Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates
Abstract
1. Introduction
2. Definition of an Effective Diagnostic Biomarker
3. EVs as Effective Diagnostic Biomarkers
Methods of EV Characterization and miRNA Extraction
4. EV-miRNAs in Necrotizing Enterocolitis
5. EV-miRNAs in Bronchopulmonary Dysplasia
6. EV-miRNAs in Hypoxic-Ischemic Brain Damage
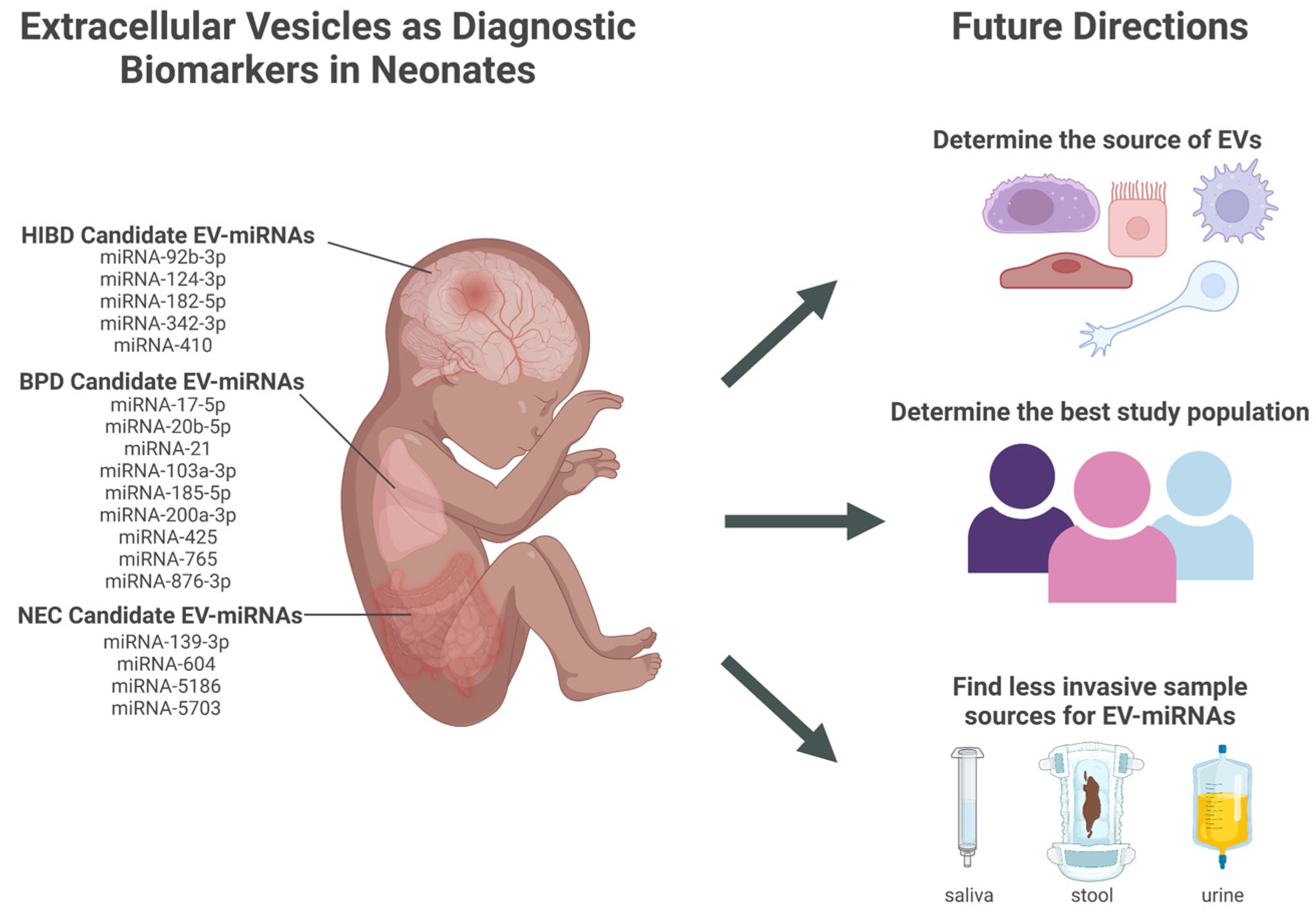
7. Next Steps in EV-miRNAs Biomarker Development in Premature Infants
7.1. Determine the Best Sample Source for Discovering Diagnostic Biomarkers from Neonatal EVs
7.2. Validation of EV-miRNAs as Reliable Diagnostic Biomarkers in the Premature Population
7.3. Determine the Feasibility of Implementing EV Diagnostic Testing: Testing Population and Role of EV Biomarkers in Clinical Decision Making
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheong, J.L.Y.; Doyle, L.W. An Update on Pulmonary and Neurodevelopmental Outcomes of Bronchopulmonary Dysplasia. Semin. Perinatol. 2018, 42, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Finder, M.; Boylan, G.B.; Twomey, D.; Ahearne, C.; Murray, D.M.; Hallberg, B. Two-Year Neurodevelopmental Outcomes After Mild Hypoxic Ischemic Encephalopathy in the Era of Therapeutic Hypothermia. Jama Pediatr. 2020, 174, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm Birth Lifetime Costs in the United States in 2016: An Update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef] [PubMed]
- Purdy, I.B.; Craig, J.W.; Zeanah, P. NICU Discharge Planning and beyond: Recommendations for Parent Psychosocial Support. J. Perinatol. 2015, 35, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.C.; Antounians, L.; Zani, A. Extracellular Vesicles as a Potential Therapy for Neonatal Conditions: State of the Art and Challenges in Clinical Translation. Pharmaceutics 2019, 11, 404. [Google Scholar] [CrossRef]
- Murphy, C.A.; O’Reilly, D.P.; Neary, E.; EL-Khuffash, A.; NíAinle, F.; McCallion, N.; Maguire, P.B. A Review of the Role of Extracellular Vesicles in Neonatal Physiology and Pathology. Pediatr. Res. 2021, 90, 289–299. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 16 September 2022).
- Byrnes, S.A.; Weigl, B.H. Selecting Analytical Biomarkers for Diagnostic Applications: A First Principles Approach. Expert Rev. Mol. Diagn. 2017, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging Biomarkers for Prediction and Early Diagnosis of Necrotizing Enterocolitis in the Era of Metabolomics and Proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef]
- Yen, E.; Kaneko-Tarui, T.; Maron, J.L. Technical Considerations and Protocol Optimization for Neonatal Salivary Biomarker Discovery and Analysis. Front. Pediatr. 2021, 8, 618553. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Extracellular Vesicles Isolation and Their Biomarker Potential: Are We Ready for Testing? Ann. Transl. Med. 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Whiteside, T.L. Information Transfer by Exosomes: A New Frontier in Hematologic Malignancies. Blood Rev. 2015, 29, 281–290. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular Vesicles: A New Communication Paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid Biopsy of Cancer: A Multimodal Diagnostic Tool in Clinical Oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Kamal, N.N.S.B.N.M.; Shahidan, W.N.S. Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers? Front. Pharmacol. 2020, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The Exosome Encapsulated MicroRNAs as Circulating Diagnostic Marker for Hepatocellular Carcinoma with Low Alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, W.; Liu, C.; Na, Q. Umbilical Cord Blood-Derived Exosomes in Maternal–Fetal Disease: A Review. Reprod. Sci. 2022, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Bajracharya, S.; Yuen, P.; Zhou, H.; Star, R.; Illei, G.; Alevizos, I. Exosomes from Human Saliva as a Source of MicroRNA Biomarkers. Oral. Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 Is a Marker of Exosomes Secreted into Urine and Amniotic Fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. New Engl. J. Med. 2018, 379, 2179–2181. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Linxweiler, J.; Junker, K. Extracellular Vesicles in Urological Malignancies: An Update. Nat. Rev. Urol. 2020, 17, 11–27. [Google Scholar] [CrossRef]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R. Distinct Mechanisms of MicroRNA Sorting into Cancer Cell-Derived Extracellular Vesicle Subtypes. Elife 2019, 8, e47544. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 Selectively Regulates MicroRNA Sorting into Microvesicles after Noxious Stimuli. J. Exp. Med. 2019, 216, 2202–2220. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Costa, M.C.; Catarino, S.; Simoes, I.; Aasen, T.; Enguita, F.J.; Girao, H. Cx43-mediated Sorting of MiRNAs into Extracellular Vesicles. Embo Rep. 2022, 23, e54312. [Google Scholar] [CrossRef] [PubMed]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular Vesicle Measurements with Nanoparticle Tracking Analysis—An Accuracy and Repeatability Comparison between NanoSight NS300 and ZetaView. J. Extracell Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bustoros, M.; Lin, X.; Mejia, C.M.D.; Gurzenda, E.; Chavez, M.; Hanna, I.; Aguiari, P.; Perin, L.; Hanna, N. Placental Extracellular Vesicles–Associated MicroRNA-519c Mediates Endotoxin Adaptation in Pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 681.e1–681.e20. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Araújo-Filho, I.; Piffoux, M.; Nicolás-Boluda, A.; Grangier, A.; Boucenna, I.; Real, C.C.; Marques, F.L.N.; Faria, D.d.P.; do Rego, A.C.M.; et al. Local Administration of Stem Cell-Derived Extracellular Vesicles in a Thermoresponsive Hydrogel Promotes a pro-Healing Effect in a Rat Model of Colo-Cutaneous Post-Surgical Fistula. Nanoscale 2020, 13, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hardij, J.; Cecchet, F.; Berquand, A.; Gheldof, D.; Chatelain, C.; Mullier, F.; Chatelain, B.; Dogné, J. Characterisation of Tissue Factor-bearing Extracellular Vesicles with AFM: Comparison of Air-tapping-mode AFM and Liquid Peak Force AFM. J. Extracell Vesicles 2013, 2, 21045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kao, Y.; Zhou, Q.; Wuethrich, A.; Stark, M.S.; Schaider, H.; Soyer, H.P.; Lin, L.L.; Trau, M. An Integrated Microfluidic-SERS Platform Enables Sensitive Phenotyping of Serum Extracellular Vesicles in Early Stage Melanomas. Adv. Funct. Mater. 2022, 32, 2010296. [Google Scholar] [CrossRef]
- Zadka, Ł.; Buzalewicz, I.; Ulatowska-Jarża, A.; Rusak, A.; Kochel, M.; Ceremuga, I.; Dzięgiel, P. Label-Free Quantitative Phase Imaging Reveals Spatial Heterogeneity of Extracellular Vesicles in Select Colon Disorders. Am. J. Pathol. 2021, 191, 2147–2171. [Google Scholar] [CrossRef]
- Guo, Y.; Vickers, K.; Xiong, Y.; Zhao, S.; Sheng, Q.; Zhang, P.; Zhou, W.; Flynn, C.R. Comprehensive Evaluation of Extracellular Small RNA Isolation Methods from Serum in High Throughput Sequencing. Bmc Genom. 2017, 18, 50. [Google Scholar] [CrossRef]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of Circulating MicroRNA Biomarkers in Plasma and Serum Using Quantitative Reverse Transcription-PCR (QRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Su, X.; Gao, X.; Dai, Z.; Zou, X. A Label-Free and PCR-Free Electrochemical Assay for Multiplexed MicroRNA Profiles by Ligase Chain Reaction Coupling with Quantum Dots Barcodes. Biosens. Bioelectron. 2014, 53, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pall, G.S.; Hamilton, A.J. Improved Northern Blot Method for Enhanced Detection of Small RNA. Nat. Protoc. 2008, 3, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, H.; Hahn, S.A.; Maghnouj, A. Quantitative RT-PCR Specific for Precursor and Mature MiRNAs. Methods Mol. Biol. Clifton N. J. 2013, 1095, 121–134. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-Time Quantification of MicroRNAs by Stem–Loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Kappel, A.; Keller, A. MiRNA Assays in the Clinical Laboratory: Workflow, Detection Technologies and Automation Aspects. Clin. Chem. Lab. Med. Cclm 2017, 55, 636–647. [Google Scholar] [CrossRef]
- Shi, R.; Chiang, V.L. Facile Means for Quantifying MicroRNA Expression by Real-Time PCR. Biotechniques 2005, 39, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.D.; Carletti, M.Z.; Christenson, L.K. Quantitative RT-PCR Methods for Mature MicroRNA Expression Analysis. Methods Mol. Biology Clifton N. J. 2010, 630, 49–64. [Google Scholar] [CrossRef]
- Carrascosa, L.G.; Huertas, C.S.; Lechuga, L.M. Prospects of Optical Biosensors for Emerging Label-Free RNA Analysis. Trac. Trends Anal. Chem. 2016, 80, 177–189. [Google Scholar] [CrossRef]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-Protein Biosensors: New Tools for Drug Discovery. Trends Biotechnol. 1998, 16, 135–140. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of New Label-Free Techniques for Biosensors: A Review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Optical Biosensors in Drug Discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive Optical Biosensors for Unlabeled Targets: A Review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, W.; Liu, S.; Huang, C.; Ghalandari, B.; Divsalar, A.; Ding, X. Recent Progresses in Electrochemical DNA Biosensors for MicroRNA Detection. Phenomics 2022, 2, 18–32. [Google Scholar] [CrossRef]
- Dave, V.P.; Ngo, T.A.; Pernestig, A.-K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA Amplification and Detection Technologies: Opportunities and Challenges for Point of Care Diagnostics. Lab. Investig. 2019, 99, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Cacheux, J.; Bancaud, A.; Leichlé, T.; Cordelier, P. Technological Challenges and Future Issues for the Detection of Circulating MicroRNAs in Patients With Cancer. Front. Chem. 2019, 7, 815. [Google Scholar] [CrossRef]
- Lai, M.; Slaughter, G. Label-Free MicroRNA Optical Biosensors. Nanomaterials 2019, 9, 1573. [Google Scholar] [CrossRef]
- Denning, T.W.; Bhatia, A.M.; Kane, A.F.; Patel, R.M.; Denning, P.W. Pathogenesis of NEC: Role of the Innate and Adaptive Immune Response. Semin. Perinatol. 2017, 41, 15–28. [Google Scholar] [CrossRef]
- Hunter, C.J.; Plaen, I.G.D. Inflammatory Signaling in NEC: Role of NF-ΚB, Cytokines and Other Inflammatory Mediators. Pathophysiol 2014, 21, 55–65. [Google Scholar] [CrossRef]
- D’Angelo, G.; Impellizzeri, P.; Marseglia, L.; Montalto, A.S.; Russo, T.; Salamone, I.; Falsaperla, R.; Corsello, G.; Romeo, C.; Gitto, E. Current Status of Laboratory and Imaging Diagnosis of Neonatal Necrotizing Enterocolitis. Ital. J. Pediatr. 2018, 44, 84. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, A.; Devette, C.; Levin, S.; Chaaban, H. Biomarkers of Necrotizing Enterocolitis: The Search Continues. Clin. Perinatol. 2022, 49, 181–194. [Google Scholar] [CrossRef]
- Galley, J.D.; Mar, P.; Wang, Y.; Han, R.; Rajab, A.; Besner, G.E. Urine-Derived Extracellular Vesicle MiRNAs as Possible Biomarkers for and Mediators of Necrotizing Enterocolitis: A Proof of Concept Study. J. Pediatr. Surg. 2021, 56, 1966–1975. [Google Scholar] [CrossRef]
- Sannigrahi, M.K.; Sharma, R.; Singh, V.; Panda, N.K.; Rattan, V.; Khullar, M. Role of Host MiRNA Hsa-MiR-139-3p in HPV-16–Induced Carcinomas. Clin. Cancer Res. 2017, 23, 3884–3895. [Google Scholar] [CrossRef]
- Go, H.; Maeda, H.; Miyazaki, K.; Maeda, R.; Kume, Y.; Namba, F.; Momoi, N.; Hashimoto, K.; Otsuru, S.; Kawasaki, Y.; et al. Extracellular Vesicle MiRNA-21 Is a Potential Biomarker for Predicting Chronic Lung Disease in Premature Infants. Am J. Physiol.-lung. C 2020, 318, L845–L851. [Google Scholar] [CrossRef]
- Zhong, X.; Yan, Q.; Chen, Z.; Jia, C.; Li, X.; Liang, Z.; Gu, J.; Wei, H.; Lian, C.; Zheng, J.; et al. Umbilical Cord Blood-Derived Exosomes From Very Preterm Infants With Bronchopulmonary Dysplasia Impaired Endothelial Angiogenesis: Roles of Exosomal MicroRNAs. Front. Cell Dev. Biol. 2021, 9, 637248. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Olave, N.; Travers, C.; Rezonzew, G.; Dolma, K.; Simpson, A.; Halloran, B.; Aghai, Z.; Das, P.; Sharma, N.; et al. Exosomal MicroRNA Predicts and Protects against Severe Bronchopulmonary Dysplasia in Extremely Premature Infants. Jci Insight 2018, 3, e93994. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Yuan, R.; Deng, Z.; Wu, X. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Hyperoxia-Induced Lung Injury via the Manipulation of MicroRNA-425. Arch. Biochem. Biophys. 2021, 697, 108712. [Google Scholar] [CrossRef] [PubMed]
- Peeples, E.S.; Sahar, N.; Snyder, W.; Mirnics, K. Temporal Brain MicroRNA Expression Changes in a Mouse Model of Neonatal Hypoxic–Ischemic Injury. Pediatr. Res. 2022, 91, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.; Goasdoue, K.; Miller, S.M.; Brennan, G.P.; Cowin, G.; O’Mahony, A.G.; Burke, C.; Hallberg, B.; Boylan, G.B.; Sullivan, A.M.; et al. Temporally Altered MiRNA Expression in a Piglet Model of Hypoxic Ischemic Brain Injury. Mol. Neurobiol. 2020, 57, 4322–4344. [Google Scholar] [CrossRef]
- Lawson, A.; Snyder, W.; Peeples, E.S. Intranasal Administration of Extracellular Vesicles Mitigates Apoptosis in a Mouse Model of Neonatal Hypoxic-Ischemic Brain Injury. Neonatology 2022, 119, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, P.; Lu, Y. MicroRNA-410 Serves as a Candidate Biomarker in Hypoxic-ischemic Encephalopathy Newborns and Provides Neuroprotection in Oxygen-glucose Deprivation-injured PC12 and SH-SY5Y Cells. Brain Behav. 2021, 11, e2293. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Wu, Y.; Fang, Y.; Hong, B.; Dai, D.; Zhou, Y.; Liu, J.; Li, Q. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MicroRNA-124-3p Attenuates Hypoxic-Ischemic Brain Damage through Depressing Tumor Necrosis Factor Receptor Associated Factor 6 in Newborn Rats. Bioengineered 2022, 13, 3195–3207. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary Dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.G.; Procianoy, R.S.; Neto, E.C.; Silveira, R.C. Preterm Neonates with Respiratory Distress Syndrome: Ventilator-Induced Lung Injury and Oxidative Stress. J. Immunol. Res. 2018, 2018, 6963754. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, J.; Tao, Y.; Guo, M.; Ya, Z.; Chen, C.; Qin, N.; Zheng, J.; Luo, J.; Xu, L. MicroRNA-21: A Promising Biomarker for the Prognosis and Diagnosis of Non-Small Cell Lung Cancer. Oncol. Lett. 2018, 16, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Wei Wei Li, J.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal Stromal Cells-Derived Exosomes Alleviate Ischemia/Reperfusion Injury in Mouse Lung by Transporting Anti-Apoptotic MiR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Zhang, X.; Weng, X.; Sheng, A.; Zhu, Y.; Chen, S.; Zheng, X.; Lu, C. High Neutrophil-to-Lymphocyte Ratio Is an Early Predictor of Bronchopulmonary Dysplasia. Front. Pediatr. 2019, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy: A Review for the Clinician. Jama Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef]
- Bonifacio, S.L.; Chalak, L.F.; Meurs, K.P.V.; Laptook, A.R.; Shankaran, S. Neuroprotection for Hypoxic-Ischemic Encephalopathy: Contributions from the Neonatal Research Network. Semin. Perinatol. 2022, 46, 151639. [Google Scholar] [CrossRef] [PubMed]
- Sabir, H.; Bonifacio, S.L.; Gunn, A.J.; Thoresen, M.; Chalak, L.F.; Committee, N.B.S.G. and P. Unanswered Questions Regarding Therapeutic Hypothermia for Neonates with Neonatal Encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101257. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.; Yip, P.K. The Role of MicroRNAs in Newborn Brain Development and Hypoxic Ischaemic Encephalopathy. Neuropharmacology 2019, 149, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, S.; Hao, X.; Zhang, B.; Zhang, H.; Xin, C.; Hao, Y. Extracellular Vesicle-Derived MicroRNA-410 From Mesenchymal Stem Cells Protects Against Neonatal Hypoxia-Ischemia Brain Damage Through an HDAC1-Dependent EGR2/Bcl2 Axis. Front. Cell Dev. Biol. 2021, 8, 579236. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Shi, L.; Kuhnell, D.; Borra, V.J.; Langevin, S.M.; Nakamura, T.; Esfandiari, L. Rapid and Label-Free Isolation of Small Extracellular Vesicles from Biofluids Utilizing a Novel Insulator Based Dielectrophoretic Device. Lab. Chip. 2019, 19, 3726–3734. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Théry, C. Why the Need and How to Approach the Functional Diversity of Extracellular Vesicles. Philos. Trans. R. Soc B Biological Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; Royen, M.E. van Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef]
- Simonsen, J.B. What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circ. Res. 2017, 121, 920–922. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential Detergent Sensitivity of Extracellular Vesicle Subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [PubMed]
- SZATANEK, R.; BARAN, J.; SIEDLAR, M.; BAJ-KRZYWORZEKA, M. Isolation of Extracellular Vesicles: Determining the Correct Approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Northrop-Albrecht, E.J.; Taylor, W.R.; Huang, B.Q.; Kisiel, J.B.; Lucien, F. Assessment of Extracellular Vesicle Isolation Methods from Human Stool Supernatant. J. Extracell Vesicles 2022, 11, e12208. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Chan, K.Y.Y.; Lam, H.S.; Wong, R.P.O.; Ma, T.P.Y.; Sit, T.; Leung, K.T.; Chan, L.C.N.; Pang, Y.L.I.; Cheung, H.M.; et al. A Prospective Cohort Study of Fecal MiR-223 and MiR-451a as Noninvasive and Specific Biomarkers for Diagnosis of Necrotizing Enterocolitis in Preterm Infants. Neonatology 2021, 117, 555–561. [Google Scholar] [CrossRef]
- Turunen, J.; Tejesvi, M.V.; Suokas, M.; Virtanen, N.; Paalanne, N.; Kaisanlahti, A.; Reunanen, J.; Tapiainen, T. Bacterial Extracellular Vesicles in the Microbiome of First-Pass Meconium in Newborn Infants. Pediatr. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary Extracellular Vesicles: A Position Paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Barreiro, K.; Dwivedi, O.P.; Valkonen, S.; Groop, P.; Tuomi, T.; Holthofer, H.; Rannikko, A.; Yliperttula, M.; Siljander, P.; Laitinen, S.; et al. Urinary Extracellular Vesicles: Assessment of Pre-analytical Variables and Development of a Quality Control with Focus on Transcriptomic Biomarker Research. J. Extracell Vesicles 2021, 10, e12158. [Google Scholar] [CrossRef] [PubMed]
- Cheshmi, B.; Cheshomi, H. Salivary Exosomes: Properties, Medical Applications, and Isolation Methods. Mol. Biol. Rep. 2020, 47, 6295–6307. [Google Scholar] [CrossRef]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef]
- Iyengar, A.; Maron, J.L. Detecting Infection in Neonates: Promises and Challenges of a Salivary Approach. Clin. Ther. 2015, 37, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Johnson, K.; Maron, J. Optimal Reference Genes for RT-QPCR Normalization in the Newborn. Biotech. Histochem. 2017, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, J.; Ibarz, M.; Cornes, M.; Nybo, M.; Haschke-Becher, E.; von Meyer, A.; Lippi, G.; Simundic, A.-M. Managing Inappropriate Utilization of Laboratory Resources. Diagnosis 2019, 6, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mrazek, C.; Simundic, A.-M.; Salinas, M.; von Meyer, A.; Cornes, M.; Bauçà, J.M.; Nybo, M.; Lippi, G.; Haschke-Becher, E.; Keppel, M.H.; et al. Inappropriate Use of Laboratory Tests: How Availability Triggers Demand—Examples across Europe. Clin. Chim. Acta 2020, 505, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Kiefer, D. A Translational View of Biomarkers in Preterm Labor. Am J Reprod Immunol 2012, 67, 268–272. [Google Scholar] [CrossRef]
- Singh, H.; Cho, S.J.; Gupta, S.; Kaur, R.; Sunidhi, S.; Saluja, S.; Pandey, A.K.; Bennett, M.V.; Lee, H.C.; Das, R.; et al. Designing a Bed-Side System for Predicting Length of Stay in a Neonatal Intensive Care Unit. Sci. Rep. 2021, 11, 3342. [Google Scholar] [CrossRef]
- Ding, L.; Wang, H.; Geng, H.; Cui, N.; Huang, F.; Zhu, X.; Zhu, X. Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Postnatal Risk Factors. Front. Pediatr. 2020, 8, 349. [Google Scholar] [CrossRef]
- Lure, A.C.; Du, X.; Black, E.W.; Irons, R.; Lemas, D.J.; Taylor, J.A.; Lavilla, O.; de la Cruz, D.; Neu, J. Using Machine Learning Analysis to Assist in Differentiating between Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Novel Predictive Analytic Tool. J. Pediatr. Surg. 2021, 56, 1703–1710. [Google Scholar] [CrossRef]
- López-Martínez, F.; Núñez-Valdez, E.R.; Gomez, J.L.; García-Díaz, V. A Neural Network Approach to Predict Early Neonatal Sepsis. Comput. Electr. Eng. 2019, 76, 379–388. [Google Scholar] [CrossRef]
- Gehle, D.B.; Chapman, A.; Gregoski, M.; Brunswick, M.; Anderson, E.; Ramakrishnan, V.; Muhammad, L.N.; Head, W.; Lesher, A.P.; Ryan, R.M. A Predictive Model for Preterm Babies Born < 30 Weeks Gestational Age Who Will Not Attain Full Oral Feedings. J. Perinatol. 2022, 42, 126–131. [Google Scholar] [CrossRef] [PubMed]
| Condition | Study | Study Population (n) | EV Source | EV Isolation (I) & Analysis (A) | EV-miRNA Isolation | miRNA | Statistical Performance |
|---|---|---|---|---|---|---|---|
| NEC | [64] | Neonates < 34 weeks GA NEC (12) Age-matched healthy controls (22) | Urine | I: ExoUrine EV Isolation Kit (System Biosciences) A: NTA, western blot, TEM | ExoRNEasy Midi Kits & Qiagen Qiaquick small RNA Kit (Qiagen Inc.) | 139-3p 604 5186 5703 | p < 0.05 p < 0.05 p < 0.05 p < 0.05 |
| BPD | [66] | Neonates < 32 weeks GA, DOL 28 BPD (39) Non-BPD controls (34) Neonatal Mice Exposed to hyperoxia (4) Exposed to air (controls) (3) | Serum | I: ExoQuick precipitation solution (System Biosciences) A: NTA & ExoScreen, western blot | mirVana miRNA Isolation kit (Ambion Applied Biosystems) | 21 | p = 0.001 AUC = 0.850 p < 0.01 |
| [67] | Neonates < 32 weeks GA BPD (12) Non-BPD controls (14) | UC Serum | I: PEG precipitation A: NTA & ExoScreen, western blot, TEM | SeraMir Exosome RNA Purification Kit (System Biosciences) | 17-5p 20b-5p 103a-3p 185-5p 200a-3p 765 | p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 | |
| [68] | Neonates 36 weeks PMA with BPD (25) GA-matched, FT controls, intubated for surgery (25) Neonates < 28 weeks GA BPD (15) Non-BPD controls (15) Neonatal Mice Exposed to hyperoxia (5-7) Exposed to air (controls) (5-7) | TA TA BALF | I: Ultracentrifugation A: NTA | miRCURY RNA Isolation Kit Cell and Plant with miRNA primers (Exiqon) | 876-3p | p = 0.001 p < 0.05 AUC = 0.917 p < 0.05 | |
| [69] | Neonatal Rats Exposed to hyperoxia (10) Exposed to air (controls) (10) | Lung Homogenate | I: Total exosome isolation reagent (Thermo Scientific) A: NTA, western blot, TEM | Trizol kits with miR primer (Beijing Dingguo Changsheng Biotechnology Co.) | 425 | p < 0.01 | |
| HIBD | [70] | Neonatal Mice, 24 hours post-surgery Unilateral carotid ligation + hypoxia (HIBD) (12) Sham surgery + normoxia (controls) (12) | Brain Homogenate | NA | NA | 182-5p * 342-3p * | p < 0.05 p < 0.05 |
| [71] | Neonates, FT Moderate to severe HIBD (7) Healthy control (7) | UC Serum | NA | NA | 92b-3p * 342-3p * | p = 0.016793 p = 0.00059 | |
| [72] | Neonatal Mice Hypoxia-preconditioned (3) | Brain homogenate | I: Ultracentrifugation and Sucrose Step Gradient A: NTA, western blot, electron microscopy | RNeasy Lipid Tissue Mini Kit (Qiagen) miRNA-Seq with NEXTFLEX small RNA kit (PerkinElmer) | 92b-3p 182-5p 342-3p | NA | |
| [73] | Neonates, FT HIBD (102) Healthy controls (60) | Serum | NA | NA | 410 * | p < 0.01 AUC = 0.886 | |
| [74] | Neonatal Rats Unilateral carotid ligation + hypoxia (HIBD) (12) Sham surgery + normoxia (controls) (12) | Brain homogenate | NA | NA | 124-3p * | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiller, E.A.; Cohen, K.; Lin, X.; El-Khawam, R.; Hanna, N. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. Int. J. Mol. Sci. 2023, 24, 2622. https://doi.org/10.3390/ijms24032622
Schiller EA, Cohen K, Lin X, El-Khawam R, Hanna N. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. International Journal of Molecular Sciences. 2023; 24(3):2622. https://doi.org/10.3390/ijms24032622
Chicago/Turabian StyleSchiller, Emily A., Koral Cohen, Xinhua Lin, Rania El-Khawam, and Nazeeh Hanna. 2023. "Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates" International Journal of Molecular Sciences 24, no. 3: 2622. https://doi.org/10.3390/ijms24032622
APA StyleSchiller, E. A., Cohen, K., Lin, X., El-Khawam, R., & Hanna, N. (2023). Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. International Journal of Molecular Sciences, 24(3), 2622. https://doi.org/10.3390/ijms24032622






