Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization
Abstract
1. Introduction
2. Results
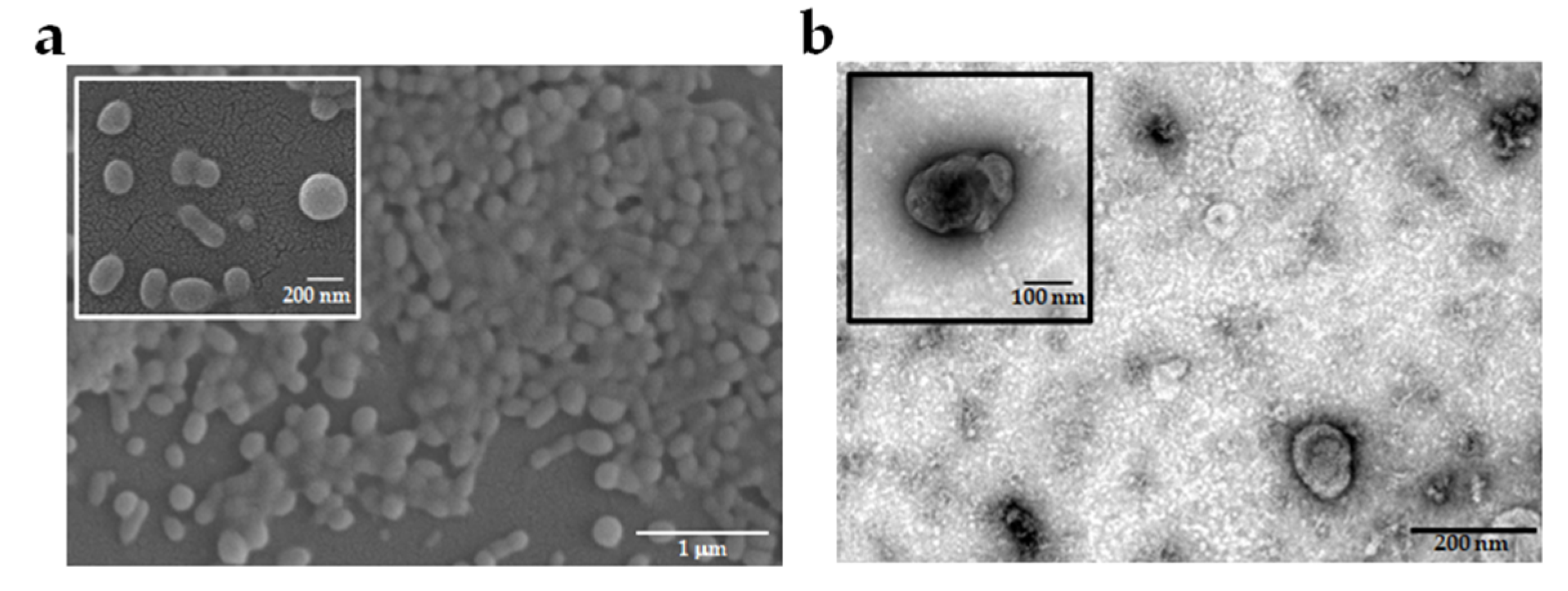
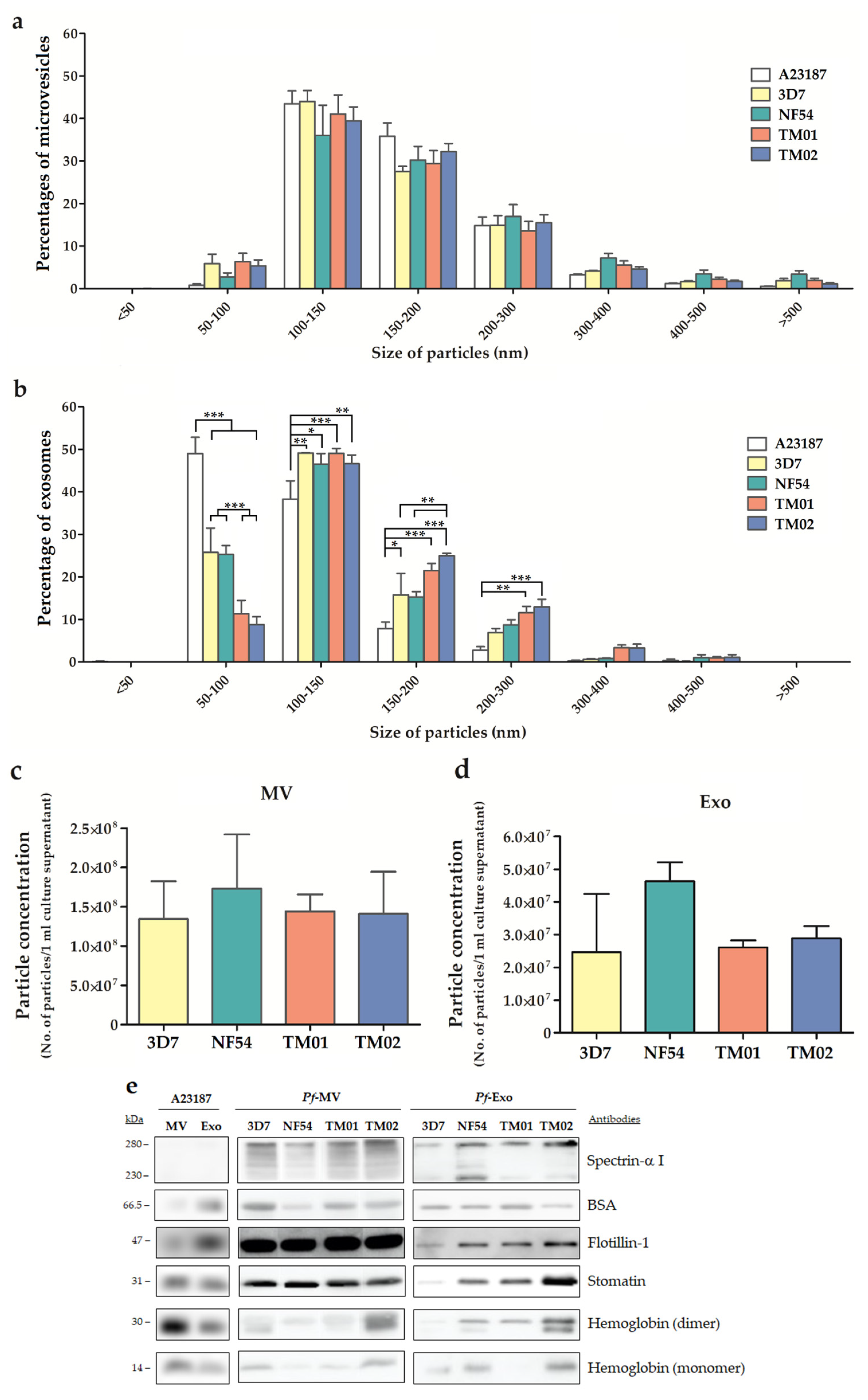
2.1. Characteristics of EVs Derived from Different P. falciparum Strains
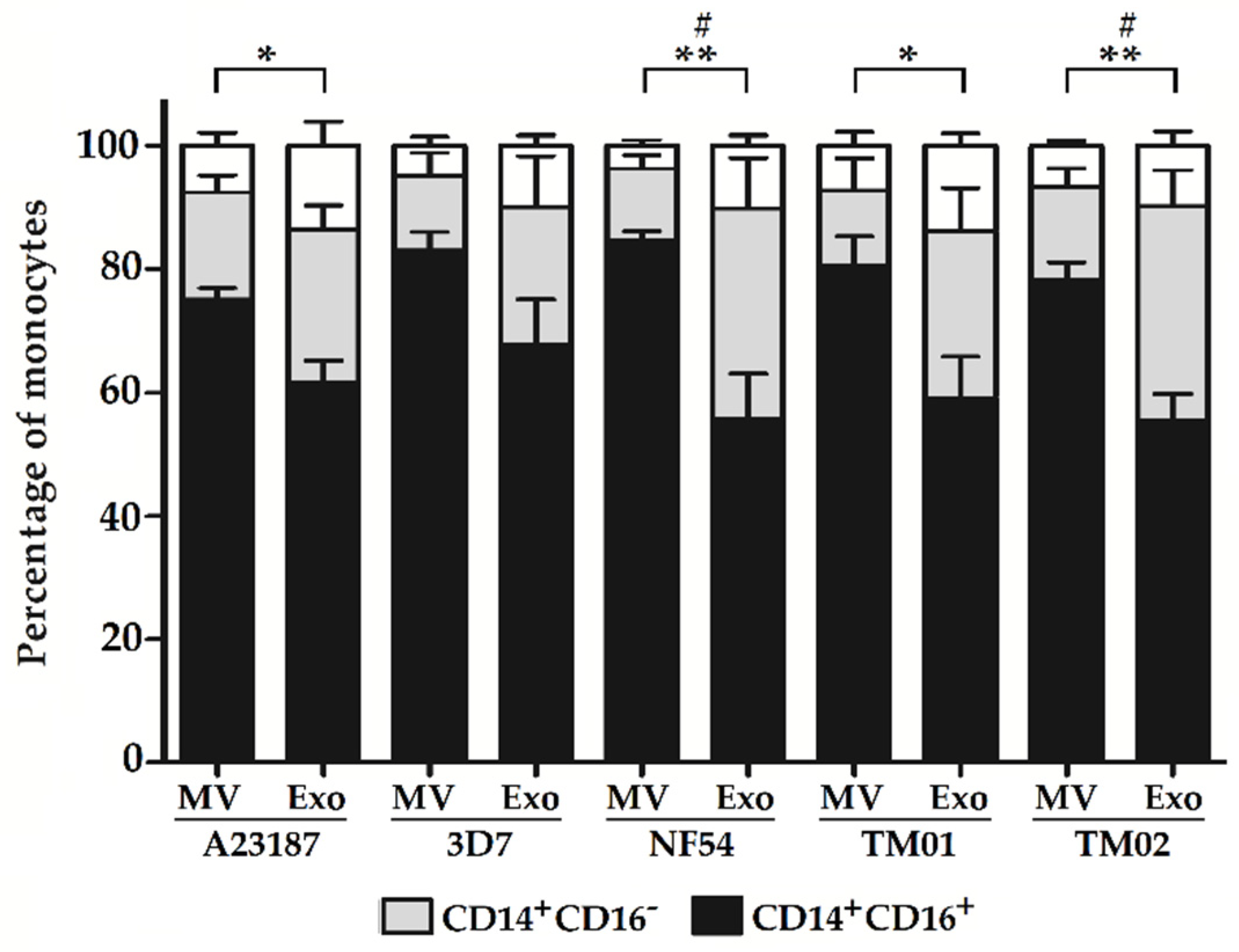
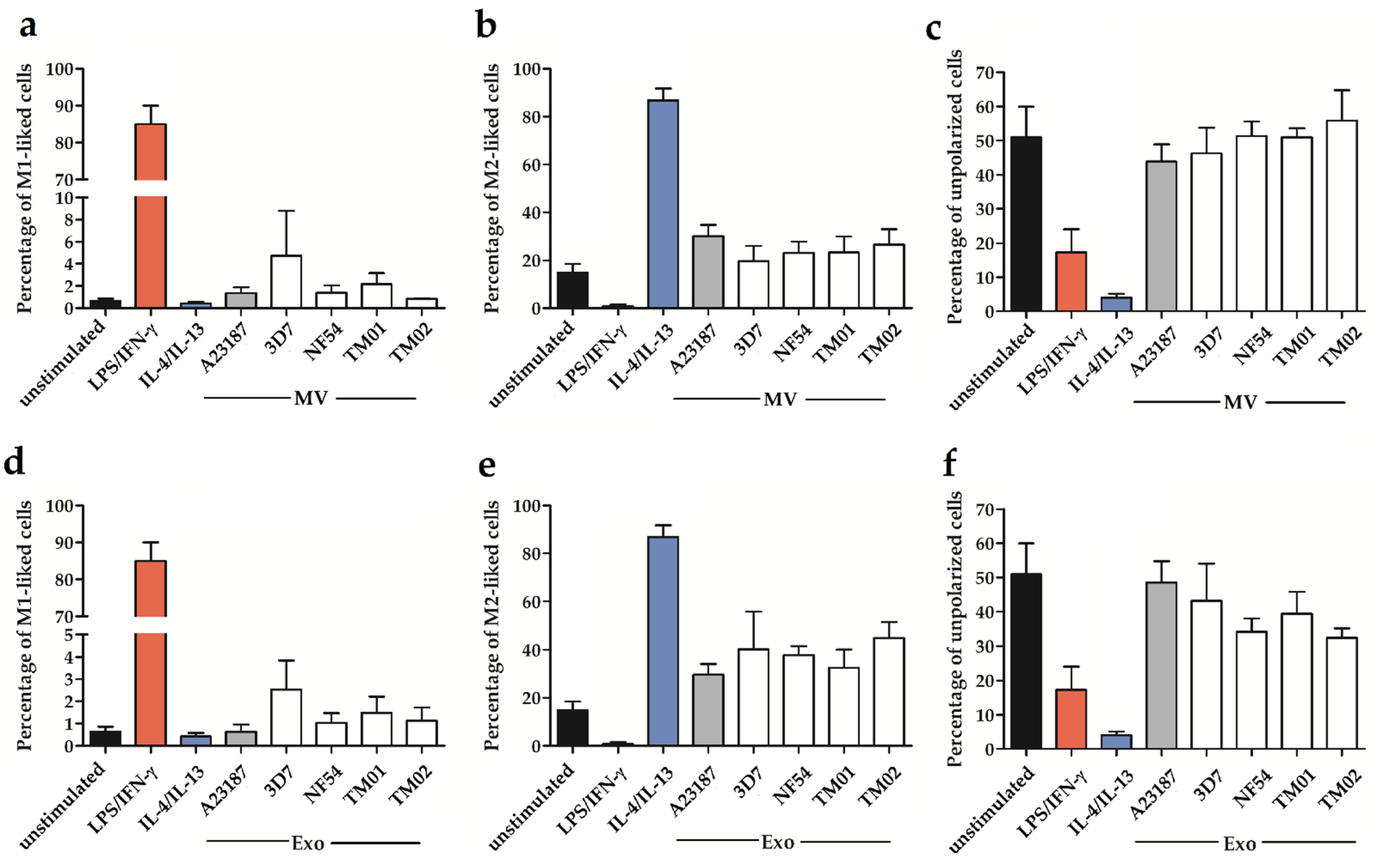
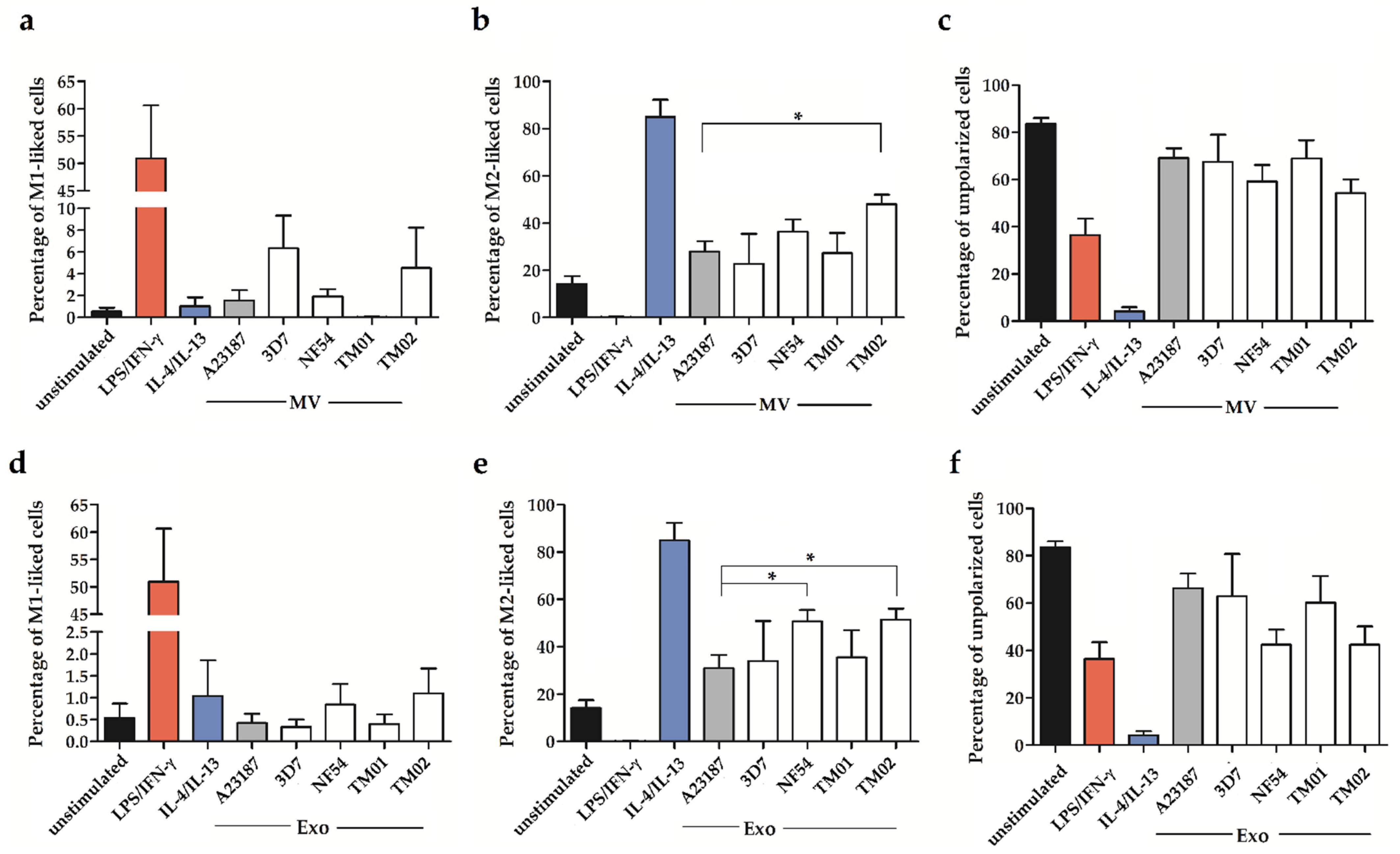
2.2. Pf-EV Stimulation of Primary Monocytes
2.3. The miRNA Levels in Pf-EVs
3. Discussion
4. Materials and Methods
4.1. Ethical Approved
4.2. Plasmodium falciparum Culture
4.3. EVs Isolation and Characterization
4.4. Assessment of EVs Morphology by Electron Microscopy
4.5. Nanoparticle Tracking Analysis (NTA)
4.6. Western Blotting Analysis
4.7. miRNA Determination
4.8. Primary Monocyte Isolation
4.9. Coculture of Pf-EVs with Primary Monocytes
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Overstreet, M.G.; Cockburn, I.A.; Chen, Y.C.; Zavala, F. Protective CD8 T cells against Plasmodium liver stages: Immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 2008, 225, 272–283. [Google Scholar] [CrossRef]
- Riley, E.M.; Stewart, V.A. Immune mechanisms in malaria: New insights in vaccine development. Nat. Med. 2013, 19, 168–178. [Google Scholar] [CrossRef]
- Ortega-Pajares, A.; Rogerson, S.J. The Rough Guide to Monocytes in Malaria Infection. Front. Immunol. 2018, 9, 2888. [Google Scholar] [CrossRef]
- Chua, C.L.; Brown, G.; Hamilton, J.A.; Rogerson, S.; Boeuf, P. Monocytes and macrophages in malaria: Protection or pathology? Trends Parasitol. 2013, 29, 26–34. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Dobbs, K.R.; Embury, P.; Vulule, J.; Odada, P.S.; Rosa, B.A.; Mitreva, M.; Kazura, J.W.; Dent, A.E. Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria. JCI Insight 2017, 2, e95352. [Google Scholar] [CrossRef]
- Loughland, J.R.; Woodberry, T.; Field, M.; Andrew, D.W.; SheelaNair, A.; Dooley, N.L.; Piera, K.A.; Amante, F.H.; Kenangalem, E.; Price, R.N.; et al. Transcriptional profiling and immunophenotyping show sustained activation of blood monocytes in subpatent Plasmodium falciparum infection. Clin. Transl. Immunol. 2020, 9, e1144. [Google Scholar] [CrossRef]
- Chimma, P.; Roussilhon, C.; Sratongno, P.; Ruangveerayuth, R.; Pattanapanyasat, K.; Perignon, J.L.; Roberts, D.J.; Druilhe, P. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 2009, 5, e1000631. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, G.; Beeson, J.; Hogarth, P.M.; Rogerson, S.J.; Yan, Y.; Jaworowski, A. CD14(hi)CD16+ monocytes phagocytose antibody-opsonised Plasmodium falciparum infected erythrocytes more efficiently than other monocyte subsets, and require CD16 and complement to do so. BMC Med. 2015, 13, 154. [Google Scholar] [CrossRef]
- Royo, J.; Rahabi, M.; Kamaliddin, C.; Ezinmegnon, S.; Olagnier, D.; Authier, H.; Massougbodji, A.; Alao, J.; Ladipo, Y.; Deloron, P.; et al. Changes in monocyte subsets are associated with clinical outcomes in severe malarial anaemia and cerebral malaria. Sci. Rep. 2019, 9, 17545. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Wei, Y.; Schober, A. MicroRNA regulation of macrophages in human pathologies. Cell. Mol. Life Sci. 2016, 73, 3473–3495. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Weinberg, J.B.; Volkheimer, A.D.; Rubach, M.P.; Florence, S.M.; Mukemba, J.P.; Kalingonji, A.R.; Langelier, C.; Chen, Y.; Bush, M.; Yeo, T.W.; et al. Monocyte polarization in children with falciparum malaria: Relationship to nitric oxide insufficiency and disease severity. Sci. Rep. 2016, 6, 29151. [Google Scholar] [CrossRef]
- Mandala, W.L.; Msefula, C.L.; Gondwe, E.N.; Drayson, M.T.; Molyneux, M.E.; MacLennan, C.A. Monocyte activation and cytokine production in Malawian children presenting with P. falciparum malaria. Parasite Immunol. 2016, 38, 317–325. [Google Scholar] [CrossRef]
- Parroche, P.; Lauw, F.N.; Goutagny, N.; Latz, E.; Monks, B.G.; Visintin, A.; Halmen, K.A.; Lamphier, M.; Olivier, M.; Bartholomeu, D.C.; et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad.Sci. USA 2007, 104, 1919–1924. [Google Scholar] [CrossRef]
- Krishnegowda, G.; Hajjar, A.M.; Zhu, J.; Douglass, E.J.; Uematsu, S.; Akira, S.; Woods, A.S.; Gowda, D.C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 2005, 280, 8606–8616. [Google Scholar] [CrossRef]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Eriksson, E.M.; Schofield, L. Plasmodium falciparum PfEMP1 Modulates Monocyte/Macrophage Transcription Factor Activation and Cytokine and Chemokine Responses. Infect. Immun. 2018, 86, e00447-17. [Google Scholar] [CrossRef]
- Campos, F.M.; Franklin, B.S.; Teixeira-Carvalho, A.; Filho, A.L.; de Paula, S.C.; Fontes, C.J.; Brito, C.F.; Carvalho, L.H. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar. J. 2010, 9, 327. [Google Scholar] [CrossRef]
- Pankoui Mfonkeu, J.B.; Gouado, I.; Fotso Kuate, H.; Zambou, O.; Amvam Zollo, P.H.; Grau, G.E.; Combes, V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE 2010, 5, e13415. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Mantel, P.Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef]
- Sisquella, X.; Ofir-Birin, Y.; Pimentel, M.A.; Cheng, L.; Abou Karam, P.; Sampaio, N.G.; Penington, J.S.; Connolly, D.; Giladi, T.; Scicluna, B.J.; et al. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat. Commun. 2017, 8, 1985. [Google Scholar] [CrossRef]
- Hila Ben Ami Pilo, S.K.K.; Lühle, J.; Biskup, K.; Gal, B.L.; Goldian, I.R.; Alfandari, D.; Revach, O.-Y.; Kiper, E.; Rotkopf, R.; Porat, Z.; et al. Sialylated N-glycans mediate monocyte uptake of extracellular vesicles secreted from Plasmodium falciparum-infected red blood cells. J. Extracell. Biol. 2022, 1, e33. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, Q.; Huang, Y.; Feng, L.; Pan, W. No miRNA were found in Plasmodium and the ones identified in erythrocytes could not be correlated with infection. Malar. J. 2008, 7, 47. [Google Scholar] [CrossRef]
- Doss, J.F.; Corcoran, D.L.; Jima, D.D.; Telen, M.J.; Dave, S.S.; Chi, J.T. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genom. 2015, 16, 952. [Google Scholar] [CrossRef]
- Vu, L.; Ragupathy, V.; Kulkarni, S.; Atreya, C. Analysis of Argonaute 2-microRNA complexes in ex vivo stored red blood cells. Transfusion 2017, 57, 2995–3000. [Google Scholar] [CrossRef]
- Jee, D.; Yang, J.S.; Park, S.M.; Farmer, D.T.; Wen, J.; Chou, T.; Chow, A.; McManus, M.T.; Kharas, M.G.; Lai, E.C. Dual Strategies for Argonaute2-Mediated Biogenesis of Erythroid miRNAs Underlie Conserved Requirements for Slicing in Mammals. Mol. Cell 2018, 69, 265–278.e266. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Fan, L.; Lin, Q.; Fu, D.; Wei, B.; Wei, S. MicroRNA Profiling of Exosomes Derived from Red Blood Cell Units: Implications in Transfusion-Related Immunomodulation. Biomed. Res. Int. 2019, 2019, 2045915. [Google Scholar] [CrossRef]
- Mantel, P.Y.; Hjelmqvist, D.; Walch, M.; Kharoubi-Hess, S.; Nilsson, S.; Ravel, D.; Ribeiro, M.; Gruring, C.; Ma, S.; Padmanabhan, P.; et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat. Commun. 2016, 7, 12727. [Google Scholar] [CrossRef]
- Wang, Z.; Xi, J.; Hao, X.; Deng, W.; Liu, J.; Wei, C.; Gao, Y.; Zhang, L.; Wang, H. Red blood cells release microparticles containing human argonaute 2 and miRNAs to target genes of Plasmodium falciparum. Emerg. Microbes Infect. 2017, 6, e75. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Kroh, E.; Wood, B.; Arroyo, J.D.; Dougherty, K.J.; Miyaji, M.M.; Tait, J.F.; Tewari, M. Blood cell origin of circulating microRNAs: A cautionary note for cancer biomarker studies. Cancer Prev. Res. 2012, 5, 492–497. [Google Scholar] [CrossRef]
- Vimonpatranon, S.; Roytrakul, S.; Phaonakrop, N.; Lekmanee, K.; Atipimonpat, A.; Srimark, N.; Sukapirom, K.; Chotivanich, K.; Khowawisetsut, L.; Pattanapanyasat, K. Extracellular Vesicles Derived from Early and Late Stage Plasmodium falciparum-Infected Red Blood Cells Contain Invasion-Associated Proteins. J. Clin. Med. 2022, 11, 4250. [Google Scholar] [CrossRef]
- Opadokun, T.; Agyapong, J.; Rohrbach, P. Protein Profiling of Malaria-Derived Extracellular Vesicles Reveals Distinct Subtypes. Membranes 2022, 12, 397. [Google Scholar] [CrossRef]
- Polimeni, M.; Valente, E.; Aldieri, E.; Khadjavi, A.; Giribaldi, G.; Prato, M. Haemozoin induces early cytokine-mediated lysozyme release from human monocytes through p38 MAPK- and NF-kappaB-dependent mechanisms. PLoS ONE 2012, 7, e39497. [Google Scholar] [CrossRef]
- Hirako, I.C.; Gallego-Marin, C.; Ataide, M.A.; Andrade, W.A.; Gravina, H.; Rocha, B.C.; de Oliveira, R.B.; Pereira, D.B.; Vinetz, J.; Diamond, B.; et al. DNA-Containing Immunocomplexes Promote Inflammasome Assembly and Release of Pyrogenic Cytokines by CD14+ CD16+ CD64high CD32low Inflammatory Monocytes from Malaria Patients. mBio 2015, 6, e01605–e01615. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-dependent cellular uptake of exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef]
- Tang, D.; Cao, F.; Yan, C.; Fang, K.; Ma, J.; Gao, L.; Sun, B.; Wang, G. Extracellular Vesicle/Macrophage Axis: Potential Targets for Inflammatory Disease Intervention. Front. Immunol. 2022, 13, 705472. [Google Scholar] [CrossRef]
- Guha, R.; Mathioudaki, A.; Doumbo, S.; Doumtabe, D.; Skinner, J.; Arora, G.; Siddiqui, S.; Li, S.; Kayentao, K.; Ongoiba, A.; et al. Plasmodium falciparum malaria drives epigenetic reprogramming of human monocytes toward a regulatory phenotype. PLoS Pathog. 2021, 17, e1009430. [Google Scholar] [CrossRef]
- Shen, D.; He, Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann. Transl. Med. 2021, 9, 1323. [Google Scholar] [CrossRef]
- He, S.; Wu, C.; Xiao, J.; Li, D.; Sun, Z.; Li, M. Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scand J. Immunol. 2018, 87, e12648. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef]
- Lv, L.L.; Feng, Y.; Wen, Y.; Wu, W.J.; Ni, H.F.; Li, Z.L.; Zhou, L.T.; Wang, B.; Zhang, J.D.; Crowley, S.D.; et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J. Am. Soc. Nephrol. 2018, 29, 919–935. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Wu, M.; Li, Z.L.; Feng, Y.; Cao, J.Y.; Yin, D.; Liu, H.; Tang, R.N.; Crowley, S.D.; et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 2020, 6, eaaz0748. [Google Scholar] [CrossRef]
- An, J.H.; Li, Q.; Ryu, M.O.; Nam, A.R.; Bhang, D.H.; Jung, Y.C.; Song, W.J.; Youn, H.Y. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS ONE 2020, 15, e0220756. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Podyminogin, R.L.; Navarro, G.; Zhao, G.W.; Askovich, P.S.; Weiss, M.J.; Aderem, A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J. Immunol. 2012, 189, 5965–5975. [Google Scholar] [CrossRef]
- Okamoto, M.; Fukushima, Y.; Kouwaki, T.; Daito, T.; Kohara, M.; Kida, H.; Oshiumi, H. MicroRNA-451a in extracellular, blood-resident vesicles attenuates macrophage and dendritic cell responses to influenza whole-virus vaccine. J. Biol. Chem. 2018, 293, 18585–18600. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, W.; Sheng, M.; Liu, T. MiR-451 inhibits cell growth and invasion by targeting CXCL16 and is associated with prognosis of osteosarcoma patients. Tumor Biol. 2015, 36, 2041–2048. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Kong, L.; Gao, H.; Zhang, Y.; Zheng, Y.; Wan, Y. miRNA-486-5p Promotes COPD Progression by Targeting HAT1 to Regulate the TLR4-Triggered Inflammatory Response of Alveolar Macrophages. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2991–3001. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Q.; Gong, Y.; Huang, L.; Lin, H.; Zhou, X.; Liang, X.; Guo, W. MiR-486 promotes proliferation and suppresses apoptosis in myeloid cells by targeting Cebpa in vitro. Cancer Med. 2018, 7, 4627–4638. [Google Scholar] [CrossRef]
- Lai, L.; Song, Y.; Liu, Y.; Chen, Q.; Han, Q.; Chen, W.; Pan, T.; Zhang, Y.; Cao, X.; Wang, Q. MicroRNA-92a negatively regulates Toll-like receptor (TLR)-triggered inflammatory response in macrophages by targeting MKK4 kinase. J. Biol. Chem. 2013, 288, 7956–7967. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Weichhart, T.; Hengstschlager, M.; Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef]
- Rocher, C.; Singla, D.K. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS ONE 2013, 8, e84009. [Google Scholar] [CrossRef]
- Reis, P.P.; Drigo, S.A.; Carvalho, R.F.; Lopez Lapa, R.M.; Felix, T.F.; Patel, D.; Cheng, D.; Pintilie, M.; Liu, G.; Tsao, M.S. Circulating miR-16-5p, miR-92a-3p, and miR-451a in Plasma from Lung Cancer Patients: Potential Application in Early Detection and a Regulatory Role in Tumorigenesis Pathways. Cancers 2020, 12, 2071. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- Ponnudurai, T.; Leeuwenberg, A.D.; Meuwissen, J.H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 1981, 33, 50–54. [Google Scholar]
- Karl, S.; Wong, R.P.; St Pierre, T.G.; Davis, T.M. A comparative study of a flow-cytometry-based assessment of in vitro Plasmodium falciparum drug sensitivity. Malar. J. 2009, 8, 294. [Google Scholar] [CrossRef]
- Mu, J.; Myers, R.A.; Jiang, H.; Liu, S.; Ricklefs, S.; Waisberg, M.; Chotivanich, K.; Wilairatana, P.; Krudsood, S.; White, N.J.; et al. Plasmodium falciparum genome-wide scans for positive selection, recombination hot spots and resistance to antimalarial drugs. Nat. Genet. 2010, 42, 268–271. [Google Scholar] [CrossRef]
- Rathod, P.K.; McErlean, T.; Lee, P.C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1997, 94, 9389–9393. [Google Scholar] [CrossRef]
- Mungchan, P.; Glab-Ampai, K.; Chruewkamlow, N.; Trakarnsanga, K.; Srisawat, C.; Nguyen, K.T.; Chaicumpa, W.; Punnakitikashem, P. Targeted Nanoparticles for the Binding of Injured Vascular Endothelium after Percutaneous Coronary Intervention. Molecules 2022, 27, 8144. [Google Scholar] [CrossRef]
- Xiao, C.T.; Lai, W.J.; Zhu, W.A.; Wang, H. MicroRNA Derived from Circulating Exosomes as Noninvasive Biomarkers for Diagnosing Renal Cell Carcinoma. OncoTargets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef]
- Lange, T.; Stracke, S.; Rettig, R.; Lendeckel, U.; Kuhn, J.; Schluter, R.; Rippe, V.; Endlich, K.; Endlich, N. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS ONE 2017, 12, e0183435. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Yuan, J.; Wu, J.; Deng, X.; Peng, J.; Wang, S.; Yang, C.; Ge, J.; Zou, Y. Evaluation of the performance of serum miRNAs as normalizers in microRNA studies focused on cardiovascular disease. J. Thorac. Dis. 2018, 10, 2599–2607. [Google Scholar] [CrossRef]

| miRBase ID | miRBase Accession | Mature miRNA Sequence |
|---|---|---|
| hsa-miR-451a | MIMAT0001631 | 5’AAACCGUUACCAUUACUGAGUU |
| hsa-miR-486-5p | MIMAT0002177 | 5’UCCUGUACUGAGCUGCCCCGAG |
| hsa-miR-92a-3p | MIMAT0000092 | 5’UAUUGCACUUGUCCCGGCCUGU |
| hsa-miR-16-5p | MIMAT0000069 | 5’UAGCAGCACGUAAAUAUUGGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khowawisetsut, L.; Vimonpatranon, S.; Lekmanee, K.; Sawasdipokin, H.; Srimark, N.; Chotivanich, K.; Pattanapanyasat, K. Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization. Int. J. Mol. Sci. 2023, 24, 2631. https://doi.org/10.3390/ijms24032631
Khowawisetsut L, Vimonpatranon S, Lekmanee K, Sawasdipokin H, Srimark N, Chotivanich K, Pattanapanyasat K. Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization. International Journal of Molecular Sciences. 2023; 24(3):2631. https://doi.org/10.3390/ijms24032631
Chicago/Turabian StyleKhowawisetsut, Ladawan, Sinmanus Vimonpatranon, Kittima Lekmanee, Hathai Sawasdipokin, Narinee Srimark, Kesinee Chotivanich, and Kovit Pattanapanyasat. 2023. "Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization" International Journal of Molecular Sciences 24, no. 3: 2631. https://doi.org/10.3390/ijms24032631
APA StyleKhowawisetsut, L., Vimonpatranon, S., Lekmanee, K., Sawasdipokin, H., Srimark, N., Chotivanich, K., & Pattanapanyasat, K. (2023). Differential Effect of Extracellular Vesicles Derived from Plasmodium falciparum-Infected Red Blood Cells on Monocyte Polarization. International Journal of Molecular Sciences, 24(3), 2631. https://doi.org/10.3390/ijms24032631







