S100B Affects Gut Microbiota Biodiversity
Abstract
:1. Introduction
2. Results
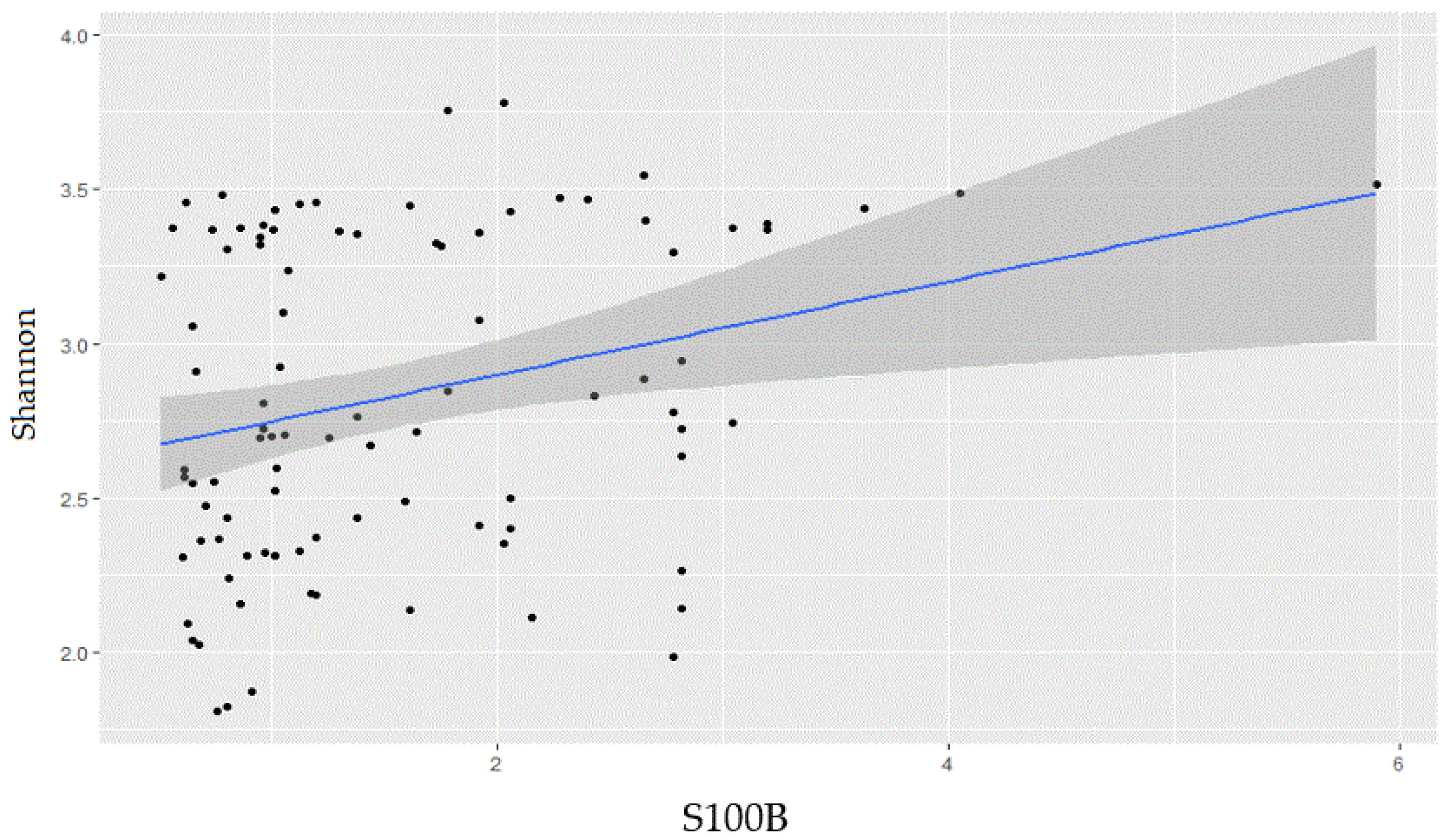
2.1. Gut Microbiota Biodiversity Increases with S100B Levels
2.2. S100B Effect on Microbiota Is Reduced by Pentamidine
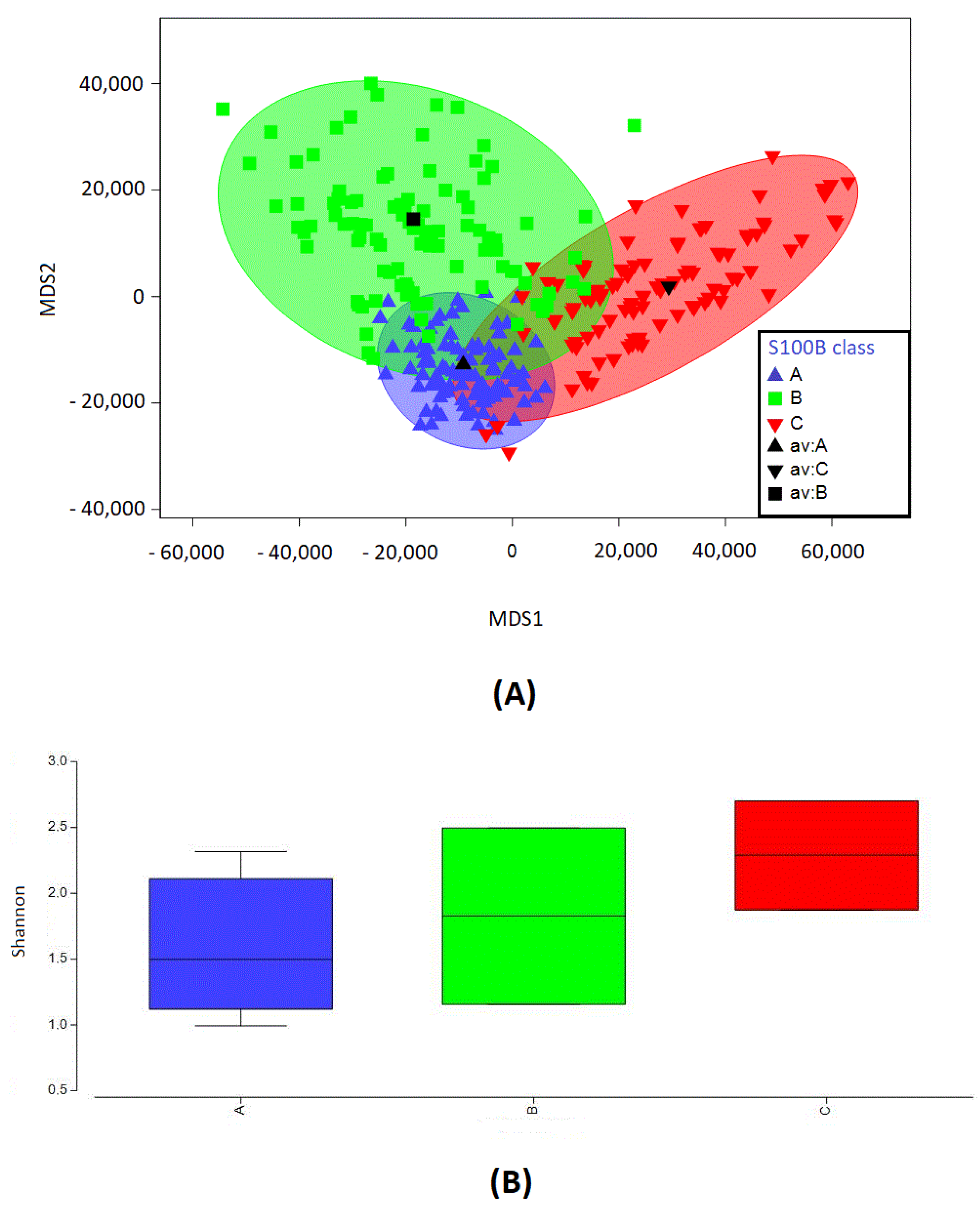
2.3. S100B Levels and Microbial Diversity Can Cluster into Three Groups
2.4. S100B Clustering and OTU
2.5. S100B Oral Administration Affects Microbiota
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Treatments
4.2. S100B ELISA Assay
4.3. DNA Extraction, 16S Ribosomal DNA (rDNA) Sequencing and Bioinformatics Analysis
4.4. Statistical Analysis and Bioinformatics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [Green Version]
- Michetti, F.; Di Sante, G.; Clementi, M.E.; Sampaolese, B.; Casalbore, P.; Volonté, C.; Romano Spica, V.; Parnigotto, P.P.; Di Liddo, R.; Amadio, S.; et al. Growing role of S100B protein as a putative therapeutic target for neurological- and nonneurological-disorders. Neurosci. Biobehav. Rev. 2021, 127, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Kwong, E.; Bajwa, E.; Klegeris, A. Resolution-Associated Molecular Patterns (RAMPs) as Endogenous Regulators of Glia Functions in Neuroinflammatory Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cirillo, C.; Sarnelli, G.; De Filippis, D.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; Petruzzelli, R.; Grosso, M.; Izzo, P.; et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 2007, 133, 918–925. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Sarnelli, G.; Scuderi, C.; Cirillo, C.; Cuomo, R.; Steardo, L. The antiprotozoal drug pentamidine ameliorates experimentally induced acute colitis in mice. J. Neuroinflammation 2012, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, H.; Deng, X.; Song, Z.; Yang, Z.; Xiong, W.; Yuan, L.; Xu, H.; Deng, S.; Deng, H. Genetic analysis of the S100B gene in Chinese patients with Parkinson disease. Neurosci Lett 2013, 555, 134–136. [Google Scholar] [CrossRef]
- Kato, H.; Kurosaki, R.; Oki, C.; Araki, T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004, 1030, 66–73. [Google Scholar] [CrossRef]
- Gazzolo, D.; Monego, G.; Corvino, V.; Bruschettini, M.; Bruschettini, P.; Zelano, G.; Michetti, F. Human milk contains S100B protein. Biochim. Biophys. Acta 2003, 1619, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Frigiola, A.; Gagliardi, L.; Ciotti, S.; Bognanno, M.; Iacopino, A.M.; Nigro, F.; Tina, G.L.; Cavallaro, D.; Mussap, M.; et al. S100B milk concentration in mammalian species. Front. Biosci. 2009, 1, 542–546. [Google Scholar]
- Di Liddo, R.; Piccione, M.; Schrenk, S.; Dal Magro, C.; Cosma, C.; Padoan, A.; Contran, N.; Scapellato, M.L.; Pagetta, A.; Romano Spica, V.; et al. S100B as a new fecal biomarker of inflammatory bowel diseases. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 323–332. [Google Scholar] [PubMed]
- Celikbilek, A.; Celikbilek, M.; Sabah, S.; Tanık, N.; Borekci, E.; Dogan, S.; Akin, Y.; Baldane, S.; Deniz, K.; Yilmaz, N.; et al. The Serum S100B Level as a Biomarker of Enteroglial Activation in Patients with Ulcerative Colitis. Int. J. Inflam. 2014, 2014, 986525. [Google Scholar] [CrossRef] [Green Version]
- Juge, N. Relationship between mucosa-associated gut microbiota and human diseases. Biochem. Soc. Trans 2022, 50, 1225–1236. [Google Scholar] [CrossRef]
- Orsini, M.; Di Liddo, R.; Valeriani, F.; Mancin, M.; D’Incà, R.; Castagnetti, A.; Aceti, A.; Parnigotto, P.P.; Romano Spica, V.; Michetti, F. In Silico Evaluation of Putative S100B Interacting Proteins in Healthy and IBD Gut Microbiota. Cells 2020, 9, 1697. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction Between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Camp, J.G.; Frank, C.L.; Lickwar, C.R.; Guturu, H.; Rube, T.; Wenger, A.M.; Chen, J.; Bejerano, G.; Crawford, G.E.; Rawls, J.F. Microbiota Modulate Transcription in the Intestinal Epithelium Without Remodeling the Accessible Chromatin Landscape. Genome Res. 2014, 24, 1504–1516. [Google Scholar] [CrossRef] [Green Version]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The Influence of the Microbiome on Respiratory Health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; de la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Brantley Hall, A.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- McKnight, S.L. Back to the future: Molecular biology meets metabolism. Cold Spring Harb. Symp Quant. Biol. 2011, 76, 403–411. [Google Scholar] [CrossRef]
- Nigro, F.; Gagliardi, L.; Ciotti, S.; Galvano, F.; Pietri, A.; Tina, G.L.; Cavallaro, D.; La Fauci, L.; Iacopino, L.; Bognanno, M.; et al. S100B Protein concentration in milk-formulas for preterm and term infants. Correlation with industrial preparation procedures. Mol. Nutr. Food Res. 2008, 52, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.V.S.; Moura-Neto, V.; Bolick, D.T.; Guerrant, R.L.; Fawad, J.A.; Shin, J.H.; Medeiros, P.H.Q.S.; Ledwaba, S.E.; Kolling, G.L.; Martins, C.S.; et al. S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides difficile Infection by Modulating Inflammatory Response. Front. Cell Infect. Microbiol. 2021, 11, 739874. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddaert, J.; Bielen, K.; Jongers, B.; Manocha, E.; Yperzeele, L.; Cras, P.; Pirici, D.; Kumar-Singh, S. CD8 Signaling in Microglia/Macrophage M1 Polarization in a Rat Model of Cerebral Ischemia. PLoS ONE 2018, 13, e0186937. [Google Scholar] [CrossRef] [PubMed]
- Masetto Antunes, M.; Godoy, G.; Masi, L.N.; Curi, R.; Barbosa Bazotte, R. Prefrontal Cortex and Hippocampus Inflammation in Mice Fed High-Carbohydrate or High-Fat Diets. J. Med. Food 2022, 25, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, S.K.; Kosek, J.C.; Eng, L.F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: An immunohistochemical study using horseradish peroxidase-labelled antibodies. J. Comp. Neurol. 1976, 165, 197–207. [Google Scholar] [CrossRef]
- Brockes, J.P.; Fields, K.L.; Raff, M.C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979, 165, 105–118. [Google Scholar] [CrossRef]
- Didier, M.; Harandi, M.; Aguera, M.; Bancel, B.; Tardy, M.; Fages, C.; Calas, A.; Stagaard, M.; Møllgård, K.; Belin, M.F. Differential immunocytochemical staining for glial fibrillary acidic (GFA) protein, S-100 protein and glutamine synthetase in the rat subcommissural organ, nonspecialized ventricular ependyma and adjacent neuropil. Cell Tissue Res. 1986, 245, 343–351. [Google Scholar] [CrossRef]
- Rickmann, M.; Wolff, J.R. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience 1995, 67, 977–991. [Google Scholar] [CrossRef]
- Yang, Q.; Hamberger, A.; Hyden, H.; Wang, S.; Stigbrand, T.; Haglid, K.G. S-100 beta has a neuronal localisation in the rat hindbrain revealed by an antigen retrieval method. Brain Res. 1995, 696, 49–61. [Google Scholar] [CrossRef]
- Hosseinifard, E.S.; Morshedi, M.; Bavafa-Valenlia, K.; Saghafi-Asl, M. The novel insight into anti-inflammatory and anxiolytic effects of psychobiotics in diabetic rats: Possible link between gut microbiota and brain regions. Eur. J. Nutr. 2019, 58, 3361–3375. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Gasbarrini, A.; Mele, M.C. Food Additives, Gut Microbiota, and Irritable Bowel Syndrome: A Hidden Track. Int. J. Environ. Res. Public Health 2020, 17, 8816. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper From the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Camponeschi, C.; De Carluccio, M.; Amadio, S.; Clementi, M.E.; Sampaolese, B.; Volonté, C.; Tredicine, M.; Romano Spica, V.; Di Liddo, R.; Ria, F.; et al. S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021, 22, 13558. [Google Scholar] [CrossRef]
- Di Sante, G.; Amadio, S.; Sampaolese, B.; Clementi, M.E.; Valentini, M.; Volonté, C.; Casalbore, P.; Ria, F.; Michetti, F. The S100B Inhibitor Pentamidine Ameliorates Clinical Score and Neuropathology of Relapsing-Remitting Multiple Sclerosis Mouse Model. Cells 2020, 9, 748. [Google Scholar] [CrossRef] [Green Version]
- Marchese, E.; Di Maria, V.; Samengo, D.; Pani, G.; Michetti, F.; Geloso, M.C. Post-natal Deletion of Neuronal cAMP Responsive-Element Binding (CREB)-1 Promotes Pro-inflammatory Changes in the Mouse Hippocampus. Neurochem. Res. 2017, 42, 2230–2245. [Google Scholar] [CrossRef]
- Valeriani, F.; Agodi, A.; Casini, B.; Cristina, M.L.; D’Errico, M.M.; Gianfranceschi, G.; Liguori, G.; Liguori, R.; Mucci, N.; Mura, I.; et al. Potential testing of reprocessing procedures by real-time polymerase chain reaction: A multicenter study of colonoscopy devices. Am. J. Infect. Control 2018, 46, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Valeriani, F.; Crognale, S.; Protano, C.; Gianfranceschi, G.; Orsini, M.; Vitali, M.; Romano Spica, V. Metagenomic analysis of bacterial community in a travertine depositing hot spring. New Microbiol. 2018, 41, 126–135. [Google Scholar]
- Valeriani, F.; Protano, C.; Gianfranceschi, G.; Leoni, E.; Galasso, V.; Mucci, N.; Vitali, M.; Romano Spica, V. Microflora Thermarum Atlas project: Biodiversity in thermal spring waters and natural SPA pools. Water Sci. Technol. Water Supply 2018, 18, 1472–1483. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Studer, M. Validating Sequence Analysis Typologies Using Parametric Bootstrap. Sociol. Methodol. 2021, 51, 290–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bernau, C.; Parmigiani, G.; Waldron, L. The impact of different sources of heterogeneity on loss of accuracy from genomic prediction models. Biostatistics 2020, 21, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.2-0. 2014. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 1 January 2023).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano Spica, V.; Valeriani, F.; Orsini, M.; Clementi, M.E.; Seguella, L.; Gianfranceschi, G.; Di Liddo, R.; Di Sante, G.; Ubaldi, F.; Ria, F.; et al. S100B Affects Gut Microbiota Biodiversity. Int. J. Mol. Sci. 2023, 24, 2248. https://doi.org/10.3390/ijms24032248
Romano Spica V, Valeriani F, Orsini M, Clementi ME, Seguella L, Gianfranceschi G, Di Liddo R, Di Sante G, Ubaldi F, Ria F, et al. S100B Affects Gut Microbiota Biodiversity. International Journal of Molecular Sciences. 2023; 24(3):2248. https://doi.org/10.3390/ijms24032248
Chicago/Turabian StyleRomano Spica, Vincenzo, Federica Valeriani, Massimiliano Orsini, Maria Elisabetta Clementi, Luisa Seguella, Gianluca Gianfranceschi, Rosa Di Liddo, Gabriele Di Sante, Francesca Ubaldi, Francesco Ria, and et al. 2023. "S100B Affects Gut Microbiota Biodiversity" International Journal of Molecular Sciences 24, no. 3: 2248. https://doi.org/10.3390/ijms24032248
APA StyleRomano Spica, V., Valeriani, F., Orsini, M., Clementi, M. E., Seguella, L., Gianfranceschi, G., Di Liddo, R., Di Sante, G., Ubaldi, F., Ria, F., Esposito, G., & Michetti, F. (2023). S100B Affects Gut Microbiota Biodiversity. International Journal of Molecular Sciences, 24(3), 2248. https://doi.org/10.3390/ijms24032248











