Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies
Abstract
1. Introduction
2. Results
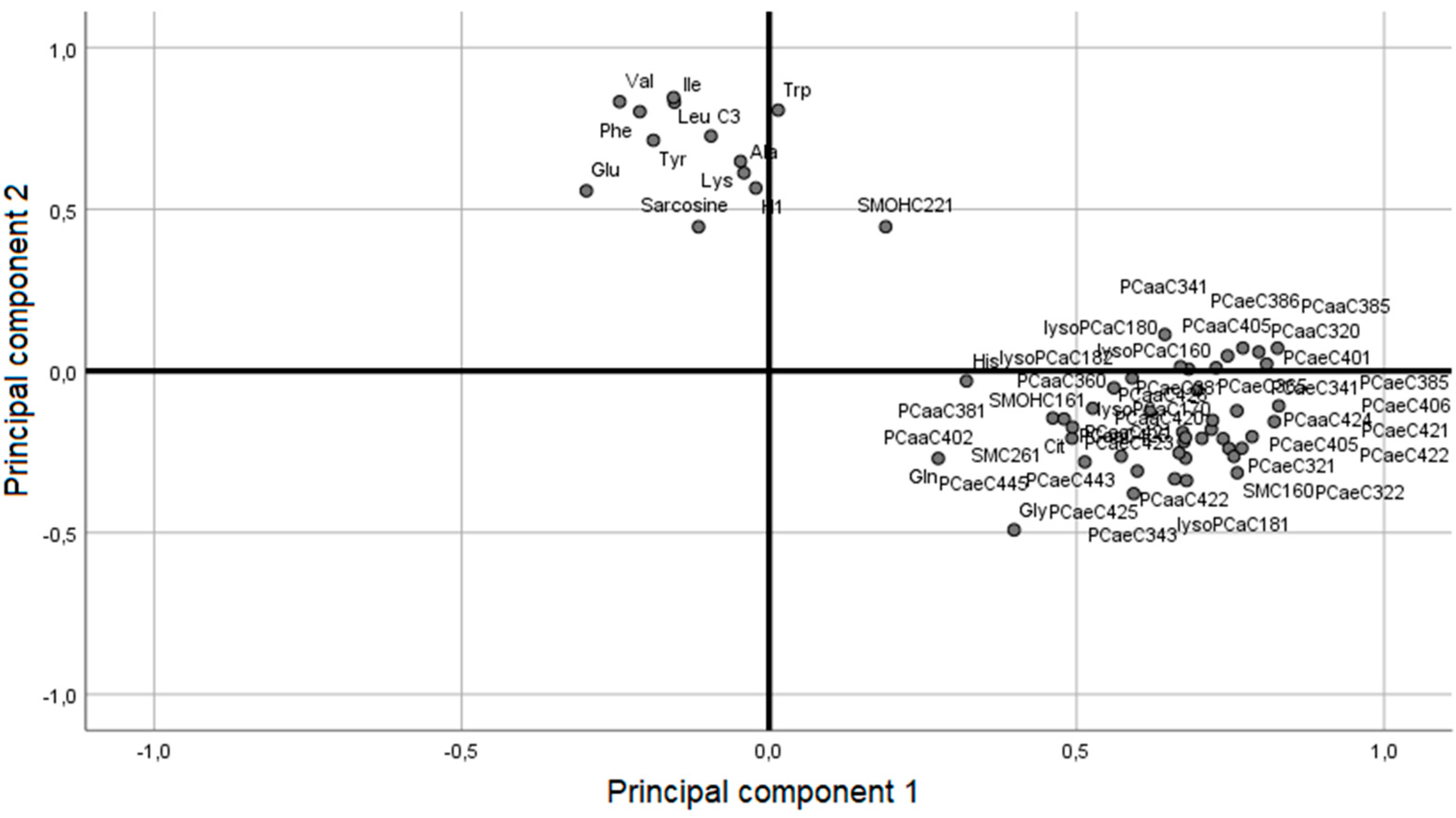
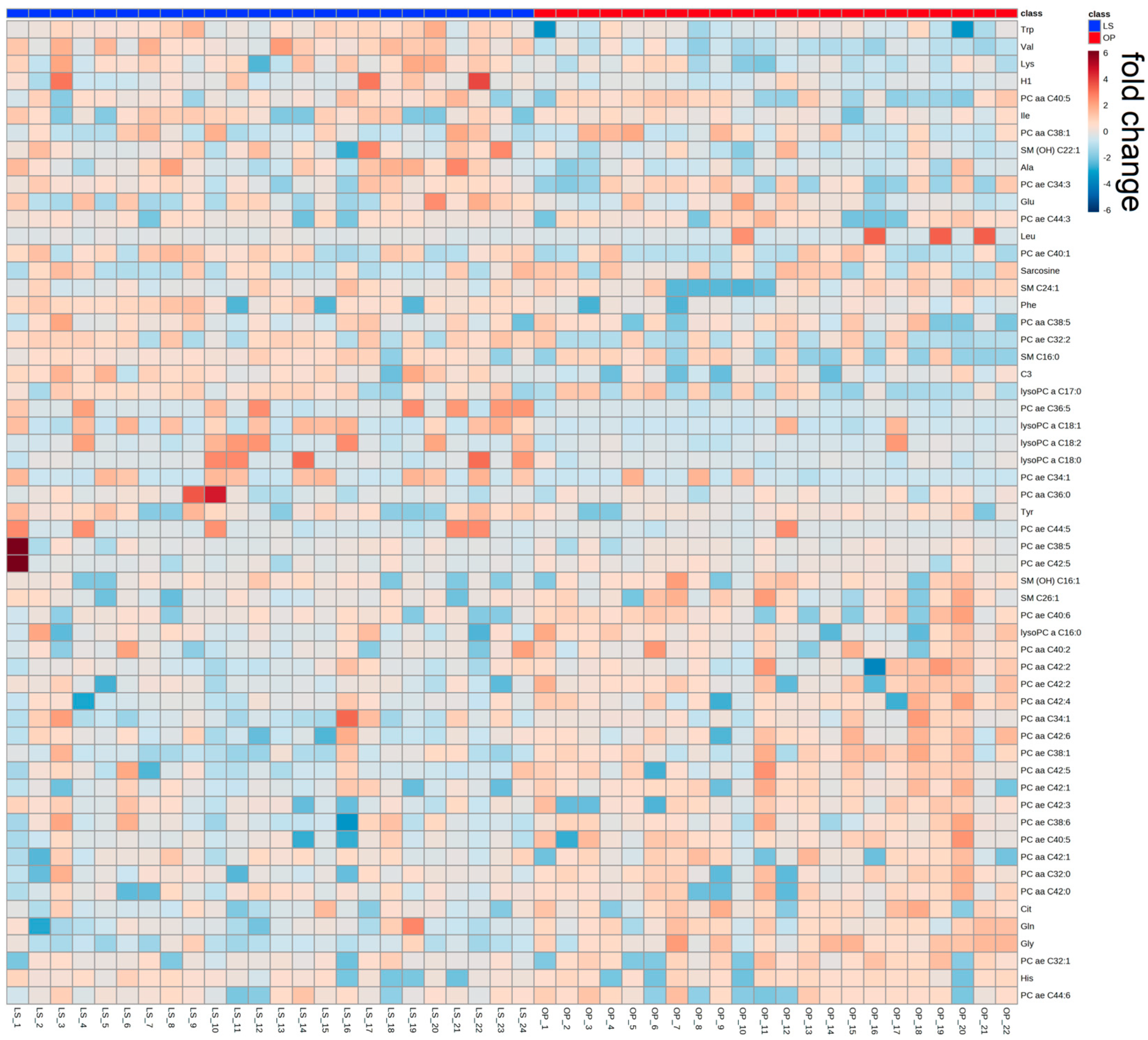
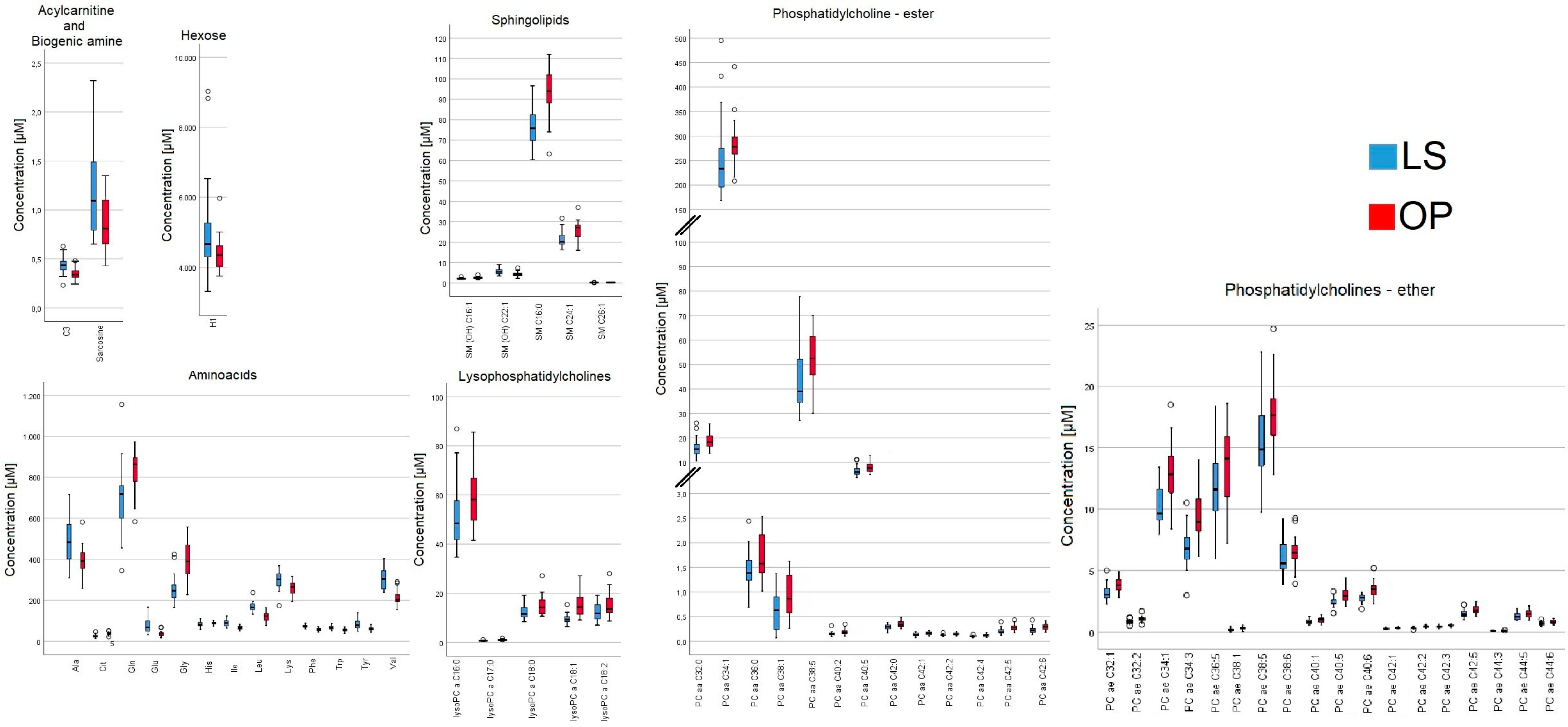
2.1. Participants from the WAS Trial
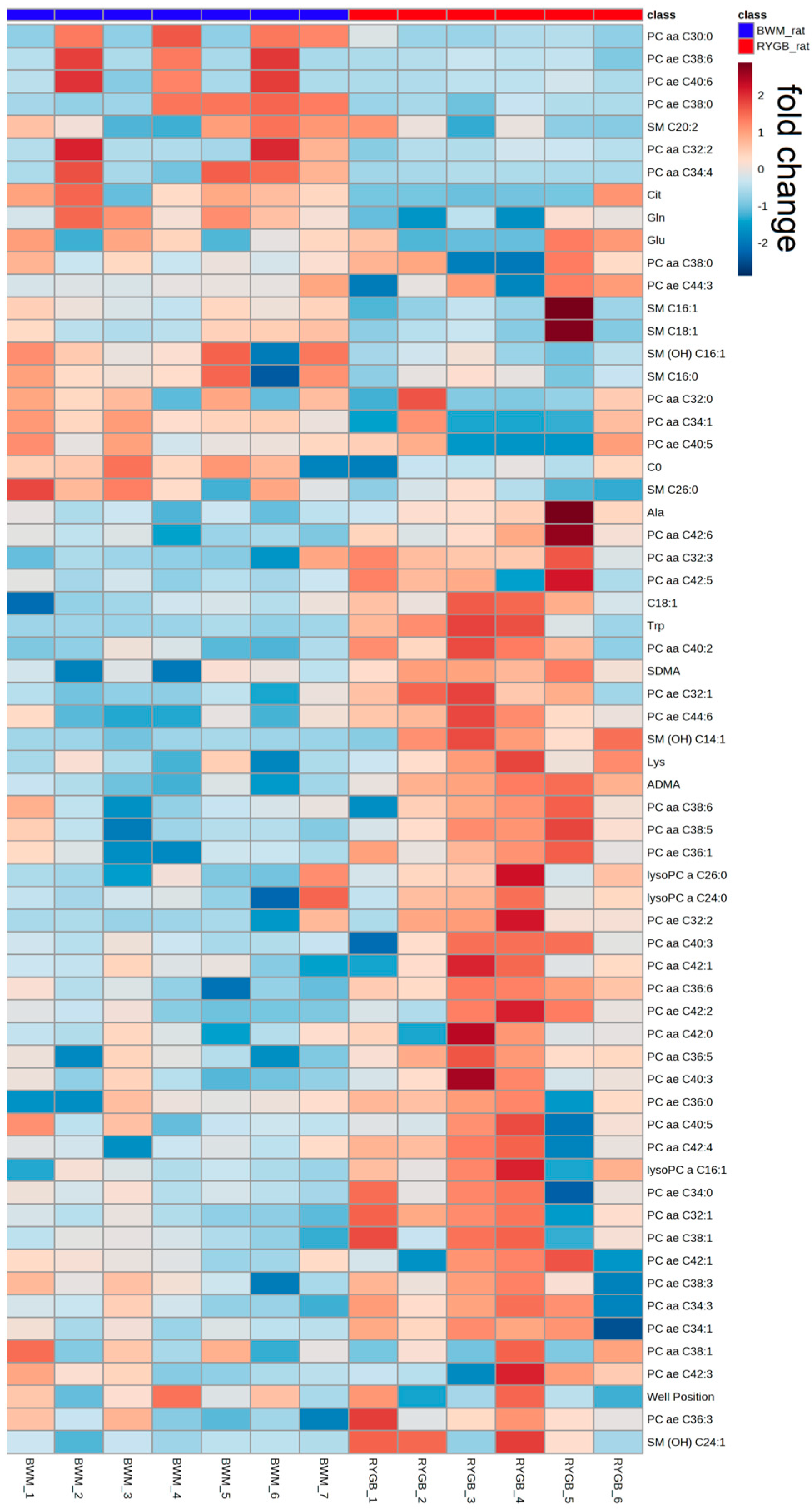
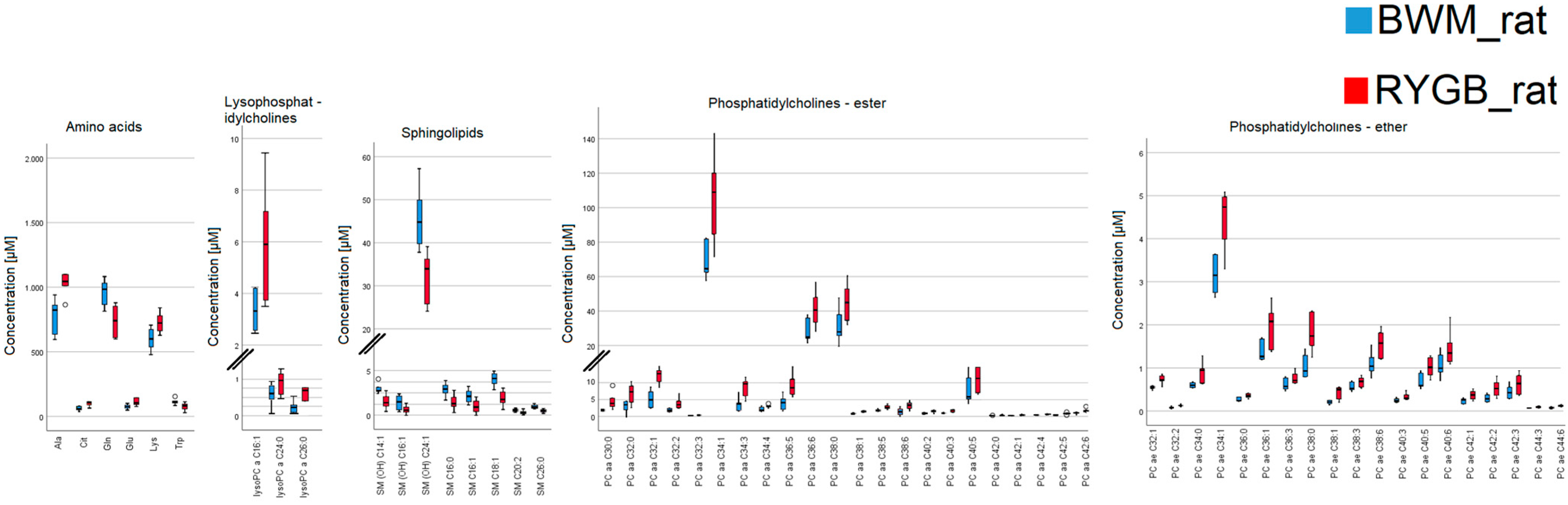
2.2. Rodent Model
2.3. Analysis of Overlapping Metabolomic Profiles in the Human OP and the Rat RYGB Group
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Animals
4.3. Laboratory Measurements
4.4. Targeted Metabolomics
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2021. Available online: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 September 2022).
- The GDB 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Ford, E.S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care 2005, 28, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Wollert, K.C.; Larson, M.G.; Coglianese, E.; McCabe, E.L.; Cheng, S.; Ho, J.E.; Fradley, M.G.; Ghorbani, A.; Xanthakis, V.; et al. Prognostic utility of novel biomarkers of cardiovascular stress: The framingham heart study. Circulation 2012, 126, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Overview: Metabolomics and lipidomics in nutrition and metabolism research. Essays Biochem 2016, 60, 407. [Google Scholar] [CrossRef] [PubMed]
- Chissini, R.D.B.C.; Kuschnir, M.C.; de Oliveira, C.L.; Giannini, D.T.; Santos, B. Cutoff values for HOMA-IR associated with metabolic syndrome in the Study of Cardiovascular Risk in Adolescents (ERICA Study). Nutrition 2020, 71, 110608. [Google Scholar] [CrossRef]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef]
- Neto, R.M.L.; Herbella, F.A.; Tauil, R.M.; Silva, F.S.; de Lima, S.E. Comorbidities remission after roux-en-y gastric bypass for morbid obesity is sustained in a long-term Follow-up and correlates with weight regain. Obes. Surg. 2012, 22, 1580–1585. [Google Scholar] [CrossRef]
- Rauschert, S.; Mori, T.A.; Beilin, L.J.; Jacoby, P.; Uhl, O.; Koletzko, B.; Oddy, W.H.; Hellmuth, C. Early life factors, obesity risk, and the metabolome of young adults. Obesity 2017, 25, 1549–1555. [Google Scholar] [CrossRef]
- Hameed, A.; Mojsak, P.; Buczynska, A.; Suleria, H.A.R.; Kretowski, A.; Ciborowski, M. Altered metabolome of lipids and Amino acids species: A source of early signature biomarkers of T2DM. J. Clin. Med. 2020, 9, 2257. [Google Scholar] [CrossRef]
- Newgard, C.B. Metabolomics and metabolic diseases: Where do we stand? Cell. Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]
- Adamski, J. Key elements of metabolomics in the study of biomarkers of diabetes. Diabetologia 2016, 59, 2497–2502. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 1–31. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.; Wang, Y.; Pedram, P.; Cahill, F.; Zhai, G.; Randell, E.; Gulliver, W.; Sun, G. Serum metabolic biomarkers distinguish metabolically healthy peripherally obese from unhealthy centrally obese individuals. Nutr. Metab. 2016, 13, 33. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef]
- Carayol, M.; Leitzmann, M.F.; Ferrari, P.; Zamora-Ros, R.; Achaintre, D.; Stepien, M.; Schmidt, J.A.; Travis, R.C.; Overvad, K.; Tjønneland, A.; et al. Blood metabolic signatures of body mass index: A targeted metabolomics study in the epic cohort. J. Proteome Res. 2017, 16, 3137–3146. [Google Scholar] [CrossRef]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19–34. [Google Scholar]
- Koschker, A.C.; Warrings, B.; Morbach, C.; Seyfried, F.; Rickert, N.; Jung, P.; Geier, A.; Dischinger, U.; Krauthausen, M.; Herrmann, M.J.; et al. Cardio-psycho-metabolic outcomes of bariatric surgery: Design and baseline of the WAS trial. Endocr. Connect 2022, 11, e210338. [Google Scholar] [CrossRef]
- Dischinger, U.; Heckel, T.; Bischler, T.; Hasinger, J.; Königsrainer, M.; Schmitt-Böhrer, A.; Otto, C.; Fassnacht, M.; Seyfried, F.; Hankir, M.K. Roux-en-Y gastric bypass and caloric restriction but not gut hormone-based treatments profoundly impact the hypothalamic transcriptome in obese rats. Nutrients 2021, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Blais, E.M.; Rawls, K.D.; Dougherty, B.V.; Li, Z.I.; Kolling, G.L.; Ye, P.; Wallqvist, A.; Papin, J.A. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat. Commun. 2017, 8, 14250. [Google Scholar] [CrossRef] [PubMed]
- Haid, M.; Muschet, C.; Wahl, S.; Römisch-Margl, W.; Prehn, C.; Möller, G.; Adamski, J. Long-term stability of human plasma metabolites during storage at −80 °C. J. Proteome Res. 2018, 17, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Fève, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE 2017, 12, e0173615. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Paulweber, B.; Zandanell, S.; Stechemesser, L.; Schranz, M.; Strebinger, G.; Huber-Schönauer, U.; Niederseer, D.; et al. Clinical and metabolic characterization of obese subjects without non-alcoholic fatty liver: A targeted metabolomics approach. Diabetes Metab. 2019, 45, 132–139. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Wahl, S.; Holzapfel, C.; Yu, Z.; Breier, M.; Kondofersky, I.; Fuchs, C.; Singmann, P.; Prehn, C.; Adamski, J.; Grallert, H.; et al. Metabolomics reveals determinants of weight loss during lifestyle intervention in obese children. Metabolomics 2013, 9, 1157–1167. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Kadam, W.; Wei, B.; Li, F. Metabolomics of Gastric Cancer. Adv. Exp. Med. Biol. 2021, 1280, 291–301. [Google Scholar]
- Hendruch, A.; Michalak, K. Lipids as a target for drugs modulations multidrug resistanceof cancer cells. Curr. Drug Targets 2003, 4, 23–30. [Google Scholar] [CrossRef]
- Vieira, E.E.S.; Pereira, I.C.; Braz, A.F.; Nascimento-Ferreira, M.V.; de Oliveira Torres, L.R.; de Freitas Brito, A.; do Nascimento Marreiro, D.; e Sousa, J.M.D.C.; da Silva, F.C.C.; Torres-Leal, F.L. Food consumption of branched chain amino acids and insulin resistance: A systematic review of observational studies in humans. Clin. Nutr. ESPEN 2020, 40, 277–281. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain amino acids: Beyond nutrition metabolism. Int. J. Mo.l Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef]
- Crossland, H.; Smith, K.; Idris, I.; Phillips, B.E.; Atherton, P.J.; Wilkinson, D.J. Exploring mechanistic links between extracellular branched-chain amino acids and muscle insulin resistance: An in vitro approach. Am. J. Physiol. Cell. Physiol. 2020, 319, C1151–C1157. [Google Scholar] [CrossRef]
- Klaus, V.S.; Schriever, S.C.; Kuhn, J.M.M.; Peter, A.; Irmler, M.; Tokarz, J.; Prehn, C.; Kastenmüller, G.; Beckers, J.; Adamski, J.; et al. Correlation guided Network Integration (CoNI) reveals novel genes affecting hepatic metabolism. Mol. Metab. 2021, 53, 101295. [Google Scholar] [CrossRef]
- Boulet, M.M.; Chevrier, G.; Grenier-Larouche, T.; Pelletier, M.; Nadeau, M.; Scarpa, J.; Prehn, C.; Marette, A.; Adamski, J.; Tchernof, A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E736–E746. [Google Scholar] [CrossRef]
- Lau, L.H.Y.; Nano, J.; Cecil, A.; Schederecker, F.; Rathmann, W.; Prehn, C.; Zeller, T.; Lechner, A.; Adamski, J.; Peters, A.; et al. Cross-sectional and prospective relationships of endogenous progestogens and estrogens with glucose metabolism in men and women: A KORA F4/FF4 Study. BMJ Open Diabetes Res. Care 2021, 9. [Google Scholar] [CrossRef]
- Dreyfuss, J.M.; Yuchi, Y.; Dong, X.; Efthymiou, V.; Pan, H.; Simonson, D.C.; Vernon, A.; Halperin, F.; Aryal, P.; Konkar, A.; et al. High-throughput mediation analysis of human proteome and metabolome identifies mediators of post-bariatric surgical diabetes control. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Yoshino, M.; Kayser, B.D.; Yoshino, J.; Stein, R.I.; Reeds, D.; Eagon, J.C.; Eckhouse, S.R.; Watrous, J.D.; Jain, M.; Knight, R.; et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N. Engl. J. Med. 2020, 383, 721–732. [Google Scholar] [CrossRef]
- Reinehr, T.; Wolters, B.; Knop, C.; Lass, N.; Hellmuth, C.; Harder, U.; Peissner, W.; Wahl, S.; Grallert, H.; Adamski, J.; et al. Changes in the serum metabolite profile in obese children with weight loss. Eur. J. Nutr. 2015, 54, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Even, P.C.; Virtue, S.; Morton, N.M.; Fromentin, G.; Semple, R.K. Editorial: Are rodent models fit for investigation of human obesity and related diseases? Front. Nutr. 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Dischinger, U.; Hasinger, J.; Königsrainer, M.; Corteville, C.; Otto, C.; Fassnacht, M.; Hankir, M.; Seyfried, F.J.D. Toward a medical gastric bypass: Chronic feeding studies with liraglutide + PYY3-36 combination therapy in diet-induced obese rats. Front. Endocrinol. 2020, 11, 598843. [Google Scholar] [CrossRef] [PubMed]
- Dischinger, U.; Corteville, C.; Otto, C.; Fassnacht, M.; Seyfried, F.; Hankir, M.K. GLP-1 and PYY3-36 reduce high-fat food preference additively after roux-en-Y gastric bypass in diet-induced obese rats. Surg. Obes. Relat. Dis. 2019, 15, 1483–1492. [Google Scholar] [CrossRef]
- Seyfried, F.; Bueter, M.; Spliethoff, K.; Miras, A.D.; Abegg, K.; Lutz, T.A.; Le Roux, C.W. Roux-en Y gastric bypass is superior to duodeno-jejunal bypass in improving glycaemic control in Zucker diabetic fatty rats. Obes. Surg. 2014, 24, 1888–1895. [Google Scholar] [CrossRef]
- Zukunft, S.; Sorgenfrei, M.; Prehn, C. Targeted Metabolomics of Dried Blood Spot Extracts. Chromatographia 2013, 76, 1295–1305. [Google Scholar] [CrossRef]
- European Medicines Agency. Guedline on Bioanalitycal Method Validation. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 31 March 2021).
- März, J.; Kurlbaum, M.; Roche-Lancaster, O.; Deutschbein, T.; Peitzsch, M.; Prehn, C.; Weismann, D.; Robledo, M.; Adamski, J.; Fassnacht, M. Plasma metabolome profiling for the diagnosis of catecholamine producing tumors. Front. Endocrinol. 2021, 12, 722656. [Google Scholar] [CrossRef]
- Vogg, N.; Müller, T.; Floren, A.; Dandekar, T.; Scherf-Clavel, O.; Fassnacht, M.; Kroiss, M.; Kurlbaum, M. Targeted metabolic profiling of urinary steroids with a focus on analytical accuracy and sample stability. J. Mass Spectrom. Adv. Clin. Lab. 2022, 25, 44–52. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]

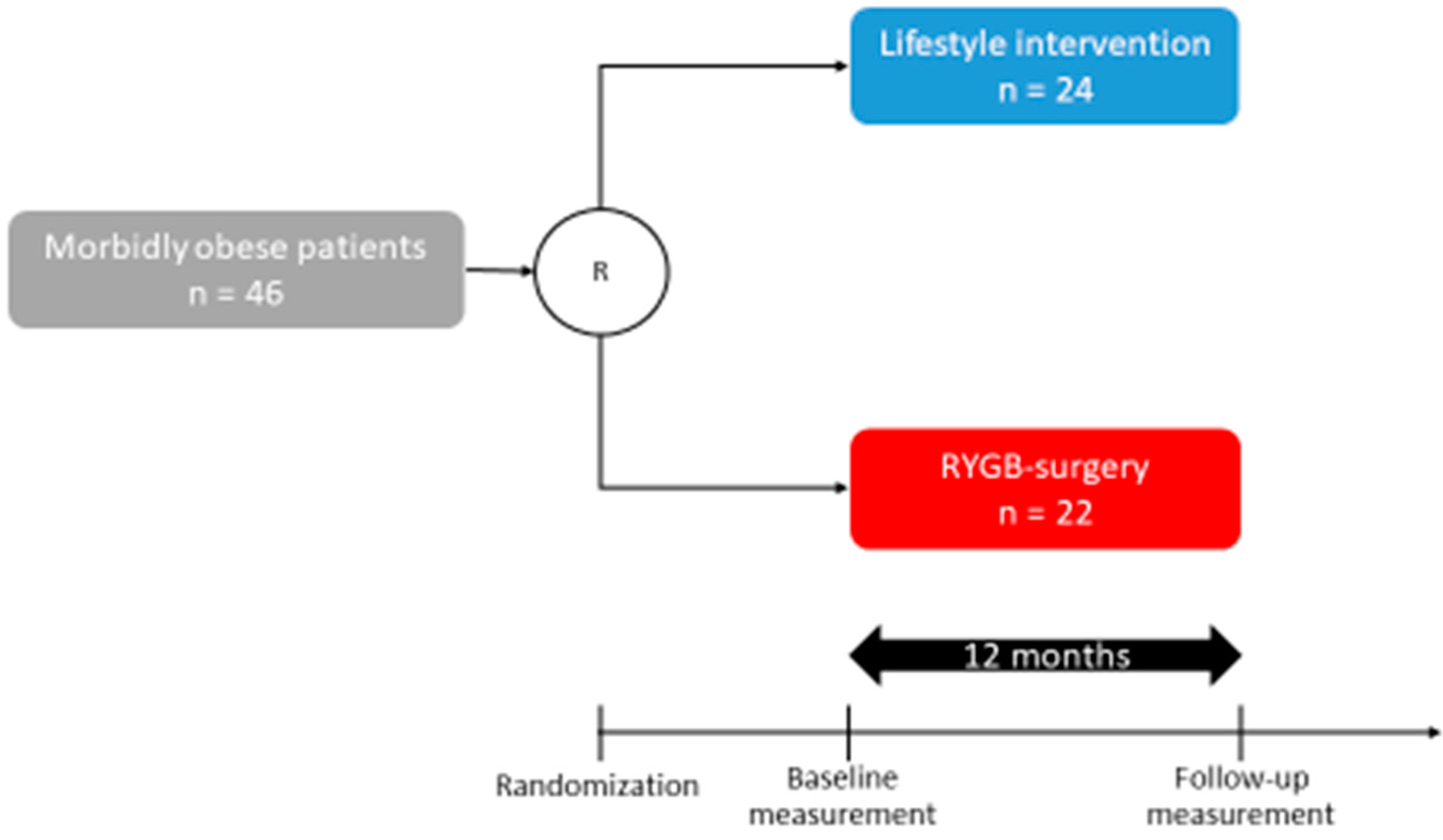
| Study Cohort (n = 46) | LS (n = 24) | OP (n = 22) | p-Value | |
|---|---|---|---|---|
| Women | 39 | 18 | 21 | 0.32 |
| Men | 7 | 6 | 1 | <0.01 |
| Age (years) | 41.2 ± 1.5 | 39.3 ± 2 | 43.1 ± 2 | 0.13 |
| Body height (cm) | 172 ± 1 | 172 ± 2 | 167 ± 2 | 0.05 |
| Body weight (kg) | 134.9 ± 4.2 | 138.9 ± 3.1 | 128.6 ± 2.4 | 0.06 |
| BMI (kg/m2) | 45.0 ± 1.4 | 47.4 ± 1.2 | 42 ± 1 | 0.09 |
| HbA1c (%) | 5.9 ± 0.2 | 6.1 ± 0.3 | 5.7 ± 0.1 | 0.17 |
| HOMA-IR | 6.8 ± 1.3 | 7.1 ± 2.3 | 6.5 ± 0.2 | 0.07 |
| Metabolites | LS (µM) | OP (µM) | Difference (µM) | Difference (%) | p-Value |
|---|---|---|---|---|---|
| C3 | 0.43 ± 0.019 | 0.35 ± 0.01 | 0.08 | 22.9 | <0.01 |
| Ala | 485.67 ± 21.8 | 392.64 ± 14.74 | 93.03 | 23.7 | <0.01 |
| Cit | 25.57 ± 1.63 | 35.84 ± 1.55 | 10.27 | 28.7 | <0.01 |
| Gln | 702 ± 30.73 | 834 ± 22.24 | 132 | 15.8 | <0.01 |
| Glu | 73.68 ± 6.25 | 38.51 ± 3.50 | 35.17 | 91.3 | <0.01 |
| Gly | 249.33 ± 13.55 | 394.68 ± 17.90 | 145.35 | 36.8 | <0.01 |
| His | 82.86 ± 2.53 | 88.2 ± 1.37 | 5.34 | 6.1 | 0.04 |
| Ile | 89.55 ± 3.48 | 65.14 ± 2.05 | 24.41 | 37.5 | <0.01 |
| Leu | 168.08 ± 4.52 | 121.61 ± 4.36 | 46.47 | 38.2 | <0.01 |
| Lys | 296.96 ± 8.84 | 260.5 ± 7.09 | 36.46 | 14 | <0.01 |
| Phe | 73.2 ± 1.38 | 56.72 ± 1.36 | 16.48 | 29.1 | <0.01 |
| Trp | 65.55 ± 1.80 | 54.53 ± 1.76 | 11.02 | 20.2 | <0.01 |
| Tyr | 82.83 ± 4.33 | 60.14 ± 1.68 | 22.69 | 37.7 | <0.01 |
| Val | 305.04 ± 9.83 | 212.09 ± 7.48 | 92.95 | 43.8 | <0.01 |
| Sarcosine | 1.14 ± 0.09 | 0.85 ± 0.06 | 0.29 | 34.1 | 0.04 |
| lysoPC a C16:0 | 51.14 ± 2.61 | 58.45 ± 2.42 | 7.31 | 12.5 | 0.04 |
| lysoPC a C17:0 | 0.74 ± 0.04 | 1.03 ± 0.07 | 0.29 | 28.2 | <0.01 |
| lysoPC a C18:0 | 12.43 ± 0.63 | 15.11 ± 0.89 | 2.68 | 17.7 | 0.03 |
| lysoPC a C18:1 | 9.48 ± 0.41 | 15.22 ± 0.93 | 5.74 | 37.7 | <0.01 |
| lysoPC a C18:2 | 12.13 ± 0.70 | 15.17 ± 1.00 | 3.04 | 20 | 0.04 |
| PC aa C32:0 | 16.01 ± 0.75 | 18.59 ± 0.65 | 2.58 | 13.9 | 0.03 |
| PC aa C34:1 | 257 ± 16.70 | 283.64 ± 9.72 | 26.64 | 9.4 | 0.03 |
| PC aa C36:0 | 1.44 ± 0.08 | 1.73 ± 0.1 | 0.29 | 16.8 | 0.04 |
| PC aa C38:1 | 0.6 ± 0.08 | 0.97 ± 0.09 | 0.37 | 38.1 | 0.03 |
| PC aa C38:5 | 44.18 ± 2.54 | 52.19 ± 2.28 | 8.01 | 15.3 | 0.02 |
| PC aa C40:2 | 0.16 ± 0.01 | 0.19 ± 0.01 | 0.03 | 15.8 | 0.04 |
| PC aa C40:5 | 6.56 ± 0.40 | 8.03 ± 0.45 | 1.47 | 18.3 | 0.03 |
| PC aa C42:0 | 0.29 ± 0.01 | 0.35 ± 0.01 | 0.06 | 17.1 | 0.03 |
| PC aa C42:1 | 0.13 ± 0.01 | 0.16 ± 0.01 | 0.03 | 18.8 | <0.01 |
| PC aa C42:2 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.02 | 13.3 | 0.04 |
| PC aa C42:4 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.03 | 25 | <0.01 |
| PC aa C42:5 | 0.21 ± 0.01 | 0.28 ± 0.01 | 0.07 | 25 | <0.01 |
| PC aa C42:6 | 0.24 ± 0.01 | 0.29 ± 0.02 | 0.05 | 17.2 | 0.04 |
| PC ae C32:1 | 3.18 ± 0.12 | 3.85 ± 0.13 | 0.67 | 17.4 | <0.01 |
| PC ae C32:2 | 0.85 ± 0.03 | 1.04 ± 0.04 | 0.19 | 18.3 | <0.01 |
| PC ae C34:1 | 10.58 ± 0.50 | 12.69 ± 0.44 | 2.11 | 16.6 | <0.01 |
| PC ae C34:3 | 6.8 ± 0.32 | 9.45 ± 0.5 | 2.65 | 28 | <0.01 |
| PC ae C36:5 | 11.52 ± 0.60 | 13.83 ± 0.61 | 2.31 | 16.7 | 0.02 |
| PC ae C38:1 | 0.17 ± 0.03 | 0.31 ± 0.03 | 0.14 | 45.2 | <0.01 |
| PC ae C38:5 | 15.55 ± 0.70 | 17.64 ± 0.63 | 2.09 | 11.8 | 0.04 |
| PC ae C38:6 | 5.89 ± 0.29 | 6.5 ± 0.26 | 0.61 | 9.4 | 0.04 |
| PC ae C40:1 | 0.82 ± 0.04 | 0.98 ± 0.05 | 0.16 | 16.3 | 0.03 |
| PC ae C40:5 | 2.32 ± 0.08 | 2.93 ± 0.11 | 0.61 | 20.8 | <0.01 |
| PC ae C40:6 | 2.79 ± 0.1 | 3.44 ± 0.14 | 0.65 | 18.9 | <0.01 |
| PC ae C42:1 | 0.26 ± 0.01 | 0.33 ± 0.01 | 0.07 | 21.2 | <0.01 |
| PC ae C42:2 | 0.35 ± 0.01 | 0.45 ± 0.02 | 0.1 | 22.2 | <0.01 |
| PC ae C42:3 | 0.44 ± 0.02 | 0.52 ± 0.02 | 0.08 | 15.4 | 0.02 |
| PC ae C42:5 | 1.46 ± 0.06 | 1.8 ± 0.07 | 0.34 | 18.9 | <0.01 |
| PC ae C44:3 | 0.1 ± 0.002 | 0.11 ± 0.003 | 0.01 | 9.1 | <0.01 |
| PC ae C44:5 | 1.25 ± 0.05 | 1.52 ± 0.07 | 0.27 | 17.8 | 0.02 |
| PC ae C44:6 | 0.68 ± 0.03 | 0.81 ± 0.03 | 0.13 | 16 | 0.01 |
| SM (OH) C16:1 | 2.27 ± 0.09 | 2.54 ± 0.11 | 0.27 | 10.6 | 0.02 |
| SM (OH) C22:1 | 5.68 ± 0.30 | 4.45 ± 0.24 | 1.23 | 27.6 | <0.01 |
| SM C16:0 | 77.54 ± 2.22 | 93.49 ± 2.63 | 15.95 | 17.1 | <0.01 |
| SM C24:1 | 21.58 ± 0.83 | 25.51 ± 1.01 | 3.93 | 15.4 | 0.02 |
| SM C26:1 | 0.18 ± 0.01 | 0.24 ± 0.02 | 0.06 | 25 | 0.01 |
| H1 | 5286.63 ± 337.92 | 4399.09 ± 101.08 | 887.54 | 20.2 | 0.02 |
| Metabolites | BWM_rat (µM) | RYGB_rat (µM) | Difference (µM) | Difference (%) | p-Value |
|---|---|---|---|---|---|
| C0 | 49 ± 2.33 | 34.15 ± 2.25 | 14.85 | 43.5 | <0.01 |
| C18:1 | 0.099 ± 0.005 | 0.1465 ± 0.01 | 0.0475 | 32.4 | 0.02 |
| Ala | 829 ± 44.15 | 1044.5 ± 129.02 | 215.5 | 20.6 | <0.01 |
| Cit | 73.3 ± 5.95 | 123.5 ± 7.36 | 50.2 | 40.6 | <0.01 |
| Gln | 946 ± 35.40 | 735.5 ± 45.28 | 210.5 | 28.6 | 0.03 |
| Glu | 67 ± 6.17 | 99.25 ± 10.21 | 32.25 | 32.5 | 0.04 |
| Lys | 602 ± 26.79 | 722.5 ± 30.22 | 120.5 | 16.7 | 0.04 |
| Trp | 113 ± 7.39 | 82.35 ± 11.24 | 30.65 | 37.2 | 0.04 |
| ADMA | 0.37 ± 0.03 | 0.66 ± 0.03 | 0.29 | 43.9 | 0.04 |
| SDMA | 0.22 ± 0.01 | 0.32 ± 0.02 | 0.1 | 31.3 | 0.03 |
| lysoPC a C16:1 | 3.08 ± 0.3 | 5.9 ± 0.83 | 2.82 | 47.8 | 0.03 |
| lysoPC a C24:0 | 0.422 ± 0.06 | 0.6115 ± 0.05 | 0.1895 | 31.0 | 0.04 |
| lysoPC a C26:0 | 0.064 ± 0.02 | 0.1265 ± 0.02 | 0.0625 | 49.4 | 0.04 |
| PC aa C30:0 | 0.992 ± 0.04 | 1.65 ± 0.31 | 0.658 | 39.9 | <0.01 |
| PC aa C32:0 | 5.73 ± 0.21 | 11.45 ± 1.29 | 5.72 | 50.0 | 0.04 |
| PC aa C32:1 | 3.28 ± 0.34 | 7.72 ± 0.47 | 4.44 | 57.5 | <0.01 |
| PC aa C32:2 | 0.855 ± 0.09 | 1.515 ± 0.2 | 0.66 | 43.6 | <0.01 |
| PC aa C32:3 | 0.079 ± 0.01 | 0.1165 ± 0.01 | 0.0375 | 32.2 | 0.02 |
| PC aa C34:1 | 63.6 ± 4.05 | 109 ± 9.61 | 45.4 | 41.7 | <0.01 |
| PC aa C34:3 | 2.57 ± 0.28 | 4.57 ± 0.33 | 2 | 43.8 | <0.01 |
| PC aa C34:4 | 0.872 ± 0.09 | 1.32 ± 0.06 | 0.448 | 33.9 | 0.03 |
| PC aa C36:5 | 3 ± 0.28 | 4.525 ± 0.41 | 1.525 | 33.7 | 0.02 |
| PC aa C36:6 | 0.23 ± 0.02 | 0.4595 ± 0.03 | 0.2295 | 49.9 | <0.01 |
| PC aa C38:0 | 0.837 ± 0.03 | 1.74 ± 0.08 | 0.903 | 51.9 | <0.01 |
| PC aa C38:1 | 0.436 ± 0.12 | 1.084 ± 0.14 | 0.648 | 59.8 | 0.02 |
| PC aa C38:5 | 24.3 ± 2.53 | 40.55 ± 4.05 | 16.25 | 40.1 | 0.04 |
| PC aa C38:6 | 29.2 ± 3.22 | 44.85 ± 4.13 | 15.65 | 34.9 | 0.03 |
| PC aa C40:2 | 0.269 ± 0.02 | 0.519 ± 0.05 | 0.25 | 48.2 | 0.02 |
| PC aa C40:3 | 0.319 ± 0.01 | 0.6175 ± 0.05 | 0.2985 | 48.3 | <0.01 |
| PC aa C40:5 | 5.22 ± 0.5 | 7.105 ± 2.22 | 1.885 | 26.5 | 0.04 |
| PC aa C42:0 | 0.061 ± 0.01 | 0.1055 ± 0.02 | 0.0445 | 42.2 | 0.02 |
| PC aa C42:1 | 0.08 ± 0.01 | 0.109 ± 0.02 | 0.029 | 26.6 | 0.04 |
| PC aa C42:4 | 0.132 ± 0.01 | 0.2005 ± 0.02 | 0.0685 | 34.2 | <0.01 |
| PC aa C42:5 | 0.132 ± 0.01 | 0.2805 ± 0.03 | 0.1485 | 52.9 | 0.03 |
| PC aa C42:6 | 0.311 ± 0.03 | 0.4905 ± 0.08 | 0.1795 | 36.6 | <0.01 |
| PC ae C32:1 | 0.545 ± 0.02 | 0.7225 ± 0.04 | 0.1775 | 24.6 | 0.01 |
| PC ae C32:2 | 0.096 ± 0.01 | 0.124 ± 0.01 | 0.028 | 22.6 | 0.03 |
| PC ae C34:0 | 0.597 ± 0.02 | 0.944 ± 0.09 | 0.347 | 36.8 | 0.02 |
| PC ae C34:1 | 2.97 ± 0.14 | 4.735 ± 0.26 | 1.765 | 37.3 | 0.03 |
| PC ae C36:0 | 0.254 ± 0.01 | 0.372 ± 0.02 | 0.118 | 31.7 | 0.02 |
| PC ae C36:1 | 1.21 ± 0.09 | 2.08 ± 0.18 | 0.87 | 41.8 | 0.02 |
| PC ae C36:3 | 0.545 ± 0.06 | 0.707 ± 0.05 | 0.162 | 22.9 | 0.04 |
| PC ae C38:0 | 0.566 ± 0.10 | 0.8355 ± 0.16 | 0.2695 | 32.3 | <0.01 |
| PC ae C38:1 | 0.193 ± 0.02 | 0.4965 ± 0.06 | 0.3035 | 61.1 | 0.02 |
| PC ae C38:3 | 0.506 ± 0.04 | 0.6865 ± 0.04 | 0.1805 | 26.3 | 0.04 |
| PC ae C38:6 | 1.13 ± 0.09 | 1.575 ± 0.12 | 0.445 | 28.3 | 0.04 |
| PC ae C40:3 | 0.225 ± 0.01 | 0.2915 ± 0.03 | 0.0665 | 22.8 | 0.04 |
| PC ae C40:5 | 0.593 ± 0.06 | 1.014 ± 0.09 | 0.421 | 41.5 | 0.02 |
| PC ae C40:6 | 1.05 ± 0.09 | 1.345 ± 0.15 | 0.295 | 21.9 | 0.04 |
| PC ae C42:1 | 0.262 ± 0.02 | 0.373 ± 0.04 | 0.111 | 29.8 | 0.04 |
| PC ae C42:2 | 0.238 ± 0.03 | 0.521 ± 0.08 | 0.283 | 54.3 | 0.03 |
| PC ae C42:3 | 0.368 ± 0.05 | 0.6365 ± 0.09 | 0.2685 | 42.2 | 0.04 |
| PC ae C44:3 | 0.067 ± 0.004 | 0.102 ± 0.01 | 0.035 | 34.3 | 0.02 |
| PC ae C44:6 | 0.059 ± 0.01 | 0.1155 ± 0.01 | 0.0565 | 48.9 | <0.01 |
| SM (OH) C14:1 | 1.25 ± 0.04 | 0.7465 ± 0.07 | 0.5035 | 67.4 | <0.01 |
| SM (OH) C16:1 | 0.771 ± 0.05 | 0.3935 ± 0.03 | 0.3775 | 95.9 | <0.01 |
| SM (OH) C24:1 | 1.94 ± 0.1 | 0.7155 ± 0.15 | 1.2245 | 171.1 | <0.01 |
| SM C16:0 | 49 ± 2.54 | 33.95 ± 2.27 | 15.05 | 44.3 | <0.01 |
| SM C16:1 | 3.89 ± 0.18 | 2.555 ± 0.3 | 1.335 | 52.3 | <0.01 |
| SM C18:1 | 2.84 ± 0.23 | 1.37 ± 0.18 | 1.47 | 107.3 | 0.02 |
| SM C20:2 | 0.095 ± 0.01 | 0.0455 ± 0.02 | 0.0495 | 108.8 | 0.04 |
| SM C26:0 | 0.148 ± 0.02 | 0.074 ± 0.01 | 0.074 | 100.0 | <0.01 |
| Amino-Acids | Lysophosphatidylcholines | Phosphatidylcholines—Ester | Phosphatidylcholines—Ether | Sphingolipids | |||||
|---|---|---|---|---|---|---|---|---|---|
| WAS | Rat | WAS | Rat | WAS | Rat | WAS | Rat | WAS | Rat |
| Gly ↑ | lysoPC a C16:0 ↑ | PC aa C36:0 ↑ | PC ae C34:3 ↑ | SM (OH) C22:1 ↓ | |||||
| His ↑ | lysoPC a C17:0 ↑ | PC aa C42:2 ↑ | PC ae C36:5 ↑ | SM C24:1 ↑ | |||||
| Ile ↓ | lysoPC a C18:0 ↑ | PC aa C32:0 ↑ | ↑ PC aa C32:0 | PC ae C38:5 ↑ | SM C26:1 ↑ | ||||
| Leu ↓ | lysoPC a C18:1 ↑ | PC aa C34:1 ↑ | ↑ PC aa C34:1 | PC ae C40:1 ↑ | SM (OH) C16:1 ↑ | ↓ SM (OH) C16:1 | |||
| Val ↓ | lysoPC a C18:2 ↑ | PC aa C38:1 ↑ | ↑ PC aa C38:1 | PC ae C42:5 ↑ | SM C16:0 ↑ | ↓ SM C16:0 | |||
| Phe ↓ | ↑ lysoPC a C16:1 | PC aa C38:5 ↑ | ↑ PC aa C38:5 | PC ae C44:5 ↑ | ↓ SM (OH) C14:1 | ||||
| Tyr ↓ | ↑ lysoPC a C24:0 | PC aa C40:2 ↑ | ↑ PC aa C40:2 | PC ae C32:1 ↑ | ↑ PC ae C32:1 | ↓ SM (OH) C24:1 | |||
| Trp ↓ | ↓ Trp | ↑ lysoPC a C26:0 | PC aa C40:5 ↑ | ↑ PC aa C40:5 | PC ae C32:2 ↑ | ↑ PC ae C32:2 | ↓ SM C16:1 | ||
| Lys ↓ | ↑ Lys | PC aa C42:0 ↑ | ↑ PC aa C42:0 | PC ae C34:1 ↑ | ↑ PC ae C34:1 | ↓ SM C18:1 | |||
| Ala ↓ | ↑ Ala | PC aa C42:1 ↑ | ↑ PC aa C42:1 | PC ae C38:1 ↑ | ↑ PC ae C38:1 | ↓ SM C20:2 | |||
| Cit ↑ | ↑ Cit | PC aa C42:4 ↑ | ↑ PC aa C42:4 | PC ae C38:6 ↑ | ↑ PC ae C38:6 | ↓ SM C26:0 | |||
| Gln ↑ | ↓ Gln | PC aa C42:5 ↑ | ↑ PC aa C42:5 | PC ae C40:5 ↑ | ↑ PC ae C40:5 | ||||
| Glu ↓ | ↑ Glu | PC aa C42:6 ↑ | ↑ PC aa C42:6 | PC ae C40:6 ↑ | ↑ PC ae C40:6 | ||||
| ↑ PC aa C30:0 | PC ae C42:1 ↑ | ↑ PC ae C42:1 | |||||||
| ↑ PC aa C32:1 | PC ae C42:2 ↑ | ↑ PC ae C42:2 | |||||||
| ↑ PC aa C32:2 | PC ae C42:3 ↑ | ↑ PC ae C42:3 | |||||||
| ↑ PC aa C32:3 | PC ae C44:3 ↑ | ↑ PC ae C44:3 | |||||||
| ↑ PC aa C34:3 | PC ae C44:6 ↑ | ↑ PC ae C44:6 | |||||||
| ↑ PC aa C34:4 | ↑ PC ae C34:0 | ||||||||
| ↑ PC aa C36:5 | ↑ PC ae C36:0 | ||||||||
| ↑ PC aa C36:6 | ↑ PC ae C36:1 | ||||||||
| ↑ PC aa C38:0 | ↑ PC ae C36:3 | ||||||||
| ↑ PC aa C38:6 | ↑ PC ae C38:0 | ||||||||
| ↑ PC aa C40:3 | ↑ PC ae C38:3 | ||||||||
| ↑ PC ae C40:3 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balonov, I.; Kurlbaum, M.; Koschker, A.-C.; Stier, C.; Fassnacht, M.; Dischinger, U. Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies. Int. J. Mol. Sci. 2023, 24, 2354. https://doi.org/10.3390/ijms24032354
Balonov I, Kurlbaum M, Koschker A-C, Stier C, Fassnacht M, Dischinger U. Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies. International Journal of Molecular Sciences. 2023; 24(3):2354. https://doi.org/10.3390/ijms24032354
Chicago/Turabian StyleBalonov, Ilja, Max Kurlbaum, Ann-Cathrin Koschker, Christine Stier, Martin Fassnacht, and Ulrich Dischinger. 2023. "Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies" International Journal of Molecular Sciences 24, no. 3: 2354. https://doi.org/10.3390/ijms24032354
APA StyleBalonov, I., Kurlbaum, M., Koschker, A.-C., Stier, C., Fassnacht, M., & Dischinger, U. (2023). Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies. International Journal of Molecular Sciences, 24(3), 2354. https://doi.org/10.3390/ijms24032354







