Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin
Abstract
1. Introduction
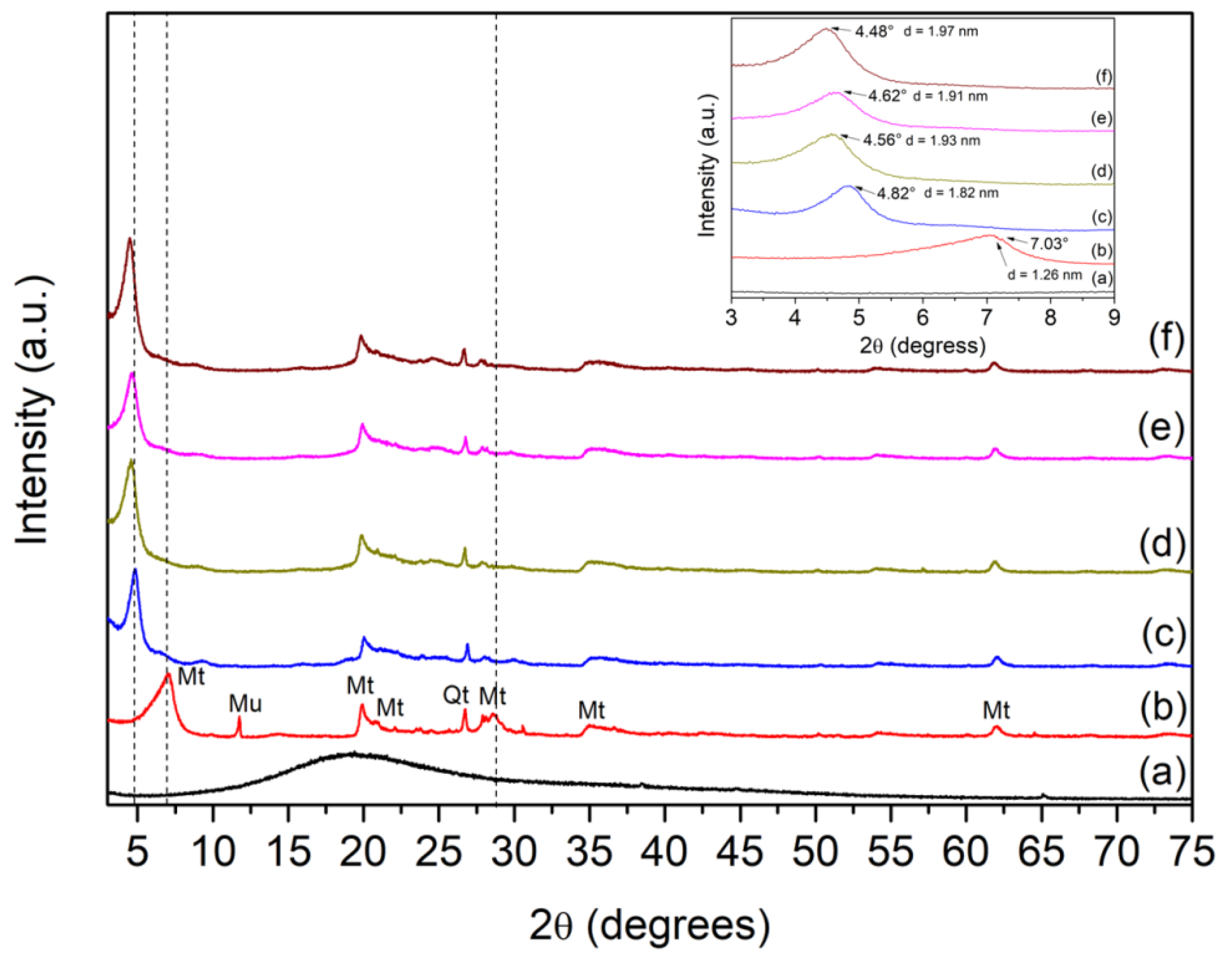
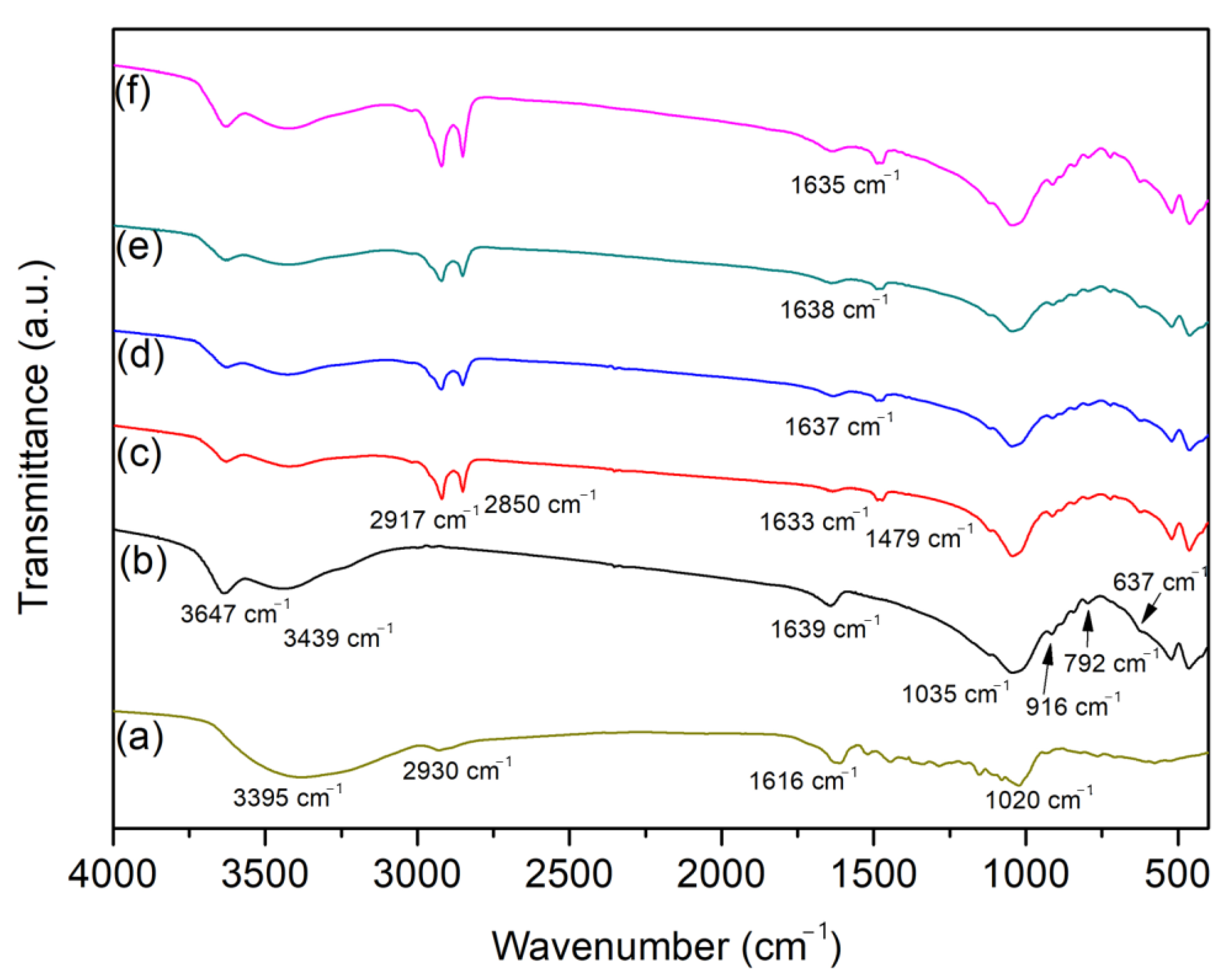
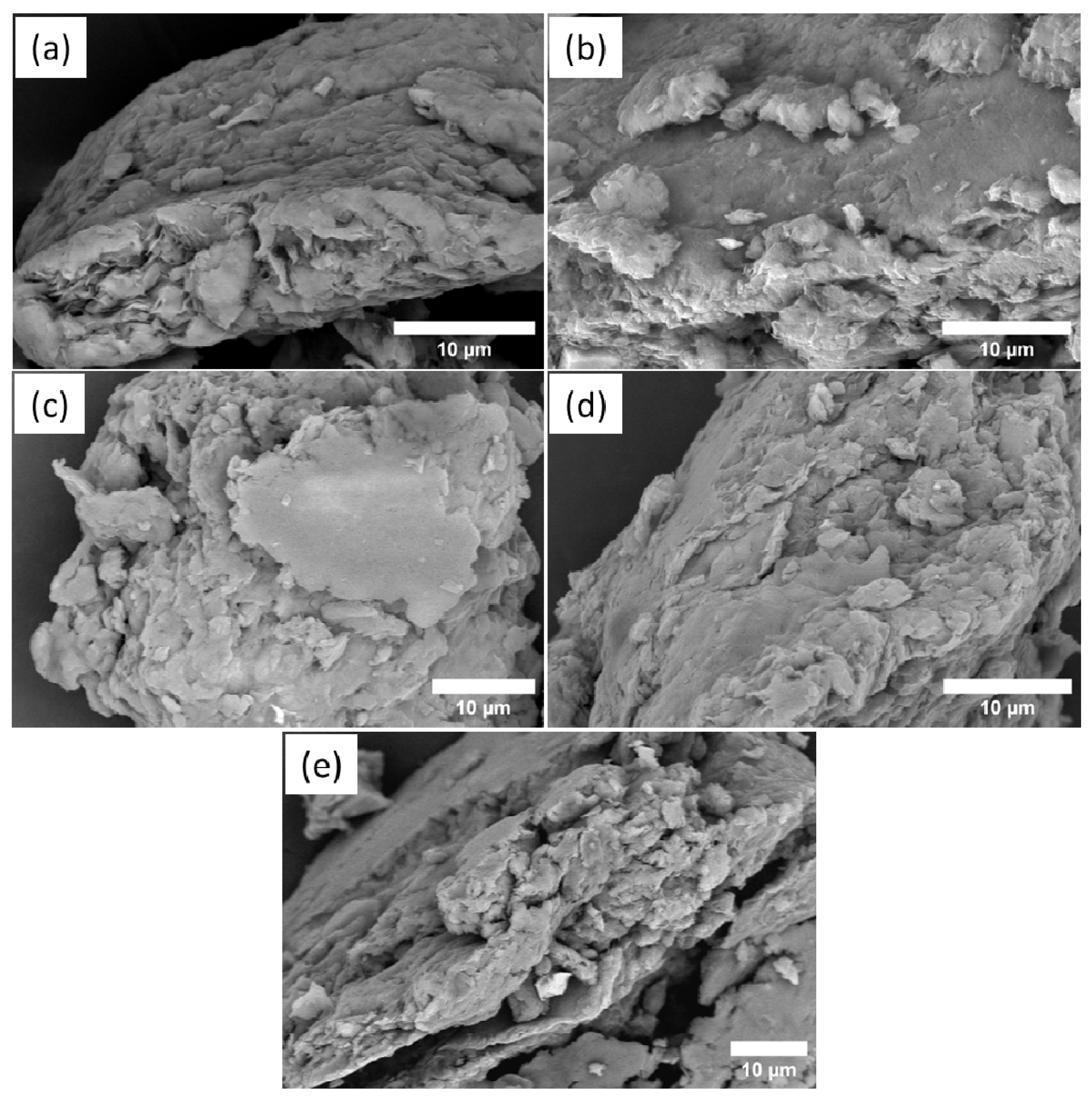
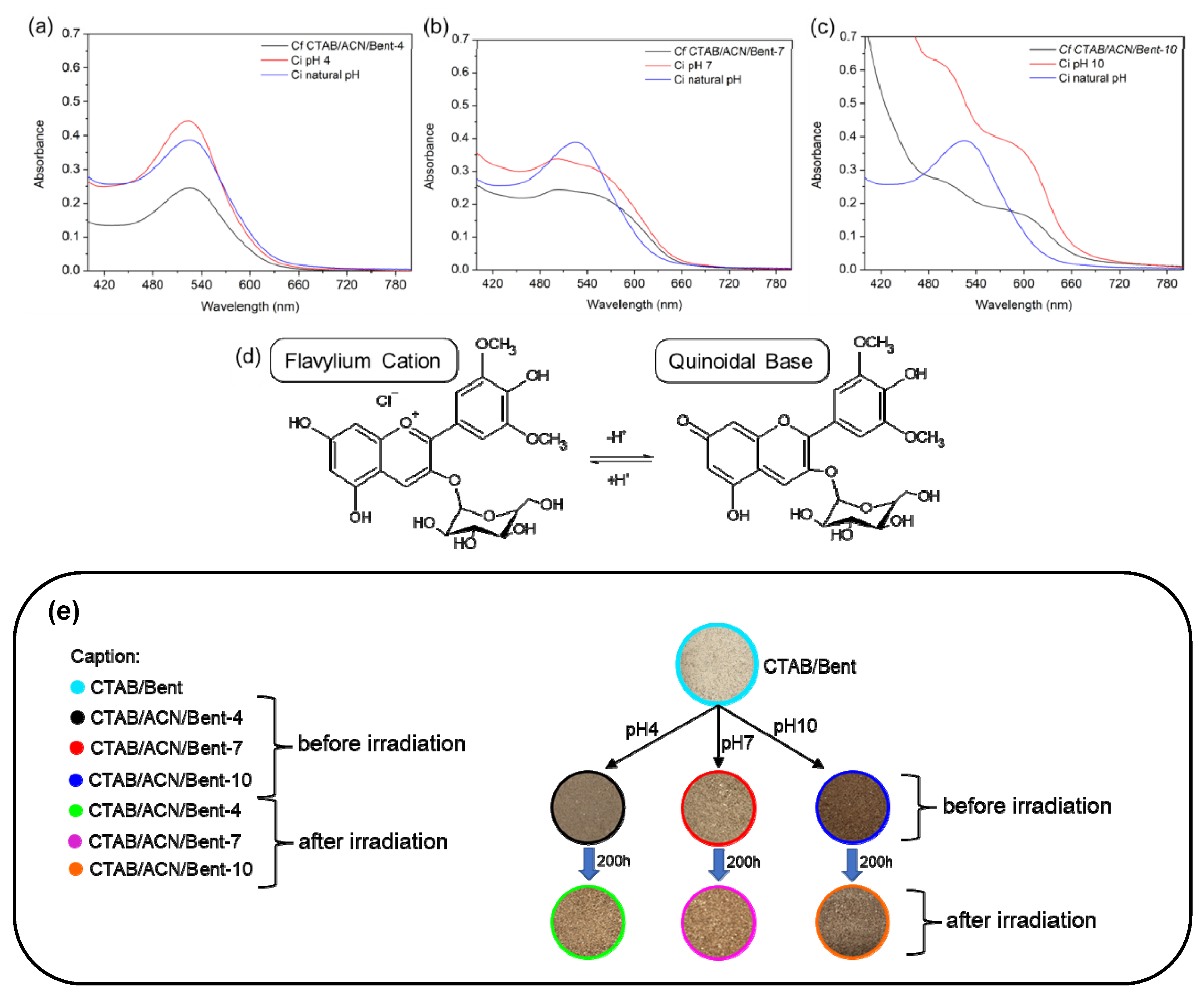
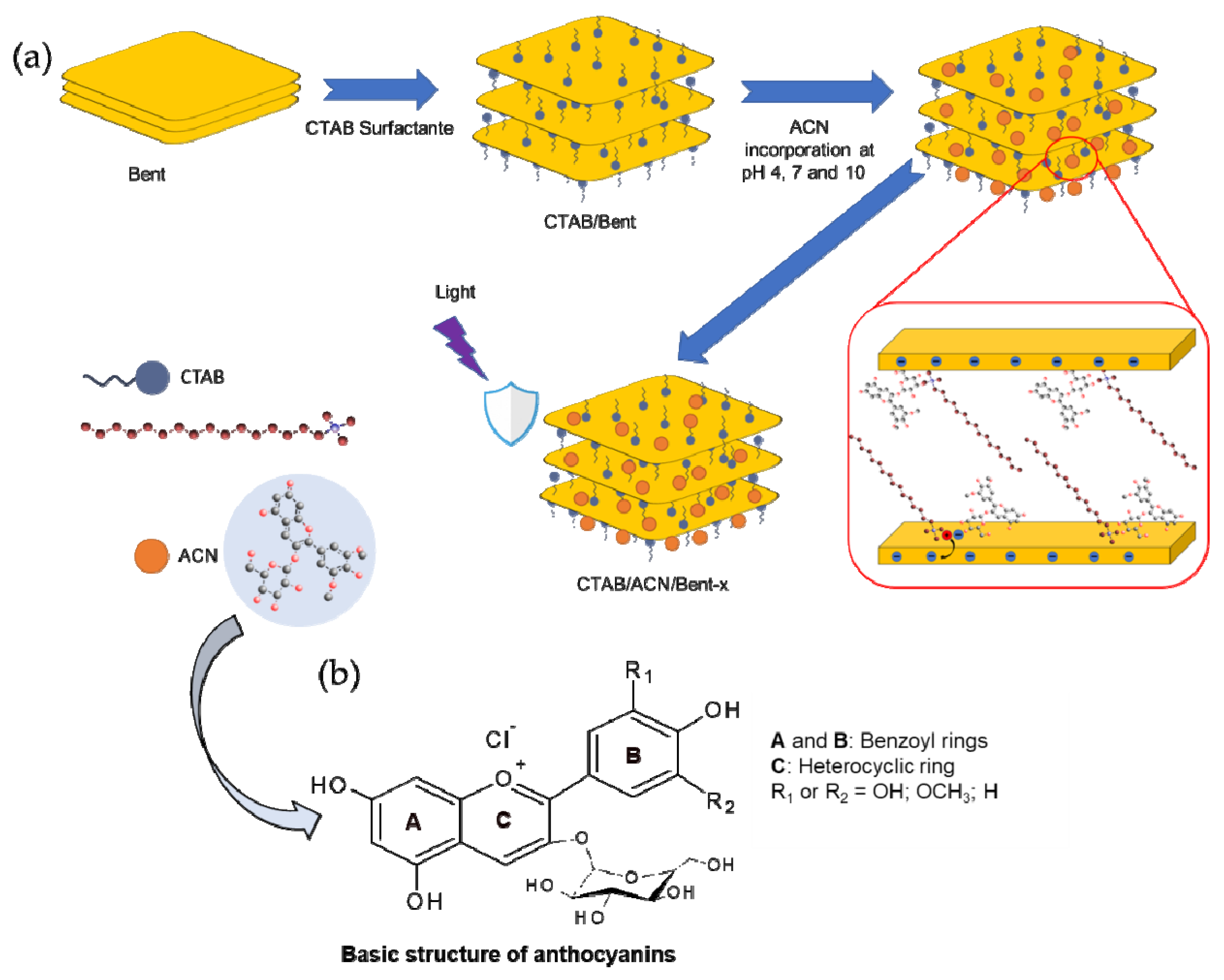
2. Results and Discussion
3. Materials and Methods
3.1. Material and Chemicals
3.2. Cation Exchange Capacity (CEC)
3.3. Synthesis of the CTAB/Bent Hybrid
3.4. Sequential Infusion of ACN to Produce ACN in the CTAB/Bent Hybrid
3.5. Photostability Test
3.6. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, L.C.B.; Silva, F.C.; Silva-Filho, E.C.; Fonseca, M.G.; Zhuang, G.; Jaber, M. Saponite-anthocyanin derivatives: The role of organoclays in pigment photostability. Appl. Clay Sci. 2020, 191, 105604. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Viqueira, V.; Luzi, F.; Dominici, F.; Terenzi, A.; Maron, E.; Hamzaoui, M.; Kohnen, S.; Torre, L.; et al. Anthocyanin Hybrid Nanopigments from Pomegranate Waste: Colour, Thermomechanical Stability and Environmental Impact of Polyester-Based Bionanocomposites. Polymers 2021, 13, 1966. [Google Scholar] [CrossRef]
- Ribeiro, H.L.; Oliveira, A.V.; de Brito, E.S.; de Ribeiro, P.R.V.; Souza Filho, M.M.; Azeredo, H.M.C. Stabilizingeffectofmontmorilloniteon acerola juiceanthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef]
- Lima, L.C.B.; Castro-Silva, F.; Silva-Filho, E.C.; Fonseca, M.G.; Jaber, M. Saponite-anthocyanin pigments: Slipping between the sheets. Microporous Mesoporous Mater. 2020, 300, 110148. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, K.M.; Silva, C.P.; Gonçalves, J.M.; Quina, F.H. Hybrid Pigments from Anthocyanin Analogues and Synthetic Clay Minerals. ACS Omega. 2020, 5, 26592–26600. [Google Scholar] [CrossRef]
- Kohno, Y.; Kinoshita, R.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, C.P.; Gehlen, M.H.; Oake, J.; Bohne, C.; Quina, F.H. Organic/inorganic hybrid pigments from flavylium cations and palygorskite. Appl. Clay Sci. 2018, 162, 478–486. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Bello, V.E.; Amoo, K.O. Production and characterization of composite nanoparticles derived from chitosan, CTAB and bentonite clay. Chem. Pap. 2022, 76, 5063–5086. [Google Scholar] [CrossRef]
- Pinos, J.Y.M.; de Melo, L.B.; de Souza, S.D.; Marçal, L.; de Faria, E.H. Bentonite functionalized with amine groups by the sol-gel route as efficient adsorbent of rhodamine-B and nickel (II). Appl. Clay Sci. 2022, 223, 106494. [Google Scholar] [CrossRef]
- Resende, R.F.; Silva, T.F.B.; Santos, N.A.V.; Papini, R.M.; Magriotis, Z.M. Anionic collector adsorption onto bentonites and potential applications in the treatment of mining wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127401. [Google Scholar] [CrossRef]
- Santos, S.S.G.; França, D.B.; Castellano, L.R.C.; Trigueiro, P.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G. Novel modified bentonites applied to the removal of an anionic azo-dye from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 1585, 24152. [Google Scholar] [CrossRef]
- Brito, D.F.; da Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef]
- França, D.B.; Trigueiro, P.; Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyridinium bentonites. Chemosphere 2019, 242, 125109. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Queiroga, L.N.F.; Pereira, M.B.B.; Silva, L.S.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Microwave bentonite silylation for dye removal: Influence of the solvent. Appl. Clay Sci. 2019, 168, 478–487. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.S.; Fonseca, M.G.; da Silva Filho, E.C.; Jaber, M. Thiabendazole/bentonites hybrids as controlled release systems. Colloids Surf. B Biointerfaces 2019, 176, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Shugulí, C.; Rodríguez, F.J.; Bruna, J.E.; Galotto, M.J.; Sarantópoulos, C.; Favaro Perez, M.A.; Padula, M. Cetylpyridinium bromide-modified montmorillonite as filler in low density polyethylene nanocomposite films. Appl. Clay Sci. 2019, 168, 203–210. [Google Scholar] [CrossRef]
- Sciascia, L.; Calabrese, I.; Cavallaro, G.; Merli, M.; Scialabba, C.; Liveri, M.L.T. Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy. Minerals 2021, 11, 1315. [Google Scholar] [CrossRef]
- Uma Maheswari, B.; Sivakumar, V.M.; Thirumarimurugan, M. Sequester adsorption of Cr(VI) and Pb(II) ions from aqueous solution by green synthesized nanocomposite (OB/ZnO): Characterization, kinetics, isotherm and mechanism. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100680. [Google Scholar] [CrossRef]
- Ulhaq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M.; Ilyas, M. Engineering TiO2 supported CTAB modified bentonite for treatment of refinery wastewater through simultaneous photocatalytic oxidation and adsorption. J. Water Process Eng. 2021, 43, 102239. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials 2019, 12, 3287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, J.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. Acid/base reversible allochroic anthocyanin/palygorskite hybrid pigments:Preparation, stability and potential applications. Dyes Pigm. 2019, 171, 107738. [Google Scholar] [CrossRef]
- Wang, T.L.; Yin, X.; Wei, X. Application of thiabendazole/bentonites hybrids as efficient antibacterial agent against Escherichia coli and Staphylococcus aureus. Mater. Res. 2021, 8, 125306. [Google Scholar]
- Zhu, Y.; Cui, Y.; Shan, Z.; Dai, R.; Shi, L.; Chen, H. Fabrication and characterization of a multi-functional and environmentally-friendly starch/organo-bentonite composite liquid dust suppressant. Powder Technol. 2021, 391, 532–543. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhong, L.; Cheng, H.; Wang, H.; Ma, Q. Adsorption of reactive red 136 onto chitosan/montmorillonite intercalated composite from aqueous solution. Appl. Clay Sci. 2019, 167, 9–22. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and characteristics of alginate and anthocyanin complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Dos Santos, A.; Viante, M.F.; Pochapski, D.J.; Downs, A.J.; Almeida, C.A.P. Enhanced removal of p-nitrophenol from aqueous media by montmorillonite clay modified with a cationic surfactant. J. Hazard. Mater. 2018, 355, 136–144. [Google Scholar] [CrossRef]
- Vallova, S.; Plevova, E.; Smutna, K.; Sokolova, B.; Vaculikova, L.; Valovicova, V.; Hundakova, M.; Praus, P. Removal of analgesics from aqueous solutions onto montmorillonite KSF. J. Therm. Anal. Calorim. 2022, 47, 1973–1981. [Google Scholar] [CrossRef]
- Yu, W.H.; Zhu, T.T.; Tong, D.S.; Wang, M.; Wu, Q.Q.; Zhou, C.H. Preparation of Organo-montmorillonites and the Relationship Between Microstructure and Swellability. Clays Clay Miner 2017, 65, 417–430. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming Montmorillonite Surfaces Using a Cationic Surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef]
- Zhuang, G.; Jaber, M.; Rodrigues, F.; Rigaud, B.; Walter, P.; Zhang, Z. A new durable pigment with hydrophobic surface based on natural nanotubes and indigo: Interactions and stability. J. Colloid Interface Sci. 2019, 552, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Czímerová, A.; Bujdák, J.; Dohrmann, R. Traditional and novel methods for estimating the layer charge of smectites. Appl. Clay Sci. 2006, 34, 2–13. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef] [PubMed]
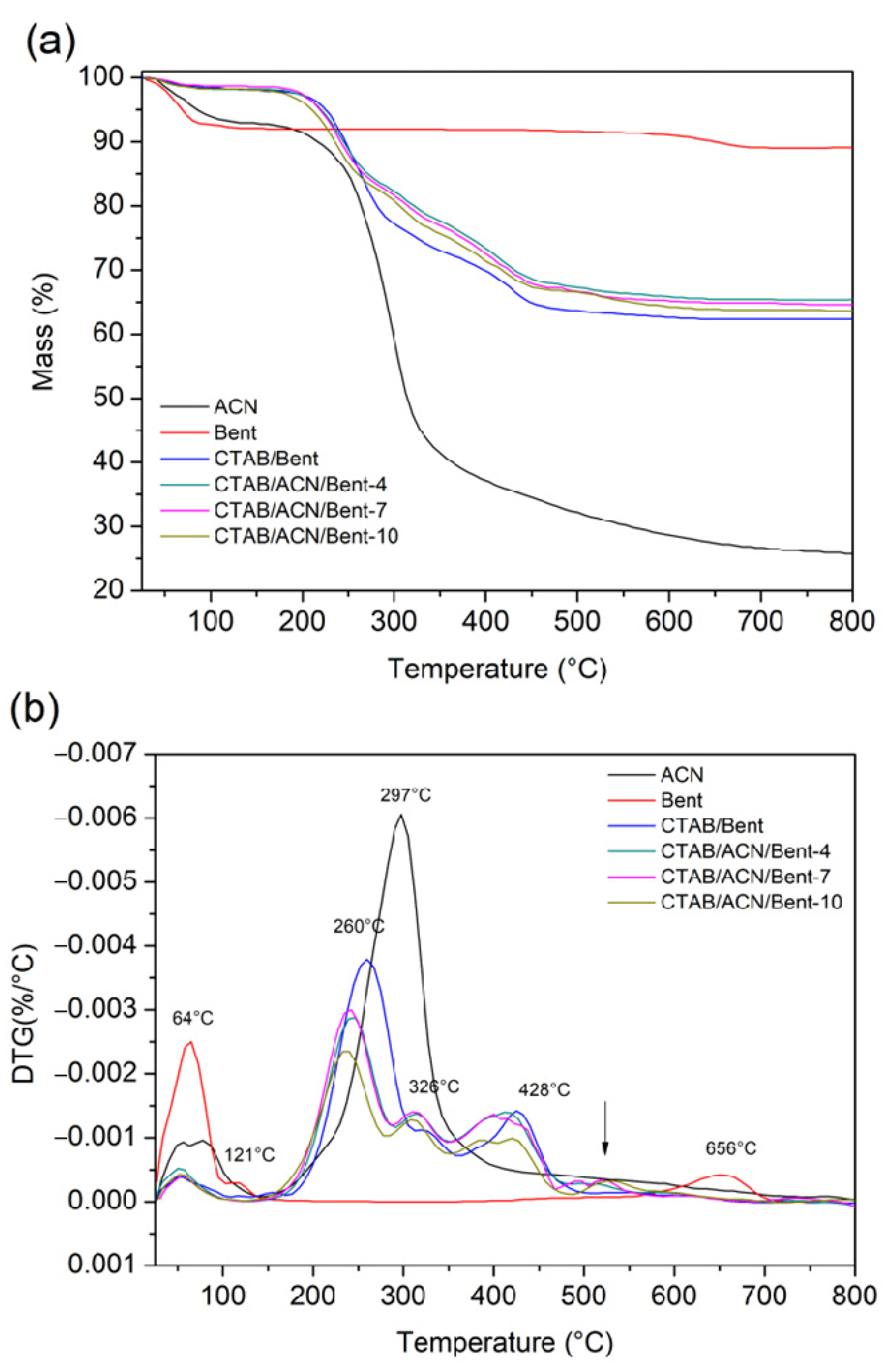
| Sample | Degradation Event | Weight Loss [%] | Temperature Range [°C] | Maximum Degradation Temperature [°C] |
|---|---|---|---|---|
| ACN | First event: Referring to the removal of water from the surface | 6.92% | 26–132 °C | 78 °C |
| Second event: Referring to the thermal decomposition of ACN | 67.20% | 132–800 °C | 297 °C | |
| Bent | First event: Referring to the removal of water from the surface | 7.32% | 30–87 °C | 64 °C |
| Second event: Referring to the removal of water from the crystal lattice | 1.00% | 88–143 °C | 121 °C | |
| Third event: Referring to the dihydroxylation of bentonite | 1.90% | 591–710 °C | 656 °C | |
| CTAB/Bent | First event: The loss of adsorbed water. | 1.65% | 38–95 °C | 53 °C |
| Second event: Decomposition of the CTAB physically adsorbed on the surface of the material | 21.31% | 200–310 °C | 260 °C | |
| Third event: Decomposition of the CTAB molecules incorporated between the layers | 2.72% | 330–370 °C | 326 °C | |
| Fourth event: Decomposition of the intercalated CTAB cations | 5.72% | 400–465 °C | 428 °C | |
| Fifth event: Referring to the dihydroxylation of bentonite | ||||
| CTAB/ACN/Bent-4 | First event: The loss of adsorbed water | 1.37% | 35–65 °C | 50 °C |
| Second event: Decomposition of the CTAB physically adsorbed on the surface of the material and ACN | 12.91% | 185–276 °C | 243 °C | |
| Third event: Decomposition of the CTAB molecules incorporated between the layers and ACN | 4.71% | 280–328 °C | 315 °C | |
| Fourth event: Decomposition of the intercalated CTAB cations and ACN | 9.61% | 335–336 °C | 418 °C | |
| Fifth event: Referring to thedihydroxylation of bentonite | 2.31% | 470–645 °C | 505 °C | |
| CTAB/ACN/Bent-7 | First event: The loss of adsorbed water | 1.42% | 35–66 °C | 54 °C |
| Second event: Decomposition of the CTAB physically adsorbed on the surface of the material and ACN | 13.25% | 186–269 °C | 239 °C | |
| Third event: Decomposition of the CTAB molecules incorporated between the layers and ACN | 5.12% | 282–330% | 310 °C | |
| Fourth event: Decomposition of the intercalated CTAB cations and ACN | 9.94% | 334–445 °C | 415 °C | |
| Fifth event: Referring to the dihydroxylation of bentonite | 2.53% | 468–663 °C | 520 °C | |
| CTAB/CAN/Bent-10 | First event: The loss of adsorbed water | 1.00% | 34–70 °C | 52 °C |
| Second event: Decomposition of the CTAB physically adsorbed on the surface of the material and ACN | 13.29% | 180–265 °C | 236 °C | |
| Third event: Decomposition of the CTAB molecules incorporated between the layers and ACN | 6.02% | 276–326 °C | 308 °C | |
| Fourth event: Decomposition of the intercalated CTAB cations and ACN | 10.14% | 327–452 °C | 405 °C | |
| Fifth event: Referring to the dihydroxylation of bentonite | 3.34% | 462–662 °C | 530 °C |
| Before Irradiation | After Irradiation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | L* | a* | b* | ΔE* Compared with CTAB/Bent | Sample | L* | a* | b* | ΔE* Compared with Non-irradiated Sample |
| CTAB/Bent | 67.7 ± 0.0 | 0.7 ± 0.0 | 11.9 ± 0.1 | - | - | - | - | - | - |
| CTAB/ACN/Bent-4 | 54.3 ± 0.1 | 1.7 ± 0.3 | 8.4 ± 0.5 | 13.6 ± 0.2 | CTAB/ACN/Bent-4 | 40.5 ± 0.3 | 4.2 ± 0.2 | 9.7 ± 0.3 | 14.1 ± 0.4 |
| CTAB/ACN/Bent-7 | 47.4 ± 0.3 | 2.2 ± 0.4 | 10.0 ± 0.2 | 20.6 ± 0.4 | CTAB/ACN/Bent-7 | 42.8 ± 0.2 | 4.5 ± 0.0 | 10.4 ± 0.2 | 5.4 ± 0.3 |
| CTAB/ACN/Bent-10 | 42.4 ± 0.3 | 5.7 ± 0.3 | 12.8 ± 0.3 | 25.6 ± 0.2 | CTAB/ACN/Bent-10 | 47.5 ± 0.3 | 4.7 ± 0.1 | 12.0 ± 0.4 | 4.2 ± 0.4 |
| Phases | Description |
|---|---|
| 1 | Initially, 1 g of clay is dispersed in 100 mL of an ammonium acetate solution with a concentration of 1.0 mol L−1 (pH = 7), and the system is kept under stirring for 72 h. |
| 2 | After 72 h, the solid is separated by centrifugation at 5000 rpm for 10 min (NI-1812 NOVA Instruments benchtop centrifuge, Brazil), 100 mL of ammonium acetate solution (1.0 mol L−1) is added again, and the system remains under agitation for another 72 h. |
| 3 | The procedure of step 2 is repeated again, and finally, the solid is washed with distilled water and ethanol, filtered off, and dried at room temperature (25 °C). |
| 4 | The CEC is calculated from the results of the CHN elemental analysis (Perkin-Elmer model PE 2400, USA) of the solid obtained. |
| Samples | Description |
|---|---|
| Bent | Bentonite clay mineral |
| ACN | Anthocyanin |
| CTAB | Surfactant hexadecyltrimethylammonium bromide |
| CTAB/Bent | Bentonite with CTAB |
| CTAB/ACN/Bent-4 | Adsorption of CTAB/Bent with ACN solution at pH 4 |
| CTAB/ACN/Bent-7 | Adsorption of CTAB/Bent with ACN solution at pH 7 |
| CTAB/ACN/Bent-10 | Adsorption of CTAB/Bent with ACN solution at pH 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, R.V.; Morais, A.I.S.; Trigueiro, P.; de Souza, J.S.N.; Damacena, D.H.L.; Brandão-Lima, L.C.; Bezerra, R.D.S.; Fonseca, M.G.; Silva-Filho, E.C.; Osajima, J.A. Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin. Int. J. Mol. Sci. 2023, 24, 2417. https://doi.org/10.3390/ijms24032417
Cunha RV, Morais AIS, Trigueiro P, de Souza JSN, Damacena DHL, Brandão-Lima LC, Bezerra RDS, Fonseca MG, Silva-Filho EC, Osajima JA. Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin. International Journal of Molecular Sciences. 2023; 24(3):2417. https://doi.org/10.3390/ijms24032417
Chicago/Turabian StyleCunha, Robson V., Alan I. S. Morais, Pollyana Trigueiro, João Sammy N. de Souza, Dihêgo H. L. Damacena, Luciano C. Brandão-Lima, Roosevelt D. S. Bezerra, Maria Gardennia Fonseca, Edson C. Silva-Filho, and Josy A. Osajima. 2023. "Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin" International Journal of Molecular Sciences 24, no. 3: 2417. https://doi.org/10.3390/ijms24032417
APA StyleCunha, R. V., Morais, A. I. S., Trigueiro, P., de Souza, J. S. N., Damacena, D. H. L., Brandão-Lima, L. C., Bezerra, R. D. S., Fonseca, M. G., Silva-Filho, E. C., & Osajima, J. A. (2023). Organic–Inorganic Hybrid Pigments Based on Bentonite: Strategies to Stabilize the Quinoidal Base Form of Anthocyanin. International Journal of Molecular Sciences, 24(3), 2417. https://doi.org/10.3390/ijms24032417











