Clay Nanotubes Loaded with Diazepam or Xylazine Permeate the Brain through Intranasal Administration in Mice
Abstract
1. Introduction
2. Results
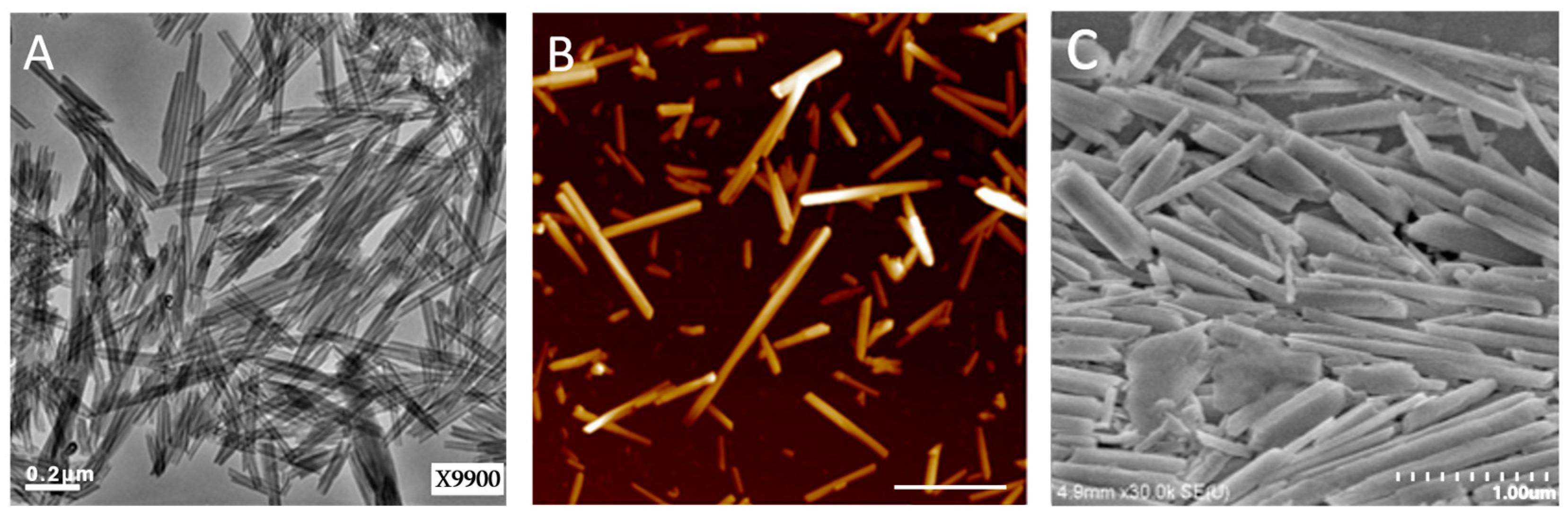
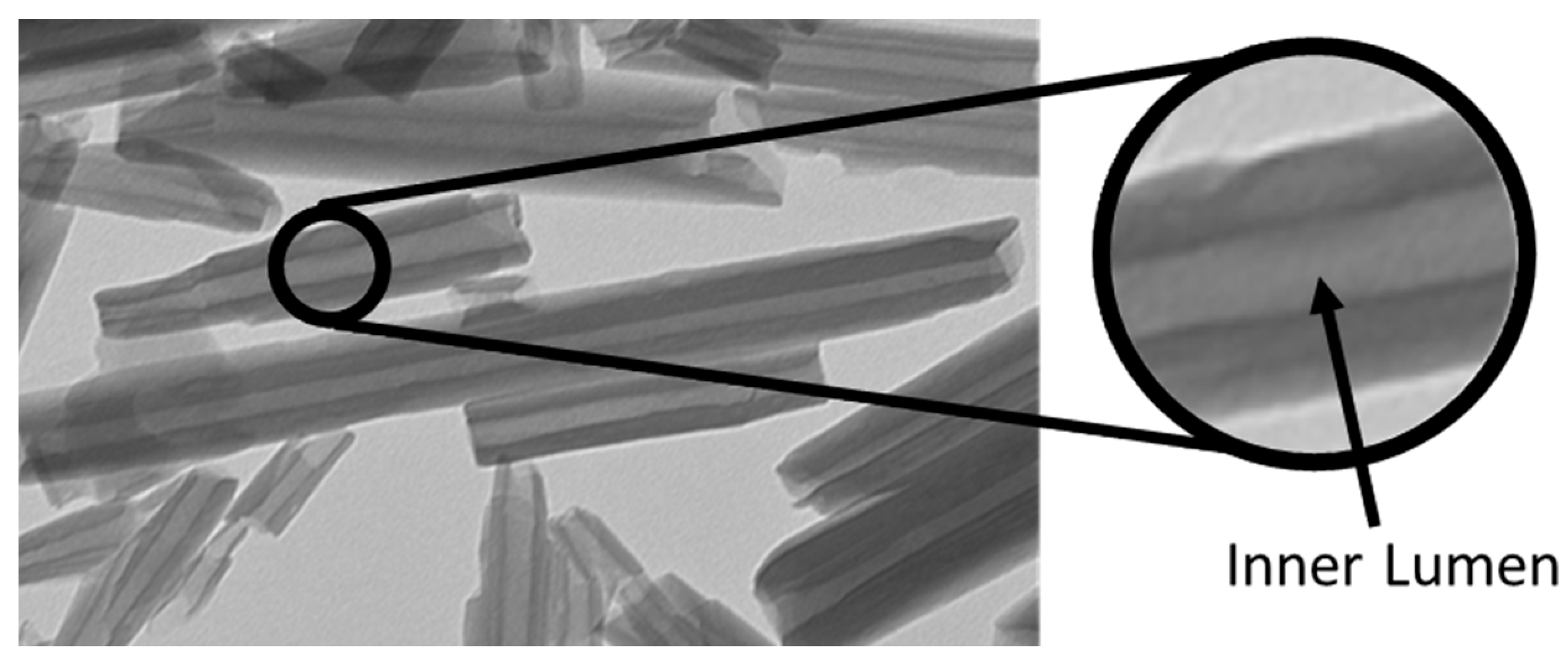
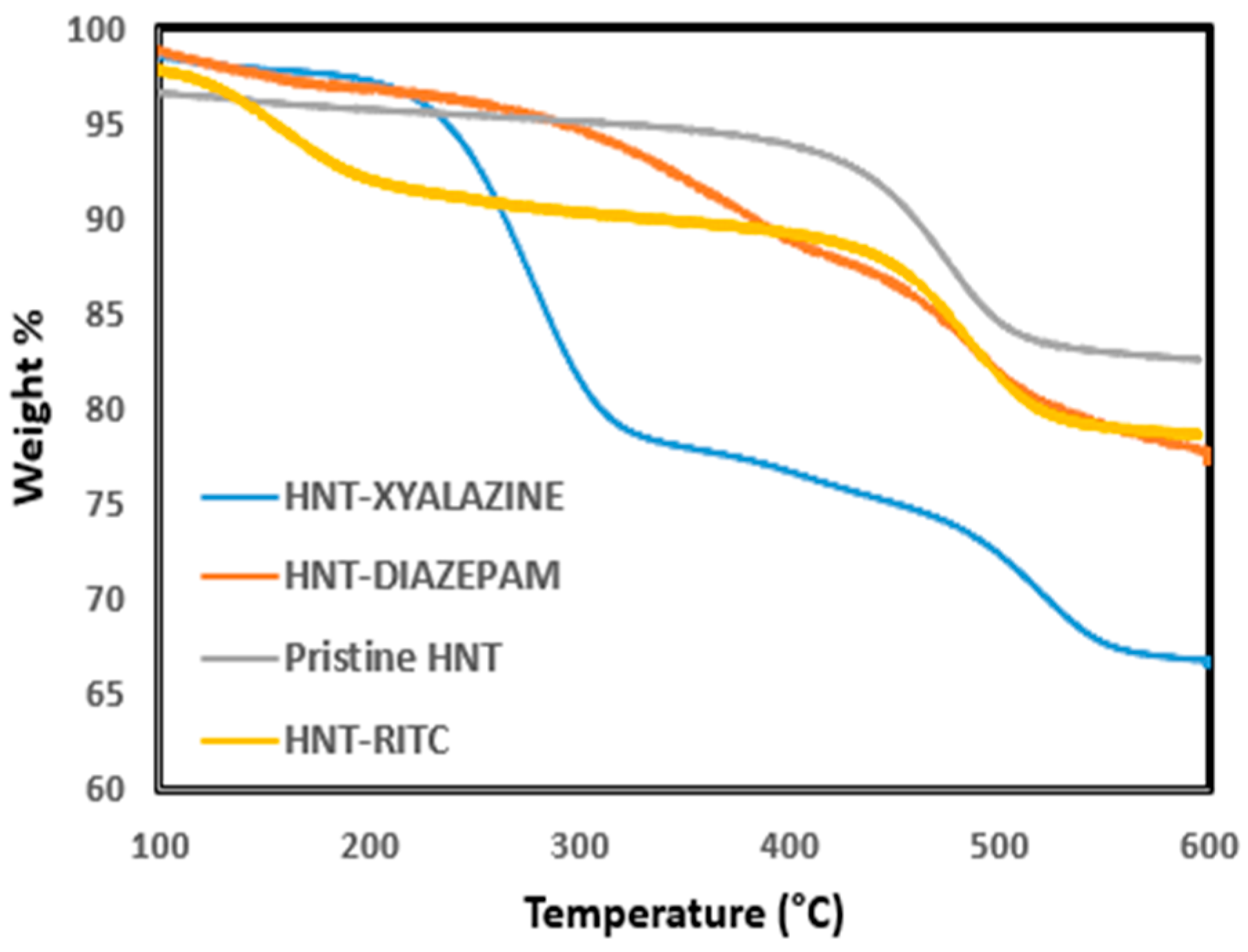
2.1. Quantification of Dye and Drugs Labeled/Loaded into Halloysite
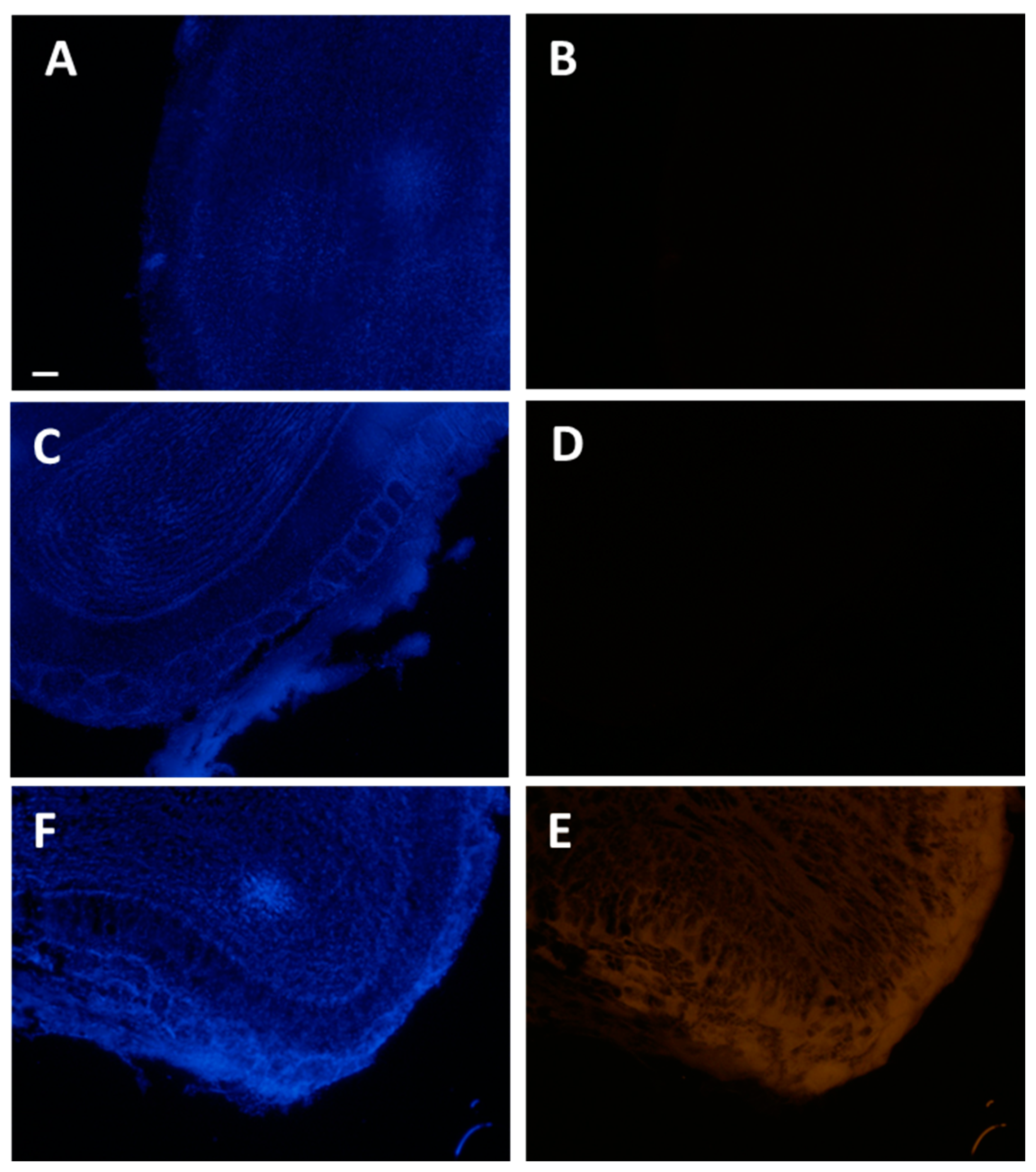
2.2. Visualization of Halloysite Delivered Non-Invasively to the Brain
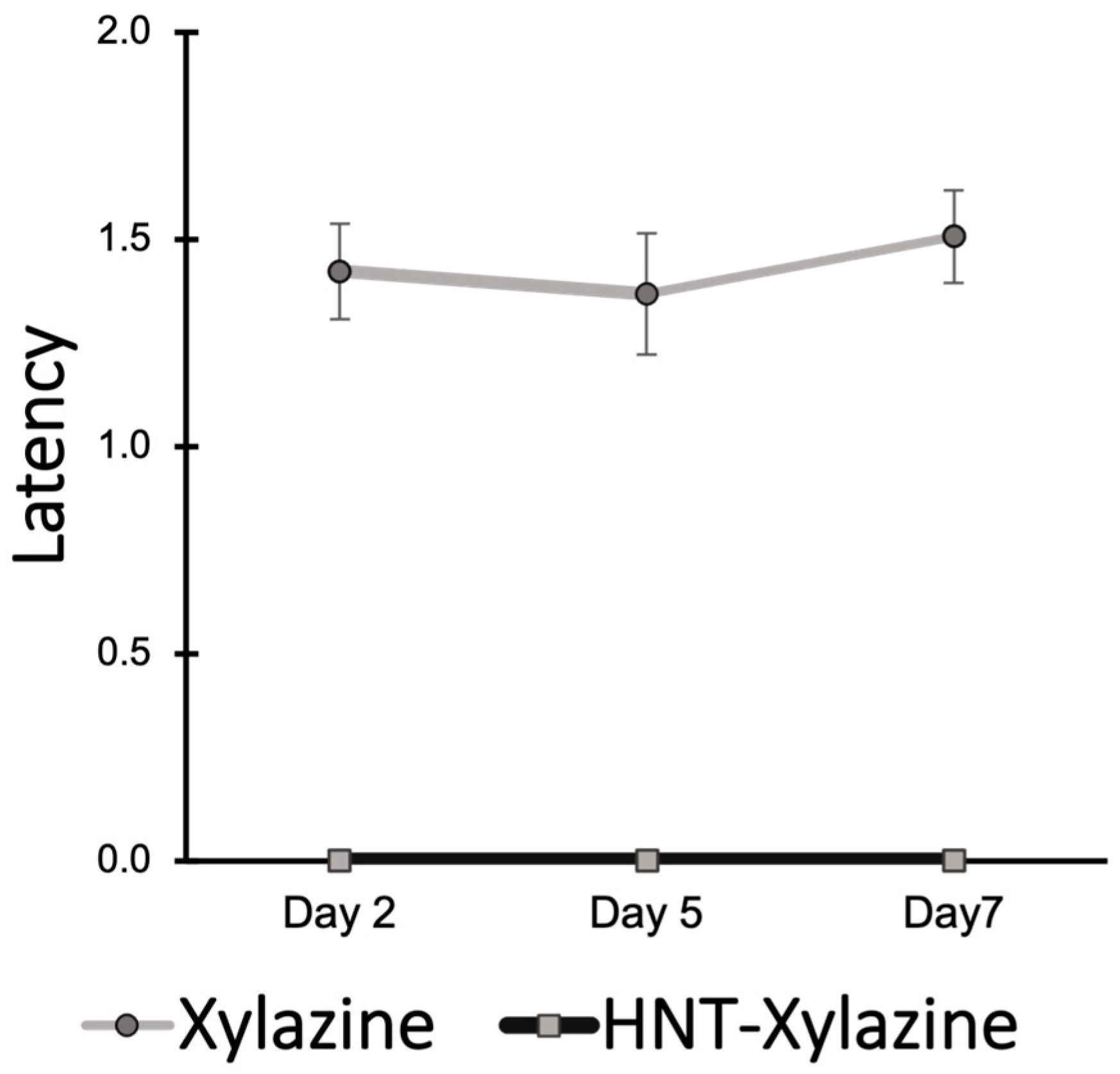
2.3. Performance in Rotarod Test Demonstrates Efficacy of Halloysite Drug Delivery
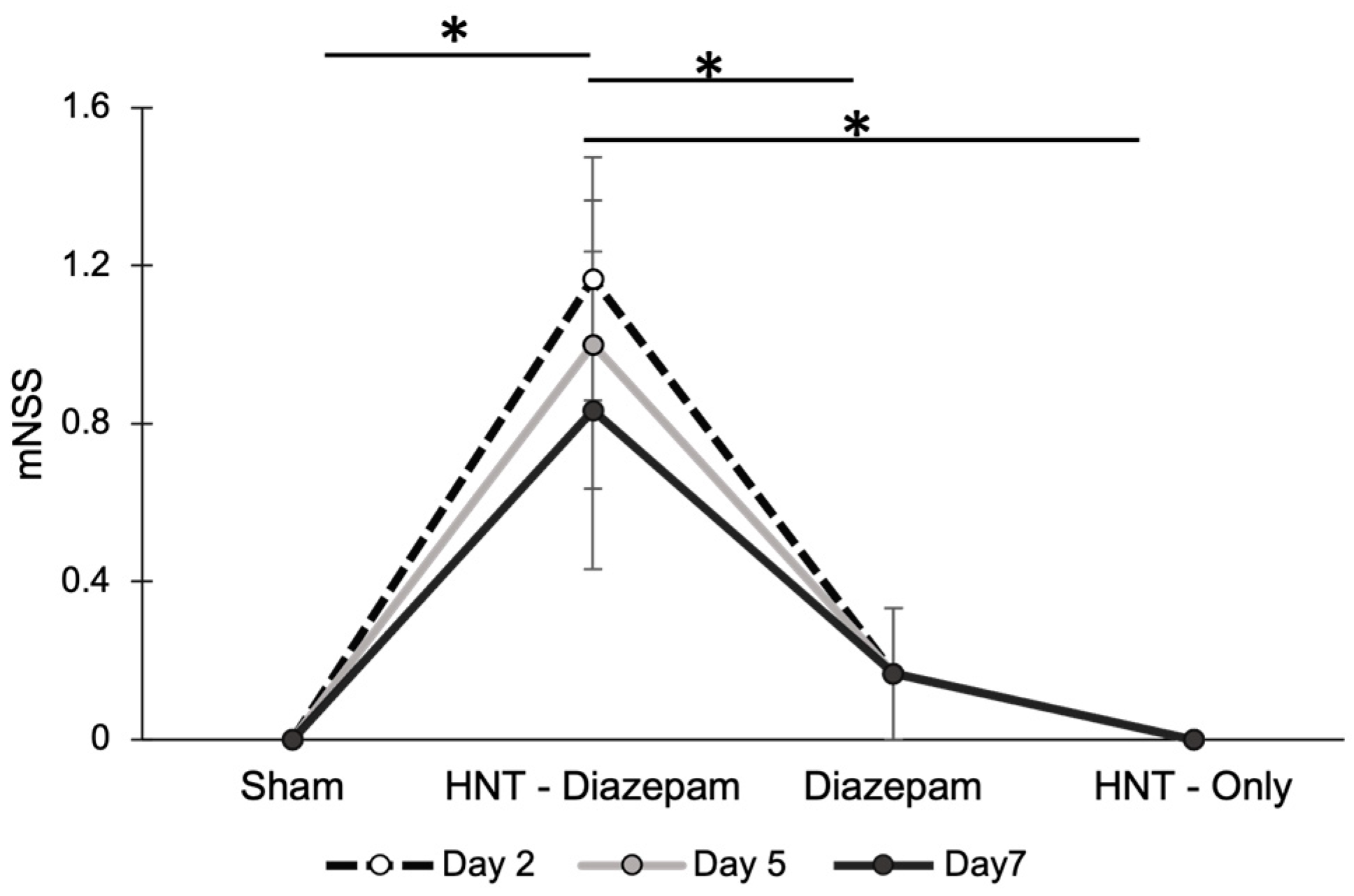
2.4. Modified Neurological Severity Scores Demonstrate the Efficacy of Halloysite Drug Delivery to CNS
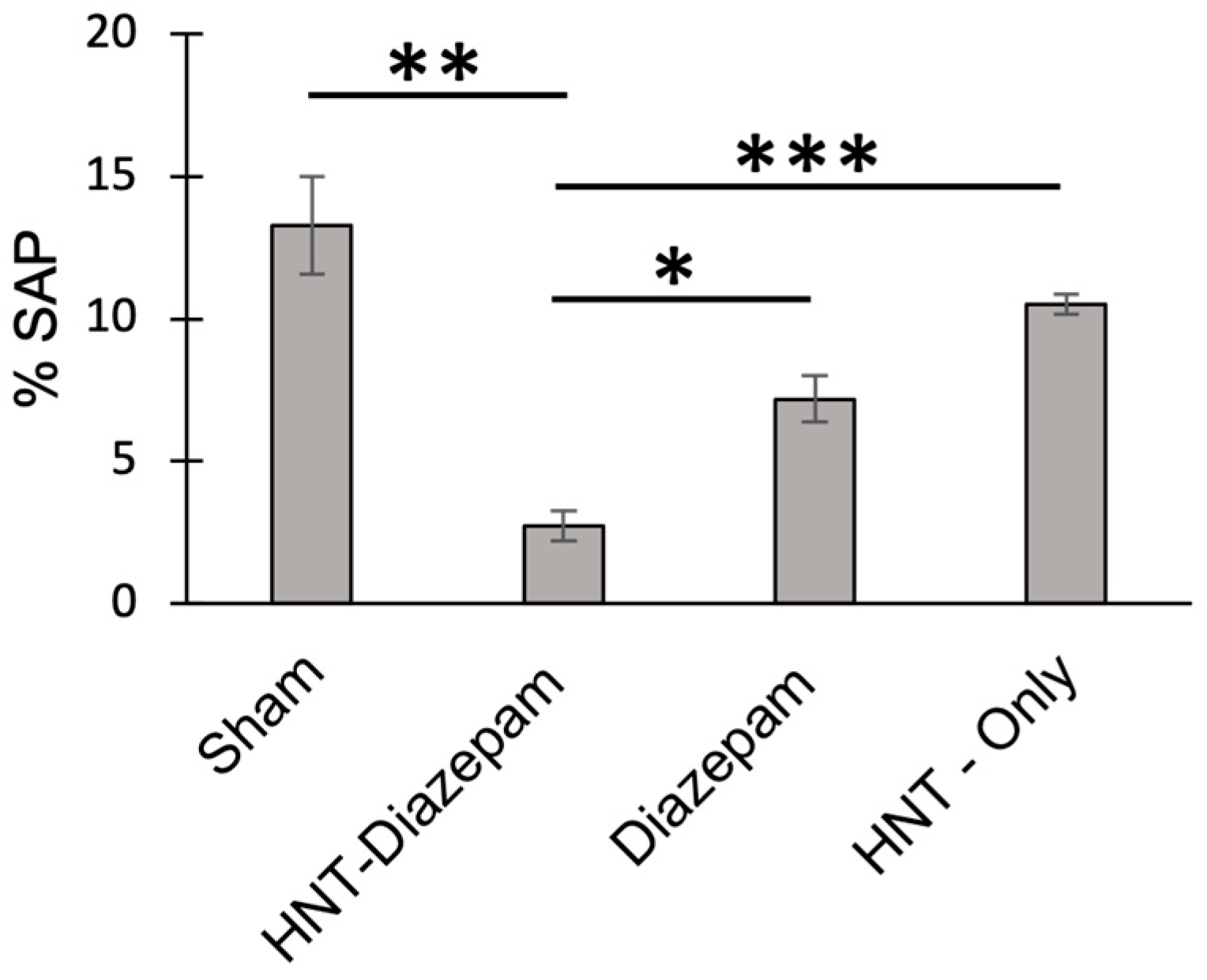
2.5. Anxiety Reduced by HNT/Diazepam Administration
2.6. Treatment Did Not Alter the Mass of Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation
4.3. Preparation of Halloysite with Labeling/Loading of RITC, Diazepam, and Xylazine
4.4. Animal Care and Handling
4.5. Intranasal Administration
4.6. Intraperitoneal Administration
4.7. Evaluation of BBB Permeability
4.8. Rotarod Test
4.9. Modified Neurological Severity Score (mNSS)
4.10. Stress Attend Posture in the Elevated Plus Maze
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Ngowi, E.E.; Wang, Y.Z.; Qian, L.; Helmy, Y.A.; Anyomi, B.; Li, T.; Zheng, M.; Jiang, E.S.; Duan, S.F.; Wei, J.S.; et al. The Application of Nanotechnology for the Diagnosis and Treatment of Brain Diseases and Disorders. Front. Bioeng. Biotechnol. 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Neurodegenerative Diseases. Available online: https://www.niehs.nih.gov/research/supported/health/neurodegenerative/index.cfm (accessed on 24 May 2023).
- Persano, F.; Batasheva, S.; Fakhrullina, G.; Gigli, G.; Leporatti, S.; Fakhrullin, R. Recent advances in the design of inorganic and nano-clay particles for the treatment of brain disorders. J. Mater. Chem. B 2021, 9, 2756–2784. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bansal, S.; Banik, A. Noninvasive Routes of Proteins and Peptides Drug Delivery. Indian J. Pharm. Sci. 2011, 73, 367. [Google Scholar]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Maggio, E.T. Intravail: Highly effective intranasal delivery of peptide and protein drugs. Expert Opin. Drug Deliv. 2006, 3, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems? J. Pharm. Sci. 2007, 96, 473–483. [Google Scholar] [CrossRef]
- Al Bakri, W.; Donovan, M.D.; Cueto, M.; Wu, Y.; Orekie, C.; Yang, Z. Overview of intranasally delivered peptides: Key considerations for pharmaceutical development. Expert Opin. Drug Deliv. 2018, 15, 991–1005. [Google Scholar] [CrossRef]
- Massaro, M.; Ciani, R.; Cinà, G.; Colletti, C.G.; Leone, F.; Riela, S. Antimicrobial Nanomaterials Based on Halloysite Clay Mineral: Research Advances and Outlook. Antibiotics 2022, 11, 1761. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Nanomaterials: A Review about Halloysite Nanotubes, Properties, and Application in the Biological Field. Int. J. Mol. Sci. 2022, 23, 11518. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Reis, S.; Veiga, F.; Saleh, M.; Lvov, Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin. Drug Deliv. 2019, 16, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Rozhina, E.V.; Trifonov, A.A.; Fakhrullin, R.F. Cell surface engineering with polyelectrolyte-stabilized magnetic nanoparticles: A facile approach for fabrication of artificial multicellular tissue-mimicking clusters. Nano Res. 2015, 8, 2515–2532. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, M.; Liu, X.; Huang, C.; Gu, Z.; Cao, Y. Evaluation of toxicity of halloysite nanotubes and multi-walled carbon nanotubes to endothelial cells in vitro and blood vessels in vivo. Nanotoxicology 2020, 14, 1017–1038. [Google Scholar] [CrossRef]
- Wang, X.; Gong, J.; Gui, Z.; Hu, T.; Xu, X. Halloysite nanotubes-induced Al accumulation and oxidative damage in liver of mice after 30-day repeated oral administration. Environ. Toxicol. 2018, 33, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kamalieva, R.F.; Ishmukhametov, I.R.; Batasheva, S.N.; Rozhina, E.V.; Fakhrullin, R.F. Uptake of halloysite clay nanotubes by human cells: Colourimetric viability tests and microscopy study. Nano Struct. Nano Objects 2018, 15, 54–60. [Google Scholar] [CrossRef]
- Mehdia, Y.M.; Fizir, M.; Itatahine, A.; Hea, H.; Dramou, P. Preparation of multifunctional PEG-graft-Halloysite Nanotubes for Controlled Drug Release, Tumor Cell Targeting, and Bio-imaging. Coll. Surfaces B Biointerf. 2018, 170, 322–329. [Google Scholar]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R. Selective antimicrobial effects of curcumin @ halloysite nanoformulation: A Caenorhabditis elegans study. ACS Appl. Mater. Interf. 2019, 11, 23050–23064. [Google Scholar] [CrossRef]
- Zhao, X.; Wan, Q.; Fu, X.; Meng, X.; Ou, X.; Zhong, R.; Zhou, Q.; Liu, M. Toxicity Evaluation of One-Dimensional Nanoparticles Using Caenorhabditis elegans: A Comparative Study of Halloysite Nanotubes and Chitin Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 18965–18975. [Google Scholar] [CrossRef]
- Saleh, M.Y.; Prajapati, N.; DeCoster, M.A.; Lvov, Y. Tagged Halloysite Nanotubes as a Carrier for Intercellular Delivery in Brain Microvascular Endothelium. Front. Bioeng. Biotechnol. 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Stanko, J.R. A Review of Oral Skeletal Muscle Relaxants for the Craniomandibular Disorder (CMD) Practitioner. CRANIO 1990, 8, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bieber, M.; Gronewold, J.; Scharf, A.C.; Schuhmann, M.K.; Langhauser, F.; Hopp, S.; Mencl, S.; Geuss, E.; Leinweber, J.; Guthmann, J.; et al. Validity and Reliability of Neurological Scores in Mice Exposed to Middle Cerebral Artery Occlusion. Stroke 2019, 50, 2875–2882. [Google Scholar] [CrossRef]
- Holly, K.S.; Orndorff, C.O.; Murray, T.A. MATSAP: An automated analysis of stretch-Attend posture in rodent behavioral experiments. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Y.; Shen, Y.; Zhou, C.; Li, Y.F.; He, R.R.; Liu, M. Enhanced Therapeutic Efficacy of Doxorubicin for Breast Cancer Using Chitosan Oligosaccharide-Modified Halloysite Nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 26578–26590. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Y.; Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharm. 2022, 14, 629. [Google Scholar]
- Li, W.; Liu, D.; Zhang, H.; Correia, A.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017, 48, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, R.; Xu, J.; Lan, Y.; Jiao, Y.; Zhou, C.; Zhao, Y. Poly(l-lactide)/halloysite nanotube electrospun mats as dual-drug delivery systems and their therapeutic efficacy in infected full-thickness burns. J. Biomater. Appl. 2015, 30, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Pádua-Reis, M.; Nôga, D.A.; Tort, A.B.L.; Blunder, M. Diazepam causes sedative rather than anxiolytic effects in C57BL/6J mice. Sci. Rep. 2021, 11, 9335. [Google Scholar] [CrossRef] [PubMed]
- Pernici, C.D.; Rowe, R.; Doughty, P.T.; Madadi, M.; Lifshitz, J.; Murray, T.A. Longitudinal in vivo imaging of progressive axonal damage and resolution with minocycline treatment after diffuse brain injury in a murine model. Sci. Rep. 2020, 10, 7815. [Google Scholar] [CrossRef]
- Pernici, C.D.; Kemp, B.S.; Murray, T.A. Time Course Images of Cellular Injury and Recovery in Murine Brain with High-Resolution GRIN Lens System. Sci. Rep. 2019, 9, 7946. Available online: www.nature.com/articles/s41598-019-44174-7 (accessed on 24 May 2023). [CrossRef]
- Lee, S.A.; Holly, K.S.; Voziyanov, V.; Villalba, S.L.; Tong, R.; Grigsby, H.E.; Glasscock, E.; Szele, F.G.; Vlachos, I.; Murray, T.A. Gradient index microlens implanted in prefrontal cortex of mouse does not affect behavioral test performance over time. PLoS ONE 2016, 11, e0146533. [Google Scholar] [CrossRef]
- Diazepam: 7 Things You Should Know—Drugs.com. Available online: https://www.drugs.com/tips/diazepam-patient-tips (accessed on 24 May 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanamadala, Y.; Saleh, M.Y.; Williams, A.A.; Lvov, Y.; Murray, T.A. Clay Nanotubes Loaded with Diazepam or Xylazine Permeate the Brain through Intranasal Administration in Mice. Int. J. Mol. Sci. 2023, 24, 9648. https://doi.org/10.3390/ijms24119648
Yanamadala Y, Saleh MY, Williams AA, Lvov Y, Murray TA. Clay Nanotubes Loaded with Diazepam or Xylazine Permeate the Brain through Intranasal Administration in Mice. International Journal of Molecular Sciences. 2023; 24(11):9648. https://doi.org/10.3390/ijms24119648
Chicago/Turabian StyleYanamadala, Yaswanthi, Mahdi Y. Saleh, Afrika A. Williams, Yuri Lvov, and Teresa A. Murray. 2023. "Clay Nanotubes Loaded with Diazepam or Xylazine Permeate the Brain through Intranasal Administration in Mice" International Journal of Molecular Sciences 24, no. 11: 9648. https://doi.org/10.3390/ijms24119648
APA StyleYanamadala, Y., Saleh, M. Y., Williams, A. A., Lvov, Y., & Murray, T. A. (2023). Clay Nanotubes Loaded with Diazepam or Xylazine Permeate the Brain through Intranasal Administration in Mice. International Journal of Molecular Sciences, 24(11), 9648. https://doi.org/10.3390/ijms24119648





