Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Needs Assessment and Topic Selection
2.3. Study Development
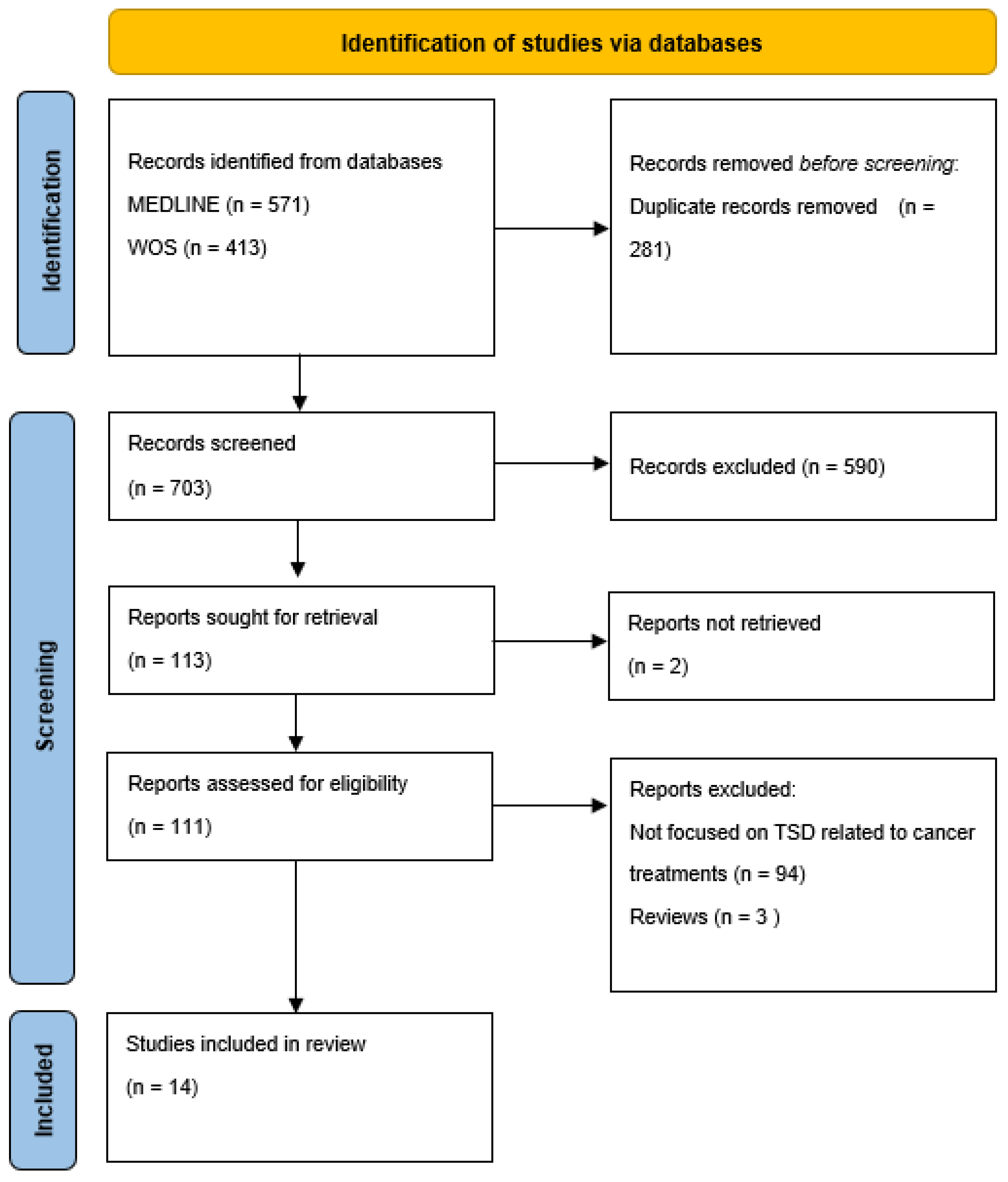
2.4. Literature Search
2.5. Screening and Study Selection
2.6. Data Extraction
2.7. Risk of Bias Assessment
| Ref. n. | Study Design | Aim (s) | Participants (N) | Assessment Tool (s) | Cancer Treatments | Key Findings |
|---|---|---|---|---|---|---|
| [9] | Prospective | Prevalence | 75 Prostate cancer | Survey regarding the taste and smell of food, appetite, and nausea. | CT and/or HT (regimen not specified) |
|
| [29] | Prospective | Self-reported TSDs based on the type of CT treatment. Impact of CT on the severity of the TSDs. | 151 | Questionnaire structured in three sections: eating habits; sensory changes (taste/smell changes and thermal sensitivity); and other clinical disorders (nausea, vomiting, dry mouth, mucositis, and dysphagia). | CT (regimen, Paclitaxel, oxaliplatin, docetaxel, carboplatin, anthracyclines, cisplatin, irinotecan, 5-FU, vinorelbine) |
|
| [30] | Cross-sectional | Prevalence, severity and self-reported characteristics of TAs induced by CT. TAs across CT regimes. | 243 | Validated TA Scale Self-reported TAs duration CiTAS. | CT (regimen: FOLFOX, paclitaxel, docetaxel, cisplatin, pemetrexed, FEC, EC, FOLFIRI, Gemcitabina, TJ, TPF, Gemcarbo, Cisgem, Gemox) |
|
| [31] | Prospective | Incidence of TAs | 41 BC | Not validated Questionnaire, filter paper disk method, CTCAE v. 4.0. | CT (regimen: Epirubicin, cyclophosphamide) |
|
| [32] | Prospective | Prevalence TAs across CT regimes. | 109 BC, gynecological | Validated TAs scale. | CT (regimen, Gemcitabina, epirubicin, docetaxel, capecitabine, epirubicin/docetaxel) |
|
| [33] | Prospective | To provide new data about TSDs. | 33 head/neck | Sniffin’ Sticks test (Determination of threshold, discrimination, and identification, TDI). | CT (regimen: Cisplatin, carboplatin, 5-FU, docetaxel) |
|
| [34] | Prospective | Prevalence of dysgeusia. | 31 males 15 females (9 did not undergo CT) | Salt-impregnated taste strips with 6 concentrations of Sodium chloride. | CT (regimen: 5-FU, platinum, Tx) |
|
| [35] | Prospective | Effect of cisplatin CT on odor perception. | 15 bronchial cancer patients and 15 control subjects | European Test of Olfactory Capabilities (ETOC). | CT (regimen: cisplatin) |
|
| [36] | Cross-sectional | TAs characteristics | 100 | Taste recognition thresholds (TRTs) via a taste disc kit PRO-CTCAE CiTAS. | CT (regimen: Tx based) |
|
| [37] | Observational | Prevalence and clinical therapeutic risk factors. | 7425 | CTCAE v5.0 | CT (not specified) |
|
| [38] | Mixed methods | To investigate whether mycotoxic and/or neurotoxic drugs compromise olfactory performance. | 44 | Sniffin’ Sticks test (Determination of threshold, discrimination, and identification, TDI). | CT (regimen: Oxaliplatin, 5-FU, capecitabine, gemcitabine, carboplatin, cisplatin, doxorubicin, liposomal doxorubicin, taxanes) |
|
| [39] | Case control | Changes in the perception of tastes. | 43 | Taste strips | CT (regimen: platinum based) |
|
| [40] | Observational | Changes in the detection (DT) and recognition (RT) thresholds of umami, sweet, and bitter tastes. | 40 (NSCLC) | Rinsing technique. Not validated Questionnaire | CT (regimen: Cisplatin, paclitaxel) |
|
| [41] | Qualitative | Patient and carer descriptions, experiences and consequences of taste and flavor changes. | 10 patients 4 carers | Semi-structured interviews | Ct (regimen: Oxaliplatin) |
|
3. Results
3.1. Study Characteristics
3.2. Prevalence, Onset, Resolution of Taste and Smell Disorders
3.3. Cancer Treatment
4. Discussion
4.1. Study Limitations
4.2. Implications for Clinical Practice and Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, B.B.; Leopold, D.A. Clinical assessment of patients with smell and taste disorders. Otolaryngol. Clin. N. Am. 2004, 37, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Sevryugin, O.; Kasvis, P.; Vigano, M.; Vigano, A. Taste and smell disturbances in cancer patients: A scoping review of available treatments. Support Care Cancer. 2021, 29, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Okada, N.; Nakamura, S.; Kagawa, K.; Fujii, S.; Miki, H.; Ishizawa, K.; Abe, M.; Sato, Y. A Genome-Wide Association Study Predicts the Onset of Dysgeusia Due to Anti-cancer Drug Treatment. Biol. Pharm. Bull. 2022, 45, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Gamper 0745, E.M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J. Pain Symptom. Manage. 2012, 44, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Taste and smell changes in patients receiving cancer chemotherapy: Distress, impact on daily life, and self-care strategies. Cancer Nurs. 2009, 32, 45–54. [Google Scholar] [CrossRef]
- Gill, S.S.; Frew, J.; Fry, A.; Adam, J.; Paleri, V.; Dobrowsky, W.; Chatterjee, S.; Kelly, C.G. Priorities for the head and neck cancer patient, their companion and members of the multidisciplinary team and decision regret. Clin. Oncol. (R Coll. Radiol). 2011, 23, 518–524. [Google Scholar] [CrossRef]
- Buttiron Webber, T.; Marra, D.; Puntoni, M.; Giuliano, S.; Briata, I.M.; Cevasco, I.; Clavarezza, M.; D’Amico, M.; Defferrari, C.; Gozza, A.; et al. Patient-versus physician-reported outcomes in a low-dose tamoxifen trial in noninvasive breast cancer. Breast. J. 2021, 27, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Amézaga 4296, J.; Alfaro, B.; Ríos, Y.; Larraioz, A.; Ugartemendia, G.; Urruticoechea, A.; Tueros, I. Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Support Care Cancer 2018, 26, 4077–4086. [Google Scholar] [CrossRef]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘alimentary’ taste. A new perspective on taste classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef]
- Witt, M. Anatomy and development of the human taste system. Handb. Clin. Neurol. 2019, 164, 147–171. [Google Scholar]
- Corremans, M.; Mortelmans, D.; Geurden, B.; Luyten, S.; Bekkering, G. Prevalence and incidence of chemotherapy-induced taste alterations in adult cancer patients: A systematic review protocol. JBI Evid. Synth. 2022, 20, 1338. [Google Scholar] [CrossRef] [PubMed]
- van Oort, S.; Kramer, E.; de Groot, J.-W.; Visser, O. Taste alterations and cancer treatment. Curr. Opin. Support Palliat 2018, 12, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Barlow, L.A.; Munger, S.D.; Millar, S.E.; Vergara, M.N.; Jiang, P.; Schwob, J.E.; Goldstein, B.J.; Boye, S.E.; Martens, J.R.; et al. Identifying Treatments for Taste and Smell Disorders: Gaps and Opportunities. Chem. Senses. 2020, 45, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D.; Holbrook, E.H.; Noell, C.A.; Mabry, R. Disorders of Taste and Smell Medscape; 2021 [Updated Aug, 2022]. Available online: https://emedicine.medscape.com/article/861242-overview (accessed on 22 November 2022).
- Liu, G.; Zong, G.; Doty, R.L.; Sun, Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: A cross-sectional study. BMJ Open 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef]
- Uí Dhuibhir, P.; Barrett, M.; O’Donoghue, N.; Gillham, C.; El Beltagi, N.; Walsh, D. Self-reported and objective taste and smell evaluation in treatment-naive solid tumour patients. Support Care Cancer 2020, 28, 2389. [Google Scholar] [CrossRef]
- Snyder 2396, D.J.; Prescott, J.; Bartoshuk, L.M. Modern psychophysics and the assessment of human oral sensation. Adv. Oto-Rhino-Laryngol. 2006, 63, 221–241. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R. The influence of chemotherapy on taste perception and food hedonics: A systematic review. Canc. Treat. Rev. 2012, 38, 152–163. [Google Scholar] [CrossRef]
- McGowan, D. Chemotherapy-induced oral dysfunction: A literature review. Br. J. Nurs. 2008, 17, 1422–1426. [Google Scholar] [CrossRef]
- Wismer, W.V. Assessing alterations in taste and their impact on cancer care. Curr. Opin. Support. Palliat. Care 2008, 2, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Fernandez, B.E.; Martinez-Michel, L.; Thorlakson, J.; Wismer, W.V. Patient-reported taste change assessment questionnaires used in the oncology setting: A narrative review. Eur. J. Oncol. Nurs. 2020, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- O’Leary 1775, D.F.; Casey, M.; O’Connor, L.; Stokes, D.; Fealy, G.M.; O’Brien, D.; Smith, R.; McNamara, M.S.; Egan, C. Using rapid reviews: An example from a study conducted to inform policy-making. J. Adv. Nurs. 2017, 73, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Langlois, E.V.; Straus, S.E. Rapid Reviews to Strengthen Health Policy and Systems: A Practical Guide; World Health Organization, Alliance for Health Policy and Systems Research: Geneva, Switzerland, 2017; ISBN 978 92 4 151276 3. [Google Scholar]
- Langlois, E.V.; Straus, S.E.; Antony, J.; King, V.J.; Tricco, A.C. Using rapid reviewto strengthen health policy and systems and progress towards universal health coverage. BMJ Glob Health 2019, 4, e001178. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 1. [Google Scholar]
- Pluddemann, A.; Aronson, J.K.; Onakpoya, I.; Heneghan, C.; Mahtani, K.R. Redefining rapid reviews: A flexible framework for restricted systematic reviews. BMJ Evid. Based Med. 2018, 23, 201–203. [Google Scholar] [CrossRef]
- Alonzi, S.; Hoerger, M.; Perry, L.M.; Chow, L.D.; Manogue, C.; Cotogno, P.; Ernst, E.M.; Ledet, E.M.; Sartor, O. Changes in taste and smell of food during prostate cancer treatment. Support Care Cancer 2021, 29, 2807–2809. [Google Scholar] [CrossRef]
- Campagna, S.; Gonella, S.; Sperlinga, R.; Giuliano, P.L.; Marchese, R.; Pedersini, R.; Berchialla, P.; Dimonte, V. Prevalence, Severity, and Self-Reported Characteristics of Taste Alterations in Patients Receiving Chemotherapy. Oncol. Nurs. Forum. 2018, 45, 342–353. [Google Scholar] [CrossRef]
- Denda, Y.; Niikura, N.; Satoh-Kuriwada, S.; Yokoyama, K.; Terao, M.; Morioka, T.; Tsuda, B.; Okamura, T.; Ota, Y.; Tokuda, Y.; et al. Taste alterations in patients with breast cancer following chemotherapy: A cohort study. Breast Cancer 2020, 27, 954–962. [Google Scholar] [CrossRef]
- Gamper, E.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Wintner, L.M.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B.; Zabernigg, A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: Prevalence, course of severity, and quality of life correlates. Acta. Oncol. 2012, 51, 490–496. [Google Scholar] [CrossRef]
- Haxel, B.R.; Berg, S.; Boessert, P.; Mann, W.J.; Fruth, K. Olfaction in chemotherapy for head and neck malignancies. Auris. Nasus. Larynx. 2016, 43, 74–78. [Google Scholar] [CrossRef]
- Imai, H.; Soeda, H.; Komine, K.; Otsuka, K.; Shibata, H. Preliminary estimation of the prevalence of chemotherapy-induced dysgeusia in Japanese patients with cancer. BMC Palliat. Care 2013, 12, 38. [Google Scholar] [CrossRef]
- Joussain, P.; Giboreau, A.; Fontas, M.; Laville, M.; Hummel, T.; Souquet, P.J.; Bensafi, M. Cisplatin chemotherapy induces odor perception changes in bronchial cancer patients. Lung. Cancer 2013, 82, 168–170. [Google Scholar] [CrossRef]
- Kaizu, M.; Komatsu, H.; Yamauchi, H.; Yamauchi, T.; Sumitani, M.; Doorenbos, A.Z. Characteristics of taste alterations in people receiving taxane-based chemotherapy and their association with appetite, weight, and quality of life. Support Care Cancer 2021, 29, 5103–5114. [Google Scholar] [CrossRef]
- Malta, C.E.N.; de Lima Martins, J.O.; Carlos, A.C.A.M.; Freitas, M.O.; Magalhães, I.A.; de Vasconcelos, H.C.A.; de Lima Silva-Fernandes, I.J.; de Barros Silva, P.G. Risk factors for dysgeusia during chemotherapy for solid tumors: A retrospective cross-sectional study. Support Care Cancer 2022, 5114, 313–325. [Google Scholar] [CrossRef]
- Riga, M.; Chelis, L.; Papazi, T.; Danielides, V.; Katotomichelakis, M.; Kakolyris, S. Hyposmia: An underestimated and frequent adverse effect of chemotherapy. Support Care Cancer 2015, 23, 3053–3058. [Google Scholar] [CrossRef]
- Sicchieri, J.M.F.; Peria, F.M.; Sartorelli, D.S.; Diez-Garcia, R.W. Recognition of taste in patients during antineoplastic therapy with platinum drugs. Nutrition 2019, 67–68, 11. [Google Scholar] [CrossRef]
- Turcott, J.G.; Juárez-Hernández, E.; De la Torre-Vallejo, M.; Sánchez-Lara, K.; Luvian-Morales, J.; Arrieta, O. Value: Changes in the Detection and Recognition Thresholds of Three Basic Tastes in Lung Cancer Patients Receiving Cisplatin and Paclitaxel and Its Association with Nutritional and Quality of Life Parameters. Nutr. Cancer 2016, 68, 241–249. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R.; Aranda, S. Experiences and consequences of altered taste, flavour and food hedonics during chemotherapy treatment. Support Care Cancer 2012, 20, 2765–2774. [Google Scholar] [CrossRef]
- Shiffman, S.S. Influence of Drugs on Taste Function. In Handbook of Olfaction and Gustation, 3rd ed.; Doty, R.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 911–926. [Google Scholar]
- Qato, D.M.; Alexander, G.C.; Conti, R.M.; Johnson, M.; Schumm, P.; Lindau, S.T. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008, 300, 2867–2878. [Google Scholar] [CrossRef]
- Hamor, G.H.; Lafdjian, A. Dualistic thiourea moiety taste response of methimazole. J. Pharm. Sci. 1967, 56, 777–778. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Age-related changes in pharmacokinetics. Curr. Drug Metab. 2011, 12, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Nafziger, A.N.; Bertino, J.S., Jr. Evaluation of inhibitory drug interactions during drug development: Genetic polymorphisms must be considered. Clin. Pharmacol. Ther. 2005, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buttiron Webber, T.; Briata, I.M.; DeCensi, A.; Cevasco, I.; Paleari, L. Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review. Int. J. Mol. Sci. 2023, 24, 2538. https://doi.org/10.3390/ijms24032538
Buttiron Webber T, Briata IM, DeCensi A, Cevasco I, Paleari L. Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review. International Journal of Molecular Sciences. 2023; 24(3):2538. https://doi.org/10.3390/ijms24032538
Chicago/Turabian StyleButtiron Webber, Tania, Irene Maria Briata, Andrea DeCensi, Isabella Cevasco, and Laura Paleari. 2023. "Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review" International Journal of Molecular Sciences 24, no. 3: 2538. https://doi.org/10.3390/ijms24032538
APA StyleButtiron Webber, T., Briata, I. M., DeCensi, A., Cevasco, I., & Paleari, L. (2023). Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review. International Journal of Molecular Sciences, 24(3), 2538. https://doi.org/10.3390/ijms24032538







