Exploration of Novel Scaffolds Targeting Cytochrome b of Pyricularia oryzae
Abstract
1. Introduction
2. Results
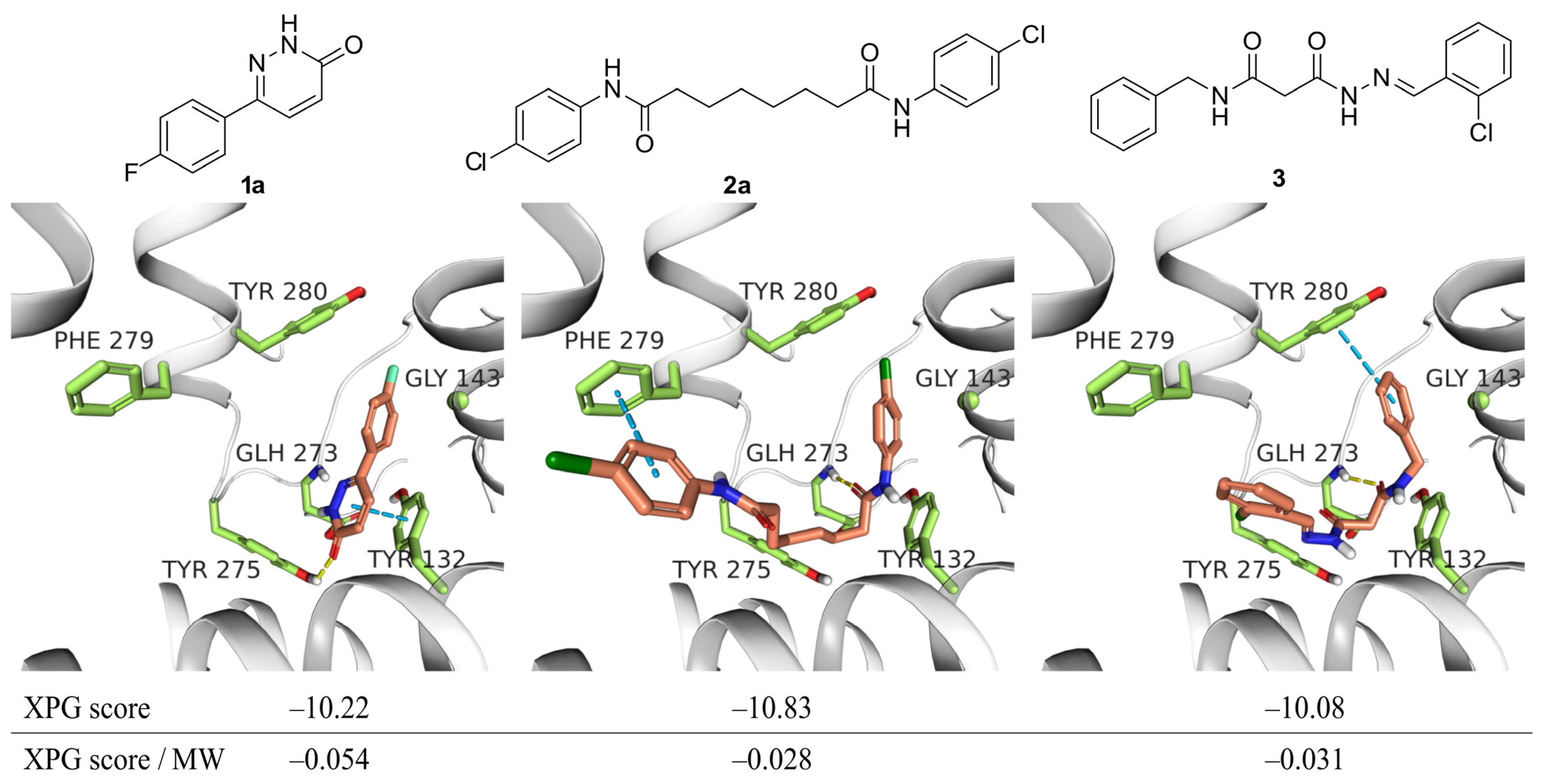
2.1. Virtual Screening and Identification of Potential Inhibitors of the Qo-Site of Cytochrome b
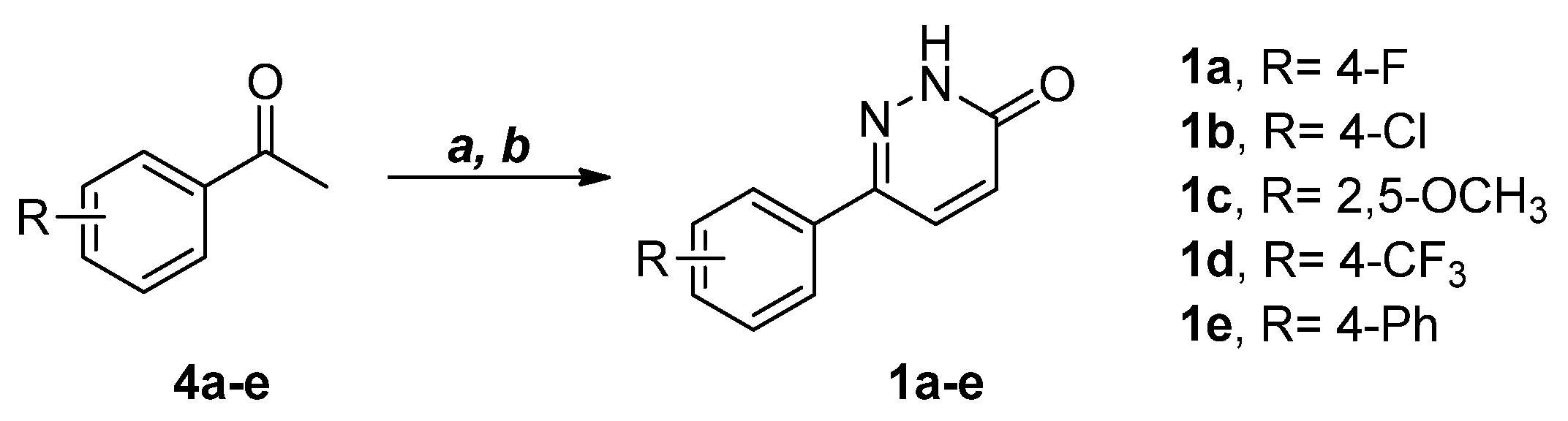
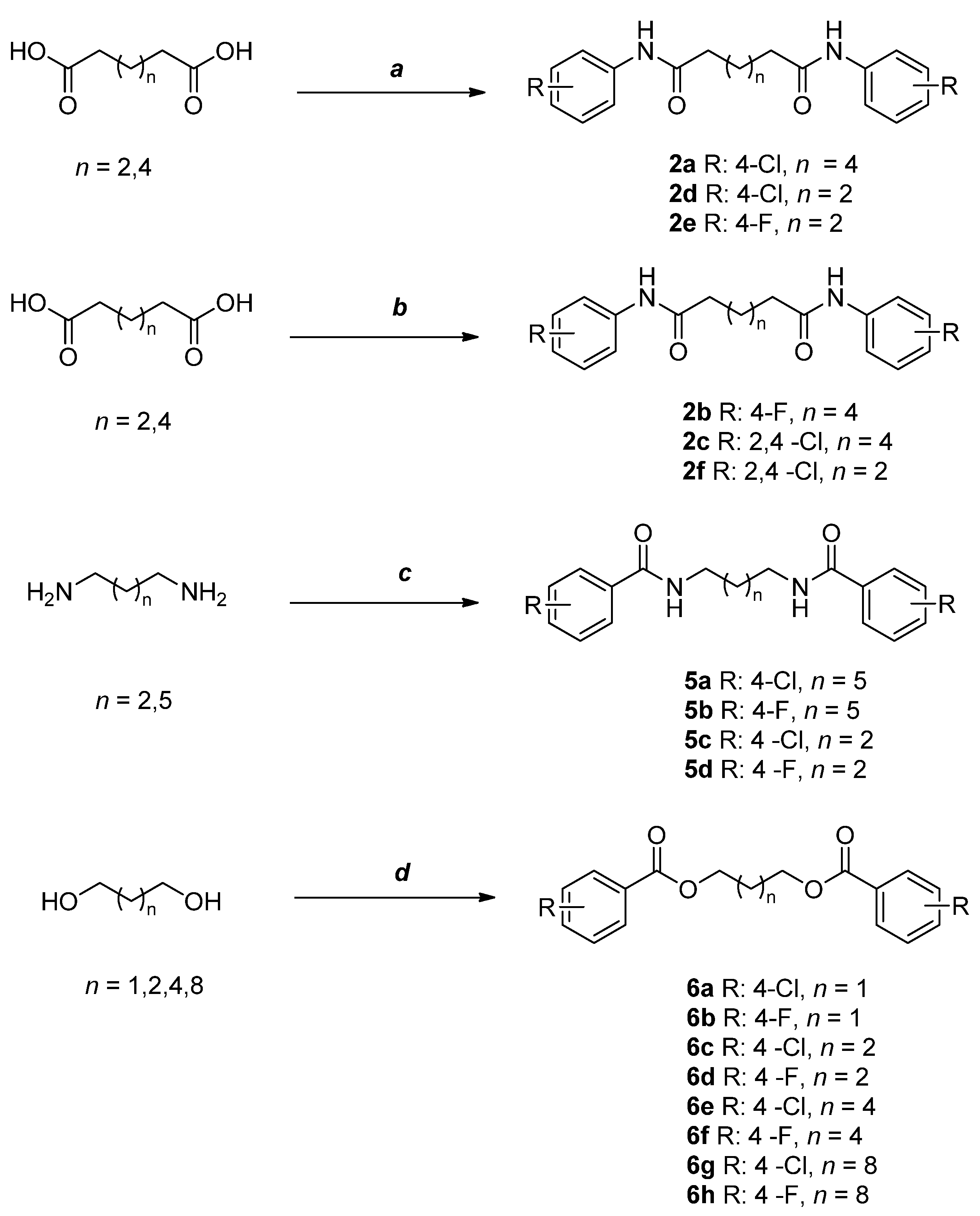
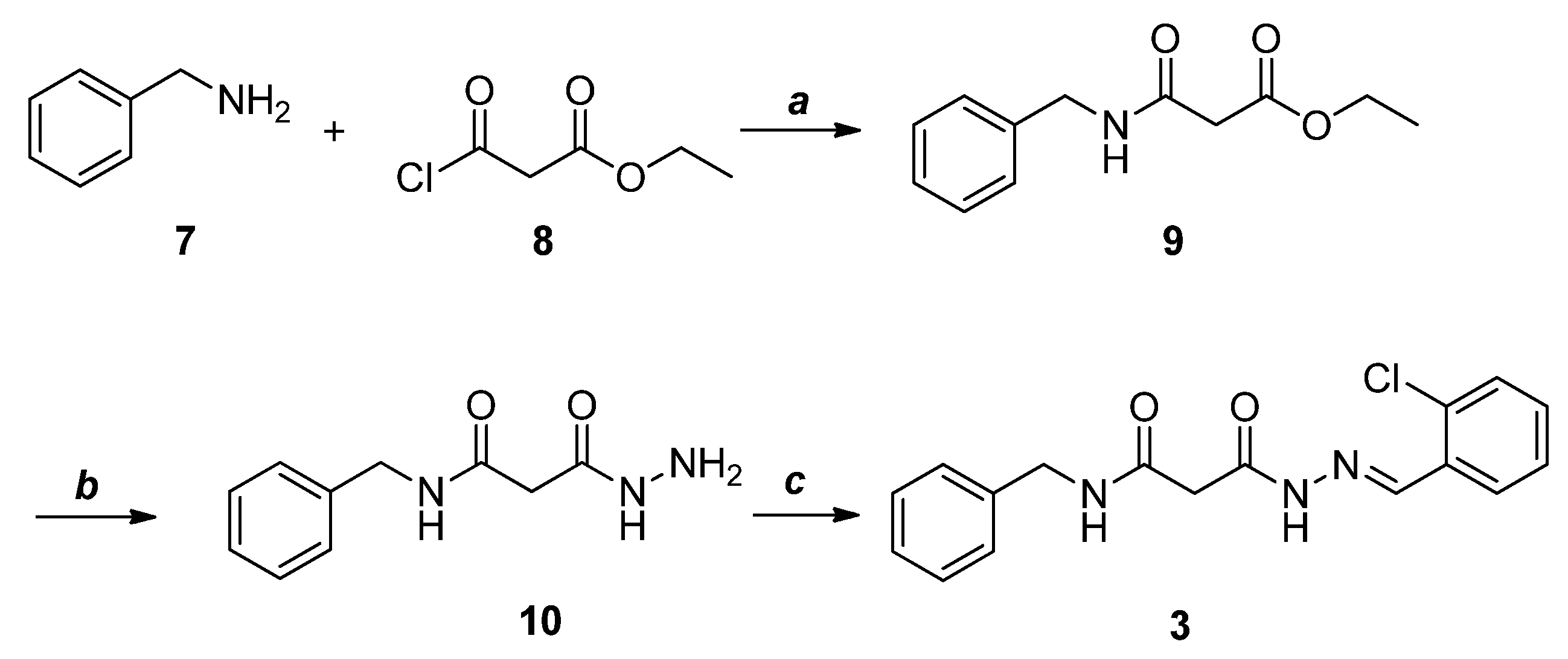
2.2. Synthesis of Selected Compounds and Their Analogues
2.3. Enzymatic Inhibition of Cytochrome bc1 Complex by the Compounds
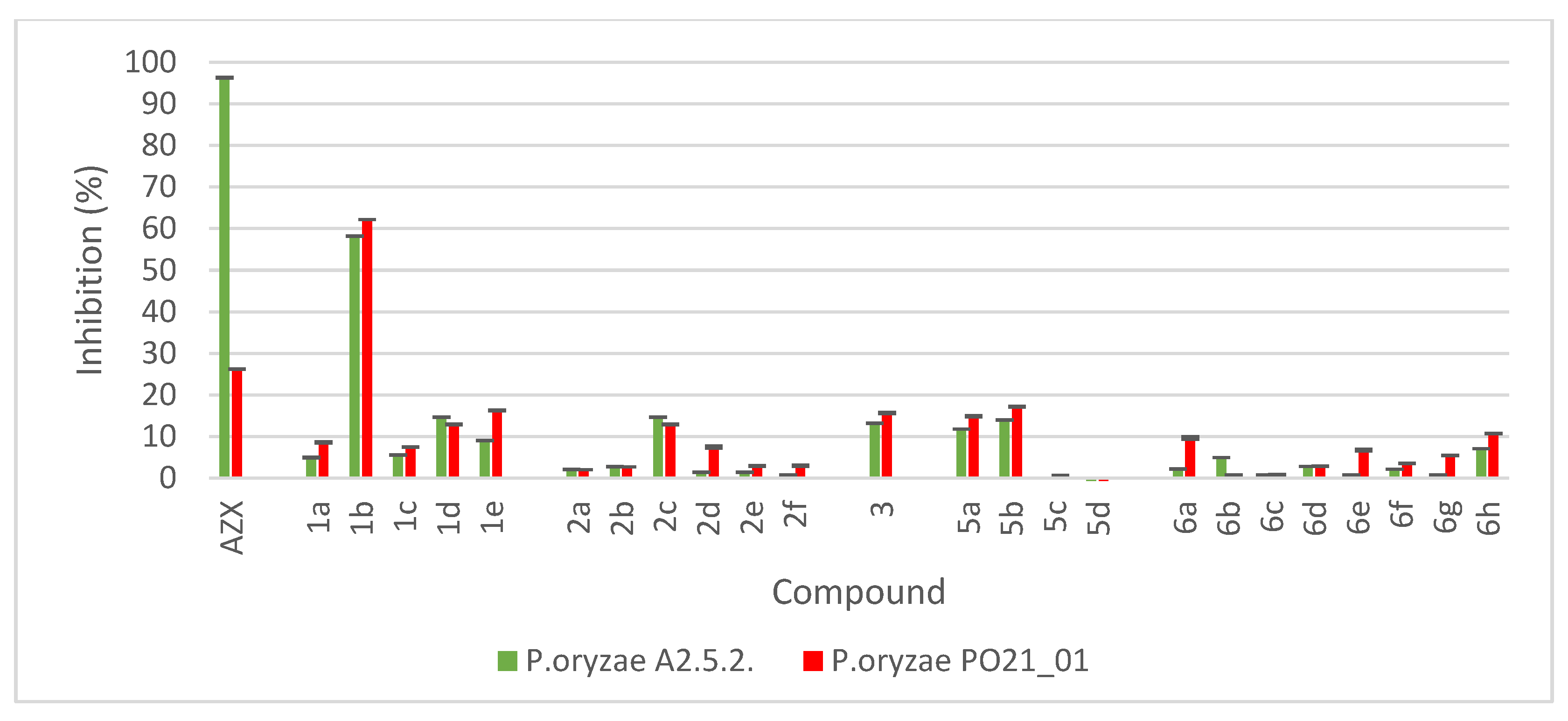
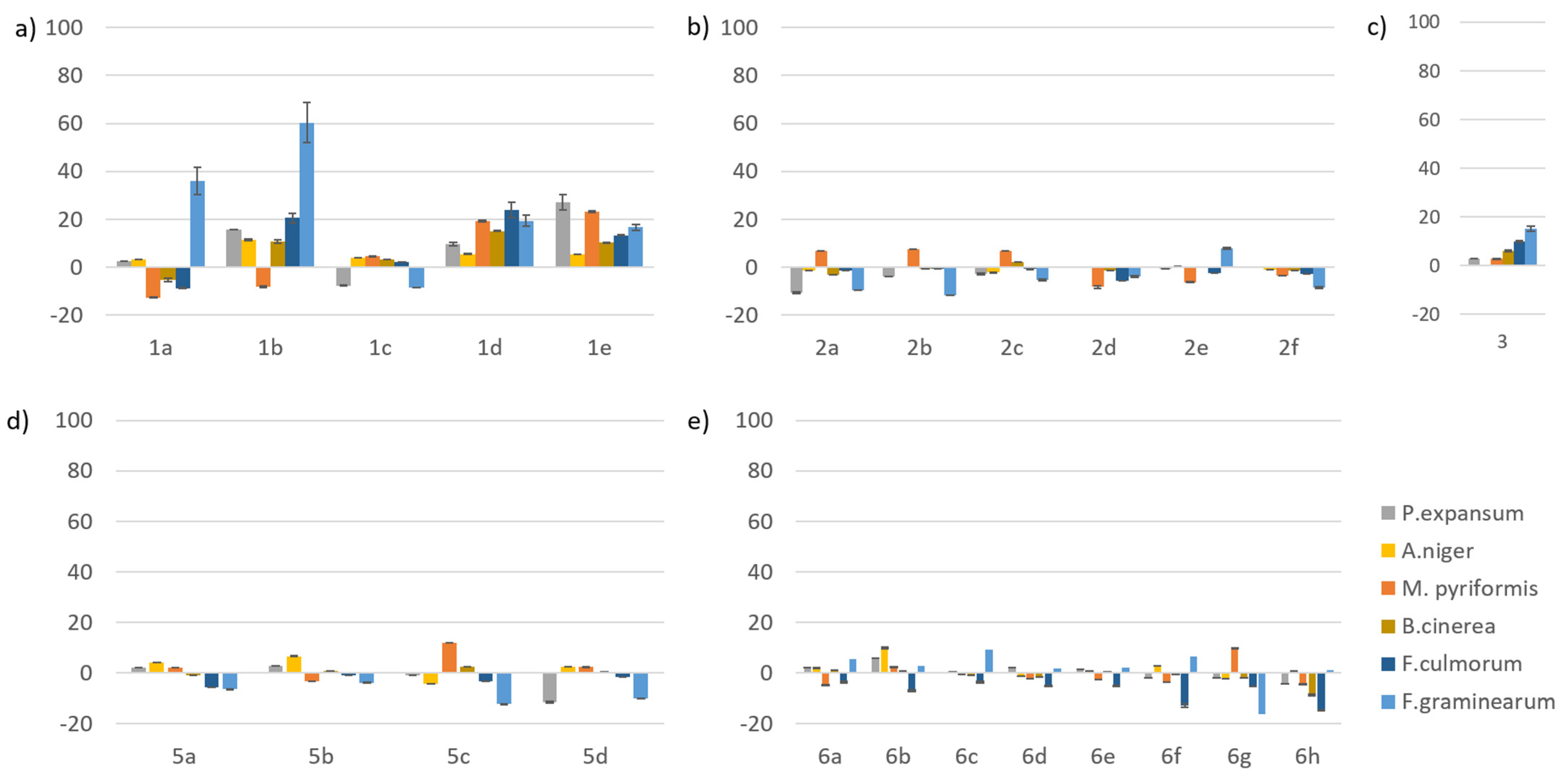
2.4. Mycelium Growth Inhibition by the Compounds
2.5. In Silico ADME/TOX Predictions
3. Discussion
4. Materials and Methods
4.1. Molecular Modelling, Virtual Screening, and ADME/TOX Analysis
4.2. Chemistry
4.3. General Procedures for the Synthesis of Compounds
4.3.1. General Procedure for the Synthesis of 6-Phenylpyridazin-3(2H)-ones (1a–e)
4.3.2. General Procedure for the Synthesis of Compounds 2b, 2c, and 2f
4.3.3. General Procedures for the Synthesis of Compounds 2a, 2d, and 2e
4.3.4. General Procedure for the Synthesis of Compounds 5a–d
4.3.5. General Procedure for the Synthesis of Compounds 6a–h
4.3.6. Synthesis of Ethyl 3-(Benzylamino)-3-oxopropanoate (9)
4.3.7. N-Benzyl-3-hydrazinyl-3-oxopropanamide (10)
4.3.8. N-Benzyl-3-(2-(2-chlorobenzylidene) Hydrazinyl)-3-oxopropanamide (3)
4.4. Fungal Strains
4.5. Enzyme Inhibition Assays for the Measurement of QoI Action
4.6. In Vitro Fungal Mycelium Growth Inhibition by the Compounds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.; Rios, J.A.; Aucique-Pérez, C.E.; Moreira, S.I.; Croll, D.; Alves, E.; de Carvalho, G.; Maciel, J.L.N.; et al. Wheat blast: From its origins in South America to its emergence as a global threat. Mol. Plant Pathol. 2018, 20, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Valent, B. The Impact of Blast Disease: Past, Present, and Future. Methods Mol. Biol. 2021, 2356, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Valent, B. Wheat blast disease: Danger on the move. Trop. Plant Pathol. 2017, 42, 210–222. [Google Scholar] [CrossRef]
- Gladieux, P.; Condon, B.; Ravel, S.; Soanes, D.; Maciel, J.L.N.; Nhani, A.; Chen, L.; Terauchi, R.; Lebrun, M.-H.; Tharreau, D.; et al. Gene Flow between Divergent Cereal- and Grass-Specific Lineages of the Rice Blast Fungus Magnaporthe oryzae. Mbio 2018, 9, e01219-17. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, J.; Pordel, A.; Ravel, S.; Tharreau, D. First Scientific Report of Pyricularia oryzae Causing Gray Leaf Spot Disease on Perennial Ryegrass (Lolium perenne) in France. Plant Dis. 2019, 103, 1024. [Google Scholar] [CrossRef]
- Valent, B.; Cruppe, G.; Stack, J.P.; Cruz, C.D.; Farman, M.L.; Paul, P.A.; Peterson, G.L.; Pedley, K.F. Recovery Plan for Wheat Blast Caused by Magnaporthe oryzae Pathotype Triticum. Plant Health Prog. 2021, 22, 182–212. [Google Scholar] [CrossRef]
- EC. Final Review Report for the Active Substance Tricyclazole; European Commission: Brussels, Belgium, 2016.
- Fitogest. Available online: https://fitogest.imagelinenetwork.com/it/ (accessed on 6 December 2022).
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Zuccolo, M.; Kunova, A.; Musso, L.; Forlani, F.; Pinto, A.; Vistoli, G.; Gervasoni, S.; Cortesi, P.; Dallavalle, S. Dual-active antifungal agents containing strobilurin and SDHI-based pharmacophores. Sci. Rep. 2019, 9, 11377. [Google Scholar] [CrossRef]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health Part B 2007, 42, 441–451. [Google Scholar] [CrossRef]
- Lamberth, C. Complex III inhibiting strobilurin esters, amides, and carbamates as broad-spectrum fungicides. In Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 371–384. [Google Scholar]
- Kunova, A.; Palazzolo, L.; Forlani, F.; Catinella, G.; Musso, L.; Cortesi, P.; Eberini, I.; Pinto, A.; Dallavalle, S. Structural Investigation and Molecular Modeling Studies of Strobilurin-Based Fungicides Active against the Rice Blast Pathogen Pyricularia oryzae. Int. J. Mol. Sci. 2021, 22, 3731. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, A.J. Plant Health Management: Fungicides and Antibiotics. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 408–424. ISBN 9780080931395. [Google Scholar]
- FRAC List of Plant Pathogenic Organisms Resistant to Disease Control Agents. Available online: www.frac.info (accessed on 16 November 2022).
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. The QoI fungicides, the rise and fall of a successful class of agricultural fungicides. In Fungicides; Carisse, O., Ed.; InTech: Rijeka, Croatia, 2010; pp. 203–220. ISBN 978-953-307-266-1. [Google Scholar]
- Kim, Y.-S.; Dixon, E.W.; Vincelli, P.; Farman, M.L. Field Resistance to Strobilurin (QoI) Fungicides in Pyricularia grisea Caused by Mutations in the Mitochondrial Cytochrome b Gene. Phytopathology 2003, 93, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Castroagudín, V.L.; Ceresini, P.C.; de Oliveira, S.C.; Reges, J.T.A.; Maciel, J.L.N.; Bonato, A.L.V.; Dorigan, A.F.; McDonald, B.A. Resistance to QoI Fungicides Is Widespread in Brazilian Populations of the Wheat Blast Pathogen Magnaporthe oryzae. Phytopathology 2015, 105, 284–294. [Google Scholar] [CrossRef]
- Miyagawa, N.; Fuji, M. Occurrence of QoI-fungicide-resistant strains of Magnaporthe oryzae on rice and fungicidal effective. In Proceedings of the 23rd Symposium of PSJ Research Committee on Fungicide Resistance, Gifu, Japan, 2013; pp. 25–35. [Google Scholar]
- Tenni, D.; Sinetti, A.; Waldner, M.; Torriani, S.F.F.; Romani, M. First report of QoI resistance in Italian population of Pyricularia oryzae. J. Plant Dis. Prot. 2021, 128, 1705–1709. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Yoshimoto, Y.; Arimori, S.; Kiguchi, S.; Harada, T.; Iwahashi, F. Discovery of metyltetraprole: Identification of tetrazolinone pharmacophore to overcome QoI resistance. Bioorg. Med. Chem. 2020, 28, 115211. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Kiguchi, S.; Suemoto, H.; Iwahashi, F. Antifungal activity of metyltetraprole against the existing QoI-resistant isolates of various plant pathogenic fungi. Pest Manag. Sci. 2019, 76, 1743–1750. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Xu, J.; Sun, M.; Tian, H.; Zuo, D.; Guan, Q.; Bao, K.; Wu, Y.; Zhang, W. Synthesis and bioevaluation of 3,6-diaryl-[1,2,4]triazolo[4,3-b] pyridazines as antitubulin agents. ACS Med. Chem. Lett. 2016, 7, 1202–1206. Available online: https://pubs.acs.org/doi/10.1021/acsmedchemlett.6b00252 (accessed on 16 November 2022).
- Eberini, I.; Rocco, A.G.; Mantegazza, M.; Gianazza, E.; Baroni, A.; Vilardo, M.C.; Donghi, D.; Galliano, M.; Beringhelli, T. Computational and Experimental Approaches Assess the Interactions between Bovine β-Lactoglobulin and Synthetic Compounds of Pharmacological Interest. J. Mol. Graph Model 2008, 26, 1004–1013. [Google Scholar] [CrossRef]
- Cotterill, J.V.; Palazzolo, L.; Ridgway, C.; Price, N.; Rorije, E.; Moretto, A.; Peijnenburg, A.; Eberini, I. Predicting Estrogen Receptor Binding of Chemicals Using a Suite of in Silico Methods–Complementary Approaches of (Q)SAR, Molecular Docking and Molecular Dynamics. Toxicol. Appl. Pharmacol. 2019, 378, 114630. [Google Scholar] [CrossRef]
- Schultes, S.; de Graaf, C.; Haaksma, E.E.J.; de Esch, I.J.P.; Leurs, R.; Krämer, O. Ligand efficiency as a guide in fragment hit selection and optimization. Drug Discov. Today Technol. 2010, 7, e157–e162. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R.; Alex, A. Ligand efficiency: A useful metric for lead selection. Drug Discov. Today 2004, 9, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Kunova, A.; Pizzatti, C.; Bonaldi, M.; Cortesi, P. Sensitivity of Nonexposed and Exposed Populations of Magnaporthe oryzae from Rice to Tricyclazole and Azoxystrobin. Plant Dis. 2014, 98, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Spanu, F.; Scherm, B.; Balmas, V.; Hoffmann, L.; Hammond-Kosack, K.E.; Beyer, M.; Migheli, Q. FcStuA from Fusarium culmorum Controls Wheat Foot and Root Rot in a Toxin Dispensable Manner. PLoS ONE 2013, 8, e57429. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Beyer, M.; Bohn, T.; Hoffmann, L. Comparative Analysis of Genetic Chemotyping Methods for Fusarium: Tri13 Polymorphism Does not Discriminate between 3- and 15-acetylated Deoxynivalenol Chemotypes in Fusarium graminearum. J. Phytopathol. 2011, 159, 700–704. [Google Scholar] [CrossRef]
- Kunova, A.; Bonaldi, M.; Saracchi, M.; Pizzatti, C.; Chen, X.; Cortesi, P. Selection of Streptomyces against soil borne fungal pathogens by a standardized dual culture assay and evaluation of their effects on seed germination and plant growth. BMC Microbiol. 2016, 16, 272. [Google Scholar] [CrossRef]
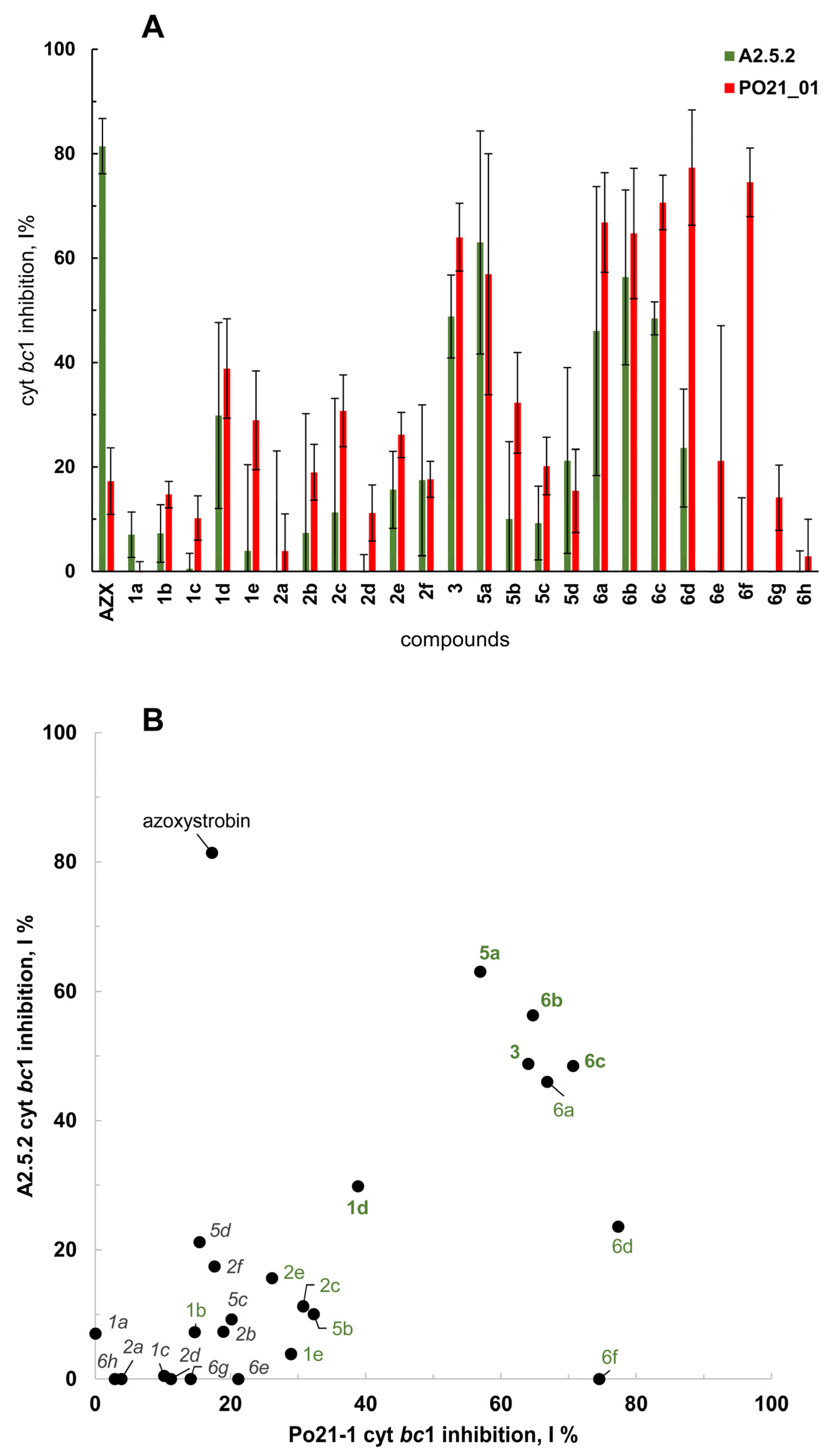
| Compound | Inhibitory Action a | |
|---|---|---|
| (200 µM) | (%; p-Value) | |
| WT | RES | |
| Azoxystrobin b | +(81.4 ± 5.3; 0.0014) | −(17.2 ± 6.4; 0.0425) |
| 1a | −(7.0 ± 4.3; 0.1073) | (−0.3 ± 2.2; 0.8192) |
| 1b | −(7.3 ± 5.5; 0.1495) | +(14.7 ± 2.5; 0.0097) |
| 1c | −(0.5 ± 2.9; 0.7980) | −(10.2 ± 4.3; 0.0536) |
| 1d | +(29.9 ± 17.8; 0.0093) | +(38.8 ± 9.5; <0.0010) |
| 1e | −(3.9 ± 16.5; 0.5891) | +(28.9 ± 9.4; <0.0010) |
| 2a | −(−2.1 ± 25.2; 0.8978) | −(3.8 ± 7.2; 0.4536) |
| 2b | −(7.3 ± 22.9; 0.6339) | −(18.9 ± 5.4; 0.0256) |
| 2c | −(11.3 ± 21.9; 0.4666) | +(30.7 ± 6.9; <0.0010) |
| 2d | −(−10.6 ± 13.7; 0.3136) | −(11.2 ± 5.4; 0.0694) |
| 2e | −(15.6 ± 7.4; 0.0673) | +(26.1 ± 4.3; 0.0090) |
| 2f | −(17.4 ± 14.5; 0.0319) | −(17.6 ± 3.5; 0.0127) |
| 3 | +(48.8 ± 7.9; <0.0010) | +(64.0 ± 6.5; <0.0010) |
| 5a | +(63.0 ± 21.4; 0.0027) | +(56.9 ± 23.1; 0.0018) |
| 5b | −(10.1 ± 14.8; 0.3591) | +(32.3 ± 9.6; <0.0010) |
| 5c | −(9.2 ± 7.1; 0.1512) | (20.2 ± 5.5; 0.0239) |
| 5d | −(21.2 ± 17.8; 0.0332) | −(15.4 ± 7.8; 0.0789) |
| 6a | −(46.0 ± 27.7; 0.0250) | +(66.8 ± 9.5; <0.0010) |
| 6b | +(56.3 ± 16.8; 0.0017) | +(64.7 ± 12.5; <0.0010) |
| 6c | +(48.5 ± 3.2; <0.0010) | +(70.6 ± 5.2; <0.0010) |
| 6d | −(23.6 ± 11.3; 0.0248) | +(77.3 ± 11.0; <0.0010) |
| 6e | −(−35.0 ± 29.5; 0.1763) | −(21.1 ± 25.9; 0.1019) |
| 6f | −(−10.0 ± 24.1; 0.5459) | +(74.5 ± 6.6; <0.0010) |
| 6g | −(−49.8 ± 44.1; 0.1898) | −(14.1 ± 6.3; 0.0599) |
| 6h | −(−2.9 ± 6.8; 0.5294) | −(2.9 ± 7.1; 0.5540) |
| Compound | Inhibitory Action a | |
|---|---|---|
| (200 µM) | (%; p-Value) | |
| WT | RES | |
| Azoxystrobin b | +(82.1 ± 9.1; <0.0010) | −(22.5 ± 17.3; 0.0440) |
| 3 | −(37.6 ± 22.6; 0.0204) | +(29.2 ± 13.3; <0.0080) |
| 5a | −(4.9 ± 23.3; 0.6601) | +(37.4 ± 11.5; 0.0019) |
| 6b | −(−4.4 ± 38.2; 0.8106) | +(44.4 ± 6.3; <0.0010) |
| 6c | −(45.9 ± 20.5; 0.0208) | +(54.1 ± 17.4; 0.0084) |
| Compound | Oral Bioavailability (%F) No First-Pass/First-Pass | Vd (L/kg) | Protein Binding | P450 Substrate | P450 Inhibition | |
|---|---|---|---|---|---|---|
| 1b | 98.9 * | 65.3 * | 0.51 | Human serum albumin | N.A. | N.A. |
| 3 | 37.3 | 33.2 | 2.1 | Human serum albumin | N.A. | N.A. |
| 5a | 33.1 | 17.9 | 5.6 | Majority of plasma proteins | N.A. | N.A. |
| 6b | 91.6 * | 80.6 * | 10 | -Lipoproteins -Albumin (less extent) | N.A. | Probable CYP1A2 efficient inhibitor (IC50 < 10 µM) |
| 6c | 42.3 | 36.4 | 5.5 | -Lipoproteins -Albumin (less extent) | N.A. | Probable CYP1A2 efficient inhibitor (IC50 < 10 µM) |
| Compound | BBB Penetration | Endocrine System Disruption | Eye/Skin Irritation | Aquatic Toxicity |
|---|---|---|---|---|
| 3 | Yes | No binding to ERα (LogRBA < −3) | N.A. | N.A. |
| 5a | Yes | Weak ERα binder (−3 < LogRBA < 0) | N.A. | Ciliate Protozoa IGC50 = 0.72 mg/L |
| 6b | Yes | Weak binding to ERα (−3 < LogRBA < 0) | N.A. | Ciliate Protozoa IGC50 = 1.1 mg/L |
| 6c | Yes | Weak ERα binder (−3 < LogRBA < 0) | N.A. | Water flea LC50 = 2.6 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, C.; Laurenzi, T.; Forlani, F.; Palazzolo, L.; Nolan, C.B.; Christodoulou, M.S.; Cortesi, P.; Pinto, A.; Eberini, I.; Kunova, A.; et al. Exploration of Novel Scaffolds Targeting Cytochrome b of Pyricularia oryzae. Int. J. Mol. Sci. 2023, 24, 2705. https://doi.org/10.3390/ijms24032705
Pinna C, Laurenzi T, Forlani F, Palazzolo L, Nolan CB, Christodoulou MS, Cortesi P, Pinto A, Eberini I, Kunova A, et al. Exploration of Novel Scaffolds Targeting Cytochrome b of Pyricularia oryzae. International Journal of Molecular Sciences. 2023; 24(3):2705. https://doi.org/10.3390/ijms24032705
Chicago/Turabian StylePinna, Cecilia, Tommaso Laurenzi, Fabio Forlani, Luca Palazzolo, Claire Beatrice Nolan, Michael S. Christodoulou, Paolo Cortesi, Andrea Pinto, Ivano Eberini, Andrea Kunova, and et al. 2023. "Exploration of Novel Scaffolds Targeting Cytochrome b of Pyricularia oryzae" International Journal of Molecular Sciences 24, no. 3: 2705. https://doi.org/10.3390/ijms24032705
APA StylePinna, C., Laurenzi, T., Forlani, F., Palazzolo, L., Nolan, C. B., Christodoulou, M. S., Cortesi, P., Pinto, A., Eberini, I., Kunova, A., & Dallavalle, S. (2023). Exploration of Novel Scaffolds Targeting Cytochrome b of Pyricularia oryzae. International Journal of Molecular Sciences, 24(3), 2705. https://doi.org/10.3390/ijms24032705









