Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients
Abstract
1. Introduction
2. Results
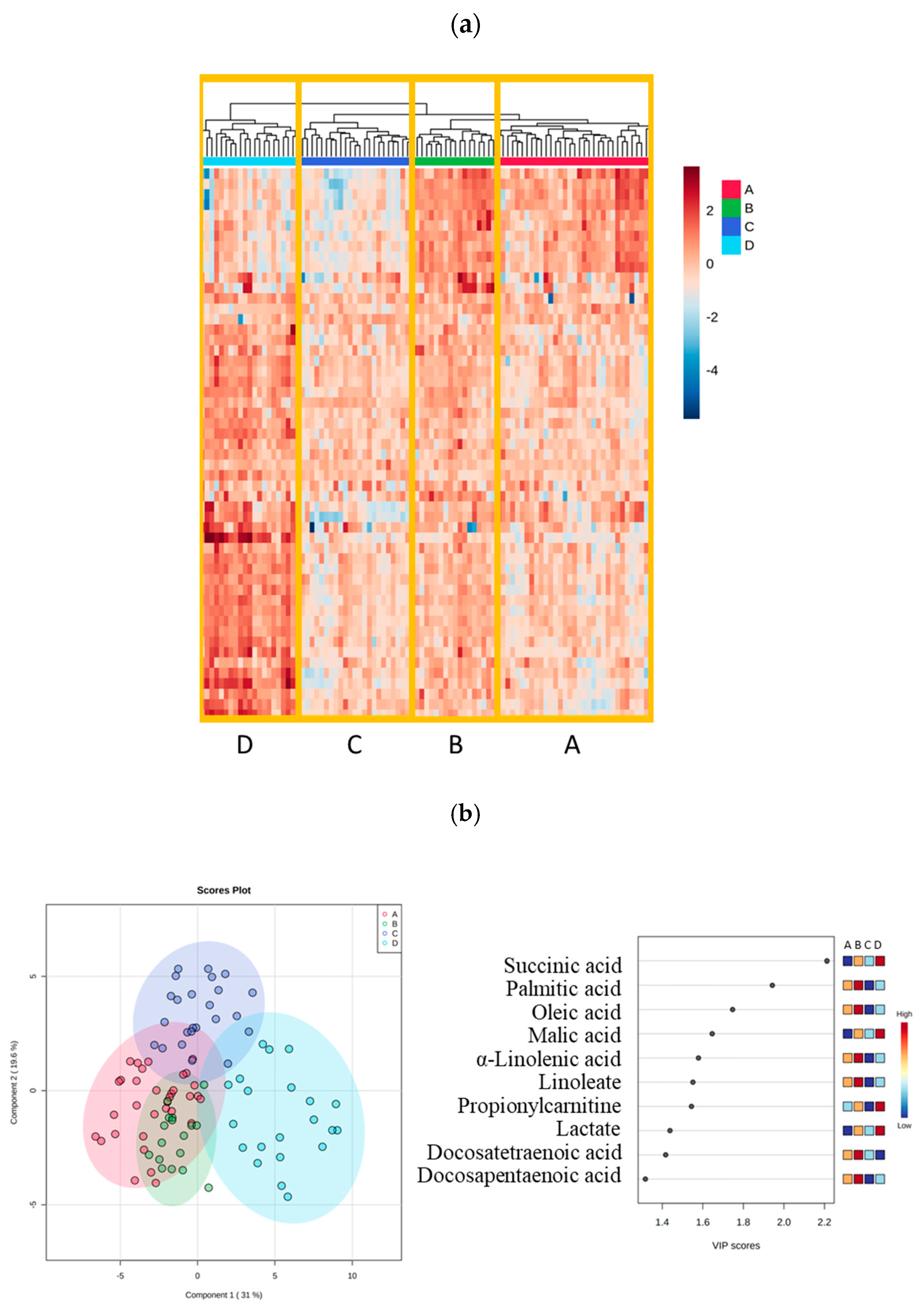
2.1. Four Phenotypes in Trauma Patients
2.2. Clinical Characteristics of the Four Phenotypes
2.3. Catecholamine and Endothelial Biomarkers
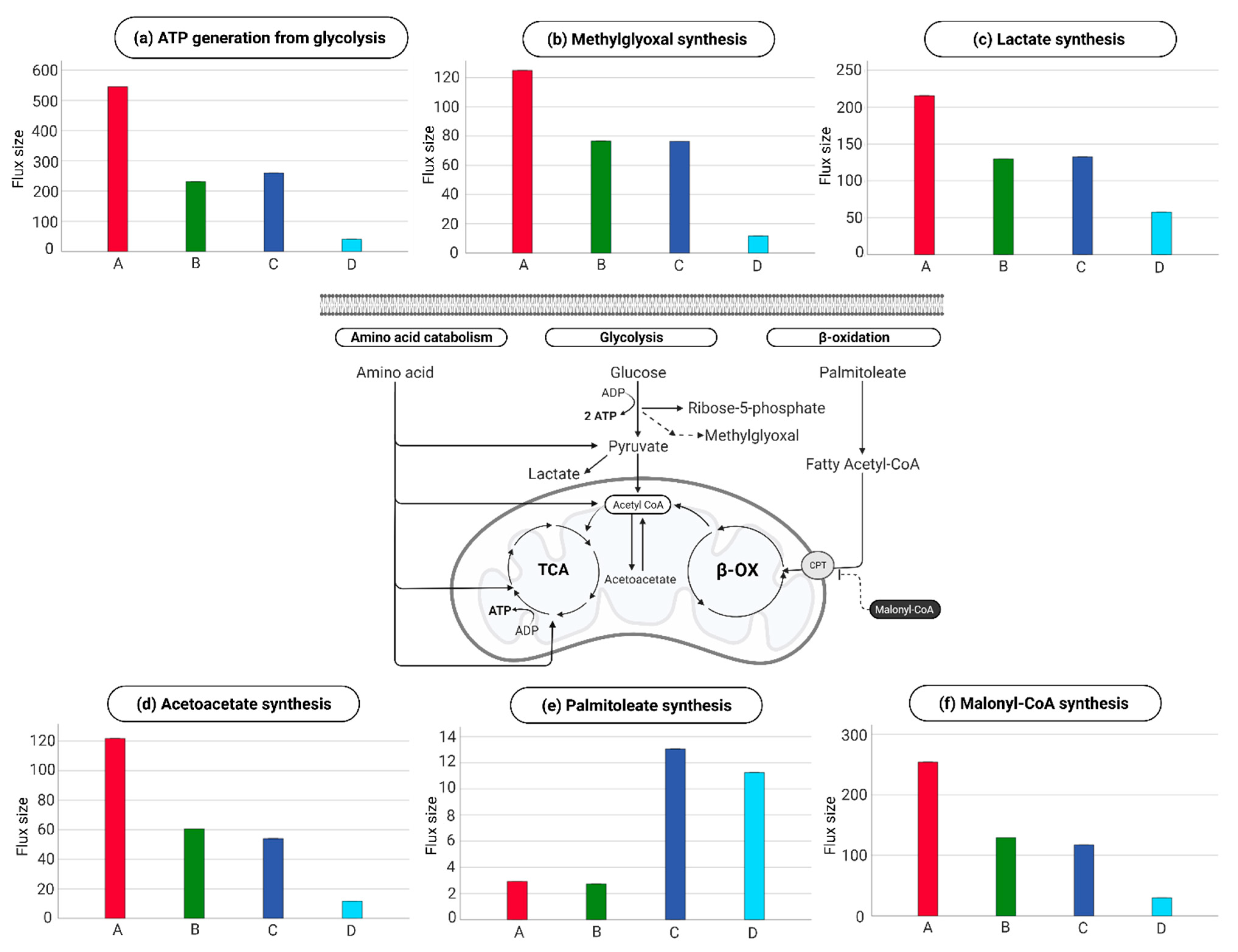
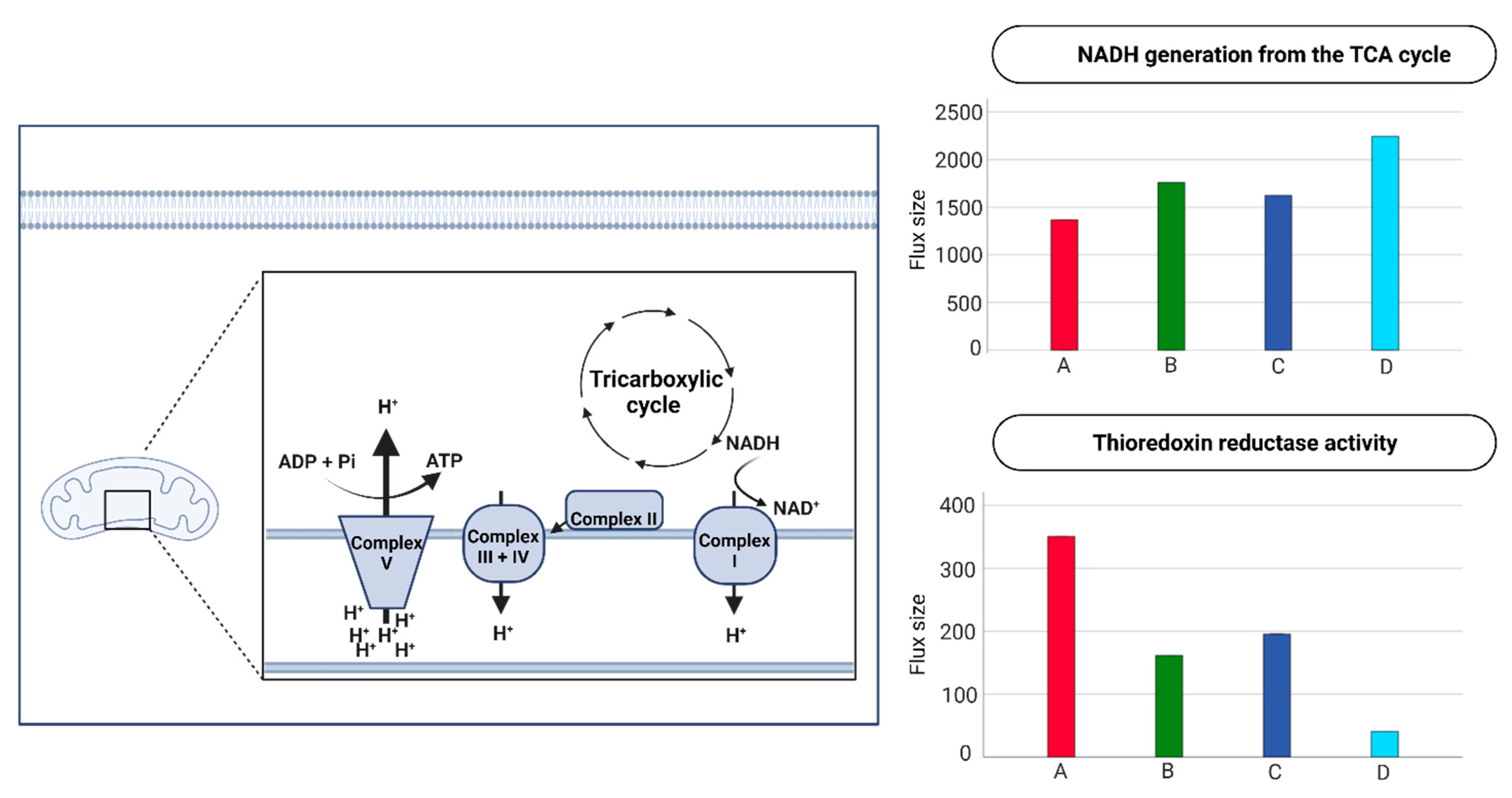
2.4. Endothelial Cell Metabolism in the Four Metabolic Phenotypes
3. Discussion
4. Materials and Methods
4.1. Setting and Patients
4.2. Patient Selection
4.3. Healthy Volunteers
4.4. Analysis of Clinical Characteristics
4.5. Sample Preparation
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Mass Spectrometry Analysis
4.8. Analysis of Mass Spectrometry Data
4.9. Analysis of Data with iEC3006 Genome-Scale Metabolic Model
4.10. Validation of GEMs Reconstruction
4.11. Inferring Metabolic Task Activity in Trauma Groups via GEMs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| ATP | Adenosine triphosphate |
| EC | Endothelial cell |
| EC-GEM | Genome-scale metabolic model of the endothelial cell |
| EoT | EoT Endotheliopathy of Trauma |
| GEMs | Genome-scale metabolic models |
| ISS | Injury Severity Score |
| NADH | Nicotinamide adenine dinucleotide + hydrogen |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least-Squares Discriminant Analysis |
| SHINE | Shock-induced endotheliopathy |
| sTM | Soluble thrombomodulin |
| TCA | Tricarboxylic acid cycle |
References
- Available online: https://www.cdc.gov/nchs/fastats/accidental-injury.htm (accessed on 7 November 2022).
- DiMaggio, C.; Ayoung-Chee, P.; Shinseki, M.; Wilson, C.; Marshall, G.; Lee, D.C.; Wall, S.; Maulana, S.; Leon Pachter, H.; Frangos, S. Traumatic injury in the United States: In-patient epidemiology 2000–2011. Injury 2016, 47, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.; Kobusingye, O. Injuries. N. Engl. J. Med. 2013, 368, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef]
- Johansson, P.I.; Henriksen, H.H.; Stensballe, J.; Gybel-Brask, M.; Cardenas, J.C.; Baer, L.A.; Cotton, B.A.; Holcomb, J.B.; Wade, C.E.; Ostrowski, S.R. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann. Surg. 2017, 265, 597–603. [Google Scholar] [CrossRef]
- Johansson, P.I.; Ostrowski, S.R. Acute coagulopathy of trauma: Balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med. Hypotheses 2010, 75, 564–567. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; Schultz, M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin. Immunopathol. 2012, 34, 167–179. [Google Scholar] [CrossRef]
- Neumar, R.W.; Nolan, J.P.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008, 118, 2452–2483. [Google Scholar] [CrossRef]
- McGarrity, S.; Anuforo, Ó.; Halldórsson, H.; Bergmann, A.; Halldórsson, S.; Palsson, S.; Henriksen, H.H.; Johansson, P.I.; Rolfsson, Ó. Metabolic systems analysis of LPS induced endothelial dysfunction applied to sepsis patient stratification. Sci. Rep. 2018, 8, 6811. [Google Scholar] [CrossRef]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. BJA Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef]
- White, N.J.; Ward, K.R.; Pati, S.; Strandenes, G.; Cap, A.P. Hemorrhagic blood failure: Oxygen debt, coagulopathy, and endothelial damage. J. Trauma Acute Care Surg. 2017, 82, S41–S49. [Google Scholar] [CrossRef]
- Henriksen, H.H.; McGarrity, S.; SigurÐardóttir, R.S.; Nemkov, T.; D’Alessandro, A.; Palsson, B.O.; Stensballe, J.; Wade, C.E.; Rolfsson, Ó.; Johansson, P.I. Metabolic Systems Analysis of Shock-Induced Endotheliopathy (SHINE) in Trauma: A New Research Paradigm. Ann. Surg. 2020, 272, 1140–1148. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Moore, H.B.; Moore, E.E.; Reisz, J.A.; Wither, M.J.; Ghasasbyan, A.; Chandler, J.; Silliman, C.C.; Hansen, K.C.; Banerjee, A. Plasma succinate is a predictor of mortality in critically injured patients. J. Trauma Acute Care Surg. 2017, 83, 491–495. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Krützfeldt, A.; Spahr, R.; Mertens, S.; Siegmund, B.; Piper, H.M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell. Cardiol. 1990, 22, 1393–1404. [Google Scholar] [CrossRef]
- Hunt, T.K.; Aslam, R.S.; Beckert, S.; Wagner, S.; Ghani, Q.P.; Hussain, M.Z.; Roy, S.; Sen, C.K. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid. Redox Signal. 2007, 9, 1115–1124. [Google Scholar] [CrossRef]
- Ruan, G.X.; Kazlauskas, A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J. Biol. Chem. 2013, 288, 21161–21172. [Google Scholar] [CrossRef]
- Sonveaux, P.; Copetti, T.; de Saedeleer, C.J.; Végran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frérart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Schulman, C.I.; Uribarri, J.; Cai, W.; Manning, R.; Landy, D.C.; Gallardo, M.; Castillo, A.; Namias, N.; Striker, G.E.; Livingstone, A.; et al. Increased circulating advanced glycation endproducts (AGEs) in acute trauma patients. Clin. Chem. Lab. Med. 2014, 52, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Saha, A.K.; Kraegen, E.W. Minireview: Malonyl CoA, AMP-Activated Protein Kinase, and Adiposity. Endocrinology 2003, 144, 5166–5171. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Olenev, V.I.; Suslova, T.B.; Cheremisina, Z.P. Lipid peroxidation in mitochondrial membrane. Adv. Lipid. Res. 1980, 17, 173–249. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Marín de Mas, I.; Torrents, L.; Bedia, C.; Nielsen, L.K.; Cascante, M.; Tauler, R. Stoichiometric gene-to-reaction associations enhance model-driven analysis performance: Metabolic response to chronic exposure to Aldrin in prostate cancer. BMC Genom. 2019, 20, 652. [Google Scholar] [CrossRef]
- Thomas, I.; Dickens, A.M.; Posti, J.P.; Czeiter, E.; Duberg, D.; Sinioja, T.; Kråkström, M.; Retel Helmrich, I.R.A.; Wang, K.K.W.; Maas, A.I.R.; et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat. Commun. 2022, 13, 2545. [Google Scholar] [CrossRef]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef]
- Ishii, H.; Uchiyama, H.; Kazama, M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. J. Thromb. Haemost. 1991, 65, 618–623. [Google Scholar] [CrossRef]
- Blann, A.D. Endothelial cell activation, injury, damage and dysfunction: Separate entities or mutual terms? Blood Coagul Fibrinolysis 2000, 11, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.; Seigneur, M. Soluble markers of endothelial cell function. Clin. Hemorheol. Microcirc. 1997, 17, 3–11. [Google Scholar] [PubMed]
- Gonzalez Rodriguez, E.; Ostrowski, S.R.; Cardenas, J.C.; Baer, L.A.; Tomasek, J.S.; Henriksen, H.H.; Stensballe, J.; Cotton, B.A.; Holcomb, J.B.; Johansson, P.I.; et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J. Am. Coll. Surg. 2017, 225, 419–427. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Slaughter, A.L.; Peltz, E.D.; Moore, E.E.; Silliman, C.C.; Wither, M.; Nemkov, T.; Bacon, A.W.; Fragoso, M.; Banerjee, A.; et al. Trauma/hemorrhagic shock instigates aberrant metabolic flux through glycolytic pathways, as revealed by preliminary (13)C-glucose labeling metabolomics. J. Transl. Med. 2015, 13, 253. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Moore, H.B.; Moore, E.E.; Wither, M.; Nydam, T.; Slaughter, A.; Silliman, C.C.; Banerjee, A.; Hansen, K.C. Metabolomics of trauma-associated death: Shared and fluid-specific features of human plasma vs lymph. Blood Transfus. 2016, 14, 185–194. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Moore, H.B.; Moore, E.E.; Wither, M.; Nemkov, T.; Gonzalez, E.; Slaughter, A.; Fragoso, M.; Hansen, K.C.; Silliman, C.C.; et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R1034–R1044. [Google Scholar] [CrossRef]
- Clendenen, N.; Nunns, G.R.; Moore, E.E.; Reisz, J.A.; Gonzalez, E.; Peltz, E.; Silliman, C.C.; Fragoso, M.; Nemkov, T.; Wither, M.J.; et al. Hemorrhagic shock and tissue injury drive distinct plasma metabolome derangements in swine. J. Trauma Acute Care Surg. 2017, 83, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Hansen, K.C.; D’Alessandro, A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass. Spectrom. 2017, 31, 663–673. [Google Scholar] [CrossRef]
- Catalin, E.; Doneanu, W.C.; Mazzeo, J.R. UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes. Waters Appl. Note 2011, 720004042en. [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Buhlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, H.H.; Marín de Mas, I.; Herand, H.; Krocker, J.; Wade, C.E.; Johansson, P.I. Metabolic systems analysis identifies a novel mechanism contributing to shock in patients with Endotheliopathy of Trauma (EoT) involving thromboxaneA2 and LTC4. Matrix Biol. Plus 2022, in press. [Google Scholar] [CrossRef]
- Airola, A.; Pahikkala, T.; Waegeman, W.; de Baets, B.; Salakoski, T. An experimental comparison of cross-validation techniques for estimating the area under the ROC curve. Comput. Stat. Data Anal. 2011, 55, 1828–1844. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Richelle, A.; Kellman, B.P.; Wenzel, A.T.; Chiang, A.W.T.; Reagan, T.; Gutierrez, J.M.; Joshi, C.; Li, S.; Liu, J.K.; Masson, H.; et al. What does your cell really do? Model-based assessment of mammalian cells metabolic functionalities using omics data. bioRxiv 2020. [Google Scholar] [CrossRef]

| Phenotype D | Phenotype C | Phenotype B | Phenotype A | |||
|---|---|---|---|---|---|---|
| (n = 21) | (n = 24) | (n = 17) | (n = 33) | p-Value | ||
| Demography | ||||||
| Age | Years | 43.0 [28.0, 50.0] | 36.0 [30.0, 46.0] | 45.0 [37.0, 54.0] | 50.0 [41.0, 60.0] | 0.021 |
| Sex | Male (%) | 19 (90.5%) | 15 (62.5%) | 13 (76.5%) | 23 (69.7%) | 0.176 |
| Race | Race [n (%)] | White = 4 (19.0%) African American = 7 (33.3%) Hispanic = 7 (33.3%) Asian = 0 (0%) Other = 3 (14.3%) | White = 8 (33.3%) African American = 7 (29.2%) Hispanic = 6 (25.0%) Asian = 0 (0%) Other = 3 (12.5%) | White = 5 (29.4%) African American = 1 (5.9%) Hispanic = 7 (41.2%) Asian = 2 (11.8%) Other = 2 (11.8%) | White = 20 (60.6%) African American = 10 (30.3%) Hispanic = 2 (6.1%) Asian = 0 (0%) Other = 1 (3.0%) | 0.004 |
| BMI | Score | 26.7 [25.0, 30.1] | 27.1 [24.8, 28.8] | 29.5 [28.1, 33.7] | 26.8 [24.7, 31.3] | 0.229 |
| Injury type and severity | ||||||
| ISS | Score | 34.0 [25.0, 45.0] | 25.0 [9.75, 29.0] | 25.0 [22.0, 29.0] | 21.0 [9.00, 25.0] | <0.001 |
| AIS Head | Score | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0.175 |
| AIS Face | Score | 0 [0, 0] | 0 [0, 0] | 0 [0, 1] | 0 [0, 0] | 0.027 |
| AIS Thorax | Score | 3.00 [3.00, 4.00] | 1.50 [0, 3.00] | 2.00 [0, 3.00] | 0 [0, 3.00] | 0.010 |
| AIS Abdomen | Score | 4.00 [3.00, 4.00] | 0 [0, 2.25] | 0 [0, 2.00] | 0 [0, 2.00] | 0.001 |
| AIS Extremity | Score | 3.00 [0, 4.00] | 2.50 [0, 3.00] | 0 [0, 2.00] | 0 [0, 0] | 0.011 |
| AIS External | Score | 1.00 [1.00, 1.00] | 1.00 [0.750, 2.75] | 1.00 [0, 5.00] | 1.00 [0, 2.00] | 0.835 |
| GCS | Score | 3.00 [3.00, 13.0] | 14.5 [3.00, 15.0] | 15.0 [3.00, 15.0] | 15.0 [7.00, 15.0] | 0.028 |
| Admission median blood pressure | ||||||
| SBP | mmHg | 98.0 [84.0, 114] | 111 [102, 140] | 119 [102, 132] | 132 [118, 142 | <0.001 |
| Heart rate | Bpm | 112 [98.0, 120] | 101 [92.0, 111] | 93.0 [84.0, 109] | 96.0 [73.5, 115] | 0.171 |
| Blood variables | ||||||
| Base excess | mEq/L | −13.0 [−16.0, −9.00] | −6.00 [−8.25, −2.00] | −3.00 [−8.00, −2.00] | −5.00 [−7.00, −2.00] | <0.001 |
| Lactate | mg/dL | 9.80 [6.85, 12.9] | 3.70 [2.75, 4.45] | 3.70 [3.10, 5.70] | 2.30 [1.60, 3.55] | <0.001 |
| Glucose | mg/dL | 229 [199, 324] | 145 [119, 165] | 173 [145, 213] | 134 [115, 196] | <0.001 |
| Transfusions pre-hospital | ||||||
| Transfused pre-hospital? | Yes [n (%)] | 6 (28.6%) | 2 (8.3%) | 3 (17.6%) | 8 (24.2%) | 0.329 |
| if yes: | ||||||
| RBC | Units | 0.500 [0, 1.00] | 0.500 [0.250, 0.750] | 0 [0, 0.500] | 1.00 [1.00, 1.50] | 0.056 |
| Plasma | Units | 1.00 [1.00, 1.00] | 0.500 [0.250, 0.750] | 1.00 [0.500, 1.00] | 1.00 [0.750, 1.25] | 0.704 |
| Whole blood | Units | 0 [0, 0] | 0.500 [0.250, 0.750] | 0 [0, 0.500] | 0 [0, 0] | 0.336 |
| Transfusions after admission | ||||||
| Transfused within 4 h? | Yes [n (%)] | 19 (90.5%) | 13 (54.2%) | 8 (47.1%) | 15 (45.5%) | 0.007 |
| if yes: | ||||||
| RBC | Units | 12.0 [5.00, 32.0] | 2.00 [1.00, 5.00] | 1.50 [0.750, 2.25] | 2.00 [2.00, 3.50] | <0.001 |
| Plasma | Units | 14.0 [4.00, 32.0] | 4.00 [1.00, 8.00] | 1.00 [1.00, 2.25] | 3.00 [1.00, 4.00] | <0.001 |
| Platelets | Units | 12.0 [0, 21.0] | 0 [0, 6.00] | 0 [0, 0] | 0 [0, 0] | 0.007 |
| Transfused within 24 h? | Yes [n (%)] | 19 (90.5%) | 16 (66.7%) | 11 (64.7%) | 21 (63.6%) | 0.156 |
| if yes: | ||||||
| RBC | Units | 14.0 [5.00, 35.0] | 2.00 [0, 5.75] | 1.00 [0, 2.00] | 2.00 [0, 3.00] | <0.001 |
| Plasma | Units | 17.0 [4.50, 35.0] | 5.00 [1.75, 10.3] | 2.00 [1.00, 4.00] | 4.00 [1.00, 9.00] | 0.001 |
| Platelets | Units | 12.0 [0, 24.0] | 0 [0, 1.50] | 0 [0, 0] | 0 [0, 0] | 0.003 |
| Outcome | ||||||
| Mortality (<24 h) | n (%) | 11 (52.4%) | 0 (0%) | 0 (0%) | 1 (3.0%) | <0.001 |
| Mortality (<72 h) | n (%) | 12 (57.1%) | 0 (0%) | 0 (0%) | 1 (3.0%) | <0.001 |
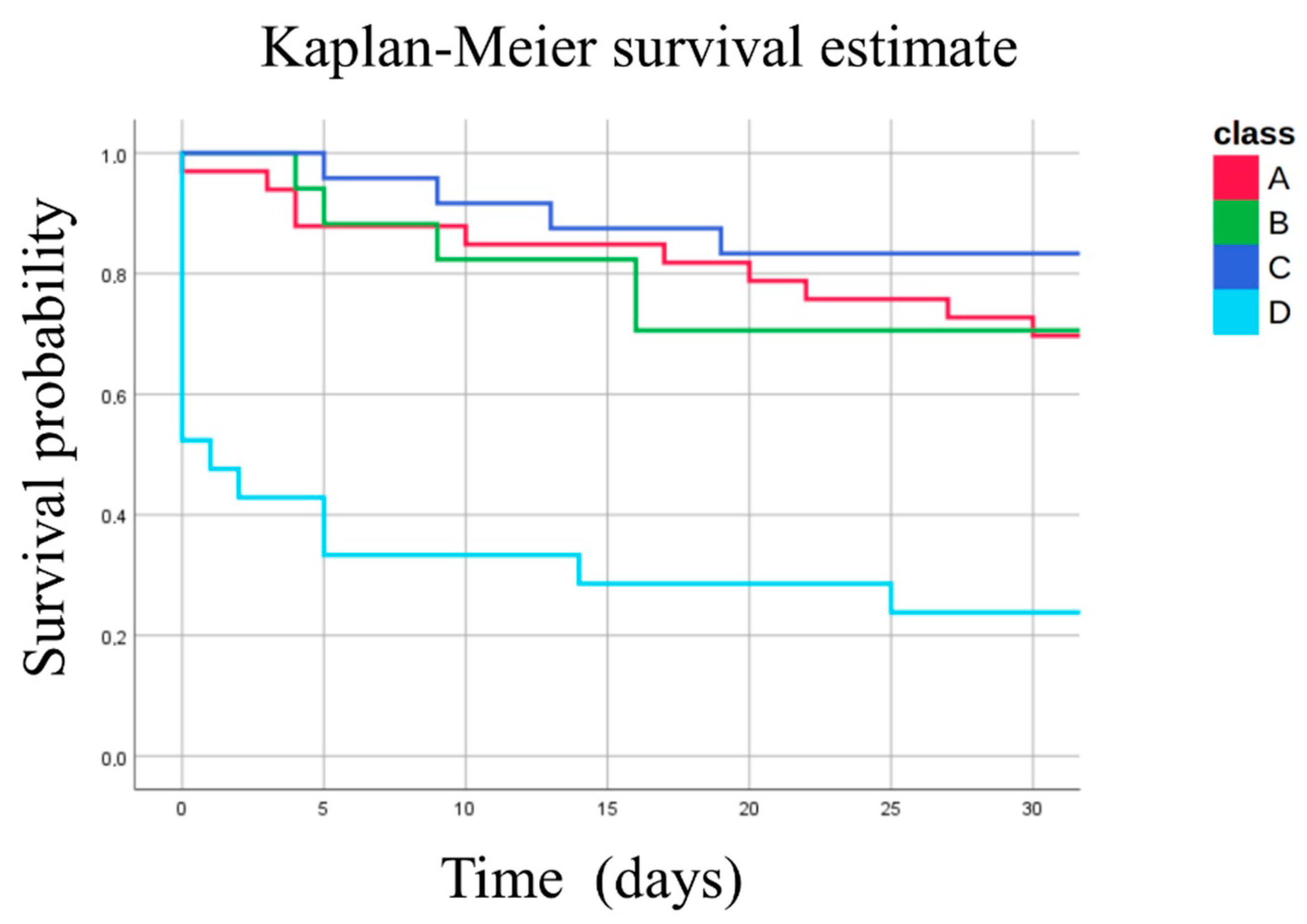
| Mortality (<30 days) | n (%) | 16 (76.2%) | 4 (16.7%) | 5 (29.4%) | 10 (30.3%) | <0.001 |
| Phenotype D | Phenotype C | Phenotype B | Phenotype A | |||
|---|---|---|---|---|---|---|
| ELISA | (n = 21) | (n = 24) | (n = 17) | (n = 33) | p-Value | |
| Epinephrine | pg/mL | 2240 [1110, 4320] | 230 [99.4, 710] | 262 [211, 363] | 271 [63.1, 426] | <0.001 |
| Norepinephrine | pg/mL | 3460 [1920, 13,100] | 741 [427, 1400] | 1180 [492, 3000] | 1180 [604, 1680] | <0.001 |
| sTM | ng/mL | 7.06 [6.01, 11.2] | 5.93 [5.01, 7.09] | 6.32 [5.15, 10.5] | 6.46 [5.15, 9.28] | 0.291 |
| Syndecan-1 | ng/mL | 190 [120, 198] | 42.3 [24.5, 120] | 49.5 [37.2, 166] | 34.4 [22.5, 101] | <0.001 |
| EoT | Yes [n (%)] | 19 (90.5%) | 13 (54.2%) | 10 (58.8%) | 16 (48.5%) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksen, H.H.; Marín de Mas, I.; Nielsen, L.K.; Krocker, J.; Stensballe, J.; Karvelsson, S.T.; Secher, N.H.; Rolfsson, Ó.; Wade, C.E.; Johansson, P.I. Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients. Int. J. Mol. Sci. 2023, 24, 2257. https://doi.org/10.3390/ijms24032257
Henriksen HH, Marín de Mas I, Nielsen LK, Krocker J, Stensballe J, Karvelsson ST, Secher NH, Rolfsson Ó, Wade CE, Johansson PI. Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients. International Journal of Molecular Sciences. 2023; 24(3):2257. https://doi.org/10.3390/ijms24032257
Chicago/Turabian StyleHenriksen, Hanne H., Igor Marín de Mas, Lars K. Nielsen, Joseph Krocker, Jakob Stensballe, Sigurður T. Karvelsson, Niels H. Secher, Óttar Rolfsson, Charles E. Wade, and Pär I. Johansson. 2023. "Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients" International Journal of Molecular Sciences 24, no. 3: 2257. https://doi.org/10.3390/ijms24032257
APA StyleHenriksen, H. H., Marín de Mas, I., Nielsen, L. K., Krocker, J., Stensballe, J., Karvelsson, S. T., Secher, N. H., Rolfsson, Ó., Wade, C. E., & Johansson, P. I. (2023). Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients. International Journal of Molecular Sciences, 24(3), 2257. https://doi.org/10.3390/ijms24032257







