Serum Amyloid A in Stable Patients with Chronic Obstructive Pulmonary Disease Does Not Reflect the Clinical Course of the Disease
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
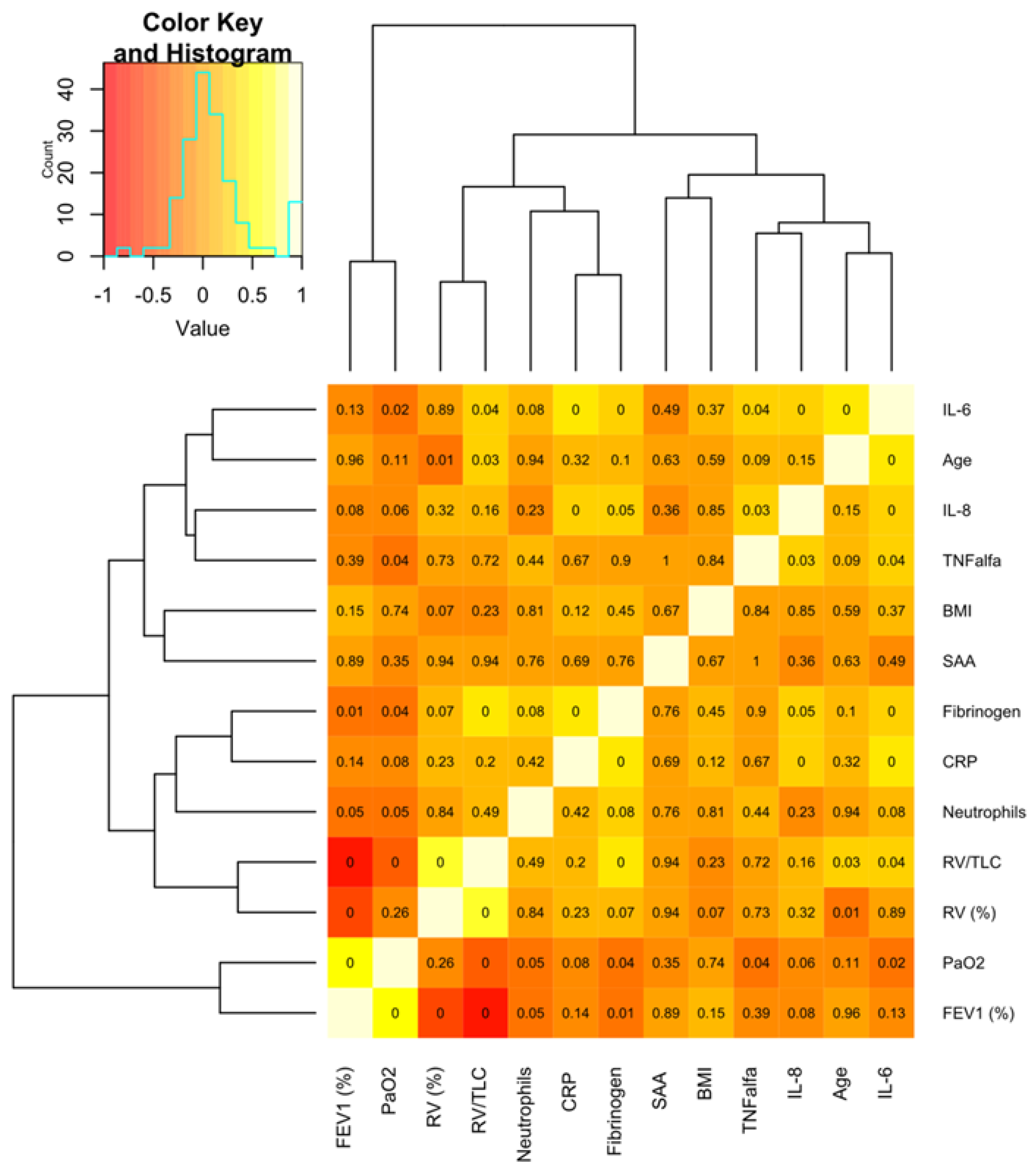
2.2. SAA and Other Markers of Inflammation in Peripheral Blood
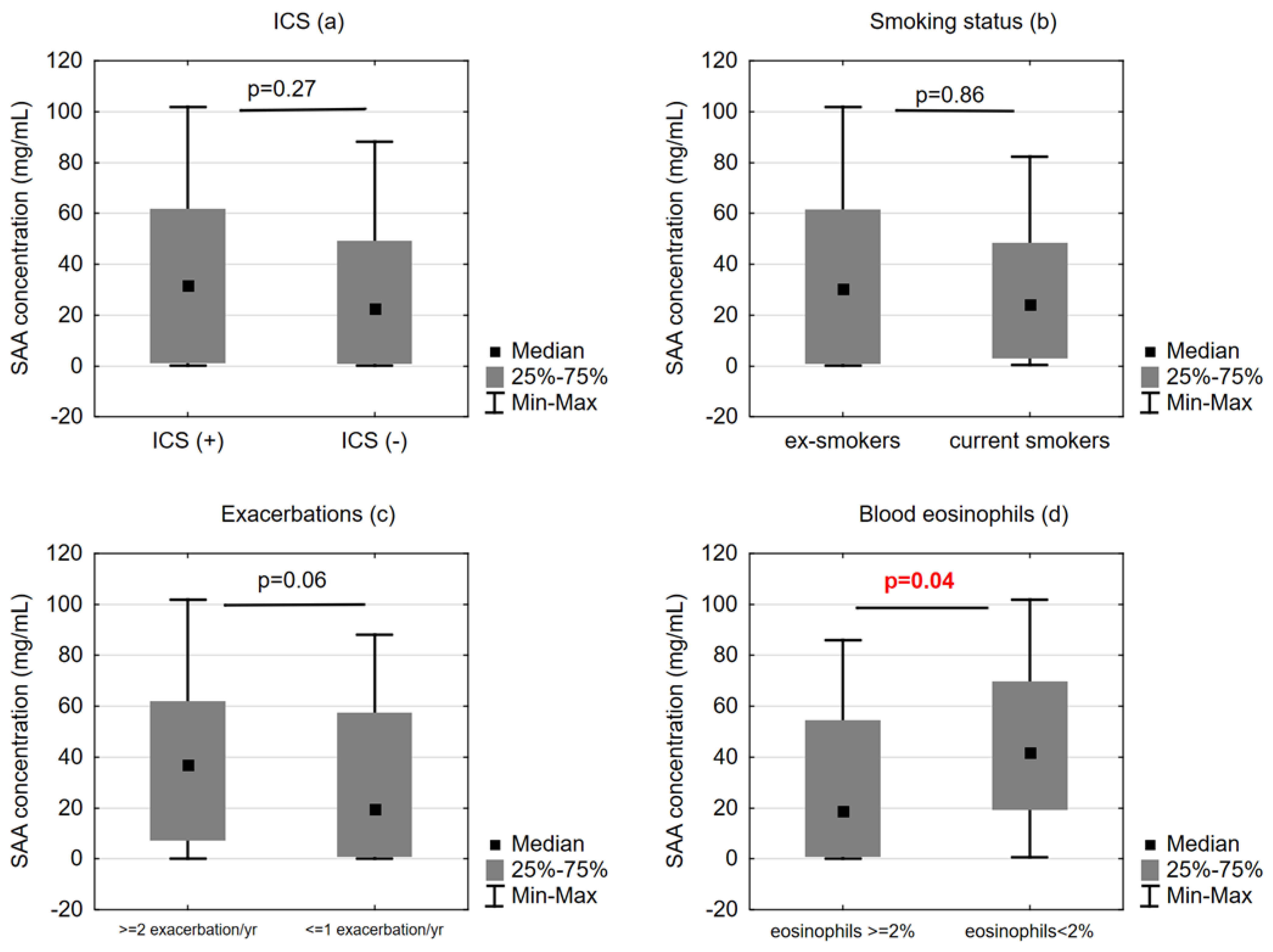
2.3. SAA Concentrations and Functional/Clinical Features of COPD
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Symptom Severity and Functional Assessment
4.3. Blood Sampling and SAA, IL-6, IL-8 and TNF-Alpha Measurement
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urieli-Shoval, S.; Linke, R.P.; Matzner, Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr. Opin. Hematol. 2000, 7, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Sun, L. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.; De Beer, F.C. Human serum amyloid A (SAA) protein: A prominent acute-phase reactant for clinical practice. Eur. J. Clin. Investig. 1996, 26, 427–435. [Google Scholar] [CrossRef] [PubMed]
- De Buck, M.; Gouwy, M.; Ming Wang, J.; Van Snick, J.; Opdenakker, G.; Struyf, S.; Van Damme, J. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions during Host Insults. Curr. Med. Chem. 2016, 23, 1725–1755. [Google Scholar] [CrossRef] [PubMed]
- He, R.L.; Zhou, J.; Hanson, C.Z.; Chen, J.; Cheng, N.; Ye, R.D. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 2009, 113, 429–437. [Google Scholar] [CrossRef]
- Bozinovski, S.; Hutchinson, A.; Thompson, M.; MacGregor, L.; Black, J.; Giannakis, E.; Karlsson, A.-S.; Silvestrini, R.; Smallwood, D.; Vlahos, R.; et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 269–278. [Google Scholar] [CrossRef]
- Smith, D.J.; Yerkovich, S.T.; Towers, M.A.; Carroll, M.L.; Thomas, R.; Uphamet, J.W. Reduced soluble receptor for advanced glycation end-products in COPD. Eur. Respir. J. 2011, 37, 516–522. [Google Scholar] [CrossRef]
- Hutchinson, A.F.; Black, J.; Thompson, M.A.; Bozinovski, S.; Brand, C.A.; Smallwood, D.M.; Irving, L.B.; Anderson, G.P. Identifying viral infections in vaccinated Chronic Obstructive Pulmonary Disease (COPD) patients using clinical features and inflammatory markers. Influ. Other Respir. Viruses 2010, 4, 33–39. [Google Scholar] [CrossRef]
- Koutsokera, A.; Kiropoulos, T.S.; Nikoulis, D.J.; Daniil, Z.D.; Tsolaki, V.; Tanou, K.; Papaioannou, A.I.; Germenis, A.; Gourgoulianis, K.I.; Kostikas, K. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir. Med. 2009, 103, 919–926. [Google Scholar] [CrossRef]
- López-Campos, J.L.; Calero, C.; Rojano, B.; López-Porras, M.; Saenz, J.; Blanco, A.I.; Sánchez-López, V.; Tobar, D.; Montes-Worboys, A.; Arellano, E. C-reactive protein and serum amyloid a overexpression in lung tissues of chronic obstructive pulmonary disease patients: A case-control study. Int. J. Med. Sci. 2013, 10, 938–947. [Google Scholar] [CrossRef]
- Sakurai, K.; Chubachi, S.; Irie, H.; Tsutsumi, A.; Kameyama, N.; Kamatani, T.; Koh, H.; Terashima, T.; Nakamura, H.; Asano, K.; et al. Clinical utility of blood neutrophil-lymphocyte ratio in Japanese COPD patients. BMC Pulm. Med. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Zilz, C.; Blaas, S.H.; Pfeifer, M.; Jorres, S.A.; Budweiser, S. Mental health, serum biomarkers and survival in severe COPD: A pilot study. Multidiscip. Respir. Med. 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Passey, S.L.; Bozinovski, S.; Vlahos, R.; Anderson, G.P.; Hansen, M.J. Serum amyloid A induces Toll-Like receptor 2-dependent inflammatory cytokine expression and atrophy in C2C12 skeletal muscle myotubes. PLoS ONE 2016, 11, e0146882. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.X.; Anthony, D.A.; Seow, H.J.; Anderson, G.P.; Bozinovski, S. Serum Amyloid A is a Candidate Mediator for Altered Macrophage Polarisation in Cigarette Smoke-Related Disease. Abstract. Proceedings of the Australian Physiological Society. Available online: http://aups.org.au/Proceedings/42/53P/53P.pdf (accessed on 5 March 2021).
- Hansen, M.J.; Chan, S.P.J.; Langenbach, S.Y.; Dousha, L.F.; Jones, J.E.; Yatmaz, S.; Seow, H.; Vlahos, R.; Anderson, G.P.; Bozinovski, S. IL-17A and serum amyloid A are elevated in a cigarette smoke cessation model associated with the persistence of pigmented macrophages, neutrophils and activated NK cells. PLoS ONE 2014, 9, e113180. [Google Scholar] [CrossRef]
- Arellano-Orden, E.; Calero-Acuña, C.; Cordero, J.A.; Abad-Arranz, M.; Sánchez-López, V.; Márquez-Martín, E.; Ortega-Ruiz, F.; López-Campos, J.L. Specific networks of plasma acute phase reactants are associated with the severity of chronic obstructive pulmonary disease: A case-control study. Int. J. Med. Sci. 2017, 14, 67–74. [Google Scholar] [CrossRef]
- Bizeto, L.; Mazzolini, A.B.; Ribeiro, M.; Stelmach, R.; Cukier, A.; Nunes, M.P. Interrelationship between serum and sputum inflammatory mediators in chronic obstructive pulmonary disease. Braz. J. Med Biol. Res. 2008, 41, 193–198. [Google Scholar] [CrossRef]
- Arellano-Orden, E.; Calero, C.; López-Ramírez, C.; Sánchez-López, V.; López-Villalobos, J.L.; Abad Arranz, M.; Blanco-Orozco, A.; Otero-Candelera, R.; López-Campos, J.L. Evaluation of lung parenchyma, blood vessels, and peripheral blood lymphocytes as a potential source of acute phase reactants in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M. Longitudinal in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration 2014, 87, 63–74. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J.; He, X.; Hao, Y.; Wang, K.; Gibson, P.G. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e57678. [Google Scholar] [CrossRef]
- Anthony, D.; Seow, H.J.; Uddin, M.; Thompson, M.; Dousha, L.; Vlahos, R.; Irving, L.B.; Levy, B.D.; Anderson, G.P.; Bozinovski, S. Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and γδ T cells. Am. J. Respir. Crit. Care Med. 2013, 188, 179–186. [Google Scholar] [CrossRef]
- Bozinovski, S.; Uddin, M.; Vlahos, R.; Thompson, M.; McQualter, J.L.; Merritt, A.-S.; Wark, P.A.B.; Hutchinson, A.; Irving, L.B.; Levy, B.D.; et al. Serum amyloid A opposes lipoxin A₄ to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2012, 109, 935–940. [Google Scholar] [CrossRef]
- Ather, J.L.; Ckless, K.; Martin, R.; Foley, K.L.; Suratt, B.T.; Boyson, J.E.; Fitzgerald, K.A.; Flavell, R.A.; Eisenbarth, S.C.; Poynter, M.E. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 2011, 187, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ozseker, F.; Buyukozturk, S.; Depboylu, B.; Yilmazbayhan, D.; Karayigit, E.; Gelincik, A.; Genc, S.; Colakoglu, B.; Dal, M.; Issever, H. Serum amyloid A (SAA) in induced sputum of asthmatics: A new look to an old marker. Int. Immunopharmacol. 2006, 6, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Keene, J.D.; Jacobson, S.; Kechris, K.; Kinney, G.L.; Foreman, M.G.; Doerschuk, C.M.; Make, B.J.; Curtis, J.L.; Rennard, S.I.; Barr, R.G.; et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am. J. Respir. Crit. Care Med. 2017, 195, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Abbasi, A.; Rossiter, H.B.; Su, X.; Liu, H.; Pi, Y.; Sang, L.; Zhong, W.; Yang, Q.; Guo, X.; et al. Serum Amyloid A in Stable COPD Patients is Associated with the Frequent Exacerbator Phenotype. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2379–2388. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Rubinsztajn, R.; Przybyłowski, T.; Maskey-Warzęchowska, M.; Paplińska-Goryca, M.; Nejman-Gryz, P.; Karwat, K.; Chazan, R. Metabolic syndrome as a factor affecting systemic inflammation in patients with chronic obstructive pulmonary disease. Adv. Exp. Med. Biol. 2017, 1021, 55–62. [Google Scholar] [CrossRef]
- Hughes, M.J.; McGettrick, H.M.; Sapey, E. Shared mechanisms of multimorbidity in COPD, atherosclerosis and type-2 diabetes: The neutrophil as a potential inflammatory target. Eur. Respir. Rev. 2020, 29, 190102. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Elmes, P.C.; Fairbairn, A.S.; Wood, C.H. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br. Med. J. 1959, 2, 257–266. [Google Scholar]
- Miller, M.R.; Hankinson, J.A.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; et al. ATS/ERS Task Force. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. ATS/ERS Task Force. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. American Thoracic Society Statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158 Pt 1, 1384–1387. [Google Scholar] [CrossRef]
- Ho, J.; He, W.; Chan, M.T.; Tse, G.; Liu, T.; Wong, S.H.; Leung, C.C.; Wong, W.T.; Tsang, S.; Zhang, L.; et al. Eosinophilia and clinical outcome of chronic obstructive pulmonary disease: A meta-analysis. Sci. Rep. 2017, 7, 13451. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available online: http://www.R-project.org/ (accessed on 29 January 2021).
| Variable | Value |
|---|---|
| Age (years) | 69.0 (61.0–75.0) |
| Sex (M/F) | 62/38 |
| BMI (kg/m2) | 26.6 (24.2–30.5) |
| Smoking history (packyears) | 41.0 (28.0–52.5) |
| Current/ex-/never smokers | 21/75/4 |
| Pulmonary function (post-bronchodilator values) | |
| FEV1 (% predicted) | 55.2 (43.0–70.0) |
| FVC (% predicted) | 86.6 (75.7–98.8) |
| FEV1/FVC (%) | 50.8 (40.8–59.1) |
| RV (% predicted) | 163.7 (136.0–202.8) |
| TLC (% predicted) | 121.1 (105.7–136.2) |
| RV/TLC (%) | 54.6 (50.6–61.9) |
| Exercise performance and symptom level | |
| 6 MWD (m) | 476.1 (435.7–527.5) |
| 6 MWD (% predicted) | 92.0 (77.3–106.1) |
| mMRC (points) | 2.0 (1.0–3.0) |
| CAT (points) | 13.2 (5.6–21.4) |
| 12 mo ΔFEV1 (mL) * | 0.06 [(−0.06)–0.16] |
| Number of exacerbations within 12 months | 1.0 (0.0–2.0) |
| Arterial blood gases (room air) | |
| PaO2 (mmHg) | 73.2 (65.3–80.4) |
| PaCO2 (mmHg) | 39.4 (36.9–42.3) |
| Biochemistry | |
| Total cholesterol | 191.0 (158.0–221.0) |
| HDL | 52.0 (43.0–63.0) |
| Triglycerides | 97.0 (80.0–134.0) |
| Fasting glucose | 87.0 (78.0–97.0) |
| Variable | Value |
|---|---|
| WBC (×109/L) | 6.3 (5.3–8.1) |
| Neutrophils (×109/L) | 3.36 (2.8–4.4) |
| Eosinophils (×109/L) | 0.21 (0.13–0.30) |
| Eosinophils (%) | 3.5 (2.3–5.2) |
| SAA (ng/mL) | 24.9 (1.0–59.1) |
| CRP (mg/L) | 3.0 (2.5–7.7) |
| Fibrinogen (mg/dL) | 373.0 (327.0–460.0) |
| IL-6 (pg/mL) | 2.6 (1.7–5.1) |
| IL-8 (pg/mL) | 8.6 (5.2–10.8) |
| TNF alpha (pg/mL) | 1.8 (1.5–2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maskey-Warzęchowska, M.; Rubinsztajn, R.; Przybyłowski, T.; Karwat, K.; Nejman-Gryz, P.; Paplińska-Goryca, M.; Chazan, R. Serum Amyloid A in Stable Patients with Chronic Obstructive Pulmonary Disease Does Not Reflect the Clinical Course of the Disease. Int. J. Mol. Sci. 2023, 24, 2478. https://doi.org/10.3390/ijms24032478
Maskey-Warzęchowska M, Rubinsztajn R, Przybyłowski T, Karwat K, Nejman-Gryz P, Paplińska-Goryca M, Chazan R. Serum Amyloid A in Stable Patients with Chronic Obstructive Pulmonary Disease Does Not Reflect the Clinical Course of the Disease. International Journal of Molecular Sciences. 2023; 24(3):2478. https://doi.org/10.3390/ijms24032478
Chicago/Turabian StyleMaskey-Warzęchowska, Marta, Renata Rubinsztajn, Tadeusz Przybyłowski, Krzysztof Karwat, Patrycja Nejman-Gryz, Magdalena Paplińska-Goryca, and Ryszarda Chazan. 2023. "Serum Amyloid A in Stable Patients with Chronic Obstructive Pulmonary Disease Does Not Reflect the Clinical Course of the Disease" International Journal of Molecular Sciences 24, no. 3: 2478. https://doi.org/10.3390/ijms24032478
APA StyleMaskey-Warzęchowska, M., Rubinsztajn, R., Przybyłowski, T., Karwat, K., Nejman-Gryz, P., Paplińska-Goryca, M., & Chazan, R. (2023). Serum Amyloid A in Stable Patients with Chronic Obstructive Pulmonary Disease Does Not Reflect the Clinical Course of the Disease. International Journal of Molecular Sciences, 24(3), 2478. https://doi.org/10.3390/ijms24032478






