Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders
Abstract
1. Introduction
2. Information Search Strategy in the Available
3. The Pathogenesis of Depressive Disorders
3.1. Inflammation in Depressive Disorders
3.2. Microbiota and Systemic Inflammation in Depression
3.3. Oxidative Stress in Depressive Disorders
4. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols
5. Foods Containing Polyphenols as Part of Diet Therapy in Depression—A Research Review
5.1. Influence of Polyphenols on Inflammation in Depression
5.1.1. Quercetin
5.1.2. Curcumin
5.1.3. Tannic Acid
5.1.4. Chrysin
5.1.5. Peoniflorin
5.1.6. Tea Polyphenols
5.2. Influence of Polyphenols on the Microbiome
5.3. Influence of Polyphenols on Antioxidant Status in Depression
5.4. Neuroprotective Action of Polyphenols
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, E.; Wendołowicz, A.; Kowzan, U.; Konarzewska, B.; Szulc, A.; Ostrowska, L. Does the usual dietary intake of patients with depression require vitamin-mineral supplementation? Psychiatr. Pol. 2014, 48, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Shadrina, M.; Bondarenko, E.A.; Slominsky, P.A. Genetics factors in major depression disease. Front. Psychiatry 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Liberman, A.C.; Dos Santos Claro, P.A.; Ugo, M.B.; Deussing, J.M.; Arzt, E. Stress-related brain neuroinflammation impact in depression: Role of the corticotropin-releasing hormone system and P2X7 receptor. Neuroimmunomodulation 2021, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Agrawal, M.; Gautam, S.; Gautam, M. Role of antioxidants in depression. Ind J. Priv. Psychiatry 2017, 11, 1–4. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatr. 2016, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Gerhard, D.; Thomas, A.; Duman, R.S. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr. Neuropharmacol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Vargas, C.; Stojanova, J.; Arancibia, M. Diet and depressive disorders. Arch. Clin. Psychiatry 2021, 48, 117–122. [Google Scholar]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Czarny, P.; Kwiatkowski, D.; Galecki, P.; Talarowska, M.; Orzechowska, A.; Bobinska, K.; Bielecka-Kowalska, A.; Szemraj, J.; Maes, M.; Su, K.P.; et al. Association between single nucleotide polymorphisms of MUTYH, hOGG1 and NEIL1 genes, and depression. J. Affect. Disord. 2015, 184, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Somani, A.; Singh, A.K.; Gupta, B.; Nagarkoti, S.; Dalal, P.K.; Dikshit, M. Oxidative and nitrosative stress in major depressive disorder: A case control study. Brain Sci. 2022, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.; Acar, S.T.; Yazici, A.; Yazici, K.; Tamer, L. Altered levels of malondialdehyde and vitamin e in major depressive disorder and generalized anxiety disorder. Dusunen Adam J. Psychiatry Neurol. Sci. 2012, 25, 206. [Google Scholar]
- Rybka, J.; Kedziora-Kornatowska, K.; Banaś-Leżańska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
- Szebeni, A.; Szebeni, K.; DiPeri, T.; Chandley, M.J.; Crawford, J.D.; Stockmeier, C.A.; Ordway, G.A. Shortened telomere length in white matter oligodendrocytes in major depression: Potential role of oxidative stress. Int. J. Neuropsychopharmacol. 2014, 17, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Perez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: Results from a meta-analysis. J. Clin. Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative stress parameters and antioxidants in patients with bipolar disorder: Results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disord. 2021, 23, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Schmidt, D.; Stein, C.M.; Morrow, J.D.; Salomon, R.M. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013, 206, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.; Selvi, Y.; Ozkol, H.; Tuluce, Y.; Besiroglu, L.; Aydin, A. Comparison of superoxide dismutase, glutathione peroxidase and adenosine deaminase activities between respiratory and nocturnal subtypes of patients with panic disorder. Neuropsychobiology 2012, 66, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Raffa, M.; Barhoumi, S.; Atig, F.; Fendri, C.; Kerkeni, A.; Mechri, A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Siwek, M.; Dudek, D.; Styczeń, K.; Nowak, G.; Szewczyk, B.; Sowa-Kućma, M. Markers of oxidation stress in the context of bipolar disorder in comparison with unipolar disorder and control group. In Proceedings of the International Review of Bipolar Disorders, Sevilla, Spain, 18–20 March 2013; pp. 1512–1518. [Google Scholar]
- Banerjee, U.; Dasgupta, A.; Rout, J.K.; Singh, O.P. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 56–61. [Google Scholar] [CrossRef]
- Gibson, S.A.; Korade, Ž.; Shelton, R.C. Oxidative stress and glutathione response in tissue cultures from persons with major depression. J. Psychiatr. Res. 2012, 46, 1326–1332. [Google Scholar] [CrossRef]
- Rawdin, B.S.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Scola, G.; McNamara, R.K.; Croarkin, P.E.; Leffler, J.M.; Cullen, K.R.; Geske, J.R.; Biernacka, J.M.; Frye, M.A.; DelBello, M.P.; Andreazza, A.C. Lipid peroxidation biomarkers in adolescents with or at high-risk for bipolar disorder. J. Affect. Disord. 2016, 192, 176–183. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Penninx, B.W.J.H. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol. Med. 2017, 47, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Ormonde do Carmo, M.B.; Mendes-Ribeiro, A.C.; Matsuura, C.; Pinto, V.L.; Mury, W.V.; Pinto, N.O.; Moss, M.B.; Ferraz, M.R.; Brunini, T.M. Major depression induces oxidative stress and platelet hyperaggregability. J. Psychiatr. Res. 2015, 61, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Saunders, E.F.H.; Santhanam, L.; Alexander, L.M. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ. Res. 2019, 15, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, B.R.; Near, J.; Cowen, P.J. Neurochemistry of major depression: A study using magnetic resonance spectroscopy. Psychopharmacology 2015, 232, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Freed, R.D.; Hollenhorst, C.N.; Weiduschat, N.; Mao, X.; Kang, G.; Shungu, D.C.; Gabbay, V. A pilot study of cortical glutathione in youth with depression. Psychiatry Res. Neuroimaging 2017, 270, 54–60. [Google Scholar] [CrossRef]
- Magalhães, P.V.; Jansen, K.; Pinheiro, R.T.; Colpo, G.D.; da Motta, L.L.; Klamt, F.; da Silva, R.A.; Kapczinski, F. Peripheral oxidative damage in early-stage mood disorders: A nested population-based case-control study. Int. J. Neuropsychopharmacol. 2012, 15, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of treatment-resistant depression: Challenges and strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Frank, M.; Szulc, A.; Gałęcka, M.; Szachta, P.; Barwinek, D. From gut to depression—The role of intestinal barrier dysfunction and activation of the immune system in the inflammatory hypothesis of depression. Neuropsychiatry Neuropsychol. 2012, 7, 76–84. [Google Scholar]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Chattu, V.K.; Aeri, B.T. Nutritional aspects of depression in adolescents—A systematic review. Int. J. Prev. Med. 2019, 10, 42. [Google Scholar]
- Skarupski, K.A.; Tangney, C.C.; Li, H.; Evans, D.A.; Morris, M.C. Mediterranean diet and depressive symptoms among older adults over time. J. Nutr. Health Aging 2013, 17, 441–445. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Holland, T.; Agarwal, P.; Aggarwal, N.; Morris, M.C. DASH and Mediterranean-Dash intervention for neurodegenerative delay (MIND) diets are associated with fewer depressive symptoms over time. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.; Watkins, E.; Bot, M.; Brouwer, I.A.; Roca, M.; Kohls, E.; Pennix, B.W.J.H.; van Grootheest, G.; Hegerl, U.; Gili, M.; et al. Nutrition and depression: Summary of findings from the EU-funded MooDFOOD depression prevention randomised controlled trial and a critical review of the literature. Nutr. Bull. 2020, 45, 403–414. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols-the positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef] [PubMed]
- Fahim, A.T.; Abd El-Fattah, A.A.; Sadik, N.A.H.; Ali, B.M. Resveratrol and dimethyl fumarate ameliorate testicular dysfunction caused by chronic unpredictable mild stress-induced depression in rats. Arch. Biochem. Biophys. 2019, 665, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress. 2016, 4, 15–22. [Google Scholar] [CrossRef]
- Kovacs, D.; Eszlari, N.; Petschner, P.; Pap, D.; Vas, S.; Kovacs, P.; Gonda, X.; Juhasz, G.; Bagdy, G. Effects of IL1B single nucleotide polymorphisms on depressive and anxiety symptoms are determined by severity and type of life stress. Brain Behav. Immun. 2016, 56, 96–104. [Google Scholar] [CrossRef]
- Barnes, J.; Mondelli, V.; Pariante, C.M. Genetic contributions of inflammation to depression. Neuropsychopharmacology 2017, 42, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Doz-Deblauwe, E.; Winter, N.; Briard, B. Specific NLRP3 inflammasome assembling and regulation in neutrophils: Relevance in inflammatory and infectious diseases. Cells 2022, 11, 1188. [Google Scholar] [CrossRef]
- Feng, M.; Wei, S.; Zhang, S.; Yang, Y. Anti-inflammation and anti-pyroptosis activities of mangiferin via suppressing NF-κB/NLRP3/GSDMD signaling cascades. Int. J. Mol. Sci. 2022, 23, 10124. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Afonina, I.S.; Müller, C.; Martin, S.J.; Beyaert, R. Proteolytic processing of interleukin-1 family cytokines: Variations on a common theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Puig, L. IL-1 family cytokines in inflammatory dermatoses: Pathogenetic role and potential therapeutic implications. Int. J. Mol. Sci. 2022, 23, 9479. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.A.; El Feky, M.; Hussein, A.A.R.M.; Mahmoud, M.A.; Idriss, N.K.; Abdelwahab, S.F.; Ibrahim, M.A. Interleukin-18 and interferon-γ single nucleotide polymorphisms in Egyptian patients with tuberculosis. PLoS ONE 2021, 16, e0244949. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Brann, D.W. NADPH Oxidase 2 regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid. Med. Cell. Longev. 2017, 2017, 6057609. [Google Scholar] [CrossRef]
- Moon, J.S.; Nakahira, K.; Chung, K.P.; DeNicola, G.M.; Koo, M.J.; Pabón, M.A.; Rooney, K.T.; Yoon, J.H.; Ryter, S.W.; Stout-Delgado, H.; et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016, 22, 1002–1012. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, T.; Liu, M.; Cao, D.; Li, J.; Li, Y.; Xia, M.; Wang, X.; Zheng, T.; Liu, C.; et al. Targeting NLRP3 inflammasome in translational treatment of nervous system diseases: An update. Front. Pharmacol. 2021, 12, 707696. [Google Scholar] [CrossRef] [PubMed]
- Licandro, G.; Ling Khor, H.; Beretta, O.; Lai, J.; Derks, H.; Laudisi, F.; Conforti-Andreoni, C.; Qian, H.L.; Teng, G.G.; Ricciardi-Castagnoli, P.; et al. The NLRP3 inflammasome affects DNA damage responses after oxidative and genotoxic stress in dendritic cells. Eur. J. Immunol. 2013, 43, 2126–2137. [Google Scholar] [CrossRef]
- Inserra, A.; Mastronardi, C.A.; Rogers, G.; Licinio, J.; Wong, M.L. Neuroimmunomodulation in major depressive disorder: Focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol. Neurobiol. 2019, 56, 4288–4305. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.A.; Rocha-Guzmán, N.E.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Bravo-Muñoz, M. Ancestral food sources rich in polyphenols, their metabolism, and the potential influence of gut microbiota in the management of depression and anxiety. J. Agric. Food Chem. 2022, 70, 944–956. [Google Scholar] [CrossRef]
- Mouihate, A. TLR4-mediated brain inflammation halts neurogenesis: Impact of hormonal replacement therapy. Front. Cell. Neurosci. 2014, 8, 146. [Google Scholar] [CrossRef]
- Kuzior, H.; Fiebich, B.L.; Yousif, N.M.; Saliba, S.W.; Ziegler, C.; Nickel, K.; Maier, S.J.; Suß, P.; Runge, K.; Matysik, M.; et al. Increased IL-8 concentrations in the cerebrospinal fluid of patients with unipolar depression. Compr. Psychiatry 2020, 102, 152196. [Google Scholar] [CrossRef]
- Paul, E.R.; Schwieler, L.; Erhardt, S.; Boda, S.; Trepci, A.; Kämpe, R.; Asratian, A.; Holm, L.; Yngve, A.; Dantzer, R.; et al. Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav. Immun. 2022, 101, 136–145. [Google Scholar] [CrossRef]
- Karakuła-Juchnowicz, H.; Flis, M.; Szymona, K.; Kuczyńska, M.; Stelmach, E.; Kowal-Popczak, A. New prospects for antipsychotic treatment—The role of the kynurenine pathway. Psychiatr. Pol. 2014, 48, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Pompili, S.; Valenta, S.T.; Salvi, V.; Volpe, U. C-reactive protein as a biomarker for major depressive disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Bliźniewska-Kowalska, K.; Gałecki, P.; Su, K.P.; Halaris, A.; Szemraj, J.; Gałecka, M. Expression of PON1, PON2, PON3 and MPO genes in patients with depressive disorders. J. Clin. Med. 2022, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Donaldson, J.; Jachimowicz, K. The role of nutritional factors in the modulation of the composition of the gut microbiota in people with autoimmune diabetes. Nutrients 2022, 14, 2498. [Google Scholar] [CrossRef]
- Lurie, I.; Yang, Y.X.; Haynes, K.; Mamtani, R.; Boursi, B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study. J. Clin. Psychiatry 2015, 76, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.A.; Nieuwdorp, M.; Zwinderman, A.H.; Deschasaux, M.; Radjabzadeh, D.; Kraaij, R.; Davids, M.; de Rooij, S.R.; Lok, A. The gut microbiota and depressive symptoms across ethnic groups. Nat. Commun. 2022, 13, 7129. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlokken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Neurosci. Biobehav. Rev. 2016, 64, 134–147. [Google Scholar]
- Garcia-Bueno, B.; Caso, J.R.; Madrigal, J.L.; Lezam, J.C. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 2016, 64, 134–147. [Google Scholar] [CrossRef]
- Caso, J.R.; MacDowell, K.S.; González-Pinto, A.; García, S.; de Diego-Adeliño, J.; Carceller-Sindreu, M.; Sarramea, F.; Caballero-Villarraso, J.; Gracia-García, P.; De la Cámara, C.; et al. Gut microbiota, innate immune pathways, and inflammatory control mechanisms in patients with major depressive disorder. Transl. Psychiatry 2021, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Baker, G.B.; Dursun, S.M. The relationship between the gut microbiome-immune system-brain axis and major depressive disorder. Front. Neurol. 2021, 12, 721126. [Google Scholar] [CrossRef]
- Janowska, M.; Rog, J.; Karakula-Juchnowicz, H. Disruptions within gut microbiota composition induced by improper antibiotics therapy as a probable trigger factor for development of depression—Case reports. Ann. Agric. Environ. Med. 2021, 28, 713–718. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA 2018, 115, E10539–E10547. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.P.; de Jonge, J.H.; SmitBahn, S.; Penninx, B.W. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From probiotics to psychobiotics—The gut-brain axis in psychiatric disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Greene, M.W.; Ramesh Babu, J.; Frugé, A.D. Psychobiotics as treatment for anxiety, depression, and related symptoms: A systematic review. Nutr. Neurosci. 2021, 24, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, T.; Cai, J.; Liu, B.; Zeng, Y.; Zhang, X. The microbiome-gut-brain axis, a potential therapeutic target for substance-related disorders. Front. Microbiol. 2021, 12, 738401. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Amini-Khoei, H.; Boroujeni, S.N.; Maghsoudi, F.; Rahimi-Madiseh, M.; Bijad, E.; Moradi, M.; Lorigooini, Z. Possible involvement of L-arginine-nitric oxide pathway in the antidepressant activity of Auraptene in mice. Behav. Brain Funct. 2022, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Wenske, S.; Lackmann, J.W.; Lalk, M.; von Woedtke, T.; Wende, K. On the liquid chemistry of the reactive nitrogen species peroxynitrite and nitrogen dioxide generated by physical plasmas. Biomolecules 2020, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Omidi-Ardali, H.; Badi, A.G.; Saghaei, E.; Amini-Khoei, H. Nitric oxide mediates the antidepressant-like effect of modafinil in mouse forced swimming and tail suspension tests. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Saravi, S.S.; Arefidoust, A.; Yaftian, R.; Saeedi Saravi, S.S.; Dehpour, A.R. 17α-ethinyl estradiol attenuates depressive-like behavior through GABAA receptor activation/nitrergic pathway blockade in ovariectomized mice. Psychopharmacology 2016, 233, 1467–1485. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ. Toxicol. Pharmacol. 2013, 36, 9–18. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cai, Q.; Wei, H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 1998, 24, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Benatti, C.; Blom, J.M.C.; Caraci, F.; Tascedda, F. The many faces of mitochondrial dysfunction in depression: From pathology to treatment. Front. Pharmacol. 2019, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and mood: Mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Rezin, G.T.; Cardoso, M.R.; Gonçalves, C.L.; Scaini, G.; Fraga, D.B.; Riegel, R.E.; Comim, C.M.; Quevedo, J.; Streck, E.L. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem. Int. 2008, 53, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Mazereeuw, G.; Herrmann, N.; Andreazza, A.C.; Khan, M.M.; Lanctôt, K.L. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr. Dis. Treat. 2015, 11, 2479–2491. [Google Scholar] [PubMed]
- Milaneschi, Y.; Cesari, M.; Simonsick, E.M.; Vogelzangs, N.; Kanaya, A.M.; Yaffe, K.; Patrignani, P.; Metti, A.; Kritchevsky, S.B.; Pahor, M.; et al. Lipid peroxidation and depressed mood in community-dwelling older men and women. PLoS ONE 2013, 8, e65406. [Google Scholar] [CrossRef]
- Cline, B.H.; Anthony, D.C.; Lysko, A.; Dolgov, O.; Anokhin, K.; Schroeter, C.; Malin, D.; Kubatiev, A.; Steinbusch, H.W.; Lesch, K.P.; et al. Lasting downregulation of the lipid peroxidation enzymes in the prefrontal cortex of mice susceptible to stress-induced anhedonia. Behav. Brain Res. 2015, 276, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta. 2015, 1851, 1052–1065. [Google Scholar] [CrossRef]
- Husted, K.S.; Bouzinova, E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina 2016, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Ahmed, I.; Moktadir, A.A.; Nahar, Z.; Islam, M.S.; Shahid, S.F.B.; Islam, S.N.; Islam, M.S.; Hasnat, A. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: A case-control study. SAGE Open Med. 2018, 6, 2050312118773953. [Google Scholar] [CrossRef]
- Stefanescu, C.; Ciobica, A. The relevance of oxidative stress status in first episode and recurrent depression. J. Affect. Disord. 2012, 143, 34–38. [Google Scholar] [CrossRef]
- Panwar, R.; Sivakumar, M. Comet parameters and plasma 8-Iso-prostaglandins F2α: Common markers of etiopathogenesis in major depression and indicators of antioxidant action of fluoxetine. Natl. J. Clin. Anat. 2021, 10, 118–125. [Google Scholar]
- Kodydková, J.; Vávrová, L.; Zeman, M.; Jirák, R.; Macásek, J.; Stanková, B.; Tvrzická, E.; Zák, A. Antioxidative enzymes and increased oxidative stress in depressive women. Clin. Biochem. 2009, 42, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ratti, B.A.; O’Brien, J.G.; Lautenschlager, S.O.; Gius, D.R.; Bonini, M.G.; Zhu, Y. Manganese superoxide dismutase (SOD2): Is there a center in the universe of mitochondrial redox signaling? J. Bioenerg. Biomembr. 2017, 49, 325–333. [Google Scholar] [CrossRef]
- Ait Tayeb, A.E.K.; Becquemont, L.; El-Asmar, K.; Mahmoudi, K.; Colle, R.; Trabado, S.; Gressier, F.; Feve, B.; Corruble, E.; Verstuyft, C. SOD2 genetic polymorphism (rs4880) has no impact on 6-month response to antidepressant treatment and inflammatory biomarkers in depressed patients. Basic Clin. Pharmacol. Toxicol. 2020, 126, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Kwiatkowski, D.; Kacperska, D.; Kawczyńska, D.; Talarowska, M.; Orzechowska, A.; Bielecka-Kowalska, A.; Szemraj, J.; Gałecki, P.; Śliwiński, T. Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder. Med. Sci. Monit. 2015, 21, 412–418. [Google Scholar] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J. Antagonism by bioactive polyphenols against inflammation: A systematic view. Inflamm. Allergy Drug Targets 2014, 13, 34–64. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Tan, A.A.; Ozdemir, D.; Bestepe, N.; Dellal, F.D.; Bilginer, M.C.; Faki, S.; Bicer, C.; Ersoy, R.; Cakir, B. Low rate of latent autoimmune diabetes in adults (LADA) in patients followed for type 2 diabetes: A single center’s experience in Turkey. Arch. Endocrinol. Metab. 2020, 64, 584–590. [Google Scholar]
- Simos, Y.V.; Verginadis, I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Karthikeyan, A.; Dheen, S.T. Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromolecular Med. 2016, 18, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Grubić Kezele, T.; Curko-Cofek, B. Neuroprotective panel of olive polyphenols: Mechanisms of action, anti-demyelination, and anti-stroke properties. Nutrients 2022, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Chegini, M.; Shirani, P.; Omidvar, N.; Eini-Zinab, H.; Pour-Ebrahim, F.; Rezazadeh, A. Relationship between diet quality and depression among Iranian older adults in Tehran. BMC Geriatr. 2022, 22, 708. [Google Scholar] [CrossRef]
- Pereira, G.A.; da Silva, A.; Hermsdorff, H.H.M.; Moreira, A.P.B.; de Aguiar, A.S. Association of dietary total antioxidant capacity with depression, anxiety, and sleep disorders: A systematic review of observational studies. J. Clin. Transl. Res. 2021, 27, 631–640. [Google Scholar]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary total antioxidant capacity is inversely associated with depression, anxiety and some oxidative stress biomarkers in postmenopausal women: A cross-sectional study. Ann. Gen. Psychiatry 2019, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Daneshzad, E.; Keshavarz, S.A.; Qorbani, M.; Larijani, B.; Azadbakht, L. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: A cross-sectional study among diabetic women. Clin. Nutr. ESPEN 2020, 37, 187–194. [Google Scholar] [CrossRef]
- de Oliveira, N.G.; Teixeira, I.T.; Theodoro, H.; Branco, C.S. Dietary total antioxidant capacity as a preventive factor against depression in climacteric women. Dement. Neuropsychol. 2019, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, T.; Ziaee, A.; Salehi-Abargouei, A.; Ferns, G.A.; Ghayour-Mobarhan, M.; Khayyatzadeh, S.S. Dietary total antioxidant capacity is inversely related to the prevalence of depression in adolescent girls. BMC Pediatr. 2022, 22, 535. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Omranzadeh, A.; Miri-Moghaddam, M.M.; Arekhi, S.; Naseri, A.; Ziaee, A.; Khajavi, L.; Salehkhani, F.N.; Ferns, G.A.; Ghayour-Mobarhan, M. Dietary antioxidants and fibre intake and depressive symptoms in Iranian adolescent girls. Public Health Nutr. 2021, 24, 5650–5656. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Vasmehjani, A.A.; Ghayour-Mobarhan, M.; Ferns, G.A.; Khayyatzadeh, S.S. The association between dietary phytochemical index with depression and quality of life in Iranian adolescent girls. Biopsychosoc. Med. 2022, 16, 5. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High polyphenol diet improves psychological well-being: The polyphenol intervention trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking what we eat to our mood: A review of diet, dietary antioxidants, and depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of polyphenols in a Mediterranean diet on symptoms of depression: A systematic literature review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Oddo, V.M.; Welke, L.; McLeod, A.; Pezley, L.; Xia, Y.; Maki, P.; Koenig, M.D.; Kominiarek, M.A.; Langenecker, S.; Tussing-Humphreys, L. Adherence to a Mediterranean Diet is associated with lower depressive symptoms among U.S. Adults. Nutrients 2022, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary polyphenol intake and depression: Results from the mediterranean healthy eating, lifestyle and aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.P.; Tirkey, N.; Kuhad, A.; Chopra, K. Effect of quercetin on lipopolysaccharide induced-sickness behavior and oxidative stress in rats. Indian J. Pharmacol. 2011, 43, 192–196. [Google Scholar] [CrossRef]
- Miki, T.; Eguchi, M.; Kochi, T.; Akter, S.; Hu, H.; Kashino, I.; Kuwahara, K.; Kabe, I.; Nanri, A.; Mizoue, T. Prospective study on the association between dietary non-enzymatic antioxidant capacity and depressive symptoms. Clin. Nutr. ESPEN 2020, 36, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A. Nutritional epidemiology of type 2 diabetes and depressive symptoms. J. Epidemiol. 2013, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Li, Y.; Toit, E.D.; Wendt, L.; Sun, J. Effects of polyphenol supplementations on improving depression, anxiety, and quality of life in patients with depression. Front. Psychiatry 2021, 12, 765485. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Michońska, I.; Dereń, K. The Mediterranean Diet and the Western Diet in adolescent depression-current reports. Nutrients 2022, 14, 4390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, L.; Hong, W.; Luo, M.; Zhao, N.; Hu, X.; Shi, M.; Qiu, J.; Shen, Y.; Teng, X.; et al. Inflammatory cytokines changed in patients with depression before and after repetitive transcranial magnetic stimulation treatment. Front. Psychiatry 2022, 13, 925007. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef] [PubMed]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on treatment with curcumin has antidepressive effects in Thai patients with major depression: Results of a randomized double-blind placebo-controlled study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Agah, S.; Estêvão, M.D.; Hosseini, A.S.; Heydari, H.; Toupchian, O.; Abdollahi, S.; Persad, E.; Abu-Zaid, A.; Rezamand, G.; et al. Effect of saffron supplementation on oxidative stress parameters: A systematic review and meta-analysis of randomized placebo-controlled trials. Food Sci. Nutr. 2021, 9, 5809–5819. [Google Scholar] [CrossRef]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin attenuates chronic unpredictable mild stressinduced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 signaling pathway in rats. Oxid. Med. Cell. Longev. 2020, 2020, 9268083. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015, 141, 156–169. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef]
- Pan, X.; Chen, L.; Xu, W.; Bao, S.; Wang, J.; Cui, X.; Gao, S.; Liu, K.; Avasthi, S.; Zhang, M.; et al. Activation of monoaminergic system contributes to the antidepressant- and anxiolytic-like effects of J147. Behav. Brain Res. 2021, 411, 113374. [Google Scholar] [CrossRef]
- Rubab, S.; Naeem, K.; Rana, I.; Khan, N.; Afridi, M.; Ullah, I.; Shah, F.A.; Sarwar, S.; Din, F.U.; Choi, H.I.; et al. Enhanced neuroprotective and antidepressant activity of curcumin-loaded nanostructured lipid carriers in lipopolysaccharide-induced depression and anxiety rat model. Int. J. Pharm. 2021, 603, 120670. [Google Scholar] [CrossRef] [PubMed]
- Saied, N.M.; Georgy, G.S.; Hussien, R.M.; Hassan, W.A. Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin. Exp. Pharmacol. Physiol. 2021, 48, 337–346. [Google Scholar] [CrossRef]
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; de Barros Falcao Ferraz, A.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; da Silva Moreira, L.K.; da Silva Neri, H.F.; da Silva, C.R.B.; Pruccoli, L.; Dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Abdel-Hafiz, L.; Khabour, O.F.; El-Elimat, T.; Alzubi, M.A.; Alali, F.Q. Evaluation of the effect of Hypericum triquetrifolium Turra on memory impairment induced by chronic psychosocial stress in rats: Role of BDNF. Drug Des. Dev. Ther. 2020, 14, 5299–5314. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.A.; Fahim, A.T.; Sadik, N.; Ali, B.M. Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res. 2018, 1701, 227–236. [Google Scholar] [CrossRef]
- Yang, X.H.; Song, S.Q.; Xu, Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: Involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2727–2736. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V.G.M. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced hippocampus and frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.Y.; Zeng, M.J.; Zhou, L.P.; Li, Y.Q.; Zhao, F.; Shang, Z.Y.; Deng, X.Y.; Ma, Z.Q.; Fu, Q.; Ma, S.P.; et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018, 64, 175–182. [Google Scholar] [CrossRef]
- Rai, A.; Gill, M.; Kinra, M.; Shetty, R.; Krishnadas, N.; Rao, C.M.; Sumalatha, S.; Kumar, N. Catechin ameliorates depressive symptoms in Sprague Dawley rats subjected to chronic unpredictable mild stress by decreasing oxidative stress. Biomed. Rep. 2019, 11, 79–84. [Google Scholar] [CrossRef]
- Li, M.; Shao, H.; Zhang, X.; Qin, B. Hesperidin alleviates lipopolysaccharide-induced neuroinflammation in mice by promoting the miRNA-132 pathway. Inflammation 2016, 39, 1681–1689. [Google Scholar] [CrossRef]
- Khan, K.; Najmi, A.K.; Akhtar, M. A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-HT levels in one of the animal models of depression in mice. Drug Res. 2019, 67, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Cao, C.; Hou, Y.; Li, Y.; Wei, X.; Li, S.; Jia, S.; Zhao, X. Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals 2021, 34, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, J.; Wu, X.; Song, L.; Wang, Y.; Gong, M.; Li, B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021, 1772, 147661. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Shao, T.; Ruan, L.; Wang, L.; Sun, J.; Li, J.; Zhu, X.; O’Donnell, J.M.; Pan, J. Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: Behavioral and neurobiological analyses. Metab. Brain Dis. 2013, 28, 571–583. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, L.; Chen, J.; Liu, C.; Li, S.; Hua, M.; Qu, D.; Shao, Z.; Sun, Y. Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response. Biochem. Biophys. Res. Commun. 2020, 530, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, T.; Yu, Z.; Li, T.; Zhang, J.; Zhang, Z.; Cai, M.; Zhang, W.; Xiang, J.; Cai, D. Apocynum venetum leaf extract reverses depressive-like behaviors in chronically stressed rats by inhibiting oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 100, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Le Zhai, J.; Liao, Y.H.; Aibai, S.; Yang, Y.; Liu, X.M. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J. Ethnopharmacol. 2016, 194, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Thakare, V.N.; Patil, R.R.; Oswal, R.J.; Dhakane, V.D.; Aswar, M.K.; Patel, B.M. Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice. J. Psychopharmacol. 2018, 32, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Thakare, V.N.; Aswar, M.K.; Kulkarni, Y.P.; Patil, R.R.; Patel, B.M. Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiol. Behav. 2017, 179, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.F.; Abbas, A.M.; Elsamanoudy, A.Z.; Ghoneim, F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiol. Pharmacol. 2015, 66, 515–527. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, J.; Xiong, Y.; Zhang, X.; Lin, Y.; Liu, Z. Jasmine tea attenuates chronic unpredictable mild stress-induced depressive-like behavior in rats via the gut-brain axis. Nutrients 2021, 14, 99. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, G.; Gou, L.; Sun, L.; Fu, X.; Lan, N.; Li, S.; Yin, X. Antidepressant-like effects of tea polyphenols on mouse model of chronic unpredictable mild stress. Pharmacol. Biochem. Behav. 2013, 104, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Li, C.; Li, M.; Liao, Y.; Liu, X.; Si, J.; Chang, Q.; Pan, R. Antidepressant activity of an aqueous extract from okra seeds. RSC Adv. 2018, 8, 32814–32822. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant potential of quercetin and its glycoside derivatives: A comprehensive review and update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Bramanti, P.; Mazzon, E. Role of quercetin in depressive-like behaviors: Findings from animal models. Appl. Sci. 2021, 11, 7116. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Li, H.R.; Chen, X.X.; Gao, X.R.; Huang, L.L.; Du, A.Q.; Jiang, C.; Li, H.; Ge, J.F. Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front. Pharmacol. 2020, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Sadighparvar, S.; Darband, S.G.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Babaei, G.; Mobaraki, K.; Majidinia, M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp. Physiol. 2020, 105, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Mahmoud, M.E.; El Hanafy, A.A. Anti-inflammatory potential of curcumin and quercetin in rats: Role of oxidative stress, heme oxygenase-1 and TNF-α. Toxicol. Ind. Health 2014, 30, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008, 57, S39–S46. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, F.; Saleem, S.; Naqvi, F.; Batool, Z.; Sadir, S.; Tabassum, S.; Ahmed, S.; Liaquat, L.; Haider, S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak. J. Pharm. Sci. 2019, 32, 1893–1900. [Google Scholar]
- Lamanna-Rama, N.; Romero-Miguel, D.; Desco, M.; Soto-Montenegro, M.L. An update on the exploratory use of curcumin in neuropsychiatric disorders. Antioxidants 2022, 11, 353. [Google Scholar] [CrossRef]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The emerging role of curcumin in the modulation of TLR-4 signaling pathway: Focus on neuroprotective and anti-rheumatic properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Ruggiero, M.; Panaro, M.A. Inflammaging and brain: Curcumin and its beneficial potential as regulator of microglia activation. Molecules 2022, 27, 341. [Google Scholar] [CrossRef] [PubMed]
- He, G.L.; Luo, Z.; Yang, J.; Shen, T.T.; Chen, Y.; Yang, X.S. Curcumin ameliorates the reduction effect of PGE2 on fibrillar beta-amyloid peptide (1-42)-induced microglial phagocytosis through the inhibition of EP2-PKA signaling in N9 microglial cells. PLoS ONE 2016, 11, e0147721. [Google Scholar]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical use of curcumin in depression: A meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Cosan, D.T.; Saydam, F.; Calis, I.U.; Kolac, U.K.; Koroglu, Z.O.; Degirmenci, I.; Mutlu, F.S.; Gunes, H.V. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum. Exp. Toxicol. 2019, 38, 1296–1301. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M. The effect of exposure to Cd and Pb in the form of a drinking water or feed on the accumulation and distribution of these metals in the organs of growing Wistar rats. Biol. Trace Elem. Res. 2016, 2, 230–236. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Xue, Y.; Liang, Y.; Shi, J.; Han, X.; Zhang, J.; Chu, X.; Chu, L. Tannic acid attenuates hepatic oxidative stress, apoptosis and inflammation by activating the Keap1-Nrf2/ARE signaling pathway in arsenic trioxide-toxicated rats. Oncol. Rep. 2020, 44, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, L.; Han, X.; Zhang, Y.; Zhang, X.; Chu, X.; Zhang, F.; Zhang, Y.; Chu, L. Protective effects of tannic acid on acute doxorubicin-induced cardiotoxicity: Involvement of suppression in oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2017, 93, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Huang, J.; Rasul, A.; Anwar, H.; Imran, A.; Maqbool, J.; Razzaq, A.; Aziz, N.; Makhdoom, E.U.H.; Konuk, M.; et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules 2019, 24, 2213. [Google Scholar] [CrossRef] [PubMed]
- Luduvico, K.P.; Spohr, L.; Soares, M.S.P.; Teixeira, F.C.; de Farias, A.S.; Bona, N.P.; Pedra, N.S.; de Oliveira Campello Felix, A.; Spanevello, R.M.; Stefanello, F.M. Antidepressant effect and modulation of the redox system mediated by tannic acid on lipopolysaccharide-induced depressive and inflammatory changes in mice. Neurochem. Res. 2020, 45, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jian, S.; Wen, C.; Guo, D.; Liao, P.; Wen, J.; Kuang, T.; Han, S.; Liu, Q.; Deng, B. Gallnut tannic acid exerts anti-stress effects on stress-induced inflammatory response, dysbiotic gut microbiota, and alterations of serum metabolic profile in beagle dogs. Front. Nutr. 2022, 9, 847966. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; German-Ponciano, L.J.; Puga-Olguín, A.; Olmos-Vázquez, O.J. Pharmacological, neurochemical, and behavioral mechanisms underlying the anxiolytic- and antidepressant-like effects of flavonoid chrysin. Molecules 2022, 27, 3551. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on contemporary status and future possibilities as pro-health agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin protects against memory and hippocampal neurogenesis depletion in D-galactose-induced aging in rats. Nutrients 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Bhatia, J.; Tomar, A.; Nag, T.C.; Ray, R.; Arya, D.S. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutr. Metab. 2015, 12, 11. [Google Scholar] [CrossRef]
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and chrysin synergies to improve septic mice survival. Molecules 2017, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes de Gomes, M.; Rossito Goes, A.T.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, Z.; Fan, L.; Xue, Z.; Chen, J.; Wang, X.; Huang, Z.; Men, Y.; Yu, M.; Liu, Y.; et al. Integrating metabolomics and network analysis for exploring the mechanism underlying the antidepressant activity of paeoniflorin in rats with CUMS-induced depression. Front. Pharmacol. 2022, 13, 904190. [Google Scholar] [CrossRef]
- Liu, S.C.; Hu, W.Y.; Zhang, W.Y.; Yang, L.; Li, Y.; Xiao, Z.C.; Zhang, M.; He, Z.Y. Paeoniflorin attenuates impairment of spatial learning and hippocampal long-term potentiation in mice subjected to chronic unpredictable mild stress. Psychopharmacology 2019, 236, 2823–2834. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, M.; Wan, H.Q.; Chen, X.Q.; Li, C.F.; Zhu, J.X.; Liu, Q.; Xu, G.H.; Yi, L.T. Paeoniflorin exerts antidepressant-like effects through enhancing neuronal FGF-2 by microglial inactivation. J. Ethnopharmacol. 2021, 274, 114046. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, D.O.; Zhang, L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef]
- Kim, J. Green Tea, Coffee, and caffeine consumption are inversely associated with self-report lifetime depression in the Korean population. Nutrients 2018, 10, 1201. [Google Scholar] [CrossRef]
- Dong, X.; Yang, C.; Cao, S.; Gan, Y.; Sun, H.; Gong, Y.; Yang, H.; Yin, X.; Lu, Z. Tea consumption and the risk of depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2015, 49, 334–345. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Li, Z.; Guo, Y.; Granato, D. Green tea polyphenols upregulate the Nrf2 signaling pathway and suppress oxidative stress and inflammation markers in D-galactose-induced liver aging in mice. Front. Nutr. 2022, 9, 836112. [Google Scholar] [CrossRef]
- Wang, D.X.; Cai, M.; Wang, T.T.; Zhao, G.S.; Huang, J.B.; Wang, H.S.; Qian, F.; Ho, C.T.; Wang, Y.J. Theanine supplementation prevents liver injury and heat shock response by normalizing hypothalamic-pituitaryadrenal axis hyperactivity in mice 232. subjected to whole body heat stress. J. Funct. Foods 2018, 45, 181–189. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Chen, X.; Wang, L.; Li, J.; Li, G.; Zhang, B. Antidepressant effect of EGCG through the inhibition of hippocampal neuroinflammation in chronic unpredictable mild stress-induced depression rat model. J. Funct. Foods 2020, 73, 104106. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic-pituitary-adrenal axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J. Med. Food 2018, 21, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin gallate (GCG) inhibits 3T3-L1 differentiation and lipopolysaccharide induced inflammation through MAPK and NF-kappa B signaling. J. Funct. Foods 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kita, M.; Taniguchi, Y.; Kondo, K. theaflavins improve memory impairment and depression-like behavior by regulating microglial activation. Molecules 2019, 24, 467. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Jin, F.J.; Wang, Y.L.; Li, F.; Wang, L.; Wang, Q.L.; Ren, Z.; Wang, Y.F. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3‘-digallate on lipopolysaccharide-induced inflammation. Eur. J. Pharmacol. 2017, 794, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Lo, C.Y.; Wang, B.J.; Chiou, R.Y.Y.; Lin, S.M. Theaflavin-3,3′-digallate, a black tea polyphenol, attenuates adipocyte-activated inflammatory response of macrophage associated with the switch of M1/M2-like phenotype. J. Funct. Foods 2014, 11, 36–48. [Google Scholar] [CrossRef]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Li, J.H.; Sasaki, G.Y.; Dey, P.; Chitchumroonchokchai, C.; Labyk, A.N.; McDonald, J.D.; Kim, J.B.; Bruno, R.S. Green tea extract protects against hepatic NF kappa B activation along the gut-liver axis in diet-induced obese mice with nonalcoholic steatohepatitis by reducing endotoxin and TLR4/MyD88 signaling. J. Nutr. Biochem. 2018, 53, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.S.; Qi, G.Y.; Fan, R.; Qiao, Q.L.; Sun, Y.L.; Gao, Y.Q.; Liu, X.B. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary tea saponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz, K.; Muszyński, S.; Tomaszewska, E. Bioactive compounds, antibiotics and heavy metals: Effects on the intestinal structure and microbiome of monogastric animals—A non-systematic review. Ann. Anim. Sci. 2023. [Google Scholar] [CrossRef]
- Martin, A.R.; Villegas, I.; La Casa, C.; de la Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cárdeno, A.; Alarcón de la Lastra, C. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Bąkowski, M.; Krusiński, R.; Jachimowicz-Rogowska, K.; Demkowska-Kutrzepa, M.; Kiczorowska, B.; Krupa, W. Tannic acid and tea prevents the accumulation of lead and cadmium in the lungs, heart and brain of adolescent male Wistar rats—Possible therapeutic option. Animals 2022, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Penders, J.; Hursel, R.; Budding, A.E.; Savelkoul, P.H.; Westerterp-Plantenga, M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS ONE 2016, 11, e0153134. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial metabolism of theaflavin-3,3′-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Zhu, J.; Cheng, L.; Cao, J.; Wu, Z.; Weng, P.; Zheng, X. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. 2018, 9, 1079–1087. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Zhang, C.; Cao, J.; Wu, Z. Omics analyses of gut microbiota in a circadian rhythm disorder mouse model fed with oolong tea polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [PubMed]
- Bustos, I.; García-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food. Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2019, 17, 100495. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin trans-location and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hayek, S.; Guzman, J.R.; Gillitt, N.D.; Ibrahim, S.A.; Jobin, C.; Sang, S. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS ONE 2012, 7, e51001. [Google Scholar] [CrossRef]
- Inocente-Camones, M.A.; Arias-Arroyo, G.C.; Mauricio-Alza, S.M.; Bravo-Araujo, G.T.; Capcha-Siccha, M.F.; Cabanillas-Alvitrez, E. Polyphenols, carotenoids and flavonoids in an antioxidant probiotic yogurt made with tumbo pulp (Passiflora tripartita Kunth). Braz. J. Food Technol. 2022, 25, e2021175. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Dean, O.M.; Turner, A.; Sureda, A.; Daglia, M.; Nabavi, S.M. Oxidative stress and post-stroke depression: Possible therapeutic role of polyphenols? Curr. Med. Chem. 2015, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Baharzadeh, E.; Siassi, F.; Qorbani, M.; Koohdani, F.; Pak, N.; Sotoudeh, G. Fruits and vegetables intake and its subgroups are related to depression: A cross-sectional study from a developing country. Ann. Gen. Psychiatry 2018, 17, 46. [Google Scholar] [CrossRef]
- Yuk, Y.; Han, C.R.; Jang, Y.; Hong, Y.C.; Choi, Y.J. Association between weekly fruit and vegetable consumption and depressive symptoms: Results from the Korean Elderly Environmental Panel study. Epidemiol. Health 2021, 43, e2021029. [Google Scholar] [CrossRef] [PubMed]
- Saghafian, F.; Malmir, H.; Saneei, P.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Fruit and vegetable consumption and risk of depression: Accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br. J. Nutr. 2018, 119, 1087–1101. [Google Scholar] [CrossRef]
- Bishwajit, G.; O’Leary, D.P.; Ghosh, S.; Sanni, Y.; Shangfeng, T.; Zhanchun, F. Association between depression and fruit and vegetable consumption among adults in South Asia. BMC Psychiatry 2017, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Almajwal, A. Reversal of cisplatin triggered neurotoxicity by Acacia hydaspica ethyl acetate fraction via regulating brain acetylcholinesterase activity, DNA damage, and pro-inflammatory cytokines in the rodent model. BMC Complement. Med. Ther. 2022, 22, 179. [Google Scholar] [CrossRef]
- Samad, N.; Muneer, A.; Ullah, N.; Zaman, A.; Ayaz, M.M.; Ahmad, I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: Possible role of potential antioxidants. Pak. J. Pharm. Sci. 2017, 30, 989–995. [Google Scholar]
- Imran, I.; Javaid, S.; Waheed, A.; Rasool, M.F.; Majeed, A.; Samad, N.; Saeed, H.; Alqahtani, F.; Ahmed, M.M.; Alaqil, F.A. Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 2021, 7, 587367. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.F.; Lu, K.; Lin, P.C.; Che, H.X.; Li, H.Y.; Song, L.; Yang, X.H.; Xie, W.C. Saccharina japonica ethanol extract ameliorates depression/anxiety-like behavior by inhibiting inflammation, oxidative stress, and apoptosis in dextran sodium sulfate induced ulcerative colitis mice. Front. Nutr. 2021, 8, 784532. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.B.; Shirooie, S.; Sokeng, A.J.T.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (Molina) Stuntz) in a mouse model of post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Radavelli-Bagatini, S.; Anokye, R.; Bondonno, N.P.; Sim, M.; Bondonno, C.P.; Stanley, M.J.; Harms, C.; Woodman, R.; Magliano, D.J.; Shaw, J.E.; et al. Association of habitual intake of fruits and vegetables with depressive symptoms: The AusDiab study. Eur. J. Nutr. 2021, 60, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Akomolafe, S.F.; Akinyemi, A.J.; Anadozie, S.O. Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int. Sch. Res. Not. 2014, 2014, 984709. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Camargo, A.; Dalmagro, A.P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 2017, 125, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Rodrigues, A.F.; Rós, A.S.; De Castro, A.B.; De Lima, D.D.; Magro, D.D.; Zeni, A.L. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Arcidiaco, P.; Nabavi, S.M.; Daglia, M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2016, 60, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Khanjani, S.; Moghaddam, A.H.; Braidy, N.; Nabavi, S.M. Improvement of antioxidant defences and mood status by oral GABA tea administration in a mouse model of post-stroke depression. Nutrients 2017, 9, 446. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhou, M.; Leng, C.; Tao, X.; Zhou, R.; Li, Y.; Sun, B.; Shu, X.; Liu, W. Neuroprotective effect of polyphenol extracts from Terminalia chebula Retz. against cerebral ischemia-reperfusion injury. Molecules 2022, 27, 6449. [Google Scholar] [CrossRef]
- Dordoe, C.; Wang, X.; Lin, P.; Wang, Z.; Hu, J.; Wang, D.; Fang, Y.; Liang, F.; Ye, S.; Chen, J.; et al. Non-mitogenic fibroblast growth factor 1 protects against ischemic stroke by regulating microglia/macrophage polarization through Nrf2 and NF-κB pathways. Neuropharmacology 2022, 212, 109064. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.-K. Neuroprotective effect of eco-sustainably extracted grape polyphenols in neonatal hypoxia-ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Khan, M.R.; Almajwal, A. Anti-depressant and anxiolytic potential of Acacia hydaspica R. Parker aerial parts extract: Modulation of brain antioxidant enzyme status. BMC Complement. Altern. Med. 2017, 17, 228. [Google Scholar] [CrossRef]
- Peñalver, P.; Zodio, S.; Lucas, R.; de-Paz, M.V.; Morales, J.C. Neuroprotective and anti-inflammatory effects of pterostilbene metabolites in human neuroblastoma SH-SY5Y and RAW 264.7 macrophage cells. J. Agric. Food Chem. 2020, 68, 1609–1620. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, G.D.; Song, X.Y.; Wang, J.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Neuroprotective effects of 1,2-Diarylpropane type phenylpropanoid enantiomers from red raspberry against H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2018, 66, 331–338. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef]
- Zheng, T.; Bielinski, D.F.; Fisher, D.R.; Zhang, J.; Shukitt-Hale, B. Protective effects of a polyphenol-rich blueberry extract on adult human neural progenitor cells. Molecules 2022, 27, 6152. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Pomier, K.M.; Ahmed, R.; Melacini, G. Catechins as tools to understand the molecular basis of neurodegeneration. Molecules 2020, 25, 3571. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Green tea epigallocatechin-3-gallate (EGCG) targeting protein misfolding in drug discovery for neurodegenerative diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, R.; Kujawska, M.; Ożarowski, M.; Baraniak, J.; Laskowska, H.; Nowocień, T.; Borowska, M.; Szulc, M.; Sobczak, A.; Mikołajczak, P. Perspectives for gallotannins neuroprotective potential—Current experimental evidences. JMS 2016, 85, 317–322. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.B.; Kim, H.S. Neuroprotective effects of tannic acid in the postischemic brain via direct chelation of Zn2+. Anim. Cells Syst. 2022, 26, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tuzmen, M.N.; Yucel, N.C.; Kalburcu, T.; Demiryas, N. Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol. Mech. Methods 2015, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Tabassum, H.; Parvez, S. Tannic acid provides neuroprotective effects against traumatic brain injury through the PGC-1α/Nrf2/HO-1 pathway. Mol. Neurobiol. 2020, 57, 2870–2885. [Google Scholar] [CrossRef]
- Sehati, F.; Ahmadi, I.; Farivar, N.; Ranjbaran, M.; Sadat-Shirazi, M.S.; Nabavizadeh, F.; Shavakandi, S.M.; Ashabi, G. Tannic acid protects aged brain against cerebral hypoperfusion via modulation of Nrf2 and inflammatory pathways. Neurosci. Lett. 2021, 765, 136263. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, C.; Garlapati, P.K.; Raghavan, A.K. Gallic acid from Terminalia bellirica fruit exerts antidepressant-like activity. Rev. Bras. Farm. 2020, 30, 357–366. [Google Scholar] [CrossRef]
- Sekhar, Y.C.; Kumar, G.P.; Anilakumar, K.R. Terminalia arjuna bark extract attenuates picrotoxin-induced behavioral changes by activation of serotonergic, dopaminergic, GABAergic and antioxidant systems. Chin. J. Nat. Med. 2017, 15, 584–596. [Google Scholar]
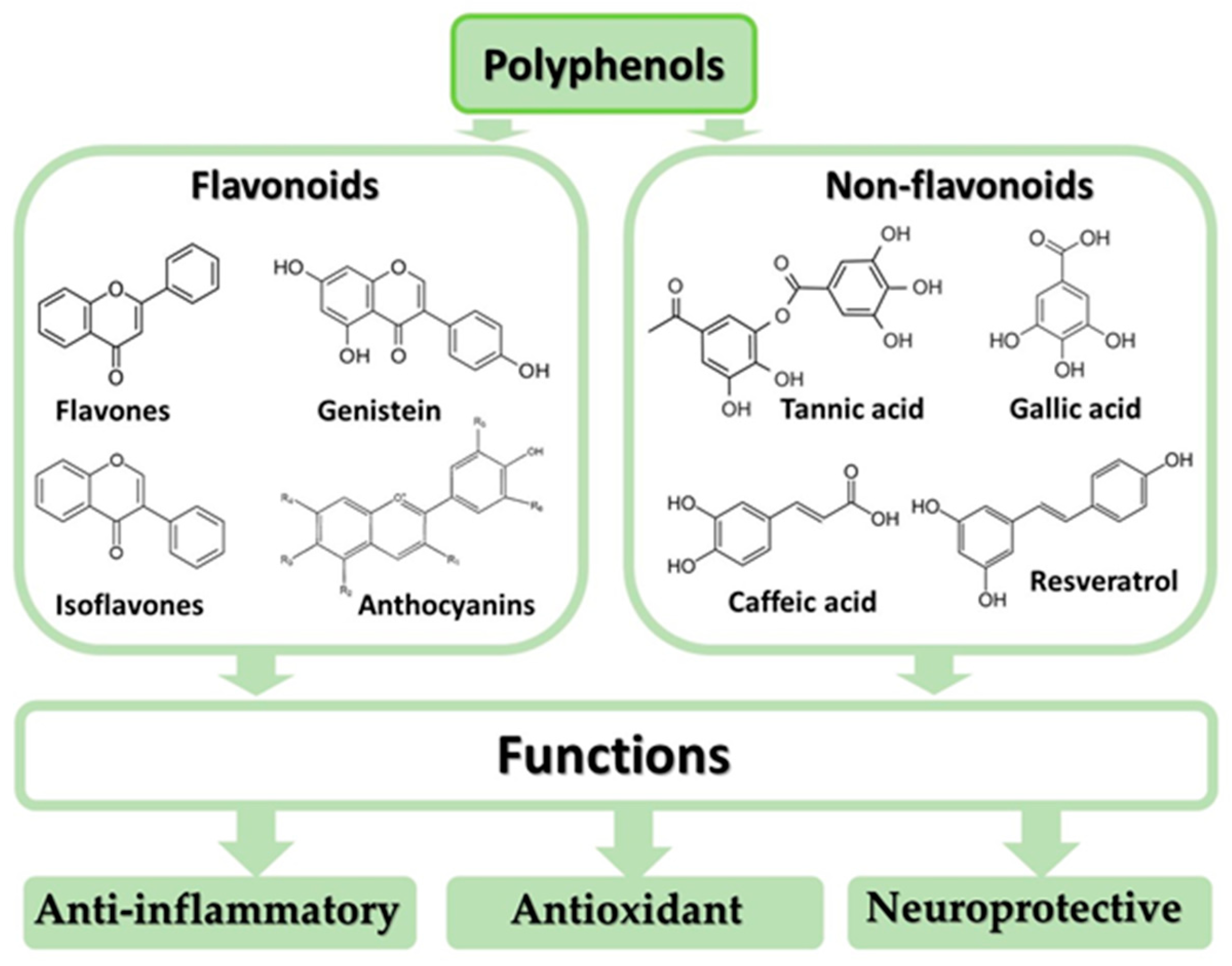
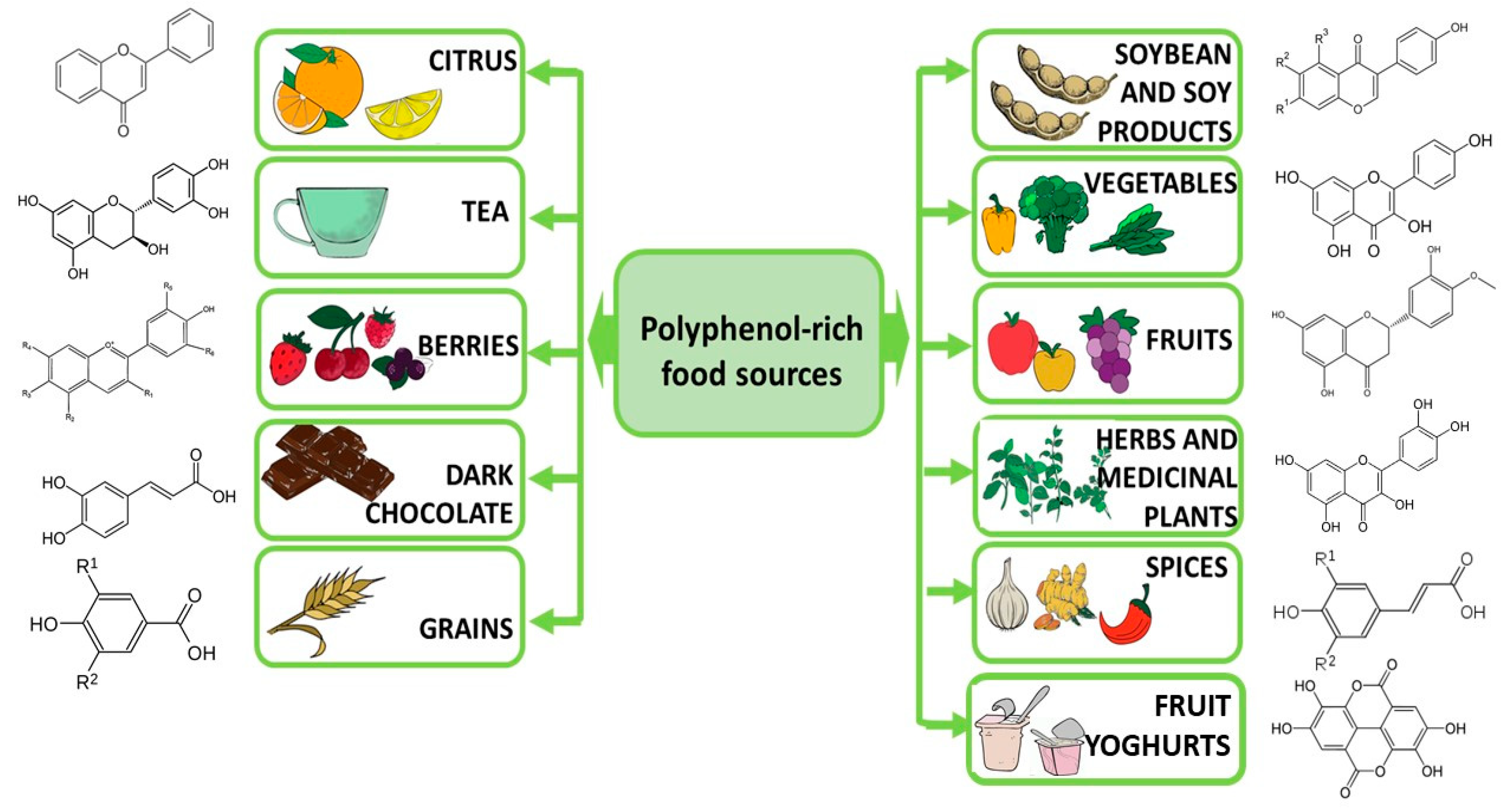
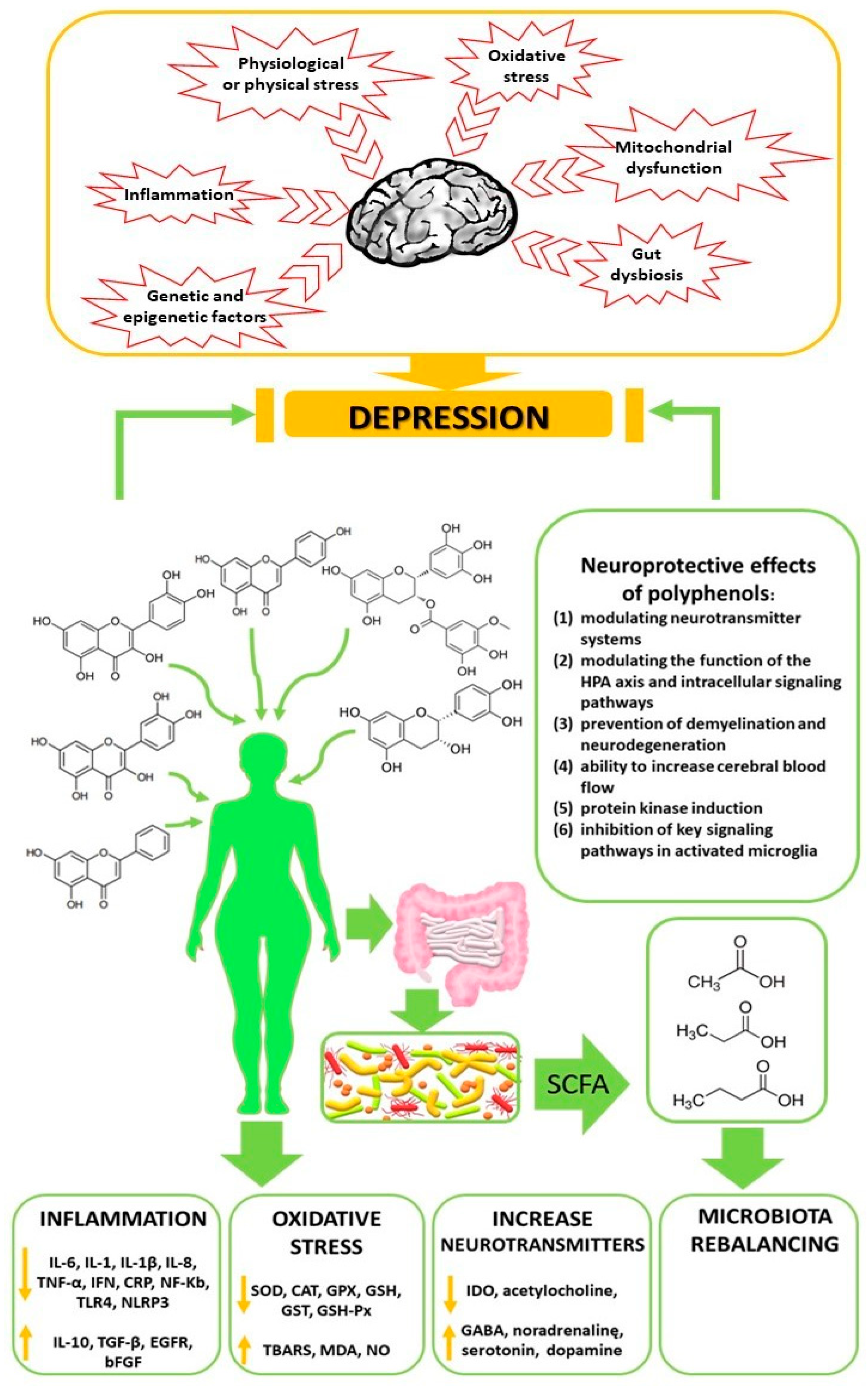
| Characteristic | Place of Collection | Antioxidant Capacity | Inflammation Parameters | Peroxidation Biomarkers | References |
|---|---|---|---|---|---|
| Control n = 20 Depression n = 40 | Serum | ↓ Vitamin A; ↓ Vitamin C; ↓ Vitamin E | [5] | ||
| Control n = 38 Depression n = 42 Generalized anxiety n = 37 | Plasma | ↓ Vitamin E | ↑ MDA | [16] | |
| Control n = 19 Depression n = 15 | Serum | ↓ GPX; ↓ SOD; ↑ RGSH | ↑ MDA | [17] | |
| Control n = 12 Depression n = 12 | Brain | ↓ SOD; ↓ CAT; ↓ GPX | [18] | ||
| Control n = 1484 Depression n = 2477 | Serum | ↑ SOD; ↓ uric acid; ↓ Zn | ↑ MDA | [19] | |
| Control n = 1788 Depression n = 1979 | Serum | ↑ GST; ↑ CAT; ↓ GSH; ↑ nitrites; ↑ uric acid; ↑ TBARS; ≈SOD; ≈GPX; ≈Zn | ↑ MDA | [20] | |
| Control n = 36 Depression n = 18 | Urine | ↑ F2 isoprostanes | [21] | ||
| Control n = 30 Depression n = 60 | Whole-blood | ↓ SOD; ↓ GPX; ↑ adenosine deaminase | [22] | ||
| Control n = 40 Depression n =30 | Plasma | ↓ GSH; ↓ RGSH; ↓ CAT; ↓ SOD; ≈GPX | [23] | ||
| Control n = 20 Depression n = 58 | Serum | ≈ SOD; ≈ CAT; ↓ GPX | [24] | ||
| Control n = 22 Depression n =55 | Serum | ↑ TBARS | [25] | ||
| Control n = 35 Depression n = 73 | Serum | ↑ TBARS | [26] | ||
| Control n = 16 Depression n = 16 | Human dermal fibroblast cultures | ↑ PC; ↑ RGSH | [27] | ||
| Control n = 20 Depression n = 20 | Plasma | ≈8-iso-PGF2a, (+) correlation with IL-6 (–) correlation with IL-10 | [28] | ||
| Control n = 13 Depression n = 51 (‘high-risk’, n = 15, ‘ultra-high-risk’, n = 20, mixed or manic symptoms that received, n = 16) | Serum | ↓ LPH | [29] | ||
| Control n = 612 Depression n = 2833 (depressive = 1619, remitted = 610, anxiety = 604) | Plasma | ↓ 8-OHdG; ≈F2-isoprostanes | [30] | ||
| Control n = 27 Depression n = 22 | Serum | ↑ PC; ↑ NADPH oxidase; ≈CAT; ≈SOD; ≈GPX | [31] | ||
| Control n = 10 Depression n = 14 | RBC | ≈SOD1; ≈SOD2; ↑ NADPH oxidase; ↑ ROS + RNS | ≈ oxidized LDL | [32] | |
| Depression = 39 Control = 31 | Brain | ↓ GSH | [33] | ||
| Depression = 19 Control = 8 | Brain | ↓ OCC; ↓ GSH, | [34] | ||
| Control n = 94 Depression n = 55 | Serum | ↑ PC; ≈TBARS | [35] |
| Polyphenols | Oxidative Stress Parameters | Inflammation Parameters | Place of Collection | References |
|---|---|---|---|---|
| Curcumin | No significant effects on blood chemistry | Blood | [147] | |
| Poliphenols | ↓ DTAC; ↓ Vitamin C; ↓ Vitamin A; ↓ Vitamin E | DTAC was calculated | [130] | |
| Curcumin | ↓IL-1β, ↓TNF-α; ↓BDNF | Serum | [148] | |
| Curcumin | ↑ MDA; ↑ TAC | Serum | [149] |
| Polyphenols | Animal Species | Target Sites | Antioxidant Factor | Oxidative Stress Parameters | Inflammation Parameters | Ref. |
|---|---|---|---|---|---|---|
| Curcumin | Sprague- Dawley rats | Hippocampus Serum | CUMS | ↓ NOx2; ↓ 4-HNE; ↓ MDA; ↑ CAT | [150] | |
| Curcumin | Rats | Brain | Intracerebroventricular injection of propanoic acid to induce autistic behavior | ↓ TBARS; ↑ CAT; ↑ SOD; ↑ GSH | [151] | |
| Curcumin | Male Wistar rats | The cortex and hippocampus | Reserpine treated | ↓ MAO | [152] | |
| Curcumin | Male mice | Hippocampus, frontal cortex, amygdala | Regular ICR | ↓ MAO-A | [153] | |
| Curcumin | Male rats | Brain | LPS administration | ↓COX-2 | [154] | |
| Curcumin | Female albino rats | Serum | OVX | ↓ MAO-B; ↑ tyrosine Hydroxylase; ↓ NO; ↑ TAC; ↓ MDA | ↓IL-1β; ↓IL-6 | [155] |
| Curcumin | Male Swiss mice | Brain | CUMS | ↑ CAT | [156] | |
| Chrysin | Mice | Brain | Male young and aged Swiss Albino mice were kept in groups during aging | ↑ SOD; ↑ CAT; ↑ GPX in PFC and hippocampus | [157] | |
| Chrysin | Male rats | Heart | Induced acute cardiotoxicity triggered by doxorubicin | ↑ GHS; ↑ CAT; ↑ SOD | [158] | |
| Chrysin | Male Swiss mice | Brain | The neurotoxicity elicited by aluminium chloride | ↓ LPO; ↑ SOD; ↑ CAT in cortex and hippocampus; ↓ ROS in neuronal SH-SY5Y and microglial THP-1 cells in vitro | [159] | |
| Hypericin | Wistar rats | Brain | Chronic psychosocial stress | ↓BDNF | [160] | |
| Dimethyl fumarate | Male Wistar rats | Testicular Serum | CUMS | ↓ MDA; ↑ GSH; ↑ TAC | [45] | |
| Dimethyl fumarate and resveratrol | Wistar rats | Brain Serum | CUMS | ↓ MDA ↑ TAC; ↑ GSH | ↑BDNF | [161] |
| Resveratrol | Male Wistar rats | Plasma | CUMS | ↑ TAC; ↑ GSH | ↑ TNF-α; ↑ IL-6; ↑ CRP | [162] |
| Hesperidin | Male Sprague- Dawley rats | Brain | CUMS | ↓ MDA; ↓ nitrite;↑ GSH; ↑ CAT, ↓ NOS; | ↓ TNF-α; ↑ IL-1β; ↓ COX-2 | [163] |
| Baicalin | Male Sprague- Dawley rats | Brain | CUMS | ↑ SOD; ↓ MDA; ↑ Bcl-2 protein; ↓ Bax | ↓ IL-1β; ↓ caspase-3 proteins; ↑ BDNF | [164] |
| Catechins | Sprague- Dawley rats | Brain | CUMS | ↑ CAT; ↑ SOD; ↑ GSH | [165] | |
| Hesperidin | Male mice | Serum | LPS | ↓ IL-1β; ↓ IL-6; ↓ TNF-α | [166] | |
| Quercetin | Male Swiss albino mice | Brain, plasma | CUMS | ↑ GSH; ↑ SOD;↑ CAT | ↓ IL-1β; ↓ TNF-α | [167] |
| Quercetin | Swiss albino mice | Brain, plasma | CUS | ↑ total thiol; ↑ CAT; ↓ TBARS; ↓ NOS; | ↓ IL-1β; ↓ IL-6; ↓ TNF-α; ↓ COX-2 | [168] |
| Quercetin | Male Sprague-Dawley rats | Serum | CUMS | ↑ SOD; ↑ CAT; ↑ GSH; ↑ GPX; ↓ MAO | ↓ IL-1β; ↓ TNF-α | [169] |
| Quercetin | Male Wistar rats | Brain | CUMS | ↓ MDA; ↓ nitrite; ↑ GSH; ↑ SOD | ↓ TNF-α; ↓ IL-6; ↓ iNOS | [170] |
| Ferulic acid | Male mice | Brain | Reserpine | ↑ SOD; ↑ GSH; ↓ nitrite; ↓ LPO | ↓ IL-1β; ↓ TNF-α | [171] |
| Salvianolic acid B | Male albino Wistar rats | Brain | CMS | ↑ CAT; ↑ SOD; ↑ GPX, ↓ MDA | ↓ IL-6; ↓ IL-1β; ↓ TNF-α; ↓ NLRP3 | [172] |
| Resveratrol | Male Wistar rats | Plasma | CUMS | ↑ TAC; ↑ GSH | ↑ TNF-α; ↑ IL-Iβ; ↑ CRP | [162] |
| Ginsenoside | Mice | Brain | Behavioral tests (forced swimming, tail suspension) | ↑ SOD | [173] | |
| Apocynum venetum leaf extract | Wistar rats | Hippocampus, serum | CUMS | ↓ ROS; ↓ MDA; ↑ SOD; ↑ CAT; ↑GPX; | ↓ Bcl-2/Bax; ↑ BDNF | [174] |
| Hemerocallis citrina Baroni | Sprague- Dawley rats | Brain | CUMS | ↓ MDA | ↑ BDNF | [175] |
| Silybum marianum (silymarin) | Swiss albino mice | Brain | Acute restraint stress induced by immobilizing | ↓ MDA; ↑ SOD; ↑ CAT; ↑ GSH | [176] | |
| Silybum marianum (silymarin) | Wistar rats | Brain | Olfactory bulbectomized technique | ↓ MDA | ↑ BDNF | [177] |
| Resveratrol | Sprague- Dawley rats | Brain | CUMS | ↓ MDA; ↑ SOD; ↑ CAT; ↑ GSH | [178] | |
| Jasminum sambac (Jasmine tea) | Sprague- Dawley rats | Brain | CUMS | ↑ GLP-1 | ↑ BDNF | [179] |
| Tea polyphenols | Mice | Brain | CUMS | ↓ MDA; ↑ SOD; ↑ CAT; ↑ GSH | [180] | |
| Okra seeds (catechin and quercetin derivatives) | Mice | Brain | Behavioral tests (open field, tail suspension, forced swimming, novelty suppressed feeding | ↓ MDA; ↑ TAC; ↑ SOD | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. https://doi.org/10.3390/ijms24032258
Winiarska-Mieczan A, Kwiecień M, Jachimowicz-Rogowska K, Donaldson J, Tomaszewska E, Baranowska-Wójcik E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. International Journal of Molecular Sciences. 2023; 24(3):2258. https://doi.org/10.3390/ijms24032258
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Małgorzata Kwiecień, Karolina Jachimowicz-Rogowska, Janine Donaldson, Ewa Tomaszewska, and Ewa Baranowska-Wójcik. 2023. "Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders" International Journal of Molecular Sciences 24, no. 3: 2258. https://doi.org/10.3390/ijms24032258
APA StyleWiniarska-Mieczan, A., Kwiecień, M., Jachimowicz-Rogowska, K., Donaldson, J., Tomaszewska, E., & Baranowska-Wójcik, E. (2023). Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. International Journal of Molecular Sciences, 24(3), 2258. https://doi.org/10.3390/ijms24032258







