry, C.; Morissette, C.; Fenart, L.; Cecchelli, R.; Tremblay, P. Mouse syngenic in vitro blood–brain barrier model: A new tool to examine inflammatory events in cerebral endothelium. Lab. Investig. 2005, 85, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Limmer, S.; Weiler, A.; Volkenhoff, A.; Babatz, F.; Klämbt, C. The Drosophila blood-brain barrier: Development and function of a glial endothelium. Front. Neurosci. 2014, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Rodrigues, B.; Kanekiyo, T.; Singh, J. In vitro and in vivo characterization of CPP and transferrin modified liposomes encapsulating pDNA. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102225. [Google Scholar] [CrossRef]
- Sá-Pereira, I.; Brites, D.; Brito, M.A. Neurovascular unit: A focus on pericytes. Mol. Neurobiol. 2012, 45, 327–347. [Google Scholar] [CrossRef]
- Zozulya, A.; Weidenfeller, C.; Galla, H.-J. Pericyte–endothelial cell interaction increases MMP-9 secretion at the blood–brain barrier in vitro. Brain Res. 2008, 1189, 1–11. [Google Scholar] [CrossRef]
- Vandenhaute, E.; Culot, M.; Gosselet, F.; Dehouck, L.; Godfraind, C.; Franck, M.; Plouët, J.; Cecchelli, R.; Dehouck, M.-P.; Ruchoux, M.-M. Brain pericytes from stress-susceptible pigs increase blood-brain barrier permeability in vitro. Fluids Barriers CNS 2012, 9, 11. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Liu, H.; Huang, L.; Meng, G.; Ding, Y.; Su, W.; Lu, J.; Gong, S.; Terstappen, G.C. Development of human in vitro brain-blood barrier model from induced pluripotent stem cell-derived endothelial cells to predict the in vivo permeability of drugs. Neurosci. Bull. 2019, 35, 996–1010. [Google Scholar] [CrossRef]
- Daniels, B.P.; Cruz-Orengo, L.; Pasieka, T.J.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Cooper, J.A.; Doering, T.L.; Klein, R.S. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods 2013, 212, 173–179. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Weidenfeller, C.; Svendsen, C.N.; Shusta, E.V. Blood–brain barrier modeling with co-cultured neural progenitor cell-derived astrocytes and neurons. J. Neurochem. 2011, 119, 507–520. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Nakao, S.; Honda, M.; Hayashi, K.; Nakaoke, R.; Kataoka, Y.; Niwa, M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell. Mol. Neurobiol. 2007, 27, 687–694. [Google Scholar] [CrossRef]
- Toyoda, K.; Tanaka, K.; Nakagawa, S.; Thuy, D.H.D.; Ujifuku, K.; Kamada, K.; Hayashi, K.; Matsuo, T.; Nagata, I.; Niwa, M. Initial contact of glioblastoma cells with existing normal brain endothelial cells strengthen the barrier function via fibroblast growth factor 2 secretion: A new in vitro blood–brain barrier model. Cell. Mol. Neurobiol. 2013, 33, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Liu, Y.; Qi, H.; Ma, Q.; Xu, L.; Chen, W.; Chen, G.; Xu, X. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int. J. Biol. Sci. 2013, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, M.; Bohacek, J. Stress does not increase blood–brain barrier permeability in mice. J. Cereb. Blood Flow Metab. 2016, 36, 1304–1315. [Google Scholar] [CrossRef]
- Wolman, M.; Klatzo, I.; Chui, E.; Wilmes, F.; Nishimoto, K.; Fujiwara, K.; Spatz, M. Evaluation of the dye-protein tracers in pathophysiology of the blood-brain barrier. Acta Neuropathol. 1981, 54, 55–61. [Google Scholar] [CrossRef]
- Majno, G.; Palade, G.; Schoefl, G.I. Studies on inflammation: II. The site of action of histamine and serotonin along the vascular tree: A topographic study. J. Cell Biol. 1961, 11, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Ek, C.J.; Dziegielewska, K.M.; Stolp, H.; Saunders, N.R. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 2006, 496, 13–26. [Google Scholar] [CrossRef]
- Clasen, R.A.; Pandolfi, S.; Hass, G.M. Vital staining, serum albumin and the blood-brain barrier. J. Neuropathol. Exp. Neurol. 1970, 29, 266–284. [Google Scholar] [CrossRef]
- Sun, H.; Hu, H.; Liu, C.; Sun, N.; Duan, C. Methods used for the measurement of blood-brain barrier integrity. Metab. Brain Dis. 2021, 36, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.E.; Peart, J.C.; Hawkins, B.T.; Campos, C.R.; Miller, D.S. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc. Natl. Acad. Sci. USA 2012, 109, 15930–15935. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Crone, C.; Christensen, O. Electrical resistance of a capillary endothelium. J. Gen. Physiol. 1981, 77, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Batto, A.F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood-Brain Barrier: A Critical Review of Methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef]
- Neuhaus, W.; Lauer, R.; Oelzant, S.; Fringeli, U.P.; Ecker, G.F.; Noe, C.R. A novel flow based hollow-fiber blood-brain barrier in vitro model with immortalised cell line PBMEC/C1–2. J. Biotechnol. 2006, 125, 127–141. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.; Cho, C.-F. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Marton, R.M.; Pașca, S.P. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 2020, 30, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Koledova, Z. 3D Cell Culture; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2017. [Google Scholar]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Lacombe, O.; Videau, O.; Chevillon, D.; Guyot, A.-C.; Contreras, C.; Blondel, S.; Nicolas, L.; Ghettas, A.; Bénech, H.; Thevenot, E. In vitro primary human and animal cell-based blood—brain barrier models as a screening tool in drug discovery. Mol. Pharm. 2011, 8, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Le Roi, B.; Kamm, R.D.; Maoz, B.M. Biology and Models of the Blood–brain Barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef]
- Sokolova, V.; Mekky, G.; van der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020, 10, 18033. [Google Scholar] [CrossRef]
- Lu, X.; Yang, J.; Xiang, Y. Modeling human neurodevelopmental diseases with brain organoids. Cell Regen. 2022, 11, 1. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the blood–brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Workman, M.J.; Svendsen, C.N. Recent advances in human iPSC-derived models of the blood–brain barrier. Fluids Barriers CNS 2020, 17, 30. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Raja, W.K.; Mungenast, A.E.; Lin, Y.-T.; Ko, T.; Abdurrob, F.; Seo, J.; Tsai, L.-H. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS ONE 2016, 11, e0161969. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab A Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Janigro, D.; Hossain, M.; Oby, E.; Rapp, E.; Cucullo, L. Side by side comparison between dynamic versus static models of blood–brain barrier in vitro: A permeability study. Brain Res. 2006, 1109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent progress in microfluidic models of the blood-brain barrier. Micromachines 2019, 10, 375. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidic blood–brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef]
- Prabhakarpandian, B.; Shen, M.-C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic blood brain barrier model. Lab A Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, S.-R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered human blood–brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef]
- Lee, S.W.L.; Campisi, M.; Osaki, T.; Possenti, L.; Mattu, C.; Adriani, G.; Kamm, R.D.; Chiono, V. Modeling nanocarrier transport across a 3D in vitro human blood-brain–barrier microvasculature. Adv. Healthc. Mater. 2020, 9, 1901486. [Google Scholar] [CrossRef]
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119. [Google Scholar] [CrossRef]
- Bhalerao, A.; Sivandzade, F.; Archie, S.R.; Chowdhury, E.A.; Noorani, B.; Cucullo, L. In vitro modeling of the neurovascular unit: Advances in the field. Fluids Barriers CNS 2020, 17, 22. [Google Scholar] [CrossRef]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Neely, M.D.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef]


| Marker | Merits | Demerits | References |
|---|---|---|---|
| Radiolabeled sucrose | Low molecular weight represents most of the small-molecule therapeutic drugs; offers quantitative permeability of the BBB | It can be metabolized | [149,150] |
| Sodium fluorescein | Low molecular weight, low limit of detection | Higher protein binding affinity than horseradish peroxidase | [144] |
| Horseradish peroxidase | Smaller protein, stable and relatively less expensive | Carcinogenic | [146] |
| Evan’s blue–albumin | Rapid, reliable, and highly sensitive assessment | It binds to albumin. Alteration of the structure in the presence of physiological buffers | [149,151] |
| Radiolabeled mannitol | Does not bind to plasma proteins, stable, uncharged. Does not interact with BBB transporters | It may contain lipophilic impurities | [15] |
| Trypan blue | Low toxicity | Binds to plasma proteins | [15] |
| Radiolabeled inulin | Does not bind to plasma proteins | It may contain lipophilic impurities | [15] |
| Dextran | It can be used for a wide range of molecular weight | Not suitable for low-molecular-weight permeability prediction | [15] |
| Albumin | It is used in the radiolabeled form, gives accurate quantification | Not suitable for low-molecular-weight permeability prediction | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaulagain, B.; Gothwal, A.; Lamptey, R.N.L.; Trivedi, R.; Mahanta, A.K.; Layek, B.; Singh, J. Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. Int. J. Mol. Sci. 2023, 24, 2710. https://doi.org/10.3390/ijms24032710
Chaulagain B, Gothwal A, Lamptey RNL, Trivedi R, Mahanta AK, Layek B, Singh J. Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. International Journal of Molecular Sciences. 2023; 24(3):2710. https://doi.org/10.3390/ijms24032710
Chicago/Turabian StyleChaulagain, Bivek, Avinash Gothwal, Richard Nii Lante Lamptey, Riddhi Trivedi, Arun Kumar Mahanta, Buddhadev Layek, and Jagdish Singh. 2023. "Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective" International Journal of Molecular Sciences 24, no. 3: 2710. https://doi.org/10.3390/ijms24032710
APA StyleChaulagain, B., Gothwal, A., Lamptey, R. N. L., Trivedi, R., Mahanta, A. K., Layek, B., & Singh, J. (2023). Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. International Journal of Molecular Sciences, 24(3), 2710. https://doi.org/10.3390/ijms24032710






