Antiproliferative Activity of Antibiotics through DNA Binding Mechanism: Evaluation and Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. General Aspects
2.2. Ex Vivo Studies
DNA Binding Studies
2.3. In Silico Studies
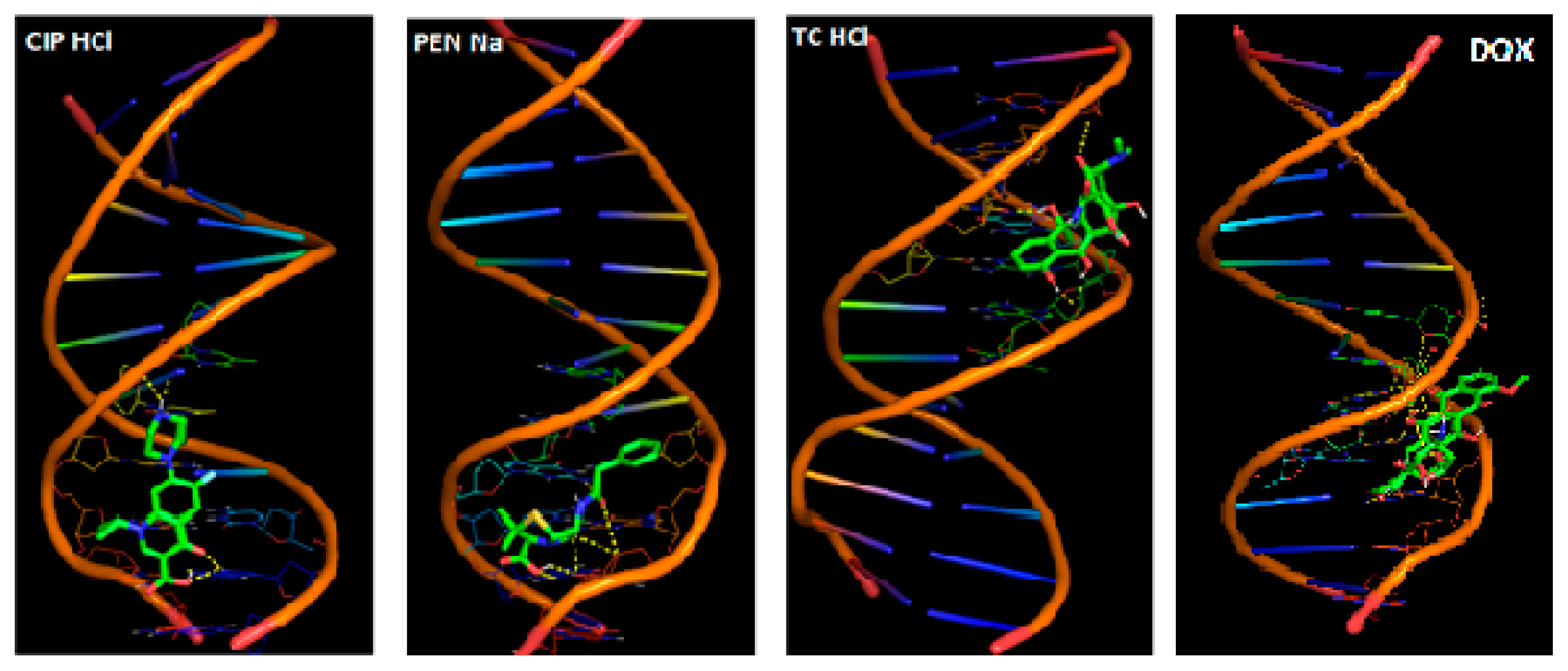
Computational Studies—Molecular Docking
2.4. In Vitro Studies
2.4.1. Anti-Proliferative Activity
2.4.2. Cell Cycle Arrest Study
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. DNA Binding Studies
4.2.1. Ultraviolet−Visible Studies
4.2.2. Viscosity Measurements
4.3. Molecular Docking
4.4. Sulforhodamine B (SRB) Assay
4.5. Cell Cycle Arrest
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S. New Approaches to Natural Anticancer Drugs. In Anticancer Antibiotics; Springer Briefs in Pharmaceutical Science & Drug Development; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 4. [Google Scholar] [CrossRef]
- Shields, M. Chapter 14—Chemotherapeutics. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 295–313. ISBN 978-0-12-802104-0. [Google Scholar]
- Hurley, L.H. DNA and Its Associated Processes as Targets for Cancer Therapy. Nat. Rev. Cancer 2002, 2, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Chrysouli, M.P.; Banti, C.N.; Kourkoumelis, N.; Moushi, E.E.; Tasiopoulos, A.J.; Douvalis, A.; Papachristodoulou, C.; Hatzidimitriou, A.G.; Bakas, T.; Hadjikakou, S.K. Ciprofloxacin Conjugated to Diphenyltin(IV): A Novel Formulation with Enhanced Antimicrobial Activity. Dalton Trans. 2020, 49, 11522–11535. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gao, H.-L.; Ashar, Y.V.; Karadkhelkar, N.M.; Yoganathan, S.; Chen, Z.-S. Ciprofloxacin Enhances the Chemosensitivity of Cancer Cells to ABCB1 Substrates. Int. J. Mol. Sci. 2019, 20, 268. [Google Scholar] [CrossRef]
- Kloskowski, T.; Szeliski, K.; Fekner, Z.; Rasmus, M.; Dąbrowski, P.; Wolska, A.; Siedlecka, N.; Adamowicz, J.; Drewa, T.; Pokrywczyńska, M. Ciprofloxacin and Levofloxacin as Potential Drugs in Genitourinary Cancer Treatment—The Effect of Dose–Response on 2D and 3D Cell Cultures. Int. J. Mol. Sci. 2021, 22, 11970. [Google Scholar] [CrossRef]
- Gurtowska, N.; Kloskowski, T.; Drewa, T. Ciprofloxacin Criteria in Antimicrobial Prophylaxis and Bladder Cancer Recurrence. Med. Sci. Monit. 2010, 16, RA218–RA223. [Google Scholar]
- Fan, M.; Chen, S.; Weng, Y.; Li, X.; Jiang, Y.; Wang, X.; Bie, M.; An, L.; Zhang, M.; Chen, B.; et al. Ciprofloxacin Promotes Polarization of CD86+CD206− Macrophages to Suppress Liver Cancer. Oncol. Rep. 2020, 44, 91–102. [Google Scholar] [CrossRef]
- Ketikidis, I.; Banti, C.N.; Kourkoumelis, N.; Tsiafoulis, C.G.; Papachristodoulou, C.; Kalampounias, A.G.; Hadjikakou, S.K. Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria. Antibiotics 2020, 9, 25. [Google Scholar] [CrossRef]
- Banti, C.N.; Ketikidis, I.; Hadjikakou, S.K.; Hatzidimitriou, A.G.; Grześkiewicz, A.M.; Kubicki, M.; Hadjiliadis, N. Study of Penicillin Degradation Mechanism upon Interaction with Silver(I) Ions. Inorg. Chim. Acta 2020, 509, 119683. [Google Scholar] [CrossRef]
- Wright, A.J.; Wilkowske, C.J. The Penicillins. Mayo Clin. Proc. 1983, 58, 21–32. [Google Scholar]
- Trialoni, P.Z.; Fyrigou, Z.-C.M.; Banti, C.N.; Hadjikakou, S.K. Conjugation of Tetracycline and Penicillin with Sb(v) and Ag(i) against Breast Cancer Cells. Main Group Met. Chem. 2022, 45, 152–168. [Google Scholar] [CrossRef]
- Friend, D.G.; Penicillin, G. Hypermethylation of CpG islands in primary and metastatic human PCa. Clin. Pharmacol. Ther. 1966, 7, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Saikali, Z.; Singh, G. Doxycycline and Other Tetracyclines in the Treatment of Bone Metastasis. Anti-Cancer Drugs 2003, 14, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Meretoudi, A.; Banti, C.N.; Siafarika, P.; Kalampounias, A.G.; Hadjikakou, S.K. Tetracycline Water Soluble Formulations with Enhanced Antimicrobial Activity. Antibiotics 2020, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Khani, H. Determination of Tetracycline at a UV-Irradiated DNA Film Modified Glassy Carbon Electrode. Electroanalysis 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Fuoco, D. Classification Framework and Chemical Biology of Tetracycline-Structure-Based Drugs. Antibiotics 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Khan, M.A.; Musarrat, J. Interactions of Tetracycline and Its Derivatives with DNA in vitro in Presence of Metal Ions. Int. J. Biol. Macromol. 2003, 33, 49–56. [Google Scholar] [CrossRef]
- Smythies, J.R.; Benington, F.; Morin, R.D. On the Molecular Mechanism of Action of the Tetracyclines. Experientia 1972, 28, 1253–1254. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, M.S. Deciphering the interaction of flavones with calf thymus DNA and octamer DNA sequence (CCAATTGG)2. RSC Adv. 2021, 11, 29354. [Google Scholar] [CrossRef]
- Banti, C.N.; Giannoulis, A.D.; Kourkoumelis, N.; Owczarzak, A.M.; Kubicki, M.; Hadjikakou, S.K. Novel Metallo-Therapeutics of the NSAID Naproxen. Interaction with Intracellular Components That Leads the Cells to Apoptosis. Dalton Trans. 2014, 43, 6848–6863. [Google Scholar] [CrossRef]
- Banti, C.N.; Giannoulis, A.D.; Kourkoumelis, N.; Owczarzak, A.M.; Poyraz, M.; Kubicki, M.; Charalabopoulos, K.; Hadjikakou, S.K. Mixed Ligand–Silver(I) Complexes with Anti-Inflammatory Agents Which Can Bind to Lipoxygenase and Calf-Thymus DNA, Modulating Their Function and Inducing Apoptosis. Metallomics 2012, 4, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA Interactions and Their Study by UV–Visible, Fluorescence Spectroscopies and Cyclic Voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal Complex Interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Jaividhya, P.; Ganeshpandian, M.; Dhivya, R.; Abdulkadher Akbarshac, M.; Palaniandavar, M. Fluorescent mixed ligand copper(II) complexes of anthracene-appended Schiff bases: Studies on DNA binding, nuclease activity and cytotoxicity. Dalton Trans. 2015, 44, 11997–12010. [Google Scholar] [CrossRef]
- Banti, C.N.; Raptopoulou, C.P.; Psycharis, V.; Hadjikakou, S.K. Novel Silver Glycinate Conjugate with 3D Polymeric Intermolecular Self-Assembly Architecture; an Antiproliferative Agent Which Induces Apoptosis on Human Breast Cancer Cells. J. Inorg. Chem. 2021, 216, 111351. [Google Scholar] [CrossRef]
- Banti, C.N.; Tsiatouras, V.; Karanicolas, K.; Panagiotou, N.; Tasiopoulos, A.J.; Kourkoumelis, N.; Hadjikakou, S.K. Antiproliferative Activity and Apoptosis Induction, of Organo-Antimony(III)–Copper(I) Conjugates, against Human Breast Cancer Cells. Mol. Divers. 2020, 24, 1095–1106. [Google Scholar] [CrossRef]
- Latsis, G.K.; Banti, C.N.; Kourkoumelis, N.; Papatriantafyllopoulou, C.; Panagiotou, N.; Tasiopoulos, A.; Douvalis, A.; Kalampounias, A.G.; Bakas, T.; Hadjikakou, S.K. Poly Organotin Acetates against DNA with Possible Implementation on Human Breast Cancer. Int. J. Mol. Sci. 2018, 19, 2055. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- El-Sofany, W.I.; El-sayed, W.A.; Abd-Rabou, A.A.; El-Shahat, M. Synthesis of New Imidazole-Triazole-Glycoside Hybrids as Anti-Breast Cancer Candidates. J. Mol. Struct. 2022, 1270, 133942. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Wahab, N.A.; Yaacob, H.; Shin, S.K.; Lai, H.S.; Serm, L.G.; Rahman, S.N.S.A. Cytotoxic Activity of Pereskia Bleo (Cactaceae) against Selected Human Cell Lines. Int. J. Cancer Res. 2008, 4, 20–27. [Google Scholar] [CrossRef]
- The PyMOL. Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Bredberg, A.; Brant, M.; Jaszyk, M. Ciprofloxacin-induced inhibition of topoisomerase II in human lymphoblastoid cells. Antimicrob. Agents Chemother. 1991, 35, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Papatriantafyllopoulou, C.; Manoli, M.; Tasiopoulos, A.J.; Hadjikakou, S.K. Nimesulide Silver Metallodrugs, Containing the Mitochondriotropic, Triaryl Derivatives of Pnictogen; Anticancer Activity against Human Breast Cancer Cells. Inorg. Chem. 2016, 55, 8681–8696. [Google Scholar] [CrossRef]
- Larsson, P.; Engqvist, H.; Biermann, J.; Werner Rönnerman, E.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T.Z. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 2020, 10, 5798. [Google Scholar] [CrossRef] [PubMed]
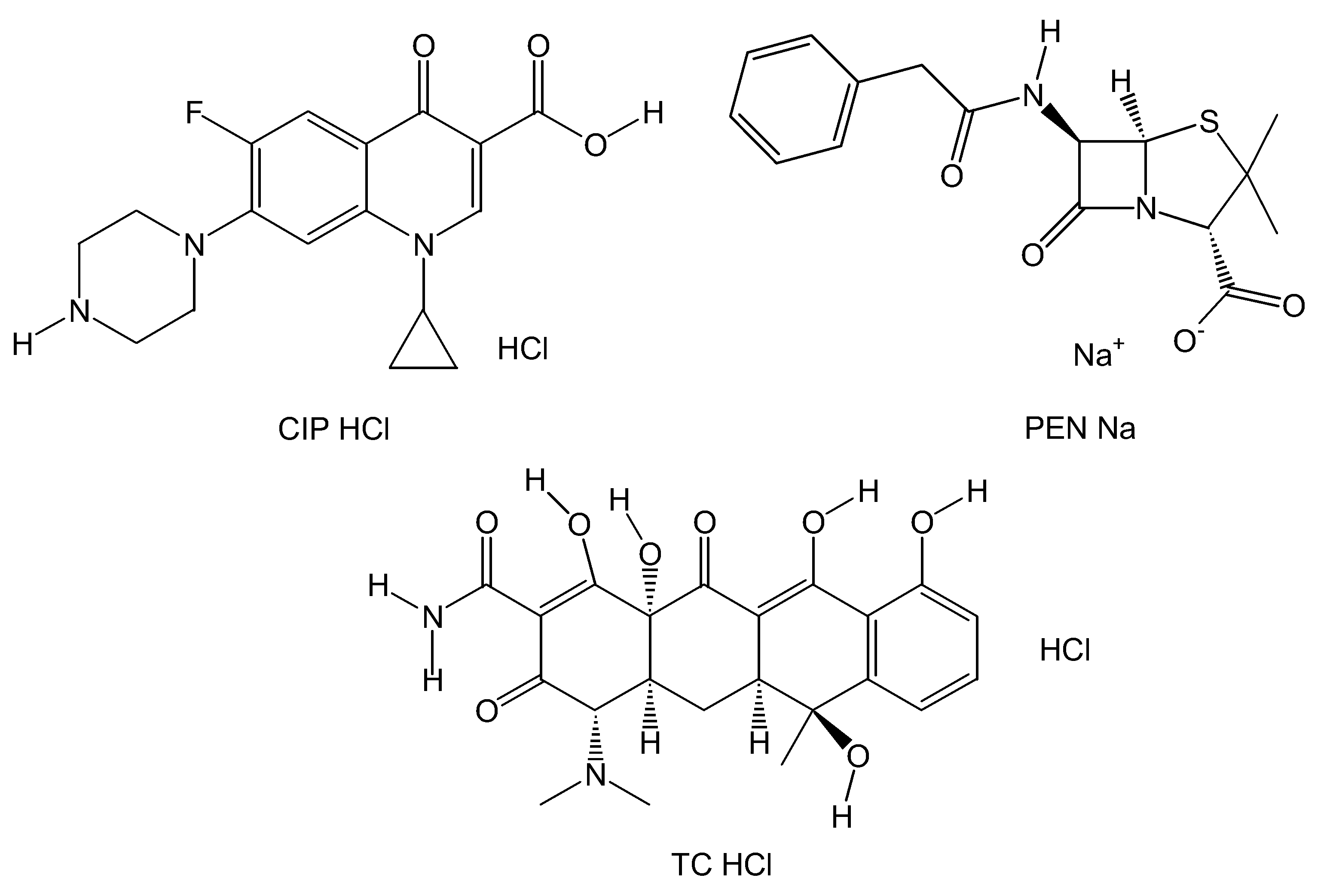


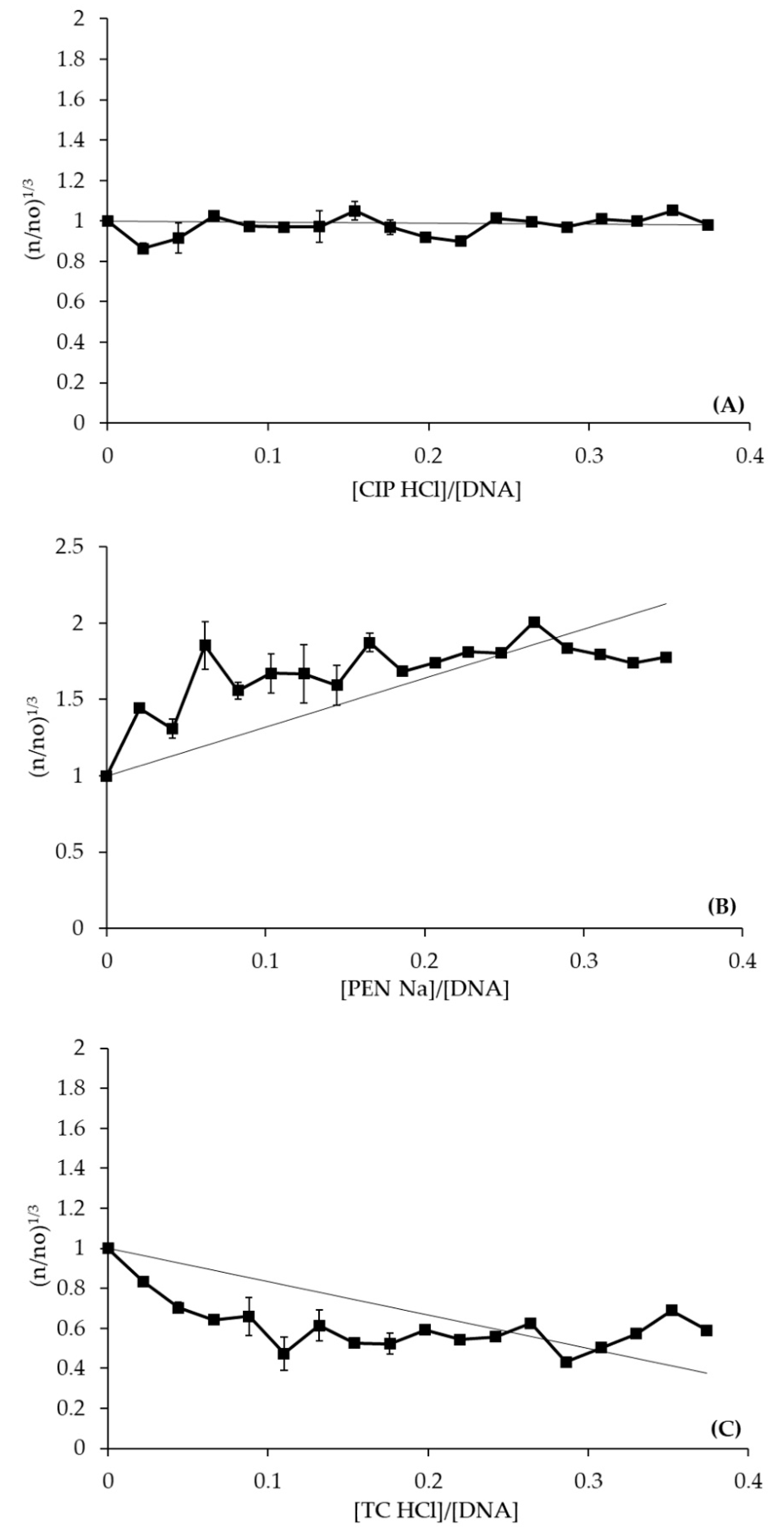

| Antibiotic | Kb (M−1) | Hyper-/Hypochromic Effect | H (%) |
|---|---|---|---|
| CIP·HCl | (2.8 ± 0.6) × 104 | Hyperchromism | 3.3 |
| PEN·Na | (0.4 ± 0.1) × 104 | Hypochromism | 1.4 |
| TC·HCl | (6.9 ± 0.3) × 104 | Hyperchromism | 8.5 |
| Antibiotic | Binding Energy (kcal/mol) | DNA Strand | Nucleotide | Nucleotide’s Atom | Atoms in Compound | Distance (Å) |
|---|---|---|---|---|---|---|
| CIP·HCl | −5.41 | B | dG14 | H7 | O2 | 2.242 |
| A | dT8 | O2 | H41 | 1.892 | ||
| PEN·Na | −6.13 | A | dG10 | H21 | O2 | 1.669 |
| B | dG16 | H22 | O5 | 2.205 | ||
| TC·HCl | −6.11 | A | dA6 | O3 | H51 | 2.038 |
| B | dC21 | O2 | H42 | 2.009 | ||
| A | dA6 | O3 | H56 | 1.809 | ||
| DOX | −9.36 | A | dG10 | H3 | O7 | 1.872 |
| Compounds | IC50 Values (μΜ) | TPI | Ref. | |
|---|---|---|---|---|
| MCF-7 | MRC-5 | |||
| CIP·HCl | 417.4 ± 28.2 | 210 ± 4.4 | 0.5 | * |
| PEN·Na | >2000 | >2000 | - | * |
| TC·HCl | 443.1 ± 17.2 | 382.1 ± 31.0 | 0.9 | * |
| DOX | 80.95 | 1.01 | 0.01 | [31,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magklaras, A.-D.C.; Banti, C.N.; Hadjikakou, S.K. Antiproliferative Activity of Antibiotics through DNA Binding Mechanism: Evaluation and Molecular Docking Studies. Int. J. Mol. Sci. 2023, 24, 2563. https://doi.org/10.3390/ijms24032563
Magklaras A-DC, Banti CN, Hadjikakou SK. Antiproliferative Activity of Antibiotics through DNA Binding Mechanism: Evaluation and Molecular Docking Studies. International Journal of Molecular Sciences. 2023; 24(3):2563. https://doi.org/10.3390/ijms24032563
Chicago/Turabian StyleMagklaras, Alexandros-Dimitrios C., Christina N. Banti, and Sotiris K. Hadjikakou. 2023. "Antiproliferative Activity of Antibiotics through DNA Binding Mechanism: Evaluation and Molecular Docking Studies" International Journal of Molecular Sciences 24, no. 3: 2563. https://doi.org/10.3390/ijms24032563
APA StyleMagklaras, A.-D. C., Banti, C. N., & Hadjikakou, S. K. (2023). Antiproliferative Activity of Antibiotics through DNA Binding Mechanism: Evaluation and Molecular Docking Studies. International Journal of Molecular Sciences, 24(3), 2563. https://doi.org/10.3390/ijms24032563








