Looking at Biomolecular Interactions through the Lens of Correlated Fluorescence Microscopy and Optical Tweezers
Abstract
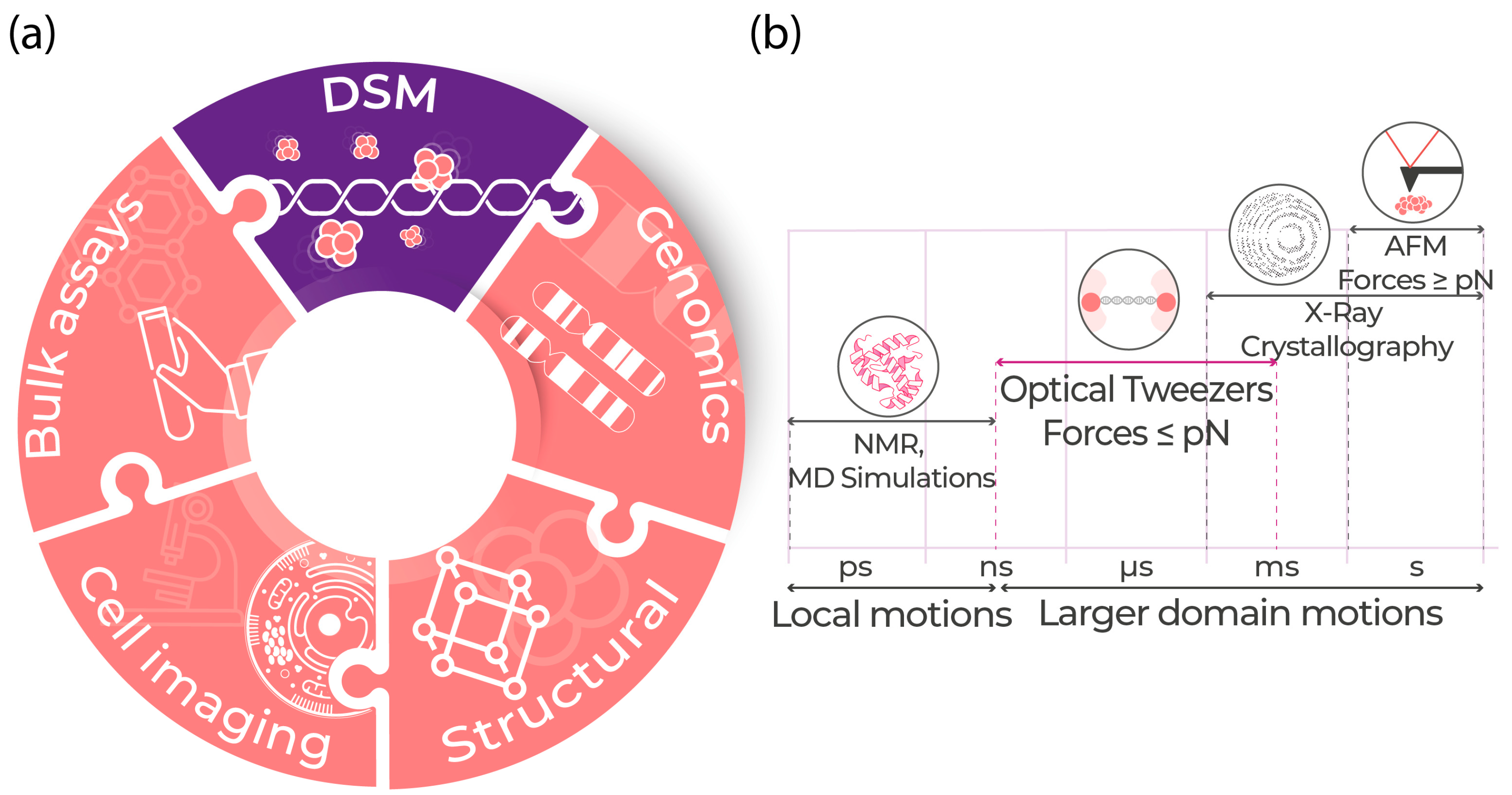
1. Introduction
2. Discussion
2.1. DNA-Protein Interactions
2.2. Gene Editing
2.3. Chromatin Organization
2.4. DNA Replication
2.5. DNA Repair

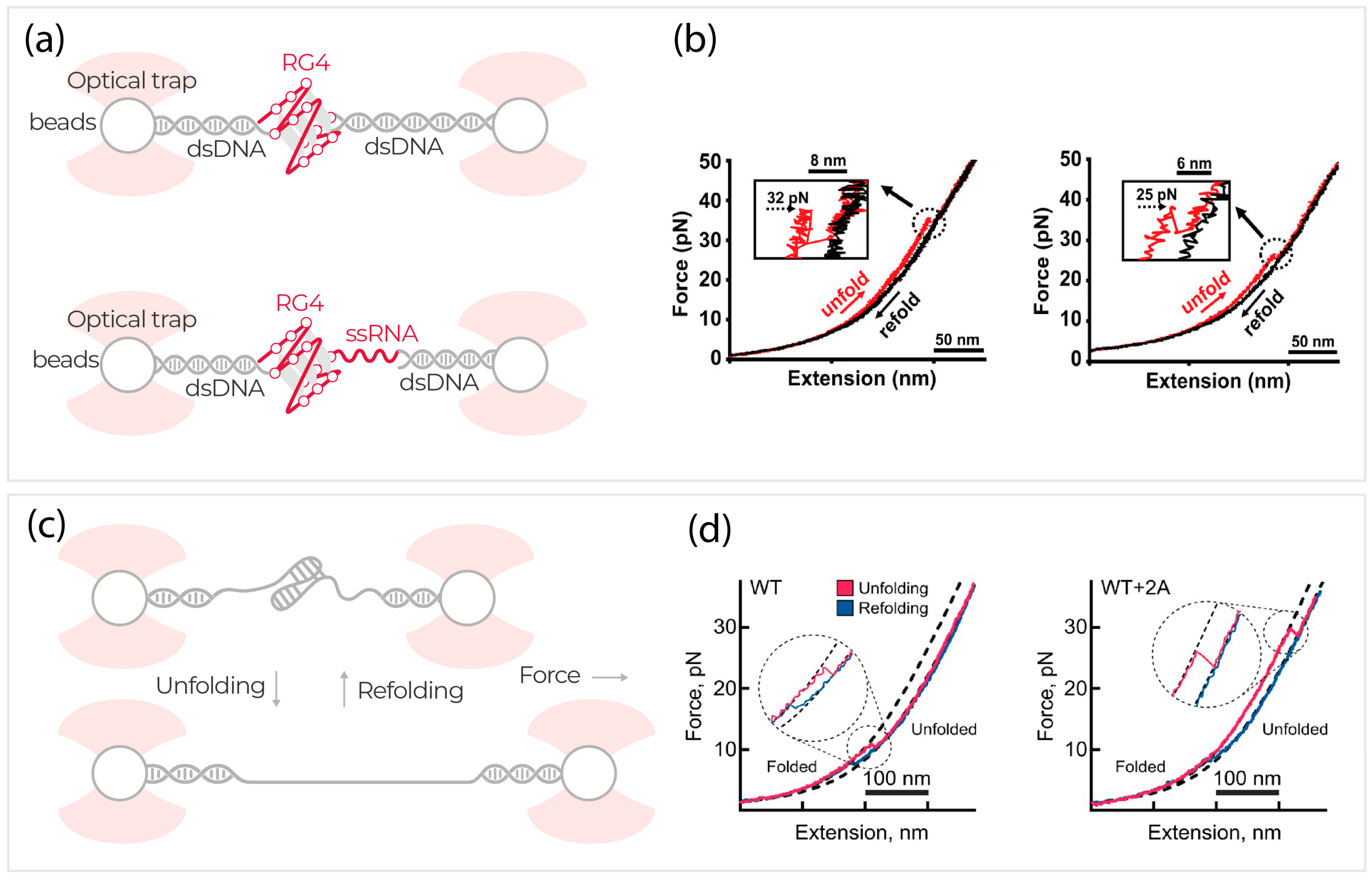
3. RNA Structure and Function
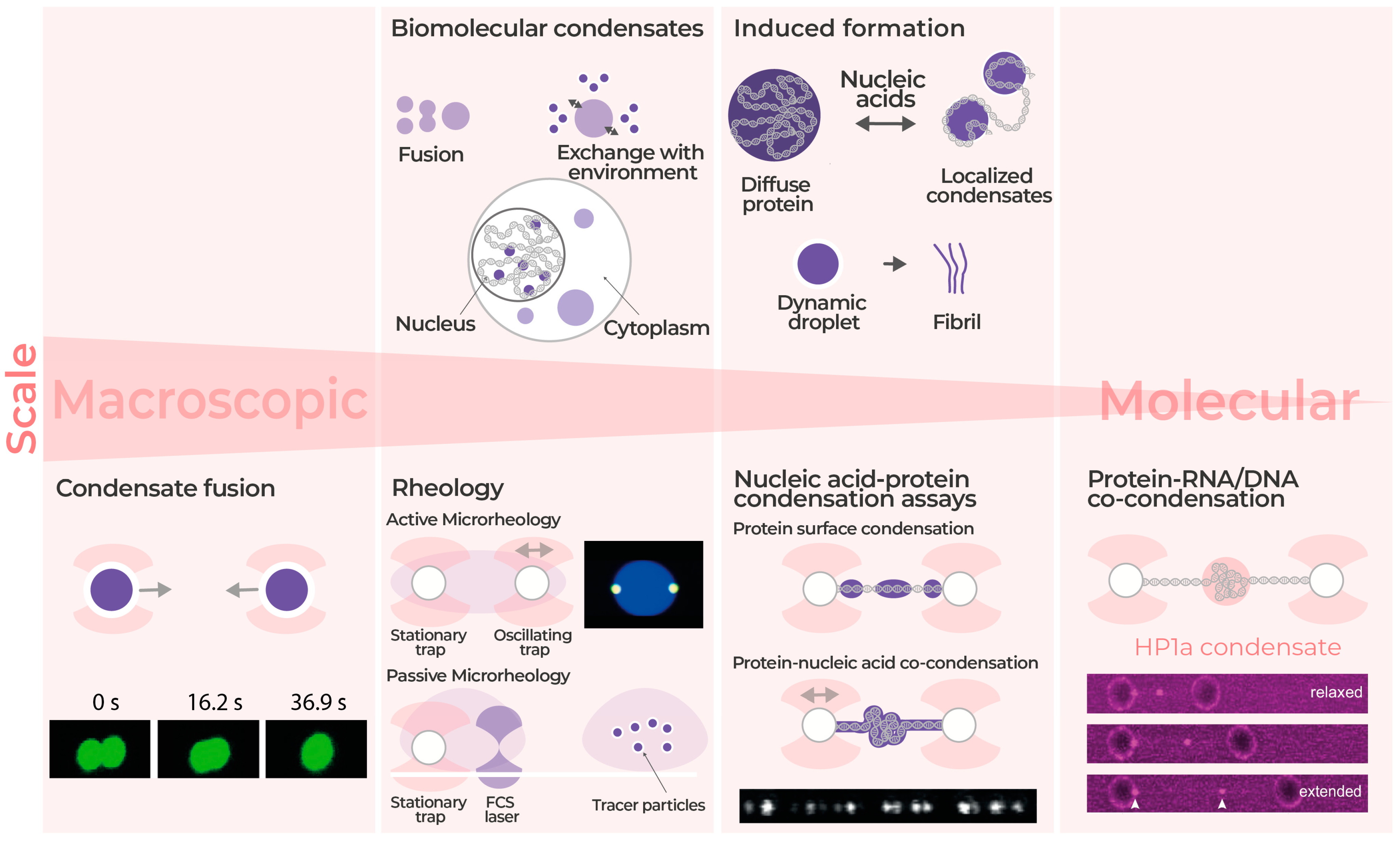
4. Biomolecular Condensates Micro-Rheology and Manipulation
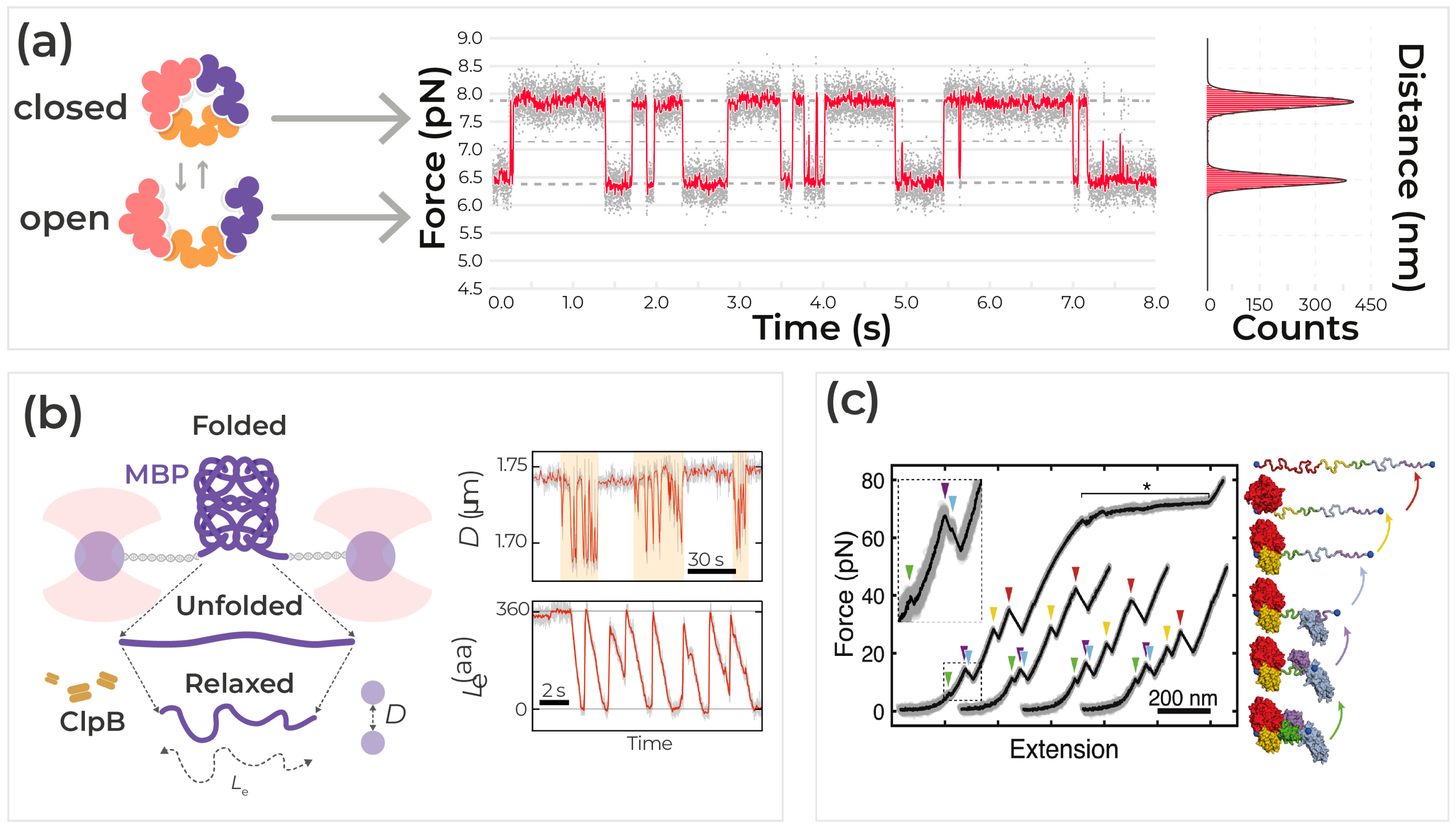
5. Protein Dynamics
6. Cellular Structure and Transport Processes
7. Future Directions
7.1. RNA Biology
7.2. Nanomaterials
7.3. Mechanobiology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, K.; Gaullier, G.; Luger, K. Nucleosome structure and dynamics are coming of age. Nat. Struct. Mol. Biol. 2019, 26, 3–13. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, K.; Zhang, N.; Wei, H.; Tan, Y.Z.; Zhang, Z.; Carragher, B.; Potter, C.S.; D’Arcy, S.; Luger, K. FACT caught in the act of manipulating the nucleosome. Nature 2020, 577, 426–431. [Google Scholar] [CrossRef]
- Pastore, A. New challenges in structural biology: Catching the complexity of dynamic nanomachines. Front. Mol. Biosci. 2014, 1, 3. [Google Scholar] [CrossRef]
- Dillard, R.S.; Hampton, C.M.; Strauss, J.D.; Ke, Z.; Altomara, D.; Guerrero-Ferreira, R.C.; Kiss, G.; Wright, E.R. Biological Applications at the Cutting Edge of Cryo-Electron Microscopy. Microsc. Microanal. 2018, 24, 406–419. [Google Scholar] [CrossRef]
- Lim, C.J.; Barbour, A.T.; Zaug, A.J.; Goodrich, K.J.; McKay, A.E.; Wuttke, D.S.; Cech, T.R. The structure of human CST reveals a decameric assembly bound to telomeric DNA. Science 2020, 368, 1081–1085. [Google Scholar] [CrossRef]
- Cai, S.W.; Zinder, J.C.; Svetlov, V.; Bush, M.W.; Nudler, E.; Walz, T.; de Lange, T. Cryo-EM structure of the human CST–Polα/primase complex in a recruitment state. Nat. Struct. Mol. Biol. 2022, 29, 813–819. [Google Scholar] [CrossRef]
- Palmer, A.G., III. NMR Characterization of the Dynamics of Biomacromolecules. Chem. Rev. 2004, 104, 3623–3640. [Google Scholar] [CrossRef]
- Ziarek, J.J.; Baptista, D.; Wagner, G. Recent developments in solution nuclear magnetic resonance (NMR)-based molecular biology. J. Mol. Med. 2018, 96, 1–8. [Google Scholar] [CrossRef]
- Walter, N.G.; Bustamante, C. Introduction to Single Molecule Imaging and Mechanics: Seeing and Touching Molecules One at a Time. Chem. Rev. 2014, 114, 3069–3071. [Google Scholar] [CrossRef]
- Leake, M.C. The physics of life: One molecule at a time. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120248. [Google Scholar] [CrossRef]
- Zlatanova, J.; van Holde, K. Single-Molecule Biology: What Is It and How Does It Work? Mol. Cell 2006, 24, 317–329. [Google Scholar] [CrossRef]
- Kapanidis, A.N.; Strick, T. Biology, one molecule at a time. Trends Biochem. Sci. 2009, 34, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.A.; Mukhopadhyay, S.; Lemke, E.A. Single-molecule biophysics: At the interface of biology, physics and chemistry. J. R. Soc. Interface 2008, 5, 15–45. [Google Scholar] [CrossRef]
- Sitters, G.; Kamsma, D.; Thalhammer, G.; Ritsch-Marte, M.; Peterman, E.J.G.; Wuite, G.J.L. Acoustic force spectroscopy. Nat. Methods 2015, 12, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Ritchie, K.; Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 1995, 68, 2580–2587. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- Ishijima, A.; Kojima, H.; Higuchi, H.; Harada, Y.; Funatsu, T.; Yanagida, T. Multiple- and single-molecule analysis of the actomyosin motor by nanometer-piconewton manipulation with a microneedle: Unitary steps and forces. Biophys. J. 1996, 70, 383–400. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Bumb, A.; Mills, M.; Neuman, K.C. SnapShot: Single-Molecule Fluorescence. Cell 2013, 153, 1408–1408.e1. [Google Scholar] [CrossRef]
- Jacobs, M.J.; Blank, K. Joining forces: Integrating the mechanical and optical single molecule toolkits. Chem. Sci. 2014, 5, 1680–1697. [Google Scholar] [CrossRef]
- Candelli, A.; Wuite, G.J.L.; Peterman, E.J.G. Combining optical trapping, fluorescence microscopy and micro-fluidics for single molecule studies of DNA–protein interactions. Phys. Chem. Chem. Phys. 2011, 13, 7263–7272. [Google Scholar] [CrossRef]
- Comstock, M.J.; Ha, T.; Chemla, Y.R. Ultrahigh-resolution optical trap with single-fluorophore sensitivity. Nat. Methods 2011, 8, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Whitley, K.D.; Comstock, M.J.; Chemla, Y.R. High-Resolution “Fleezers”: Dual-Trap Optical Tweezers Combined with Single-Molecule Fluorescence Detection. Opt. Tweezers Methods Protoc. 2017, 1486, 183–256. [Google Scholar] [CrossRef]
- Brau, R.R.; Tarsa, P.B.; Ferrer, J.M.; Lee, P.; Lang, M.J. Interlaced Optical Force-Fluorescence Measurements for Single Molecule Biophysics. Biophys. J. 2006, 91, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.J.; Fordyce, P.M.; Engh, A.M.; Neuman, K.C.; Block, S.M. Simultaneous, coincident optical trapping and single-molecule fluorescence. Nat. Methods 2004, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Farge, G.; Peterman, E.J.; Wuite, G.J. Combining Optical Tweezers, Single-Molecule Fluorescence Microscopy, and Microfluidics for Studies of DNA–Protein Interactions. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2010; Volume 475, pp. 427–453. [Google Scholar] [CrossRef]
- Hoffmann, A.; zu Hörste, G.M.; Pilarczyk, G.; Monajembashi, S.; Uhl, V.; Greulich, K. Optical tweezers for confocal microscopy. Appl. Phys. B Laser Opt. 2000, 71, 747–753. [Google Scholar] [CrossRef]
- Sirinakis, G.; Ren, Y.; Gao, Y.; Xi, Z.; Zhang, Y. Combined versatile high-resolution optical tweezers and single-molecule fluorescence microscopy. Rev. Sci. Instrum. 2012, 83, 093708. [Google Scholar] [CrossRef]
- Mehta, A.D.; Rief, M.; Spudich, J.A.; Smith, D.A.; Simmons, R.M. Single-Molecule Biomechanics with Optical Methods. Science 1999, 283, 1689–1695. [Google Scholar] [CrossRef]
- Smith, S.B.; Finzi, L.; Bustamante, C. Direct Mechanical Measurements of the Elasticity of Single DNA Molecules by Using Magnetic Beads. Science 1992, 258, 1122–1126. [Google Scholar] [CrossRef]
- Kim, S.; Blainey, P.; Schroeder, C.M.; Xie, X.S. Multiplexed single-molecule assay for enzymatic activity on flow-stretched DNA. Nat. Methods 2007, 4, 397–399. [Google Scholar] [CrossRef]
- Xiong, K.; Blainey, P.C. A Simple, Robust, and High Throughput Single Molecule Flow Stretching Assay Implementation for Studying Transport of Molecules Along DNA. J. Vis. Exp 2017, 128, e55923. [Google Scholar] [CrossRef]
- Bustamante, C.; Rivetti, C.; Keller, D.J. Scanning force microscopy under aqueous solutions. Curr. Opin. Struct. Biol. 1997, 7, 709–716. [Google Scholar] [CrossRef]
- Drake, B.; Prater, C.B.; Weisenhorn, A.L.; Gould, S.A.C.; Albrecht, T.R.; Quate, C.F.; Cannell, D.S.; Hansma, H.G.; Hansma, P.K. Imaging Crystals, Polymers, and Processes in Water with the Atomic Force Microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef]
- Cordova, J.C.; Das, D.K.; Manning, H.W.; Lang, M.J. Combining single-molecule manipulation and single-molecule detection. Curr. Opin. Struct. Biol. 2014, 28, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Veigel, C.; Schmidt, C. Moving into the cell: Single-molecule studies of molecular motors in complex environments. Nat. Rev. Mol. Cell Biol. 2011, 12, 163–176. [Google Scholar] [CrossRef]
- Maity, S.; Lyubchenko, Y.L. AFM Probing of Amyloid-Beta 42 Dimers and Trimers. Front. Mol. Biosci. 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Viazovkina, E.; Gall, A.; Lyubchenko, Y.L. Single-molecule probing of amyloid nano-ensembles using the polymer nanoarray approach. Phys. Chem. Chem. Phys. 2017, 19, 16387–16394. [Google Scholar] [CrossRef] [PubMed]
- Krasnoslobodtsev, A.V.; Zhang, Y.; Viazovkina, E.; Gall, A.; Bertagni, C.; Lyubchenko, Y.L. A Flexible Nanoarray Approach for the Assembly and Probing of Molecular Complexes. Biophys. J. 2015, 108, 2333–2339. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyubchenko, Y.L. The Structure of Misfolded Amyloidogenic Dimers: Computational Analysis of Force Spectroscopy Data. Biophys. J. 2014, 107, 2903–2910. [Google Scholar] [CrossRef]
- Maity, S.; Viazovkina, E.; Gall, A.; Lyubchenko, Y.L. Polymer Nanoarray Approach for the Characterization of Biomolecular Interactions. Nanoscale Imaging Methods Protoc. 2018, 1814, 63–74. [Google Scholar] [CrossRef]
- Ando, T.; Kodera, N.; Takai, E.; Maruyama, D.; Saito, K.; Toda, A. A high-speed atomic force microscope for studying biological macromolecules. Proc. Natl. Acad. Sci. USA 2001, 98, 12468–12472. [Google Scholar] [CrossRef]
- Pan, Y.; Shlyakhtenko, L.S.; Lyubchenko, Y.L. Insight into the dynamics of APOBEC3G protein in complexes with DNA assessed by high speed AFM. Nanoscale Adv. 2019, 1, 4016–4024. [Google Scholar] [CrossRef]
- Buzón, P.; Maity, S.; Christodoulis, P.; Wiertsema, M.J.; Dunkelbarger, S.; Kim, C.; Wuite, G.J.; Zlotnick, A.; Roos, W.H. Virus self-assembly proceeds through contact-rich energy minima. Sci. Adv. 2021, 7, eabg0811. [Google Scholar] [CrossRef]
- Banerjee, S.; Lyubchenko, Y.L. Topographically smooth and stable supported lipid bilayer for high-resolution AFM studies. Methods 2022, 197, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Plochberger, B.; Röhrl, C.; Preiner, J.; Rankl, C.; Brameshuber, M.; Madl, J.; Bittman, R.; Ros, R.; Sezgin, E.; Eggeling, C.; et al. HDL particles incorporate into lipid bilayers—A combined AFM and single molecule fluorescence microscopy study. Sci. Rep. 2017, 7, 15886. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Saleh, O.A. A high-resolution magnetic tweezer for single-molecule measurements. Nucleic Acids Res. 2009, 37, e136. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.E.; Lebel, P.; Oberstrass, F.C.; Starr, C.H.; Parente, A.C.; Ierokomos, A.; Bryant, Z. Multimodal Measurements of Single-Molecule Dynamics Using FluoRBT. Biophys. J. 2018, 114, 278–282. [Google Scholar] [CrossRef]
- Tanase, M.; Biais, N.; Sheetz, M. Magnetic Tweezers in Cell Biology. Methods Cell Biol. 2007, 83, 473–493. [Google Scholar] [CrossRef]
- Mosconi, F.; Allemand, J.F.; Bensimon, D.; Croquette, V. Measurement of the Torque on a Single Stretched and Twisted DNA Using Magnetic Tweezers. Phys. Rev. Lett. 2009, 102, 078301. [Google Scholar] [CrossRef]
- Kilinc, D.; Lee, G.U. Advances in magnetic tweezers for single molecule and cell biophysics. Integr. Biol. 2014, 6, 27–34. [Google Scholar] [CrossRef]
- Crut, A.; Koster, D.A.; Seidel, R.; Wiggins, C.H.; Dekker, N.H. Fast dynamics of supercoiled DNA revealed by single-molecule experiments. Proc. Natl. Acad. Sci. USA 2007, 104, 11957–11962. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Dekker, C. Recent Advances in Magnetic Tweezers. Annu. Rev. Biophys. 2012, 41, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Günther, K.; Mertig, M.; Seidel, R. Mechanical and structural properties of YOYO-1 complexed DNA. Nucleic Acids Res. 2010, 38, 6526–6532. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.J.; Chemla, Y.R.; Liu, S.; Wang, M.D. Optical tweezers in single-molecule biophysics. Nat. Rev. Methods Prim. 2021, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M. Optical Trapping and Manipulation of Viruses and Bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Block, S.M.; Blair, D.F.; Berg, H.C. Compliance of bacterial flagella measured with optical tweezers. Nature 1989, 338, 514–518. [Google Scholar] [CrossRef]
- Finer, J.T.; Simmons, R.M.; Spudich, J.A. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature 1994, 368, 113–119. [Google Scholar] [CrossRef]
- Smith, S.B.; Cui, Y.; Bustamante, C. Overstretching B-DNA: The Elastic Response of Individual Double-Stranded and Single-Stranded DNA Molecules. Science 1996, 271, 795–799. [Google Scholar] [CrossRef]
- Ishijima, A.; Kojima, H.; Funatsu, T.; Tokunaga, M.; Higuchi, H.; Tanaka, H.; Yanagida, T. Simultaneous Observation of Individual ATPase and Mechanical Events by a Single Myosin Molecule during Interaction with Actin. Cell 1998, 92, 161–171. [Google Scholar] [CrossRef]
- Hohng, S.; Zhou, R.; Nahas, M.K.; Yu, J.; Schulten, K.; Lilley, D.M.J.; Ha, T. Fluorescence-Force Spectroscopy Maps Two-Dimensional Reaction Landscape of the Holliday Junction. Science 2007, 318, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fu, W.; Chi, H.; Mesias, V.S.D.; Zhu, H.; Leung, C.W.; Liu, W.; Huang, J. Optical tweezers-controlled hotspot for sensitive and reproducible surface-enhanced Raman spectroscopy characterization of native protein structures. Nat. Commun. 2021, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, F.; Käll, M. On the importance of optical forces in surface-enhanced Raman scattering (SERS). Faraday Discuss. 2006, 132, 35–44. [Google Scholar] [CrossRef]
- Shabestari, M.H.; Meijering, A.; Roos, W.; Wuite, G.; Peterman, E. Recent Advances in Biological Single-Molecule Applications of Optical Tweezers and Fluorescence Microscopy. Methods Enzymol. 2017, 582, 85–119. [Google Scholar] [CrossRef]
- LUMICKS: C-Trap® Optical Tweezers—Fluorescence & Label-free Microscopy. Available online: https://lumicks.com/products/c-trap-optical-tweezers-fluorescence-label-free-microscopy/ (accessed on 23 January 2023).
- Heller, I.; Sitters, G.; Broekmans, O.D.; Farge, G.; Menges, C.; Wende, W.; Hell, S.W.; Peterman, E.; Wuite, G.J.L. STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA. Nat. Methods 2013, 10, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M.R.; Liu, S. A Tour de Force on the Double Helix: Exploiting DNA Mechanics To Study DNA-Based Molecular Machines. Biochemistry 2019, 58, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Candelli, A.; Hoekstra, T.P.; Farge, G.; Gross, P.; Peterman, E.J.G.; Wuite, G.J.L. A toolbox for generating single-stranded DNA in optical tweezers experiments. Biopolymers 2013, 99, 611–620. [Google Scholar] [CrossRef]
- Zimmer, M.M.; Kibe, A.; Rand, U.; Pekarek, L.; Ye, L.; Buck, S.; Smyth, R.P.; Cicin-Sain, L.; Caliskan, N. The short isoform of the host antiviral protein ZAP acts as an inhibitor of SARS-CoV-2 programmed ribosomal frameshifting. Nat. Commun. 2021, 12, 7193. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Z.; Zhang, X.; Li, F.; Guo, L.; Hou, X.-M.; Wu, W.-Q.; Wang, J.; Liu, C.; Zheng, K.; et al. Proximal Single-Stranded RNA Destabilizes Human Telomerase RNA G-Quadruplex and Induces Its Distinct Conformers. J. Phys. Chem. Lett. 2021, 12, 3361–3366. [Google Scholar] [CrossRef]
- Maciuba, K.; Zhang, F.; Kaiser, C.M. Facile tethering of stable and unstable proteins for optical tweezers experiments. Biophys. J. 2021, 120, 2691–2700. [Google Scholar] [CrossRef]
- Naqvi, M.M.; Avellaneda, M.J.; Roth, A.; Koers, E.J.; Roland, A.; Sunderlikova, V.; Kramer, G.; Rye, H.S.; Tans, S.J. Protein chain collapse modulation and folding stimulation by GroEL-ES. Sci. Adv. 2022, 8, eabl6293. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Alshareedah, I.; Wang, W.; Ngo, J.; Moosa, M.; Banerjee, P. Molecular Crowding Tunes Material States of Ribonucleoprotein Condensates. Biomolecules 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Alshareedah, I.; Kaur, T.; Banerjee, P.R. Methods for characterizing the material properties of biomolecular condensates. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 646, pp. 143–183. [Google Scholar] [CrossRef]
- Block, J.; Witt, H.; Candelli, A.; Danes, J.C.; Peterman, E.J.G.; Wuite, G.J.L.; Janshoff, A.; Köster, S. Viscoelastic properties of vimentin originate from nonequilibrium conformational changes. Sci. Adv. 2018, 4, eaat1161. [Google Scholar] [CrossRef]
- Budaitis, B.G.; Jariwala, S.; Rao, L.; Yue, Y.; Sept, D.; Verhey, K.J.; Gennerich, A. Pathogenic mutations in the kinesin-3 motor KIF1A diminish force generation and movement through allosteric mechanisms. J. Cell Biol. 2021, 220, e202004227. [Google Scholar] [CrossRef]
- Sheikhhassani, V.; Evers, T.M.J.; Lamba, S.; Shokri, F.; Mashaghi, A. Single cell force spectroscopy of erythrocytes at physiological and febrile temperatures reveals mechano-modulatory effects of atorvastatin. Soft Matter 2022, 18, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Evers, T.M.J.; Sheikhhassani, V.; Tang, H.; Haks, M.C.; Ottenhoff, T.H.M.; Mashaghi, A. Single-Cell Mechanical Characterization of Human Macrophages. Adv. NanoBiomed Res. 2022, 2, 2100133. [Google Scholar] [CrossRef]
- Leonard, A.C.; Méchali, M. DNA Replication Origins. Cold Spring Harb. Perspect. Biol. 2013, 5, a010116. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M.R.; Schauer, G.D.; O’Donnell, M.E.; Liu, S. Replication Fork Activation Is Enabled by a Single-Stranded DNA Gate in CMG Helicase. Cell 2019, 178, 600–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bi, L.; Hou, X.-M.; Zhang, S.; Zhang, X.; Lu, Y.; Li, M.; Modesti, M.; Xi, X.-G.; Sun, B. Human RPA activates BLM’s bidirectional DNA unwinding from a nick. Elife 2020, 9, e54098. [Google Scholar] [CrossRef]
- Synakewicz, M.; Bauer, D.; Rief, M.; Itzhaki, L.S. Bioorthogonal protein-DNA conjugation methods for force spectroscopy. Sci. Rep. 2019, 9, 13820. [Google Scholar] [CrossRef]
- Li, H.; O’Donnell, M.E. The Eukaryotic CMG Helicase at the Replication Fork: Emerging Architecture Reveals an Unexpected Mechanism. Bioessays 2018, 40, 1700208. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef]
- Rill, N.; Mukhortava, A.; Lorenz, S.; Tessmer, I. Alkyltransferase-like protein clusters scan DNA rapidly over long distances and recruit NER to alkyl-DNA lesions. Proc. Natl. Acad. Sci. USA 2020, 117, 9318–9328. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Buechelmaier, E.; Belan, O.; Newton, M.; Vancevska, A.; Kaczmarczyk, A.; Takaki, T.; Rueda, D.S.; Powell, S.N.; Boulton, S.J. HELQ is a dual-function DSB repair enzyme modulated by RPA and RAD51. Nature 2022, 601, 268–273. [Google Scholar] [CrossRef]
- Kono, S.; Berg, A.V.D.; Simonetta, M.; Mukhortava, A.; Garman, E.F.; Tessmer, I. Resolving the subtle details of human DNA alkyltransferase lesion search and repair mechanism by single-molecule studies. Proc. Natl. Acad. Sci. USA 2022, 119, e2116218119. [Google Scholar] [CrossRef]
- Belan, O.; Barroso, C.; Kaczmarczyk, A.; Anand, R.; Federico, S.; O’Reilly, N.; Newton, M.D.; Maeots, E.; Enchev, R.I.; Martinez-Perez, E.; et al. Single-molecule analysis reveals cooperative stimulation of Rad51 filament nucleation and growth by mediator proteins. Mol. Cell 2021, 81, 1058–1073.e7. [Google Scholar] [CrossRef]
- Newton, M.D.; Fairbanks, S.D.; Thomas, J.A.; Rueda, D.S. A Minimal Load-and-Lock Ru II Luminescent DNA Probe. Angew. Chem. Int. Ed. 2021, 60, 20952–20959. [Google Scholar] [CrossRef]
- Gutierrez-Escribano, P.; Newton, M.D.; Llauró, A.; Huber, J.; Tanasie, L.; Davy, J.; Aly, I.; Aramayo, R.; Montoya, A.; Kramer, H.; et al. A conserved ATP- and Scc2/4-dependent activity for cohesin in tethering DNA molecules. Sci. Adv. 2019, 5, eaay6804. [Google Scholar] [CrossRef]
- Kemmerich, F.E.; Daldrop, P.; Pinto, C.; Levikova, M.; Cejka, P.; Seidel, R. Force regulated dynamics of RPA on a DNA fork. Nucleic Acids Res. 2016, 44, 5837–5848. [Google Scholar] [CrossRef]
- Bianco, P.R. The Biochemical Mechanism of Fork Regression in Prokaryotes and Eukaryotes—A Single Molecule Comparison. Int. J. Mol. Sci. 2022, 23, 8613. [Google Scholar] [CrossRef] [PubMed]
- Manosas, M.; Perumal, S.K.; Croquette, V.; Benkovic, S.J. Direct Observation of Stalled Fork Restart via Fork Regression in the T4 Replication System. Science 2012, 338, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.; Ye, L.F.; Gergoudis, S.C.; Kwon, Y.; Niu, H.; Sung, P.; Greene, E.C. Concentration-Dependent Exchange of Replication Protein A on Single-Stranded DNA Revealed by Single-Molecule Imaging. PLoS ONE 2014, 9, e87922. [Google Scholar] [CrossRef]
- Newton, M.D.; Taylor, B.J.; Driessen, R.P.C.; Roos, L.; Cvetesic, N.; Allyjaun, S.; Lenhard, B.; Cuomo, M.E.; Rueda, D.S. DNA stretching induces Cas9 off-target activity. Nat. Struct. Mol. Biol. 2019, 26, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Schaich, M.A.; Schnable, B.L.; Kumar, N.; Roginskaya, V.; Jakielski, R.C.; Urban, R.; Zhong, Z.; Kad, N.M.; Van Houten, B. Single-Molecule Analysis of DNA-binding proteins from Nuclear Extracts (SMADNE). bioRxiv 2022. [Google Scholar] [CrossRef]
- Scull, C.E.; Dandpat, S.S.; Romero, R.A.; Walter, N.G. Transcriptional Riboswitches Integrate Timescales for Bacterial Gene Expression Control. Front. Mol. Biosci. 2021, 7, 607158. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.B.; Woodside, M.T. Probing the structural dynamics of proteins and nucleic acids with optical tweezers. Curr. Opin. Struct. Biol. 2015, 34, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.M.; Lyons, S.M.; Ivanov, P. RNA G-Quadruplexes in Biology: Principles and Molecular Mechanisms. J. Mol. Biol. 2017, 429, 2127–2147. [Google Scholar] [CrossRef]
- Li, H.; Zhang, K.; Binzel, D.W.; Shlyakhtenko, L.S.; Lyubchenko, Y.L.; Chiu, W.; Guo, P. RNA nanotechnology to build a dodecahedral genome of single-stranded RNA virus. RNA Biol. 2021, 18, 2390–2400. [Google Scholar] [CrossRef]
- Guo, P.; Haque, F. (Eds.) RNA Nanotechnology and Therapeutics; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Ding, J. High-Resolution Atomic Force Microscopy Imaging of RNA Molecules in Solution. In RNA Structure and Dynamics; Springer: Berlin/Heidelberg, Germany, 2022; pp. 133–145. [Google Scholar] [CrossRef]
- Chauvier, A.; Cabello-Villegas, J.; Walter, N.G. Probing RNA structure and interaction dynamics at the single molecule level. Methods 2019, 162–163, 3–11. [Google Scholar] [CrossRef]
- Zhuang, X. Single-Molecule RNA Science. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Kobitski, A.Y.; Nienhaus, G.U. Single-molecule Förster resonance energy transfer studies of RNA structure, dynamics and function. Biophys. Rev. 2009, 1, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, F.; Ermann, N.; Lipfert, J. Probing the mechanical properties, conformational changes, and interactions of nucleic acids with magnetic tweezers. J. Struct. Biol. 2017, 197, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bandarkar, P.; Yang, H.; Henley, R.; Wanunu, M.; Whitford, P.C. How Nanopore Translocation Experiments Can Measure RNA Unfolding. Biophys. J. 2020, 118, 1612–1620. [Google Scholar] [CrossRef]
- Bizuayehu, T.T.; Labun, K.; Jakubec, M.; Jefimov, K.; Niazi, A.M.; Valen, E. Long-read single-molecule RNA structure sequencing using nanopore. Nucleic Acids Res. 2022, 50, e120. [Google Scholar] [CrossRef]
- Kharel, P.; Becker, G.; Tsvetkov, V.; Ivanov, P. Properties and biological impact of RNA G-quadruplexes: From order to turmoil and back. Nucleic Acids Res. 2020, 48, 12534–12555. [Google Scholar] [CrossRef]
- Brierley, I.; Pennell, S.; Gilbert, R.J.C. Viral RNA pseudoknots: Versatile motifs in gene expression and replication. Nat. Rev. Genet. 2007, 5, 598–610. [Google Scholar] [CrossRef]
- Hill, C.H.; Pekarek, L.; Napthine, S.; Kibe, A.; Firth, A.E.; Graham, S.C.; Caliskan, N.; Brierley, I. Structural and molecular basis for Cardiovirus 2A protein as a viral gene expression switch. Nat. Commun. 2021, 12, 7166. [Google Scholar] [CrossRef]
- Jawerth, L.M.; Ijavi, M.; Ruer, M.; Saha, S.; Jahnel, M.; Hyman, A.A.; Jülicher, F.; Fischer-Friedrich, E. Salt-Dependent Rheology and Surface Tension of Protein Condensates Using Optical Traps. Phys. Rev. Lett. 2018, 121, 258101. [Google Scholar] [CrossRef]
- Lyon, A.S.; Peeples, W.B.; Rosen, M.K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 2021, 22, 215–235. [Google Scholar] [CrossRef]
- Sharp, P.A.; Chakraborty, A.K.; Henninger, J.E.; Young, R.A. RNA in formation and regulation of transcriptional condensates. Rna 2022, 28, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kroschwald, S.; Munder, M.C.; Maharana, S.; Franzmann, T.M.; Richter, D.; Ruer, M.; Hyman, A.A.; Alberti, S. Different Material States of Pub1 Condensates Define Distinct Modes of Stress Adaptation and Recovery. Cell Rep. 2018, 23, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Zhou, H. Determinants for Fusion Speed of Biomolecular Droplets. Angew. Chem. Int. Ed. 2020, 59, 20837–20840. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.T.; Kershner, A.M.; Bingman, C.A.; Wickens, M.; Kimble, J. PGL germ granule assembly protein is a base-specific, single-stranded RNase. Proc. Natl. Acad. Sci. USA 2016, 113, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Garaizar, A.; Espinosa, J.R.; Joseph, J.A.; Collepardo-Guevara, R. Kinetic interplay between droplet maturation and coalescence modulates shape of aged protein condensates. Sci. Rep. 2022, 12, 4390. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.M.; Kim, K.; Fritsch, A.W.; Iglesias-Artola, J.M.; Jawerth, L.M.; Wang, J.; Ruer, M.; Peychl, J.; Poznyakovskiy, A.; Guck, J.; et al. Quantitative phase microscopy enables precise and efficient determination of biomolecular condensate composition. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jawerth, L.; Fischer-Friedrich, E.; Saha, S.; Wang, J.; Franzmann, T.; Zhang, X.; Sachweh, J.; Ruer, M.; Ijavi, M.; Saha, S.; et al. Protein condensates as aging Maxwell fluids. Science 2020, 370, 1317–1323. [Google Scholar] [CrossRef]
- Dominak, L.M.; Keating, C.D. Macromolecular Crowding Improves Polymer Encapsulation within Giant Lipid Vesicles. Langmuir 2008, 24, 13565–13571. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, X.; Zhou, H.-X. Tug of War between Condensate Phases in a Minimal Macromolecular System. J. Am. Chem. Soc. 2020, 142, 8848–8861. [Google Scholar] [CrossRef]
- Ogilvie, G.; Putu, D.; Shoveller, J.; Montaner, J.; Feng, C.; Nicoletti, R.; Shannon, K. HHS Public Access. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Alshareedah, I.; Moosa, M.M.; Raju, M.; Potoyan, D.A.; Banerjee, P.R. Phase transition of RNA−protein complexes into ordered hollow condensates. Proc. Natl. Acad. Sci. USA 2020, 117, 15650–15658. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Kota, D.; Zhou, H.-X. Shear relaxation governs fusion dynamics of biomolecular condensates. Nat. Commun. 2021, 12, 5995. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.C.; Valentine, M.T.; Weeks, E.R.; Gisler, T.; Kaplan, P.D.; Yodh, A.G.; Weitz, D.A. Two-Point Microrheology of Inhomogeneous Soft Materials. Phys. Rev. Lett. 2000, 85, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Head, D.; MacKintosh, F.; Schmidt, C. Active and Passive Microrheology in Equilibrium and Nonequilibrium Systems. Macromolecules 2008, 41, 7194–7202. [Google Scholar] [CrossRef]
- Alshareedah, I.; Moosa, M.M.; Pham, M.; Potoyan, D.A.; Banerjee, P.R. Programmable viscoelasticity in protein-RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 2021, 12, 6620. [Google Scholar] [CrossRef]
- Quail, T.; Golfier, S.; Elsner, M.; Ishihara, K.; Murugesan, V.; Renger, R.; Jülicher, F.; Brugués, J. Force generation by protein–DNA co-condensation. Nat. Phys. 2021, 17, 1007–1012. [Google Scholar] [CrossRef]
- Keenen, M.M.; Brown, D.; Brennan, L.D.; Renger, R.; Khoo, H.; Carlson, C.R.; Huang, B.; Grill, S.W.; Narlikar, G.J.; Redding, S. HP1 proteins compact DNA into mechanically and positionally stable phase separated domains. Elife 2021, 10, e64563. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.A.; Wittmann, S.; Choubey, S.; Klosin, A.; Golfier, S.; Hyman, A.A.; Jülicher, F.; Grill, S.W. Sequence-dependent surface condensation of a pioneer transcription factor on DNA. Nat. Phys. 2022, 18, 271–276. [Google Scholar] [CrossRef]
- Shrinivas, K.; Sabari, B.R.; Coffey, E.L.; Klein, I.A.; Boija, A.; Zamudio, A.V.; Schuijers, J.; Hannett, N.M.; Sharp, P.A.; Young, R.A.; et al. Enhancer Features that Drive Formation of Transcriptional Condensates. Mol. Cell 2019, 75, 549–561.e7. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S.; Benkovic, S.J. Relating Protein Motion to Catalysis. Annu. Rev. Biochem. 2006, 75, 519–541. [Google Scholar] [CrossRef]
- Campbell, E.; Kaltenbach, M.; Correy, G.J.; Carr, P.D.; Porebski, B.T.; Livingstone, E.K.; Afriat-Jurnou, L.; Buckle, A.M.; Weik, M.; Hollfelder, F.; et al. The role of protein dynamics in the evolution of new enzyme function. Nat. Chem. Biol. 2016, 12, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Henzler-Wildman, K.; Kern, D. Dynamic personalities of proteins. Nature 2007, 450, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Rief, M. Exploring the energy landscape of GFP by single-molecule mechanical experiments. Proc. Natl. Acad. Sci. USA 2004, 101, 16192–16197. [Google Scholar] [CrossRef]
- Mickler, M.; Dima, R.I.; Dietz, H.; Hyeon, C.; Thirumalai, D.; Rief, M. Revealing the bifurcation in the unfolding pathways of GFP by using single-molecule experiments and simulations. Proc. Natl. Acad. Sci. USA 2007, 104, 20268–20273. [Google Scholar] [CrossRef]
- Ganim, Z.; Rief, M. Mechanically switching single-molecule fluorescence of GFP by unfolding and refolding. Proc. Natl. Acad. Sci. USA 2017, 114, 11052–11056. [Google Scholar] [CrossRef] [PubMed]
- Avellaneda, M.J.; Franke, K.B.; Sunderlikova, V.; Bukau, B.; Mogk, A.; Tans, S.J. Processive extrusion of polypeptide loops by a Hsp100 disaggregase. Nature 2020, 578, 317–320. [Google Scholar] [CrossRef]
- Moessmer, P.; Suren, T.; Majdic, U.; Dahiya, V.; Rutz, D.; Buchner, J.; Rief, M. Active unfolding of the glucocorticoid receptor by the Hsp70/Hsp40 chaperone system in single-molecule mechanical experiments. Proc. Natl. Acad. Sci. USA 2022, 119, e2119076119. [Google Scholar] [CrossRef] [PubMed]
- Suren, T.; Rutz, D.; Mößmer, P.; Merkel, U.; Buchner, J.; Rief, M. Single-molecule force spectroscopy reveals folding steps associated with hormone binding and activation of the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 2018, 115, 11688–11693. [Google Scholar] [CrossRef]
- Mandal, S.S.; Merz, D.R.; Buchsteiner, M.; Dima, R.I.; Rief, M.; Žoldák, G. Nanomechanics of the substrate binding domain of Hsp70 determine its allosteric ATP-induced conformational change. Proc. Natl. Acad. Sci. USA 2017, 114, 6040–6045. [Google Scholar] [CrossRef]
- Wruck, F.; Tian, P.; Kudva, R.; Best, R.B.; von Heijne, G.; Tans, S.J.; Katranidis, A. The ribosome modulates folding inside the ribosomal exit tunnel. Commun. Biol. 2021, 4, 523. [Google Scholar] [CrossRef]
- Avellaneda, M.J.; Koers, E.J.; Naqvi, M.M.; Tans, S.J. The chaperone toolbox at the single-molecule level: From clamping to confining. Protein Sci. 2017, 26, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Cortese, M.; Romero, P.; Iakoucheva, L.; Uversky, V.N. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 2005, 272, 5129–5148. [Google Scholar] [CrossRef] [PubMed]
- Avellaneda, M.J.; Koers, E.J.; Minde, D.P.; Sunderlikova, V.; Tans, S.J. Simultaneous sensing and imaging of individual biomolecular complexes enabled by modular DNA–protein coupling. Commun. Chem. 2020, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Diez, S.; Reuther, C.; Dinu, C.; Seidel, R.; Mertig, M.; Pompe, W.; Howard, J. Stretching and Transporting DNA Molecules Using Motor Proteins. Nano Lett. 2003, 3, 1251–1254. [Google Scholar] [CrossRef]
- Sternberg, S.H.; LaFrance, B.; Kaplan, M.; Doudna, J.A. Conformational control of DNA target cleavage by CRISPR–Cas9. Nature 2015, 527, 110–113. [Google Scholar] [CrossRef]
- Lambrughi, M.; De Gioia, L.; Gervasio, F.L.; Lindorff-Larsen, K.; Nussinov, R.; Urani, C.; Bruschi, M.; Papaleo, E. DNA-binding protects p53 from interactions with cofactors involved in transcription-independent functions. Nucleic Acids Res. 2016, 44, 9096–9109. [Google Scholar] [CrossRef]
- Presman, D.M.; Ganguly, S.; Schiltz, R.L.; Johnson, T.A.; Karpova, T.; Hager, G.L. DNA binding triggers tetramerization of the glucocorticoid receptor in live cells. Proc. Natl. Acad. Sci. USA 2016, 113, 8236–8241. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef]
- Schliwa, M.; Woehlke, G. Molecular motors. Nature 2003, 422, 759–765. [Google Scholar] [CrossRef]
- Schnitzer, M.J.; Block, S.M. Kinesin hydrolyses one ATP per 8-nm step. Nature 1997, 388, 386–390. [Google Scholar] [CrossRef]
- Schnitzer, M.J.; Visscher, K.; Block, S.M. Force production by single kinesin motors. Nature 2000, 2, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.J.; Rao, L.; Anazawa, Y.; Okada, K.; Chiba, K.; Dacy, M.; Niwa, S.; Gennerich, A.; Nowakowski, D.W.; McKenney, R.J. A highly conserved 3 10 helix within the kinesin motor domain is critical for kinesin function and human health. Sci. Adv. 2021, 7, eabf1002. [Google Scholar] [CrossRef] [PubMed]
- Block, J.; Witt, H.; Candelli, A.; Peterman, E.J.G.; Wuite, G.J.L.; Janshoff, A.; Köster, S. Nonlinear Loading-Rate-Dependent Force Response of Individual Vimentin Intermediate Filaments to Applied Strain. Phys. Rev. Lett. 2017, 118, 048101. [Google Scholar] [CrossRef] [PubMed]
- Schaedel, L.; Lorenz, C.; Schepers, A.V.; Klumpp, S.; Köster, S. Vimentin intermediate filaments stabilize dynamic microtubules by direct interactions. Nat. Commun. 2021, 12, 3799. [Google Scholar] [CrossRef]
- Schepers, A.V.; Lorenz, C.; Köster, S. Tuning intermediate filament mechanics by variation of pH and ion charges. Nanoscale 2020, 12, 15236–15245. [Google Scholar] [CrossRef]
- Lorenz, C.; Forsting, J.; Schepers, A.V.; Kraxner, J.; Bauch, S.; Witt, H.; Klumpp, S.; Köster, S. Lateral Subunit Coupling Determines Intermediate Filament Mechanics. Phys. Rev. Lett. 2019, 123, 188102. [Google Scholar] [CrossRef]
- Sorkin, R.; Marchetti, M.; Logtenberg, E.; Piontek, M.C.; Kerklingh, E.; Brand, G.; Voleti, R.; Rizo, J.; Roos, W.H.; Groffen, A.J.; et al. Synaptotagmin-1 and Doc2b Exhibit Distinct Membrane-Remodeling Mechanisms. Biophys. J. 2020, 118, 643–656. [Google Scholar] [CrossRef]
- Siahaan, V.; Tan, R.; Humhalova, T.; Libusova, L.; Lacey, S.E.; Tan, T.; Dacy, M.; Ori-McKenney, K.M.; McKenney, R.J.; Braun, M.; et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat. Chem. Biol. 2022, 18, 1224–1235. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Elston, T.; Mogilner, A.; Oster, G. Force Generation in RNA Polymerase. Biophys. J. 1998, 74, 1186–1202. [Google Scholar] [CrossRef]
- Wang, M.D.; Schnitzer, M.J.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Force and Velocity Measured for Single Molecules of RNA Polymerase. Science 1998, 282, 902–907. [Google Scholar] [CrossRef]
- Yin, H.; Wang, M.D.; Svoboda, K.; Landick, R.; Block, S.M.; Gelles, J. Transcription Against an Applied Force. Science 1995, 270, 1653–1657. [Google Scholar] [CrossRef]
- Liu, T.; Kaplan, A.; Alexander, L.; Yan, S.; Wen, J.-D.; Lancaster, L.; E Wickersham, C.; Fredrick, K.; Noller, H.; Tinoco, I., Jr.; et al. Direct measurement of the mechanical work during translocation by the ribosome. Elife 2014, 3, e03406. [Google Scholar] [CrossRef] [PubMed]
- Frieda, K.L.; Block, S.M. Direct Observation of Cotranscriptional Folding in an Adenine Riboswitch. Science 2012, 338, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Duesterberg, V.K.; Fischer-Hwang, I.T.; Perez, C.F.; Hogan, D.W.; Block, S.M. Observation of long-range tertiary interactions during ligand binding by the TPP riboswitch aptamer. Elife 2015, 4, e12362. [Google Scholar] [CrossRef]
- Uhm, H.; Kang, W.; Ha, K.S.; Kang, C.; Hohng, S. Single-molecule FRET studies on the cotranscriptional folding of a thiamine pyrophosphate riboswitch. Proc. Natl. Acad. Sci. USA 2018, 115, 331–336. [Google Scholar] [CrossRef] [PubMed]
- E Watters, K.; Strobel, E.J.; Yu, A.M.; Lis, J.T.; Lucks, J.B. Cotranscriptional folding of a riboswitch at nucleotide resolution. Nat. Struct. Mol. Biol. 2016, 23, 1124–1131. [Google Scholar] [CrossRef]
- Herbert, K.M.; Greenleaf, W.J.; Block, S.M. Single-Molecule Studies of RNA Polymerase: Motoring Along. Annu. Rev. Biochem. 2008, 77, 149–176. [Google Scholar] [CrossRef]
- Aitken, C.E.; Petrov, A.; Puglisi, J.D. Single Ribosome Dynamics and the Mechanism of Translation. Annu. Rev. Biophys. 2010, 39, 491–513. [Google Scholar] [CrossRef]
- Blanchard, S.C. Single-molecule observations of ribosome function. Curr. Opin. Struct. Biol. 2009, 19, 103–109. [Google Scholar] [CrossRef]
- Marshall, R.A.; Aitken, C.E.; Dorywalska, M.; Puglisi, J.D. Translation at the Single-Molecule Level. Annu. Rev. Biochem. 2008, 77, 177–203. [Google Scholar] [CrossRef]
- Bustamante, C.; Cheng, W.; Mejia, Y.X. Revisiting the Central Dogma One Molecule at a Time. Cell 2011, 144, 480–497. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Dandpat, S.S.; Chatterjee, S.; Walter, N.G. Precise tuning of bacterial translation initiation by non-equilibrium 5′-UTR unfolding observed in single mRNAs. Nucleic Acids Res. 2022, 50, 8818–8833. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.P.; Frank, F.; Lee, A.; Righini, M.; Lancaster, L.; Noller, H.F.; Tinoco, I.; Bustamante, C. Co-temporal Force and Fluorescence Measurements Reveal a Ribosomal Gear Shift Mechanism of Translation Regulation by Structured mRNAs. Mol. Cell 2019, 75, 1007–1019.e5. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chauvier, A.; Dandpat, S.S.; Artsimovitch, I.; Walter, N.G. A translational riboswitch coordinates nascent transcription–translation coupling. Proc. Natl. Acad. Sci. USA 2021, 118, e2023426118. [Google Scholar] [CrossRef] [PubMed]
- Marago, O.M.; Jones, P.; Gucciardi, P.G.; Volpe, G.; Ferrari, A.C. Optical trapping and manipulation of nanostructures. Nat. Nanotechnol. 2013, 8, 807–819. [Google Scholar] [CrossRef]
- Righini, M.; Zelenina, A.S.; Girard, C.; Quidant, R. Parallel and selective trapping in a patterned plasmonic landscape. Nat. Phys. 2007, 3, 477–480. [Google Scholar] [CrossRef]
- Agarwal, R.; Ladavac, K.; Roichman, Y.; Yu, G.; Lieber, C.M.; Grier, D. Manipulation and assembly of nanowires with holographic optical traps. Opt. Express 2005, 13, 8906–8912. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Z.; Liu, W.; Yan, Z. Optical trapping and manipulation for single-particle spectroscopy and microscopy. J. Chem. Phys. 2022, 157, 050901. [Google Scholar] [CrossRef]
- Shan, X.; Wang, F.; Wang, D.; Wen, S.; Chen, C.; Di, X.; Nie, P.; Liao, J.; Liu, Y.; Ding, L.; et al. Optical tweezers beyond refractive index mismatch using highly doped upconversion nanoparticles. Nat. Nanotechnol. 2021, 16, 531–537. [Google Scholar] [CrossRef]
- Prass, M.; Jacobson, K.; Mogilner, A.; Radmacher, M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J. Cell Biol. 2006, 174, 767–772. [Google Scholar] [CrossRef]
- Evers, T.M.; Sheikhhassani, V.; Haks, M.C.; Storm, C.; Ottenhoff, T.H.; Mashaghi, A. Single-cell analysis reveals chemokine-mediated differential regulation of monocyte mechanics. Iscience 2022, 25, 103555. [Google Scholar] [CrossRef] [PubMed]
- Vasse, G.F.; Buzón, P.; Melgert, B.N.; Roos, W.H.; Rijn, P. Single Cell Reactomics: Real-Time Single-Cell Activation Kinetics of Optically Trapped Macrophages. Small Methods 2021, 5, 2000849. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Shuman, H.; Bennett, J.S.; Weisel, J.W. Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7426–7431. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Zhang, C.; Chen, J.; Reihani, S.N.S.; Chen, Z. Evaluating the toxic effect of an antimicrobial agent on single bacterial cells with optical tweezers. Biomed. Opt. Express 2015, 6, 112–117. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, A.; Fishov, I.; Feingold, M. Z-ring Structure and Constriction Dynamics in E. coli. Front. Microbiol. 2017, 8, 1670. [Google Scholar] [CrossRef]
- Carmon, G.; Feingold, M. Controlled alignment of bacterial cells with oscillating optical tweezers. J. Nanophotonics 2011, 5, 051803. [Google Scholar] [CrossRef]
- Kučera, O.; Siahaan, V.; Janda, D.; Dijkstra, S.H.; Pilátová, E.; Zatecka, E.; Diez, S.; Braun, M.; Lansky, Z. Anillin propels myosin-independent constriction of actin rings. Nat. Commun. 2021, 12, 4595. [Google Scholar] [CrossRef]
- Min, T.L.; Mears, P.J.; Golding, I.; Chemla, Y.R. Chemotactic adaptation kinetics of individual Escherichia coli cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9869–9874. [Google Scholar] [CrossRef]
- Bezryadina, A.S.; Preece, D.C.; Chen, J.C.; Chen, Z. Optical disassembly of cellular clusters by tunable ‘tug-of-war’ tweezers. Light. Sci. Appl. 2016, 5, e16158. [Google Scholar] [CrossRef]
- Zhang, Z.; Kimkes, T.E.P.; Heinemann, M. Manipulating rod-shaped bacteria with optical tweezers. Sci. Rep. 2019, 9, 19086. [Google Scholar] [CrossRef]
- Le Gall, A.; Perronet, K.; Dulin, D.; Villing, A.; Bouyer, P.; Visscher, K.; Westbrook, N. Simultaneous calibration of optical tweezers spring constant and position detector response. Opt. Express 2010, 18, 26469–26474. [Google Scholar] [CrossRef]
- Storm, C.; Nelson, P.C. Measurement of the DNA Spring Constant Using Optical Tweezers. Nat. Commun. 2012, 14, 1–5. [Google Scholar]
- Viana, N.B.; Rocha, M.; Mesquita, O.N.; Mazolli, A.; Neto, P.M.; Nussenzveig, H.M. Towards absolute calibration of optical tweezers. Phys. Rev. E 2007, 75, 021914. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Tripathy, S.K.; Narayanareddy, B.R.; Mattson-Hoss, M.K.; Gross, S.P. Calibration of Optical Tweezers for In Vivo Force Measurements: How do Different Approaches Compare? Biophys. J. 2014, 107, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Arbore, C.; Perego, L.; Sergides, M.; Capitanio, M. Probing force in living cells with optical tweezers: From single-molecule mechanics to cell mechanotransduction. Biophys. Rev. 2019, 11, 765–782. [Google Scholar] [CrossRef]
- Hendricks, A.G.; Goldman, Y.E. Measuring Molecular Forces Using Calibrated Optical Tweezers in Living Cells. Opt. Tweezers: Methods Protoc. 2017, 1486, 537–552. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Zou, R.; Hu, H.; Wang, Y.; Wang, F.; Ju, L.A. Recent Advances of Optical Tweezers–Based Dynamic Force Spectroscopy and Mechanical Measurement Assays for Live-Cell Mechanobiology. Front. Phys. 2022, 10, 771111. [Google Scholar] [CrossRef]
- Wang, M.D.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Stretching DNA with optical tweezers. Biophys. J. 1997, 72, 1335–1346. [Google Scholar] [CrossRef]
- Peterman, E.J.; Gittes, F.; Schmidt, C.F. Laser-Induced Heating in Optical Traps. Biophys. J. 2003, 84, 1308–1316. [Google Scholar] [CrossRef]
- Català, F.; Marsà, F.; Montes-Usategui, M.; Farré, A.; Martín-Badosa, E. Influence of experimental parameters on the laser heating of an optical trap. Sci. Rep. 2017, 7, 16052. [Google Scholar] [CrossRef]
- Kuo, S.C. A simple assay for local heating by optical tweezers. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Visscher, K.; Gross, S.; Block, S. Construction of multiple-beam optical traps with nanometer-resolution position sensing. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 1066–1076. [Google Scholar] [CrossRef]
- Farré, A.; van der Horst, A.; Blab, G.A.; Downing, B.P.B.; Forde, N.R. Stretching single DNA molecules to demonstrate high-force capabilities of holographic optical tweezers. J. Biophotonics 2010, 3, 224–233. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghizadeh, A.; Iftikhar, M.; Dandpat, S.S.; Simpson, T. Looking at Biomolecular Interactions through the Lens of Correlated Fluorescence Microscopy and Optical Tweezers. Int. J. Mol. Sci. 2023, 24, 2668. https://doi.org/10.3390/ijms24032668
Haghizadeh A, Iftikhar M, Dandpat SS, Simpson T. Looking at Biomolecular Interactions through the Lens of Correlated Fluorescence Microscopy and Optical Tweezers. International Journal of Molecular Sciences. 2023; 24(3):2668. https://doi.org/10.3390/ijms24032668
Chicago/Turabian StyleHaghizadeh, Anahita, Mariam Iftikhar, Shiba S. Dandpat, and Trey Simpson. 2023. "Looking at Biomolecular Interactions through the Lens of Correlated Fluorescence Microscopy and Optical Tweezers" International Journal of Molecular Sciences 24, no. 3: 2668. https://doi.org/10.3390/ijms24032668
APA StyleHaghizadeh, A., Iftikhar, M., Dandpat, S. S., & Simpson, T. (2023). Looking at Biomolecular Interactions through the Lens of Correlated Fluorescence Microscopy and Optical Tweezers. International Journal of Molecular Sciences, 24(3), 2668. https://doi.org/10.3390/ijms24032668






