Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics
Abstract
1. Introduction
2. Results
2.1. Plaque Staining
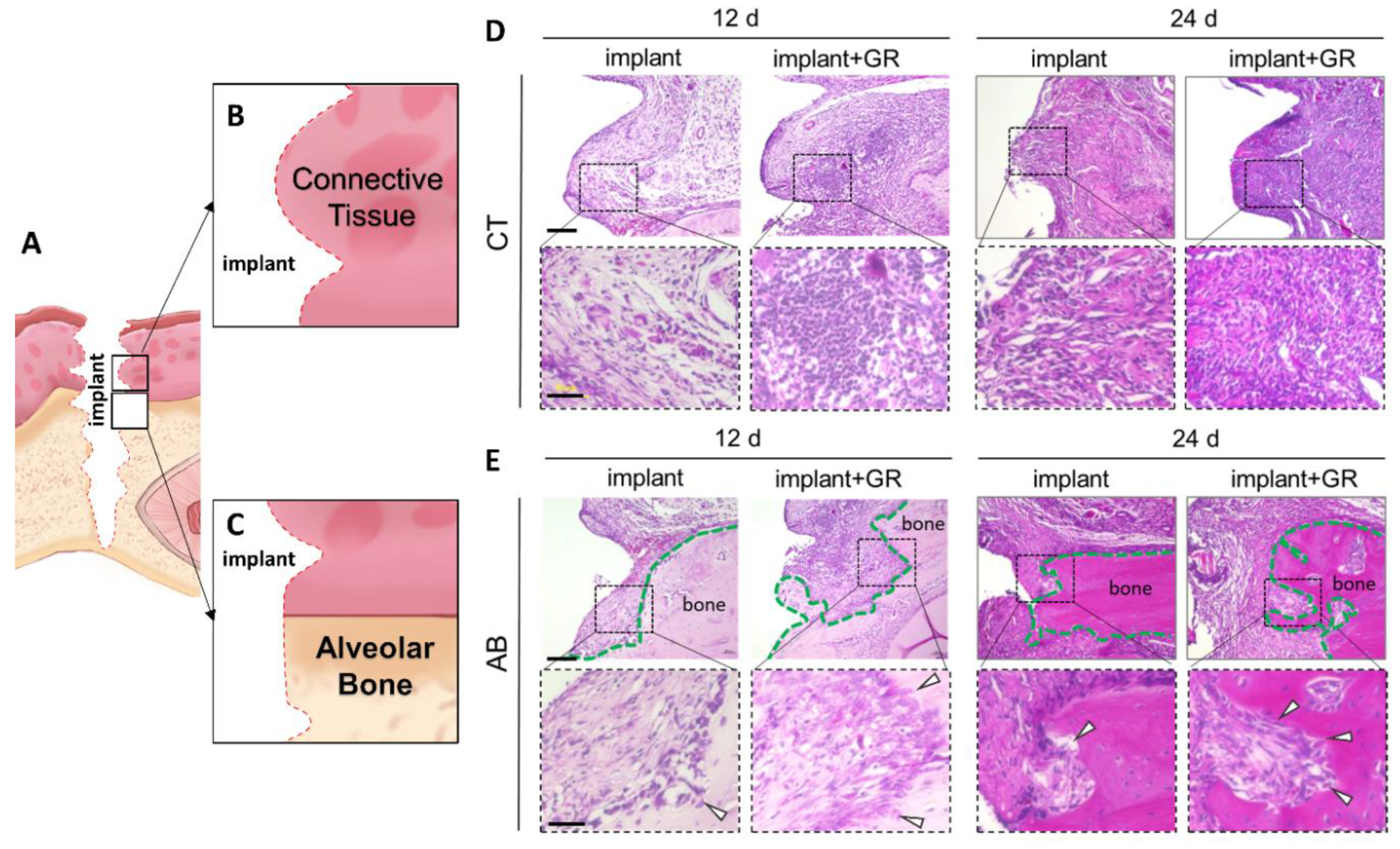
2.2. Inflammatory Cell Infiltration and Bone Resorption
2.2.1. Hematoxylin–Eosin (HE) Staining
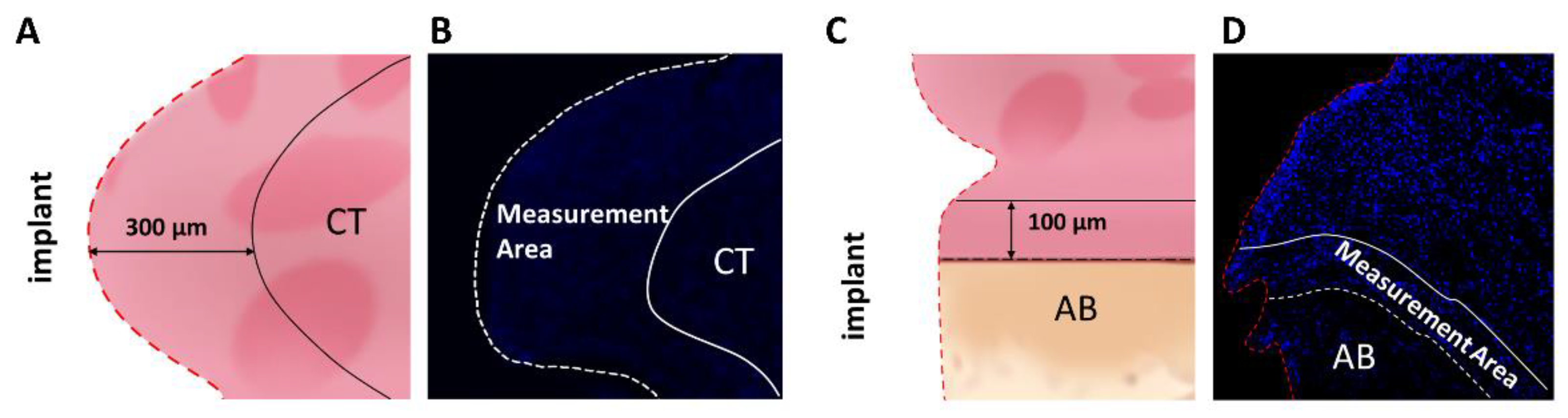
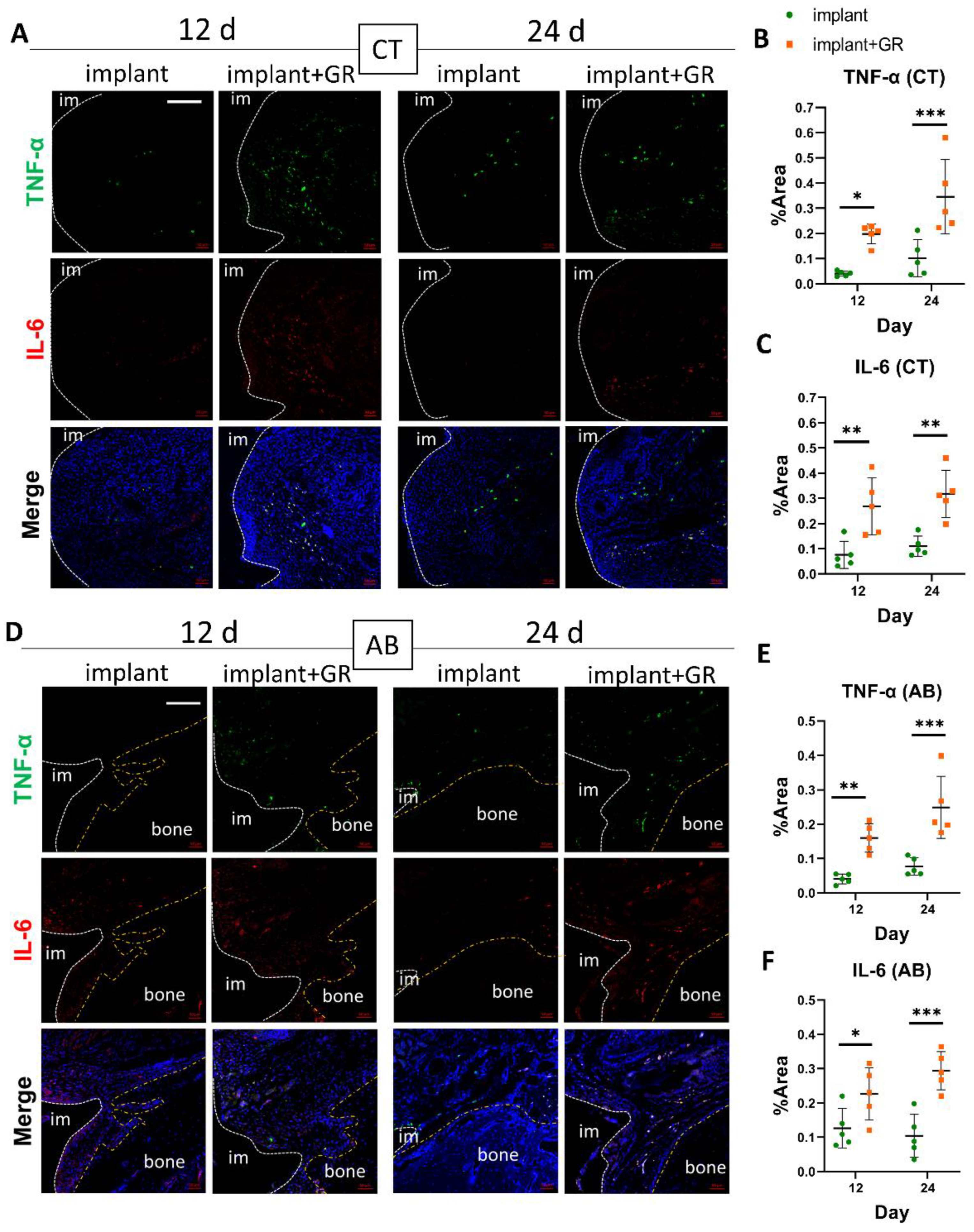
2.2.2. Inflammatory Markers by Immunohistochemistry Staining
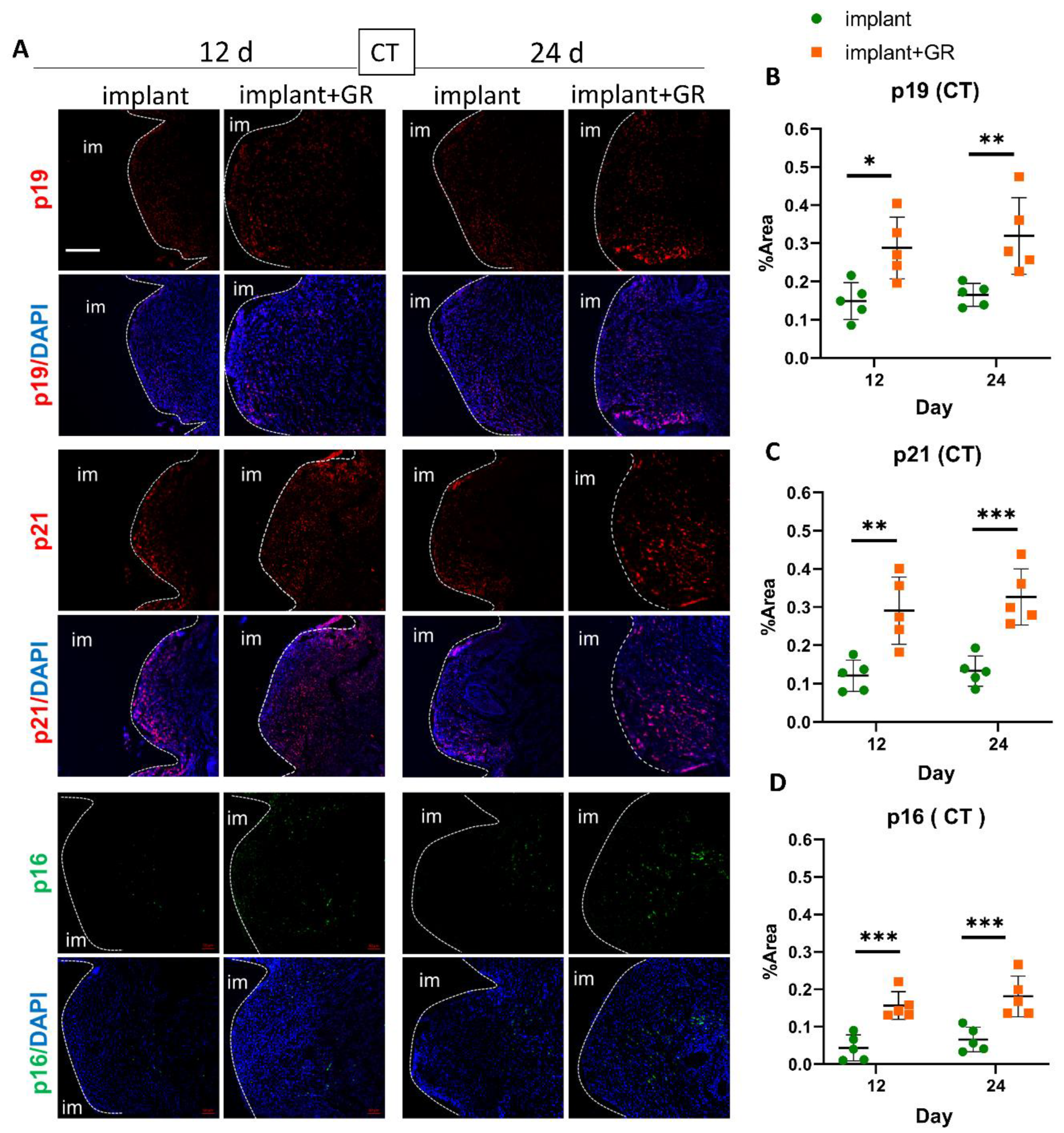
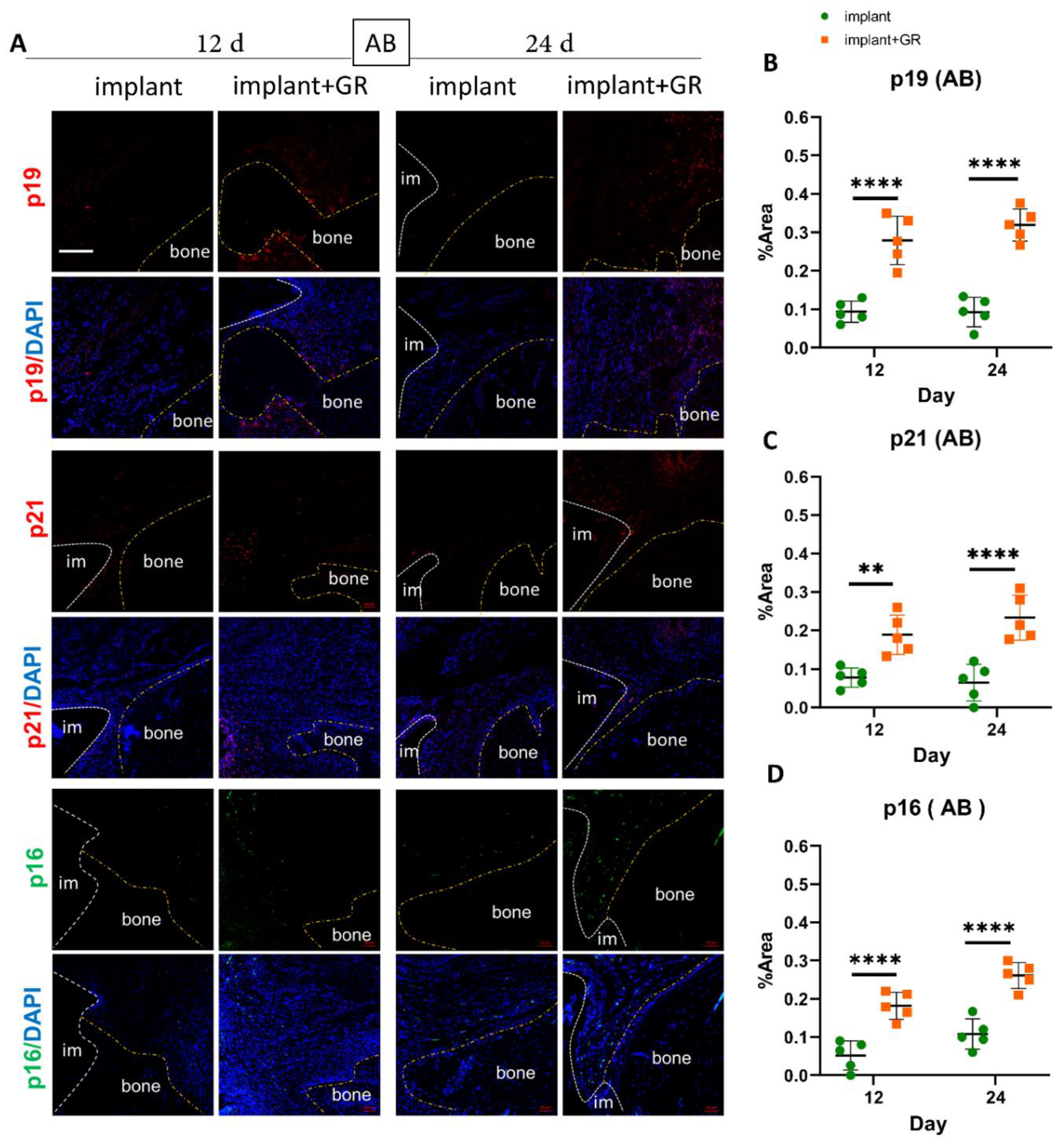
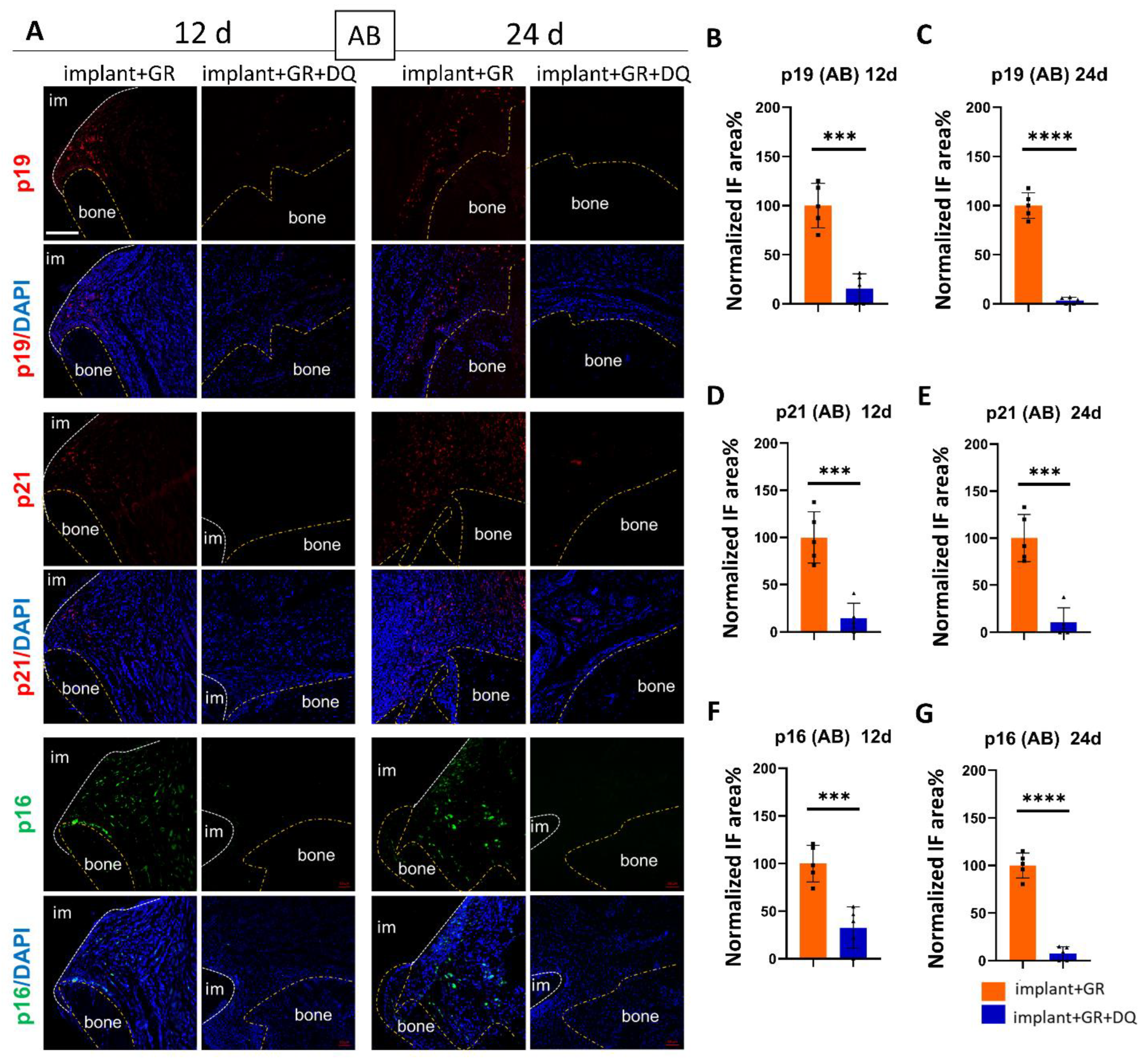
2.3. Identification of Senescent Cells by IHC Staining
2.4. Effectiveness of Senolytics for Peri-Implantitis
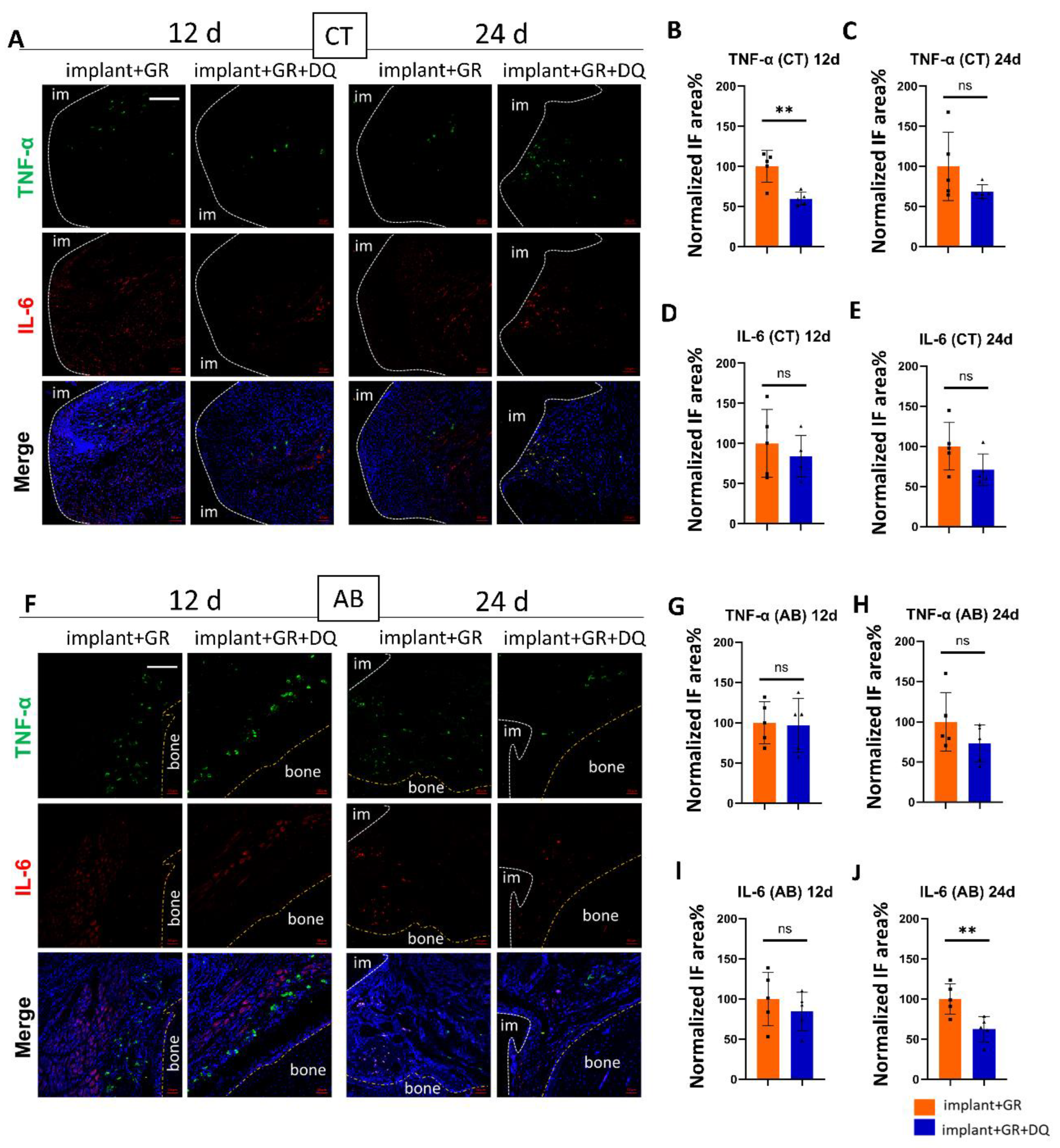
2.4.1. Inflammatory Markers by IHC Staining after Dasatinib + Quercetin (D + Q) Administration
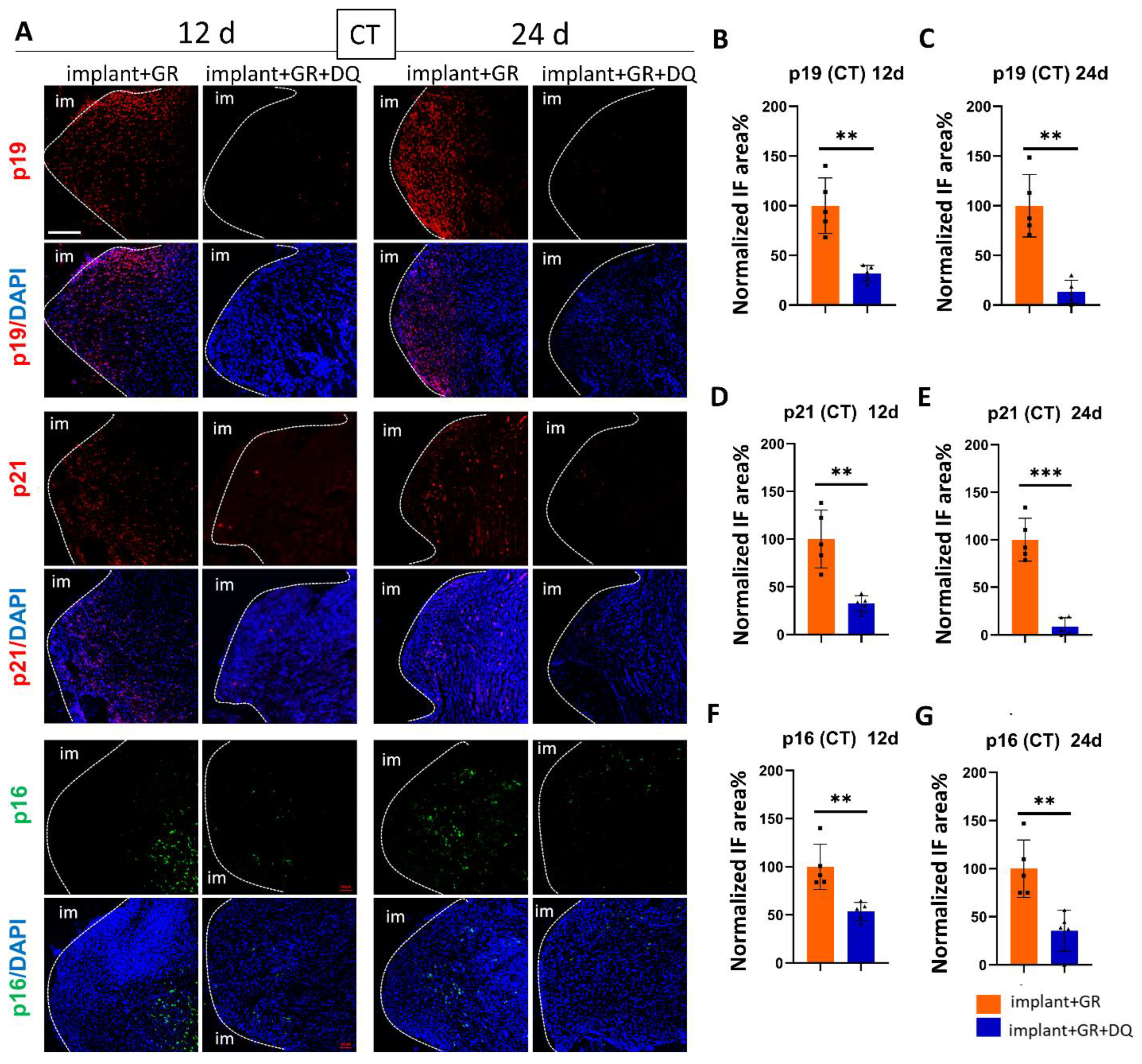
2.4.2. Identification of Senescent Cells by IHC Staining after D + Q Administration
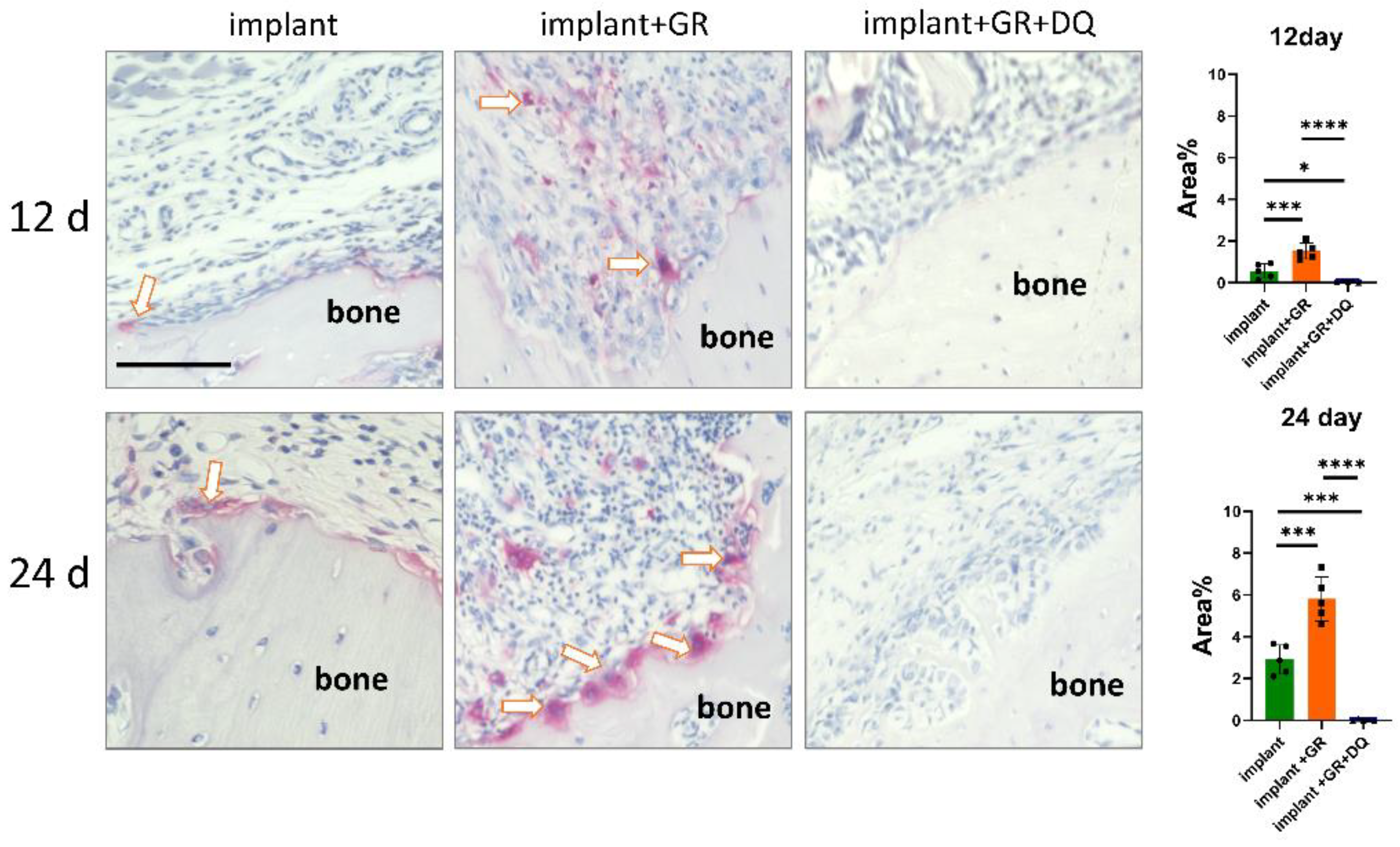
2.4.3. Osteoclast Location by Tartrate-Resistant Acid Phosphatase Staining
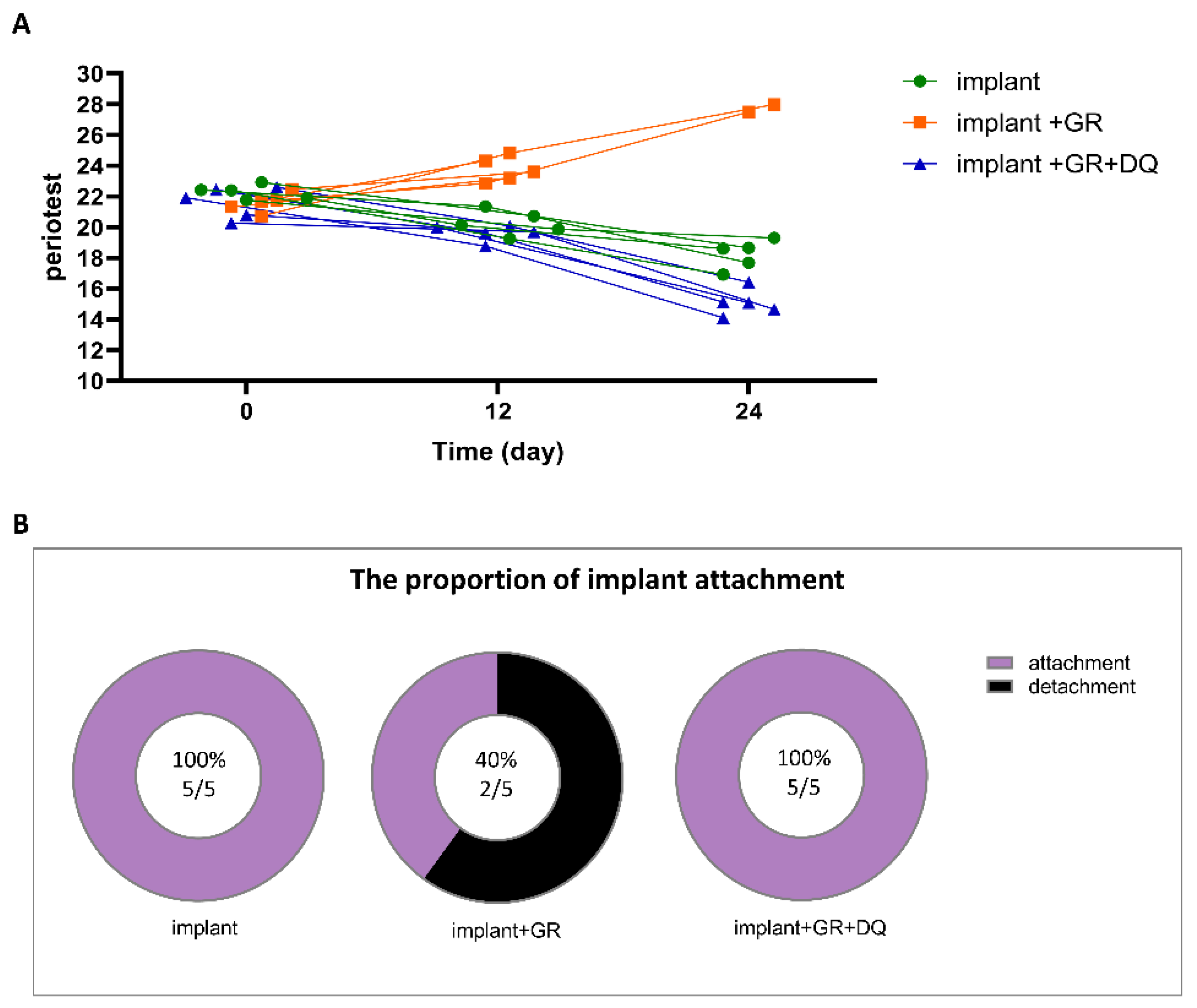
2.5. Implant Mobility and Detachment Rate
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Construction of Rat Peri-Implantitis Model
4.3. Sample Collection
4.4. Histological Staining
4.5. Immunofluorescence
4.6. Preparation and Administration of Senolytics Using Dasatinib and Quercetin
4.7. Implant Mobility and Detachment
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhart, T.; Vollgruber, A.; Gahleitner, A.; Dörtbudak, O.; Haas, R. Alternative to the median region of the palate for placement of an orthodontic implant. Clin. Oral Implant. Res. 2000, 11, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Hssieh, C.; Chen, C.; Shen, Y.; Huang, I.; Chen, C. The application of mini-implants for orthdontic anchorage. Int. J. Oral Maxillofac. Surg. 2006, 35, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Mario, S.M.P.; Leandro, C.R.; Guilherme, M.A.; Ricardo, T.C. Use of orthodontic mini-implants for maxillomandibular fixation in mandibular fracture. Craniomaxillofac. Trauma Reconstr. 2011, 4, 213–216. [Google Scholar]
- Axel, B.; Dirk, W.; Rupert, D. Mini- and micro-screws for temporary skeletal anchorage in orthodontic therapy. J. Orofac. Orthop. 2006, 6, 450–458. [Google Scholar]
- Vicioni-Marques, F.; Pimentel, D.J.B.; Matsumoto, M.A.N.; Stuani, M.B.S.; Romano, F.L. Orthodontic mini-implants: Clinical and peri-implant evaluation. J. World Fed. Orthod. 2022, 11, 22–28. [Google Scholar] [CrossRef]
- Antonino, L.G.; Lorenzo, R.; Miriam, L.; Giacomo, O.; Moschos, A.P.; Riccardo, N. Complications reported with the use of orthodontic miniscrews: A systematic review. Korean J. Orthod. 2021, 51, 199–216. [Google Scholar]
- Jayachandran, P. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516. [Google Scholar]
- Zhang, Q.; Liu, J.; Ma, L.; Bai, N.; Xu, H. LOX-1 is involved in TLR2 induced RANKL regulation in peri-implantitis. Int. Immunopharmacol. 2019, 77, 105956. [Google Scholar] [CrossRef]
- Petkovic-Curcin, A.; Matic, S.; Vojvodic, D.; Stamatovic, N.; Todorovic, T. Cytokines in pathogenesis of peri-implantitis. Vojnosanit. Pregl. 2011, 68, 435–440. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Chen, J.H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Nie, L.; Zhao, P.; Ji, N.; Liao, G.; Wang, Q. Senescence-associated secretory phenotype and its impact on oral immune homeostasis. Front. Immunol. 2022, 13, 1019313. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Zheng, L.; Wang, Q.; Hu, Y.W. The emerging role of cell senescence in atherosclerosis. Clin. Chem. Lab. Med. 2020, 59, 27–38. [Google Scholar] [CrossRef]
- Taneichi, K.; Baba, Y.; Sakai, I.; Shibaki, H. The indications for the use of salazosulfapyridine in rheumatoid arthritis patients. Ryumachi 1994, 34, 744–751. [Google Scholar]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Liu, H.; Mi, Y.; Gou, X. Astrocyte Senescence and Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2020, 12, 148. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Adam, K.; Malgorzata, W.; Gregory, P.; Agnieszka, G.; Magdalena, D.; Anna, B.Z.; Grazyna, M.; Izabela, F.; Jakub, W.; Sikora, E. Combination of dasatinib and quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile. Aging 2022, 14, 572–595. [Google Scholar]
- Deng, N.; Xie, L.; Li, Y.; Lin, H.; Luo, R. Oxymatrine alleviates periodontitis in rats by inhibiting inflammatory factor secretion and regulating MMPs/TIMP protein expression1. Acta Cir. Bras. 2018, 33, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Ogrodnik, M.B.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Marinello, C.; Ericsson, I.; Liljenberg, B. Soft tissue reaction to de novo plaque formation on implants and teeth. Clin. Oral. Implants Res. 1992, 3, 1–8. [Google Scholar] [CrossRef]
- Koutouzis, T.; Eastman, C.; Chukkapalli, S.; Larjava, H.; Kesavalu, L. A Novel Rat Model of Polymicrobial Peri-Implantitis: A Preliminary Study. J. Periodontol. 2017, 88, e32–e41. [Google Scholar] [CrossRef]
- Marcelo, O.F.; Parish, P.S.; Christoph, S.; Amita, G.; Jennifer, S.D.; Jeong-Ho, C.; Weizhen, C.; Joong-Ki, K.; Casey, C.; Steven, D.G.; et al. Development of an animal model for aggregatibacter actinomycetemcomitans. J. Periodontol. 2011, 82, 778–789. [Google Scholar]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Akiyama, S.; Amano, A.; Kato, T.; Takada, Y.; Kimura, K.R.; Morisaki, I. Relationship of periodontal bacteria and Porphyromonas gingivalis fimA variations with phenytoin-induced gingival overgrowth. Oral. Dis. 2006, 12, 51–56. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Lappalainen, R.; Laine, P.; Kitti, U.; Santavirta, S.; Teronen, O. Immunohistochemical evaluation of inflammatory mediators in failing implants. Int. J. Periodontics Restor. Dent. 2006, 26, 135–141. [Google Scholar]
- Kawamoto, T.; Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot’s film method (2012). Methods Mol. Biol. 2014, 1130, 149–164. [Google Scholar]
- Wang, X.; Honda, Y.; Zhao, J.; Morikuni, H.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Enhancement of Bone-Forming Ability on Beta-Tricalcium Phosphate by Modulating Cellular Senescence Mechanisms Using Senolytics. Int. J. Mol. Sci. 2021, 22, 12415. [Google Scholar] [CrossRef]
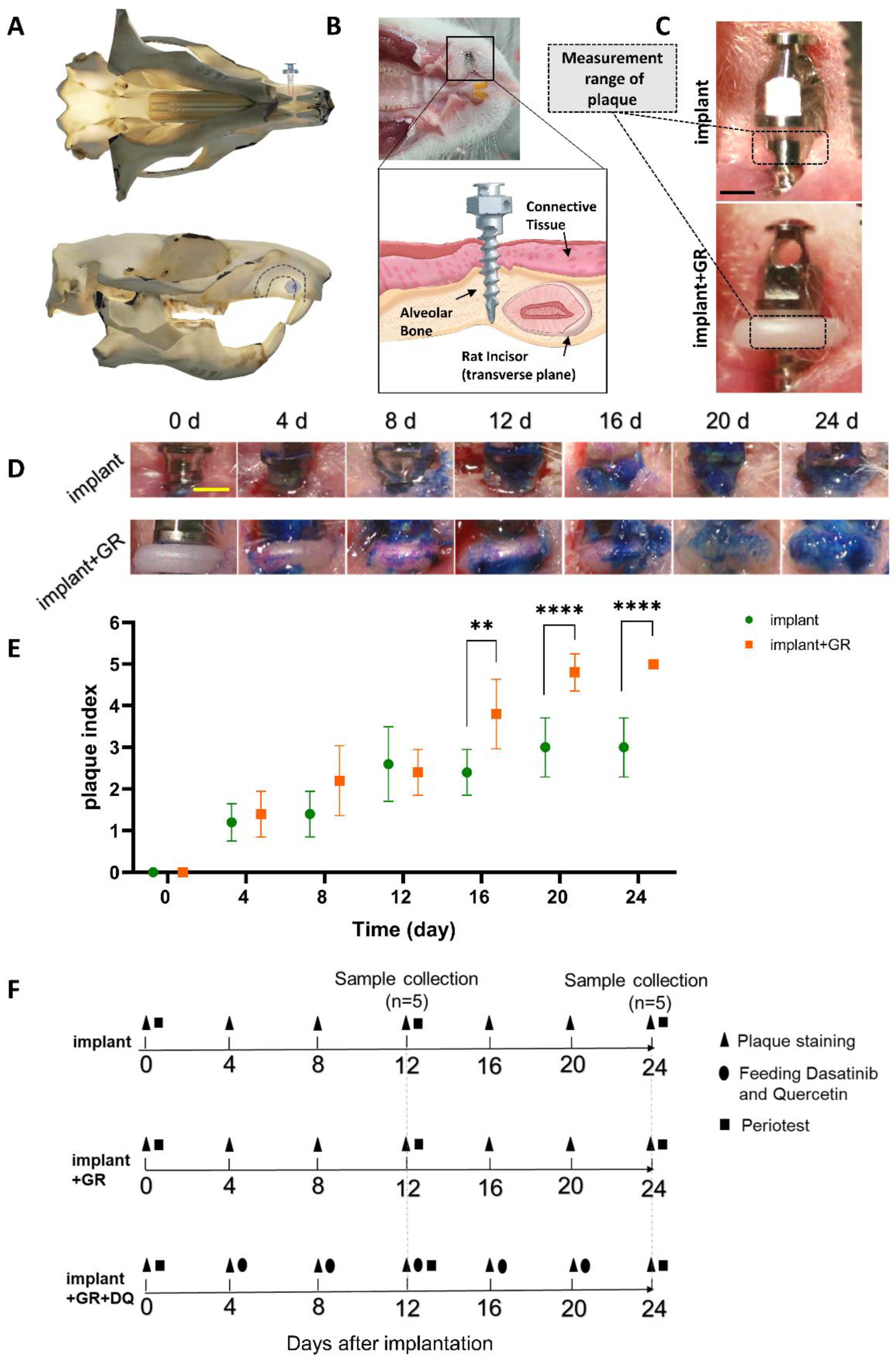
| Score | Criteria |
|---|---|
| 0 | No plaque |
| 1 | Flecks of plaque on the edge of the measurement range |
| 2 | Definitive line of plaque on the edge of the measurement range |
| 3 | A band of plaque covering less than one-third of the measurement range |
| 4 | Plaque covering at least one-third but less than two-thirds of the measurement range |
| 5 | Plaque covering two-thirds or more of the measurement range |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Nakagawa, M.; Nishiura, A.; Yamada, M.; Morikuni, H.; Honda, Y.; Matsumoto, N. Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics. Int. J. Mol. Sci. 2023, 24, 2507. https://doi.org/10.3390/ijms24032507
Yang N, Nakagawa M, Nishiura A, Yamada M, Morikuni H, Honda Y, Matsumoto N. Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics. International Journal of Molecular Sciences. 2023; 24(3):2507. https://doi.org/10.3390/ijms24032507
Chicago/Turabian StyleYang, Niuxin, Masato Nakagawa, Aki Nishiura, Masahiro Yamada, Hidetoshi Morikuni, Yoshitomo Honda, and Naoyuki Matsumoto. 2023. "Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics" International Journal of Molecular Sciences 24, no. 3: 2507. https://doi.org/10.3390/ijms24032507
APA StyleYang, N., Nakagawa, M., Nishiura, A., Yamada, M., Morikuni, H., Honda, Y., & Matsumoto, N. (2023). Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics. International Journal of Molecular Sciences, 24(3), 2507. https://doi.org/10.3390/ijms24032507






