Abstract
Anthocyanins are one of the natural pigments that humanity has employed the most and can substitute synthetic food dyes, which are considered toxic. They are responsible for most purple, blue, and red pigment nuances in tubers, fruits, and flowers. However, they have some limitations in light, pH, oxygen, and temperature conditions. Combining biomolecules and inorganic materials such as clay minerals can help to reverse these limitations. The present work aims to produce materials obtained using cetyltrimethylammonium bromide in bentonite clay for incorporation and photostabilization of anthocyanin dye. Characterizations showed that the organic molecules were intercalated between the clay mineral layers, and the dye was successfully incorporated at a different pH. Visible light-driven photostability tests were performed with 200 h of irradiation, confirming that the organic–inorganic matrices were efficient enough to stabilize the quinoidal base form of anthocyanin. The pigment prepared at pH 10 was three-fold more stable than pH 4, showing that the increase in the synthesis pH promotes more stable colors, probably due to the stronger intermolecular interaction obtained under these conditions. Therefore, organobentonite hybrids allow to stabilize the fragile color coming from the quinoidal base form of anthocyanin dyes.
1. Introduction
Anthocyanins (ACN) are organic substances responsible for most purple, blue, and red colors of flowers and fruits. ACN have antioxidant properties and can produce free radicals. Despite having great potential for use in several areas, the applicability of ACN is limited by its reactivity and degradability [1,2,3]. The main factors influencing the color and stability of ACNs are the pH, temperature, light, oxygen, copigmentation, solvent effect, and structure [2,3].
Once extracted from their natural sources, the possibility of exploitable color nuances is reduced by the instability of the molecular structure of ACNs. When in solution, the flavylium cation, which is the most stable form of ACN, is converted to a quinoid base under neutral to mildly alkaline conditions and finally to the uncolored chalcone via carbinol pseudobase form with pH increase. All these conversions are accompanied by a marked color change, making it very difficult to apply the color range present in the pH medium from near neutral to slightly alkaline, due to the instability of the flavylium cation derivatives formed [1,4].
Several studies have been conducted to produce materials that can be used as a strategy to improve the color stability of ACN. These strategies include inorganic matrices such as clays, mesoporous materials, and zeolites [4,5]. In these materials, organic molecules can be intercalated in confined spaces or adsorbed on the surface, promoting increased stability of these organic molecules [5]. In addition, recent studies have shown that clay minerals, such as montmorillonite, saponite, and palygorskite improve the photo and chemical properties of ACN through the formation of hybrid materials [1,4,6,7].
Among clays, bentonite (Bent) is the raw clay containing at least 50% of smectite, particularly montmorillonite (Mt). In addition, Bent often contains other clay minerals including illite and/or other minerals such as quartz and feldspar [8,9]. Mt is 2:1 phyllosilicate, where the octahedral sheets lie between two tetrahedral sheets. Octahedral sheets have aluminum or magnesium bonded to oxygen or hydroxyl groups, while tetrahedral sheets are formed by silicon bonded to oxygen atoms [10]. Isomorphic substitutions in the octahedral sheets of the Mt result in the presence of Na+ or Ca2+ interlayer cations to counterbalance the excess negative charge in the lamella. Mt has a high cation exchange capacity and a large surface area, and it can be functionalized with different organic molecules by chemical modification. Thus, due to the structural and physicochemical properties, Mt is used in diverse industrial applications [9,11].
Additionally, Mt can be modified in many ways with organic molecules, resulting in inorganic–organic hybrid materials. Among them, intercalation of surfactants between the layers of Mt increases the interlayer space and is an effective route to prepare new functional materials [9,12]. Intercalated surfactants of Mt are produced through an ion exchange reaction between organic alkyl ammonium salts and the interlayer cations. Cetyltrimethylammonium bromide (CTAB), [(C16H33)N(CH3)3]Br is the most commonly used cationic surfactant for Mt intercalation. CTAB is often used to provide the adequate opening of Mt layers and improve the ability of Mt to incorporate new organic molecules in subsequent reactions [8].
Concerning the hybrid formation with ACN and organoclay, LIMA et al. 2020 [1] investigated the stabilization of the ACN quinoidal base form due to interactions with CTA+ molecules previously intercalated in the clay mineral saponite. The color of the hybrid pigment they obtained refers to the quinoidal base form even though they used a buffered medium at pH 3. Thus, this work has a new route to stabilize the quinoidal base form using bentonite as mineral clay, in addition to evaluating the synthesis at pH 7 and 10, which are more favorable to the presence of the quinoidal base form; this has not yet been evaluated in previous studies.
The present work aims to produce materials derived from bentonite clay intercalated with surfactant and apply them to incorporate anthocyanin dye at different pH conditions. Finally, it investigates the photostability under visible light of the ACN incorporated into CTAB-expanded clay materials by colorimetric studies.
2. Results and Discussion
CHN elemental analysis of the Bent sample resulted in 1.04 ± 0.02, 1.34 ± 0.00, and 1.05 ± 0.03%, respectively. The nitrogen content and exchange ammonium amount was 0.75 ± 0.02, and the calculated cation exchange capacity (CEC) was 75.23 ± 1.789 cmol(+)/kg (Table S1 Supplementary Material). The CEC value was close to the bentonite clays obtained in previous studies [12,13].
X-ray diffractometry (XRD) patterns of the raw and modified bentonites are presented in Figure 1. The XRD of the ACN (Figure 1a) is typical of an amorphous material [14]. XRD patterns of the Bent clay in Figure 1b indicated that Mt is the majority phase in the clay sample. Reflections at 2θ = 7.03°, 19.88°, 20.88°, 28.62°, 34.94°, and 61.92° were indexed to Mt. Other reflections at 2θ = 11.74° and 26.74° were associated with muscovite (Mu) and quartz (Qt) phases, respectively. The reflections at 2θ = 7.06° and 61.92° are related to the crystallographic planes (001) and (060), respectively. These planes are characteristic of dioctahedral montmorillonite. Reflection at 2θ = 7.03° was associated to a basal spacing (d001) of 1.26 nm [12,13,15,16]. For CTAB/Bent, Figure 1c shows the disappearance of the reflection at 2θ = 11.74° and 28.62° and the displacement of the peak at 2θ = 7.03° to 4.82°, which corresponded to a d value of 1.83 nm. The displacement of the 001 reflections to a lower 2θ value indicated an increase in the basal space of the Mt after incorporating CTAB and confirms that CTAB was successfully intercalated into the Mt interlayer space [9,12,17]. XRD patterns of the materials after the dye incorporation are presented in Figure 1d–f. The initial reflection (001) at 4.82° for Bent was shifted to 4.56°, 4.62°, and 4.48° for CTAB/ACN/Bentx (x is referred to as pH 4, 7, or 10, respectively), representing basal spacing (d001) changes from 1.82 nm to 1.93 nm, 1.91 nm, 1.97 nm, respectively. The increases in d001 space can indicate the successful intercalation of the ACN in modified Mt at the adopted pH [1,18].
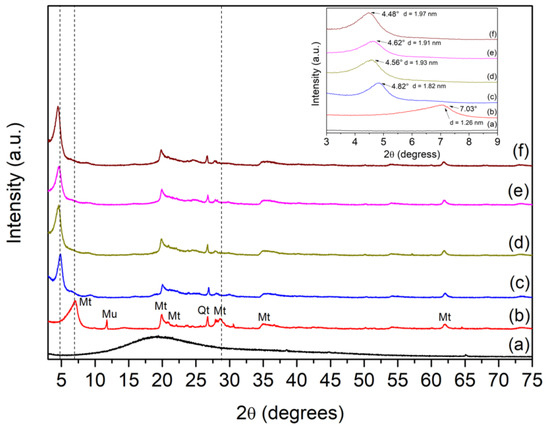
Figure 1.
XRD patterns for ACN (a), Bent (b), CTAB/Bent (c), CTAB/ACN/Bent-4 (d), CTAB/ACN/Bent-7 (e), and CTAB/ACN/Bent-10 (f).
FTIR spectra of the raw and modified clay mineral samples are presented in Figure 2. The FTIR spectrum of the Bent (Figure 2b) showed characteristic Mt bands located at 3647 and 3439 cm−1, assigned to OH stretching of the structural hydroxyl groups (AlOH, MgOH) and SiOH or OH of the interlayer water of the Mt, respectively. The band at 1639 cm−1 was related to the angular deformation of the interlayer water. The band at 1035 cm−1 was attributed to the Si-O stretching of the tetrahedral sheet of the 2:1 phyllosilicate structure. The band at approximately 916 cm−1 was related to the Al-O angular deformation. Finally, the bands at 792 and 637 cm−1 were related to the presence of quartz and the M-O-Si angular vibration of cations located at octahedral sites [15,19].
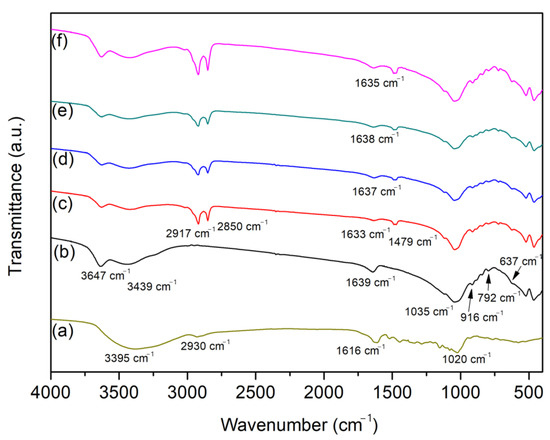
Figure 2.
FTIR spectra of ACN (a), Bent (b), CTAB-Bent (c), CTAB/ACS/Bent-4 (d), CTAB/ACS/Bent-7 (e), and CTAB/ACS/Bent-10 (f).
In the FTIR spectrum of the CTAB/Bent in Figure 2c, bands of the inorganic structure were maintained, but three new bands appeared at 2917, 2850, and 1479 cm−1. The bands at 2917 and 2850 cm−1 were assigned to the asymmetrical and symmetrical CH stretching of methylene groups, indicating the presence of alkyl moieties of the CTAB chain. The band at 1479 cm−1 was attributed to the N-H deformations in the ammonium group [1,9,20]. FTIR results corroborated the XRD patterns and confirmed that CTAB was incorporated in the Mt phase.
Figure 2a shows the FTIR spectrum of pure ACN. A broad band centered at 3395 and a low-intensity band at 2930 cm−1 were assigned to the OH stretching in the phenol group and CH asymmetric stretching, respectively. The band at 1616 cm−1 was attributed to the C=C stretching of the aromatic rings, and the band at 1020 cm−1 was related to the CCH deformation of the aromatic rings [21].
Figure 2d–f present the FTIR spectra of the materials after the incorporation of ACN at the different pH used in this study. The incorporation of ACN in the CTAB/Bent sample did not result in changes in the spectra of these materials with the presence of the dye, possibly due to the strong absorption associated to CTAB in the same region as the ACN. However, the FTIR spectra of dyed samples was observed in 1633 cm−1, which may be related to the hydrogen interactions between the nitrogen and OH groups of the CTAB/Bent and ACN molecules [1,22].
Possible alterations in the morphology of samples after the reactions were monitored by scanning electron microscopy (SEM) analysis. SEM images are in Figure 3. The morphology of raw Bent (Figure 3a) showed a heterogeneous and irregular structure and the presence of some flakes or agglomerates [20,23,24,25]. After intercalation with CTAB, the CTAB/Bent (Figure 3b) continued to have an irregular and heterogeneous morphology. In addition, the morphology of CTAB/Bent displayed a number of flakes or agglomerates of varying sizes as well as cracks and caves. This may have occurred because incorporating CTAB promoted a displacement in the layers of the Mt, which resulted in a disordered agglomeration of platelets in some parts of the sample [20,23,24]. SEM images of the materials after the ACN incorporation are in Figure 3c–e and indicate that morphology in the form of flakes or agglomerates was maintained [25].
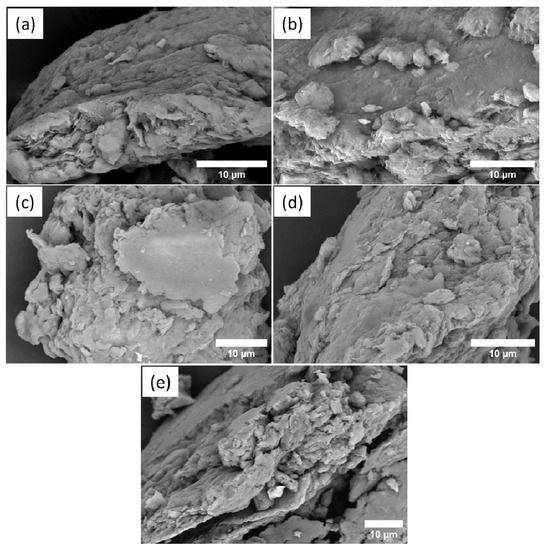
Figure 3.
SEM of Bent (a), CTAB/Bent (b), CTAB/ACN/Bent-4 (c), CTAB/ACN/Bent-7 (d), and CTAB/ACN/Bent-10 (e).
TG/DTG curves of the materials are shown in Figure 4. In addition, Table 1 presents the main thermal degradation events of ACN, Bent, and its derivatives. The ACN had two characteristic mass loss events, the first one with a maximum temperature of 78 °C, referring to the elimination of surface water, and the second event with a maximum temperature of 297 °C, referring to the thermal decomposition of ACN [26]. The raw Bent presented three mass loss events that are characteristic of the bentonite clay. The incorporation of CTAB altered the thermal characteristics of the Bent clay, as shown in the TG and DTG of the CTAB/Bent material. The graph illustrates that the degradation event related to the loss of adsorbed water (first event) in the CTAB/Bent material occurred at a lower temperature due to the increased hydrophobicity of the clay by incorporating CTAB. Thus, the CTAB/Bent material has less water content, and the amount of water eliminated reduces the dehydration temperature [27,28].
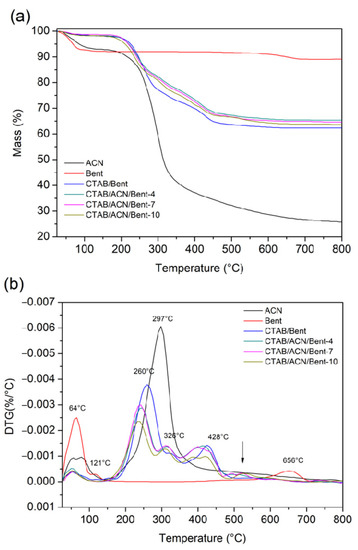
Figure 4.
TG (a) and DTG (b) curves of ACN, Bent, CTAB/Bent, and dyed solids.

Table 1.
Main thermal degradation events of Bent and its derivatives.
Furthermore, the CTAB/Bent material presented three more mass loss events after incorporating CTAB. The maximum temperatures of these three degradation events were 260 °C (second event), 326 °C (third event), and 428 °C (fourth event), which are related to the presence of CTAB in the clay structure [29,30]. The dehydroxylation thermal event (fifth event) shifted from 650 °C to 575 °C, with a decrease in intensity, as shown in the DTG. The TGs and DTGs of the CTAB/ACN/Bent-4, CTAB/ACN/Bent-7, and CTAB/ACN/Bent-10 materials showed the same four thermal degradation events as their precursor CTAB/Bent, with slight variations in degradation temperatures and percentages of mass loss due to intermolecular interactions obtained from the incorporation of ACN. Therefore, these events are related to the degradation of both CTAB and ACN. In addition to these four events, all materials had a fifth thermal degradation event in the temperature ranges of 470–645 °C (CTAB/ACN/Bent-4), 468–663 °C (CTAB/ACN/Bent-7), and 462–662 °C (CTAB/ACN/Bent-10). This thermal degradation event is related to the thermal dehydroxylation of the clay that was shifted to lower temperatures after the formation of the hybrid pigment [1,22].
The flavylium cation form of ACN majorly influenced the UV-Vis spectra of ACN solutions at natural and pH 4. When the pH was increased to pH 7 and 10 before hybrid formation, the shift and broadening of the absorption band compared with natural pH indicated the deprotonation of the flavylium cations into the quinoidal base form of ACN. Furthermore, after incorporating ACN in the CTAB/Bent, the intensity of absorption bands decreased, providing the values of adsorption capacity equal to 55.4% (Figure 5c), 26.7% (Figure 5b), and 44.7% (Figure 5a) for the pigments obtained at pH 10, 7, and 4, respectively, suggesting that the ACN molecule was about 20% more adsorbed at pH 10 (Figure 5c) [1,4].
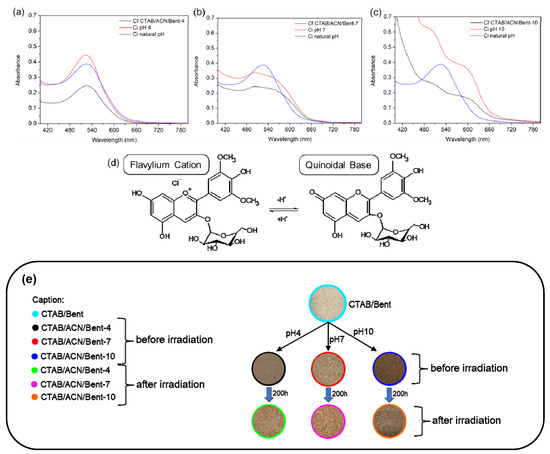
Figure 5.
UV-Vis spectra of the ACN solutions at natural pH and pH 4 (a), 7 (b), and 10 (c) before and after incorporating dye in the CTAB/Bent hybrid. Ci and Cf are the initial and final dye concentrations, respectively, (d) deprotonation of the flavylium cations into the quinoidal base form of ACN (e) Digital photographs of the hybrid pigments before and after irradiation.
The photostability of the hybrid dyed materials was evaluated under visible light for 200 h by the L*a*b* colors space parameters of solid hybrid pigments measured before and after light exposure, as seen in Figure 5e and Table 2.

Table 2.
Total color differences (ΔE*) for each pigment compared with CTAB/Bent precursor and after 200 h of irradiation.
The L* (lightness) coordinate can assume values from 0 (black) to 100 (white). The b* (yellowness) coordinate indicates the yellow–blue component of a color, where positive and negative values indicate yellow and blue, respectively. The a* (redness) coordinate indicates the red–green component of a color, where positive and negative values indicate red and green, respectively [31].
The new colors obtained after ACN incorporation at the respective pH can be seen in digital photographs of Figure 5e and as a function of L*a*b* coordinates of CIE color space in Table 2. The different colors with increased pH are a function of the more pronounced influence of the quinoidal base form of ACN. Comparing the hybrid pigment with the CTAB/Bent precursor, the total color differences ΔE* values (Table 2) calculated before the irradiation were higher when the pH increased, probably due to the increased ACN incorporation and quinoidal base form contribution [1,4].
After irradiation, the color of the hybrid pigment changes its initial CIE L*a*b* coordinates, indicating a fade of the respective color (Figure 5e and Table 2). The changes of L*a*b* coordinates were less pronounced for the CTA/ACN/Bent-10, indicating that the pigment formed at the highest pH had the least fading. The ΔE* values can quantitatively monitor these changes after irradiation, which was about 3-fold smaller for the pigment obtained at pH 10 compared to pH 4 (Table 2) and about 4.5-fold lower than the values published by LIMA et al. 2020 (ΔE* = 19.3 for a pigment based on saponite obtained at pH 3) [1], who synthesized their hybrid pigments at a lower pH. This result indicates that the pigment at pH 10 was the most photostable, probably due to the strong intermolecular interactions formed in the pigment obtained at this pH [1,4].
3. Materials and Methods
3.1. Material and Chemicals
Raw Bent sample, donated by the Bentonisa company (Boa Vista, Paraíba, Brazil), was used as precursor material. Hexadecyltrimethylammonium bromide (CTAB) (Êxodo Científica, Sumaré, Brazil), ammonium acetate (Êxodo Científica, São Paulo, Brazil), anthocyanin (ACN) (elderberry extract—SCIYU BIOTECH CO., LIMITED, Xi’an, China), hydrochloric acid (Dinâmica, São Paulo, Brazil), sodium hydroxide (Êxodo Científica, São Paulo, Brazil), and ethanol (Dinâmica, São Paulo, Brazil) were analytical grade and used without prior purification. Distilled water was used in all preparations
3.2. Cation Exchange Capacity (CEC)
The CEC of Bent sample was determined by ion exchange method using ammonium acetate solution [13,32]. The procedure was carried out with following the steps described in Table 3.

Table 3.
Cation exchange capacity (CEC) of Bent by the ammonium acetate method.
3.3. Synthesis of the CTAB/Bent Hybrid
The CTAB/Bent hybrid synthesis was performed using CTAB in the proportion of 200% of the CEC of the clay mineral. In the results obtained from the CEC in Section 3.2 (Table S1), the amount of CTAB mass corresponded to 200% of the CEC of the Bent (calculation available in Supplementary Material) [12]. According to Brito et al. 2018, this proportion favors better conditioning of CTAB in clay. Reactions were performed following the method described previously [9,12,13]. Thus, 4.0 g of Bent were suspended in 100 mL of CTAB (2.19 g) surfactant and stirred for 24 h at room temperature (25 °C). The resulting solid was centrifuged (5000 rpm for 10 min) and washed with distilled water. Finally, the produced material was dried at 100 °C for 24 h.
3.4. Sequential Infusion of ACN to Produce ACN in the CTAB/Bent Hybrid
According to previous studies, the reaction between the dye and CTAB/Bent hybrid was performed at acidic, neutral, and basic pH [1,12,13]. In a typical procedure, 1 g of CTAB/Bent was dispersed in 100 mL of 600 mg L−1aqueous dye solution at pH 4.0, 7.0, and 10.0, and the systems were stirred for 4 h at 25 °C. Next, the pH was adjusted with 1.0 mol L−1 HCl and/or 1.0 mol L−1 NaOH aqueous solutions. The initial pH of the dye solution (without solid) was ∼4.3. Subsequently, the samples were centrifuged at 5000 rpm for 10 min. The solid fraction was washed with distilled water and centrifuged under the same conditions. Finally, the samples were dried in an oven at 50 °C for 24 h. Samples were denominated as CTAB/ACN/Bentx (x is referred to as pH 4, 7 or 10, respectively) (Table 4). The procedure is summarized in the scheme of Figure 6 [33,34].

Table 4.
Summary of acronyms used in the synthesized samples.
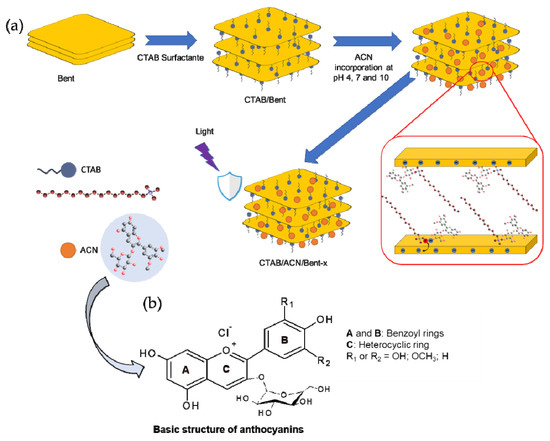
Figure 6.
(a) Scheme relating the synthesis of the CTAB/ACN/Bentx hybrids and (b) basic structure of anthocyanins (ACN).
3.5. Photostability Test
The photostability of dye in the CTAB/ACN/Bentx hybrids was performed by exposing the samples to visible light irradiation by using a 160W Hg lamp. The radiation was monitored by a radiometer (Hanna), and the value was 1.55 klux. Each hybrid pigment powder was dispersed in Petri dishes, placed at a distance of 15 cm below the lamp, and irradiated for 200 h [4]. The “Commission Internationale of l’Eclairage” (CIE) 1976 color space system was applied to evaluate the color of the pigments. Measurements were performed as a function of L*, a*, and b* coordinates obtained from a UV-Vis spectrometer, Shimadzu UV-2600i. The differences in colors between unexposed and exposed samples were calculated by equation [1,4].
3.6. Characterizations
Elemental analyses (CHN) of the samples were performed in a Perkin-Elmer PE-2400 microelemental device. The samples were characterized by X-ray diffractometry (XRD), using a Shimadzu diffractometer (model Labx-XDR 6000, Japan) operating at 40 kV and 30 mA, with Cu Kα radiation (λ = 1.5406 Å) and 3 to 75° 2θ range with a speed of first min−1. The Fourier transform infrared spectroscopy (FTIR) analyses of the samples were performed in a Bruker Vertex 70 model equipment (Germany) by preparing KBr pellets at 1% (m/m). FTIR spectra were obtained with a resolution of 4 cm−1, 120 scans between 4000 and 400 cm−1. The morphology was carried out by scanning electron microscopy (SEM), using a field emission electron source (SEM-EC) device from FEI, model Quanta FEG-250 (Eindhoven, Netherlands). The samples were mounted on stubs using double-sided carbon tape and covered in gold. The SEM analysis conditions were from 8 to 20 kV, and the working distance was 10 mm with point 3. The thermal stability of the samples was studied using the thermogravimetry technique (TG). TG curves were obtained using the TGA-50 Shimadzu (Japan) device at 10 °C min−1 heating rate in an argon atmosphere in alumina pun at 25 to 800 °C, and a mass of approximately 8 mg. Dye solutions were scanned using a UV-Vis spectrophotometer Cary 60, Agilent Technologies (USA), between 250 and 800 nm.
4. Conclusions
CTAB-intercalated bentonite was used as a matrix to improve the photostability of ACN dye. The incorporation of CTAB surfactants and ACN molecules into the Bent clay sample was confirmed by the increases in the d001 spaces of 0.11 nm, 0.09 nm, and 0.15 nm for the pigment obtained at pH 4, 7, and 10, respectively. The new absorption bands in FTIR were attributed to the organic molecules contributions, and the morphology differences observed by the SEM images and the events of mass loss observed between 150 and 550 °C in the TG/DTG analysis were associated to the respective organic molecules incorporated. In addition, the photostability of the anthocyanin incorporated into the organoclay was confirmed by the L*a*b* colors space parameters, in which the pigment obtained at pH 10 was about 3-fold more stable than the pH 4-synthesized pigment and about 4.5-fold more stable than the other previously published.
The colors of the hybrid pigments are a function of their intermolecular interaction with ACN molecules, which is influenced by the pH used during the synthesis. Previous works demonstrated that the hybrid formation at slightly acid conditions allow the CTA+ species, previously incorporated into clay, to promote the conversion of the dye molecule from flavylium cation to the quinoidal base form, enabling dye incorporation and stabilizing the weakly photostable structural form of ACN. As the quinoidal base form of ACN is more pronounced in a neutral to slightly basic medium, the increase in pH used to synthesize the pigment in this work promoted stability in colors since the more vital intermolecular interaction was easily obtained under these conditions.
A wide range of possibilities is opening to develop new hybrid pigments that explore the weakly applicable colors from the quinoidal base form of anthocyanin for different application fields, exploring other clay minerals or another surfactant at a different pH of syntheses because it is a demonstrated way to stabilize this easily breakable molecule.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24032417/s1.
Author Contributions
Conceptualization, R.V.C., R.D.S.B., A.I.S.M. and J.S.N.d.S.; data curation, R.V.C., P.T., L.C.B.-L. and A.I.S.M.; formal analysis, R.V.C., R.D.S.B., P.T. and A.I.S.M.; investigation, R.V.C., A.I.S.M. and L.C.B.-L.; methodology, R.V.C., P.T. and A.I.S.M.; project administration, J.A.O.; software, E.C.S.-F. and J.A.O.; supervision, E.C.S.-F. and J.A.O.; validation, R.V.C.; visualization, A.I.S.M.; writing—original draft, R.V.C., R.D.S.B., D.H.L.D. and M.G.F.; writing—review and editing, R.V.C., R.D.S.B., A.I.S.M., P.T. and J.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by UFPI/IFPI (Grant 45/2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Coordination Support in Higher Education (CAPES), the National Council for Scientific and Technological Development (CNPq), and the Foundation of Support to Research of Piauí (FAPEPI) for financial support. The Federal University of Piauí (UFPI) and the Federal Institute of Piauí (IFPI) provided the research conditions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lima, L.C.B.; Silva, F.C.; Silva-Filho, E.C.; Fonseca, M.G.; Zhuang, G.; Jaber, M. Saponite-anthocyanin derivatives: The role of organoclays in pigment photostability. Appl. Clay Sci. 2020, 191, 105604. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Viqueira, V.; Luzi, F.; Dominici, F.; Terenzi, A.; Maron, E.; Hamzaoui, M.; Kohnen, S.; Torre, L.; et al. Anthocyanin Hybrid Nanopigments from Pomegranate Waste: Colour, Thermomechanical Stability and Environmental Impact of Polyester-Based Bionanocomposites. Polymers 2021, 13, 1966. [Google Scholar] [CrossRef]
- Ribeiro, H.L.; Oliveira, A.V.; de Brito, E.S.; de Ribeiro, P.R.V.; Souza Filho, M.M.; Azeredo, H.M.C. Stabilizingeffectofmontmorilloniteon acerola juiceanthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef]
- Lima, L.C.B.; Castro-Silva, F.; Silva-Filho, E.C.; Fonseca, M.G.; Jaber, M. Saponite-anthocyanin pigments: Slipping between the sheets. Microporous Mesoporous Mater. 2020, 300, 110148. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, K.M.; Silva, C.P.; Gonçalves, J.M.; Quina, F.H. Hybrid Pigments from Anthocyanin Analogues and Synthetic Clay Minerals. ACS Omega. 2020, 5, 26592–26600. [Google Scholar] [CrossRef]
- Kohno, Y.; Kinoshita, R.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, C.P.; Gehlen, M.H.; Oake, J.; Bohne, C.; Quina, F.H. Organic/inorganic hybrid pigments from flavylium cations and palygorskite. Appl. Clay Sci. 2018, 162, 478–486. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Bello, V.E.; Amoo, K.O. Production and characterization of composite nanoparticles derived from chitosan, CTAB and bentonite clay. Chem. Pap. 2022, 76, 5063–5086. [Google Scholar] [CrossRef]
- Pinos, J.Y.M.; de Melo, L.B.; de Souza, S.D.; Marçal, L.; de Faria, E.H. Bentonite functionalized with amine groups by the sol-gel route as efficient adsorbent of rhodamine-B and nickel (II). Appl. Clay Sci. 2022, 223, 106494. [Google Scholar] [CrossRef]
- Resende, R.F.; Silva, T.F.B.; Santos, N.A.V.; Papini, R.M.; Magriotis, Z.M. Anionic collector adsorption onto bentonites and potential applications in the treatment of mining wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127401. [Google Scholar] [CrossRef]
- Santos, S.S.G.; França, D.B.; Castellano, L.R.C.; Trigueiro, P.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G. Novel modified bentonites applied to the removal of an anionic azo-dye from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 1585, 24152. [Google Scholar] [CrossRef]
- Brito, D.F.; da Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef]
- França, D.B.; Trigueiro, P.; Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyridinium bentonites. Chemosphere 2019, 242, 125109. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Queiroga, L.N.F.; Pereira, M.B.B.; Silva, L.S.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Microwave bentonite silylation for dye removal: Influence of the solvent. Appl. Clay Sci. 2019, 168, 478–487. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.S.; Fonseca, M.G.; da Silva Filho, E.C.; Jaber, M. Thiabendazole/bentonites hybrids as controlled release systems. Colloids Surf. B Biointerfaces 2019, 176, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Shugulí, C.; Rodríguez, F.J.; Bruna, J.E.; Galotto, M.J.; Sarantópoulos, C.; Favaro Perez, M.A.; Padula, M. Cetylpyridinium bromide-modified montmorillonite as filler in low density polyethylene nanocomposite films. Appl. Clay Sci. 2019, 168, 203–210. [Google Scholar] [CrossRef]
- Sciascia, L.; Calabrese, I.; Cavallaro, G.; Merli, M.; Scialabba, C.; Liveri, M.L.T. Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy. Minerals 2021, 11, 1315. [Google Scholar] [CrossRef]
- Uma Maheswari, B.; Sivakumar, V.M.; Thirumarimurugan, M. Sequester adsorption of Cr(VI) and Pb(II) ions from aqueous solution by green synthesized nanocomposite (OB/ZnO): Characterization, kinetics, isotherm and mechanism. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100680. [Google Scholar] [CrossRef]
- Ulhaq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M.; Ilyas, M. Engineering TiO2 supported CTAB modified bentonite for treatment of refinery wastewater through simultaneous photocatalytic oxidation and adsorption. J. Water Process Eng. 2021, 43, 102239. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials 2019, 12, 3287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, J.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. Acid/base reversible allochroic anthocyanin/palygorskite hybrid pigments:Preparation, stability and potential applications. Dyes Pigm. 2019, 171, 107738. [Google Scholar] [CrossRef]
- Wang, T.L.; Yin, X.; Wei, X. Application of thiabendazole/bentonites hybrids as efficient antibacterial agent against Escherichia coli and Staphylococcus aureus. Mater. Res. 2021, 8, 125306. [Google Scholar]
- Zhu, Y.; Cui, Y.; Shan, Z.; Dai, R.; Shi, L.; Chen, H. Fabrication and characterization of a multi-functional and environmentally-friendly starch/organo-bentonite composite liquid dust suppressant. Powder Technol. 2021, 391, 532–543. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhong, L.; Cheng, H.; Wang, H.; Ma, Q. Adsorption of reactive red 136 onto chitosan/montmorillonite intercalated composite from aqueous solution. Appl. Clay Sci. 2019, 167, 9–22. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and characteristics of alginate and anthocyanin complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Dos Santos, A.; Viante, M.F.; Pochapski, D.J.; Downs, A.J.; Almeida, C.A.P. Enhanced removal of p-nitrophenol from aqueous media by montmorillonite clay modified with a cationic surfactant. J. Hazard. Mater. 2018, 355, 136–144. [Google Scholar] [CrossRef]
- Vallova, S.; Plevova, E.; Smutna, K.; Sokolova, B.; Vaculikova, L.; Valovicova, V.; Hundakova, M.; Praus, P. Removal of analgesics from aqueous solutions onto montmorillonite KSF. J. Therm. Anal. Calorim. 2022, 47, 1973–1981. [Google Scholar] [CrossRef]
- Yu, W.H.; Zhu, T.T.; Tong, D.S.; Wang, M.; Wu, Q.Q.; Zhou, C.H. Preparation of Organo-montmorillonites and the Relationship Between Microstructure and Swellability. Clays Clay Miner 2017, 65, 417–430. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming Montmorillonite Surfaces Using a Cationic Surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef]
- Zhuang, G.; Jaber, M.; Rodrigues, F.; Rigaud, B.; Walter, P.; Zhang, Z. A new durable pigment with hydrophobic surface based on natural nanotubes and indigo: Interactions and stability. J. Colloid Interface Sci. 2019, 552, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Czímerová, A.; Bujdák, J.; Dohrmann, R. Traditional and novel methods for estimating the layer charge of smectites. Appl. Clay Sci. 2006, 34, 2–13. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).