Genome-Wide Analyses of Thaumatin-like Protein Family Genes Reveal the Involvement in the Response to Low-Temperature Stress in Ammopiptanthus nanus
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification of TLPs in A. nanus
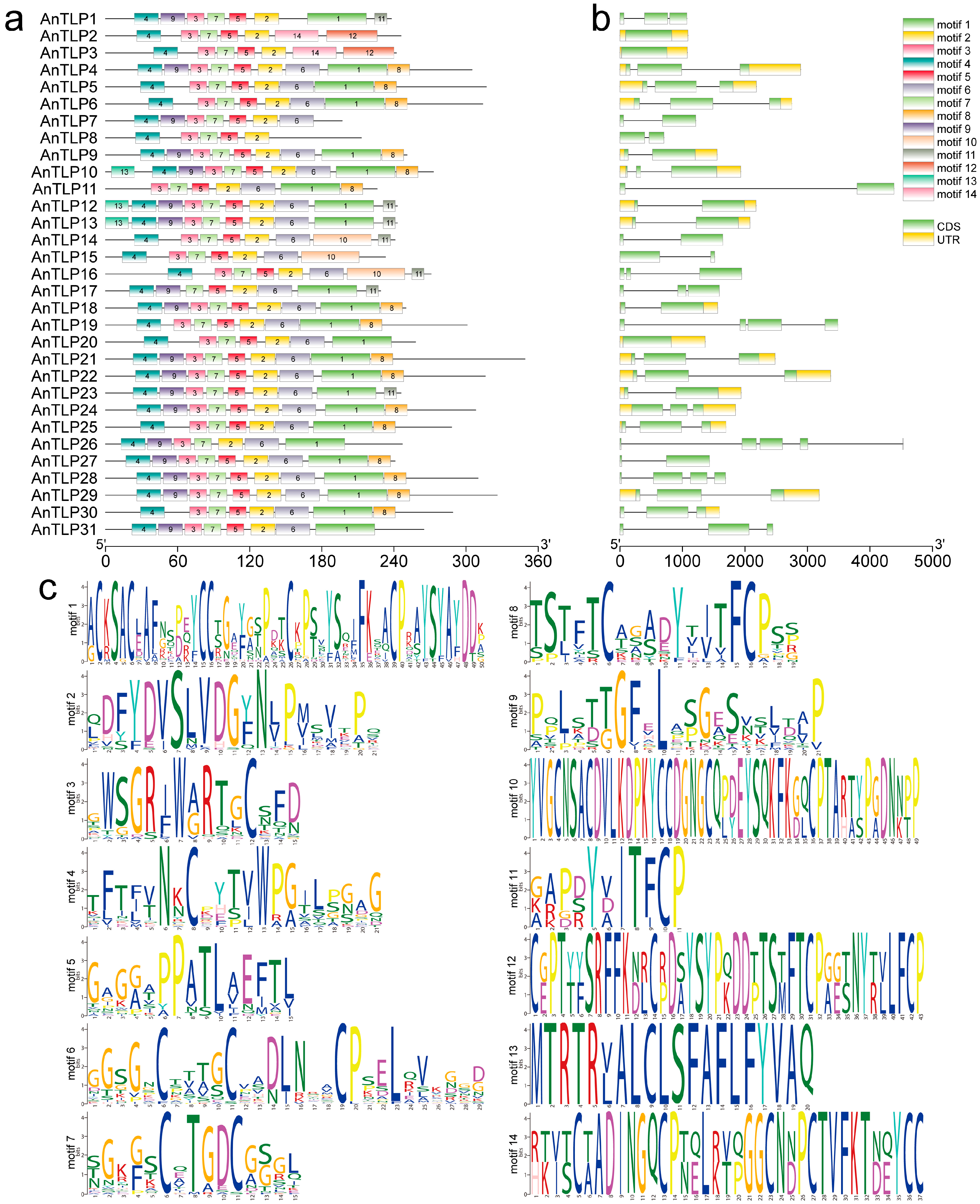
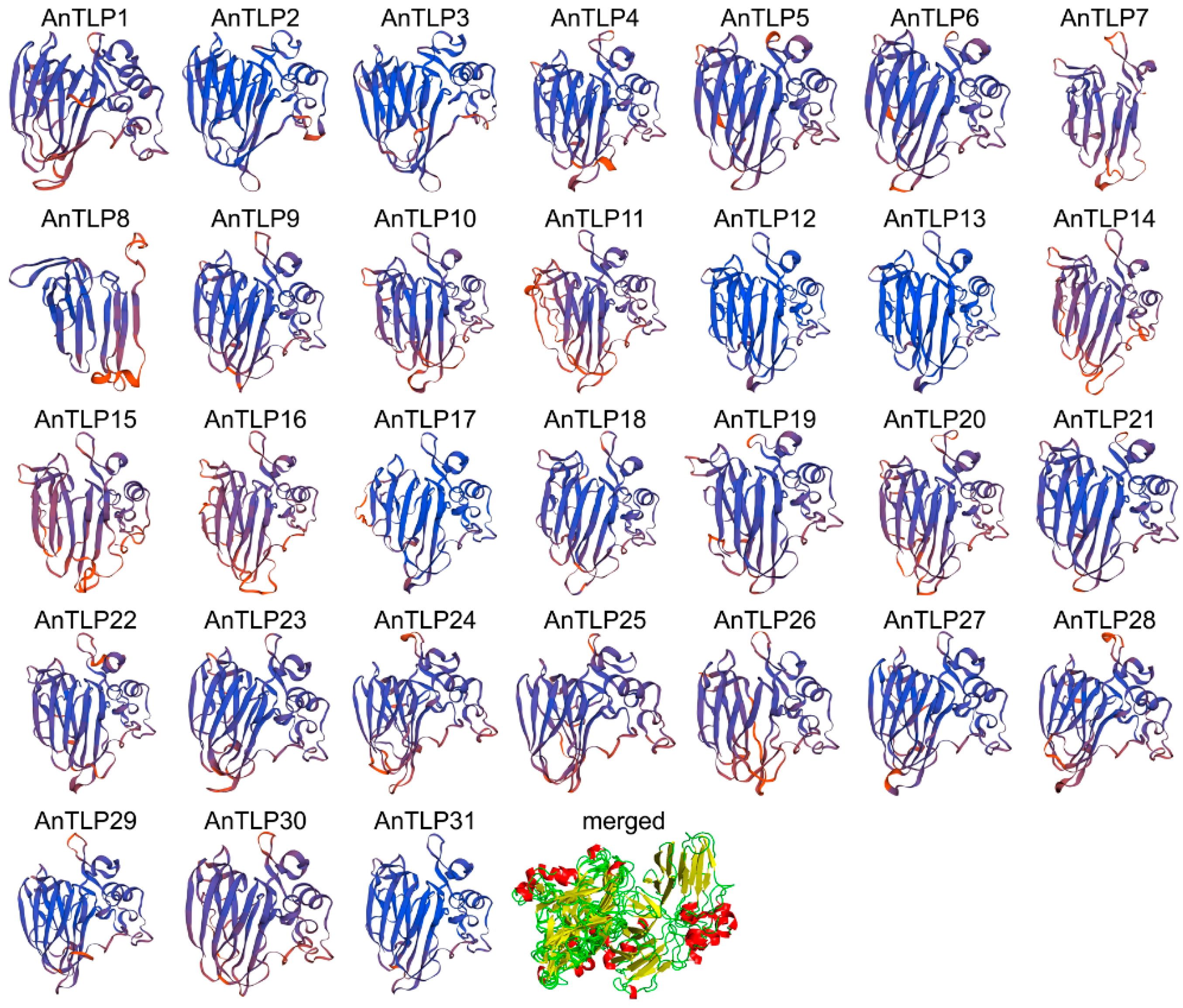
2.2. Structural Analysis of TLPs in A. nanus
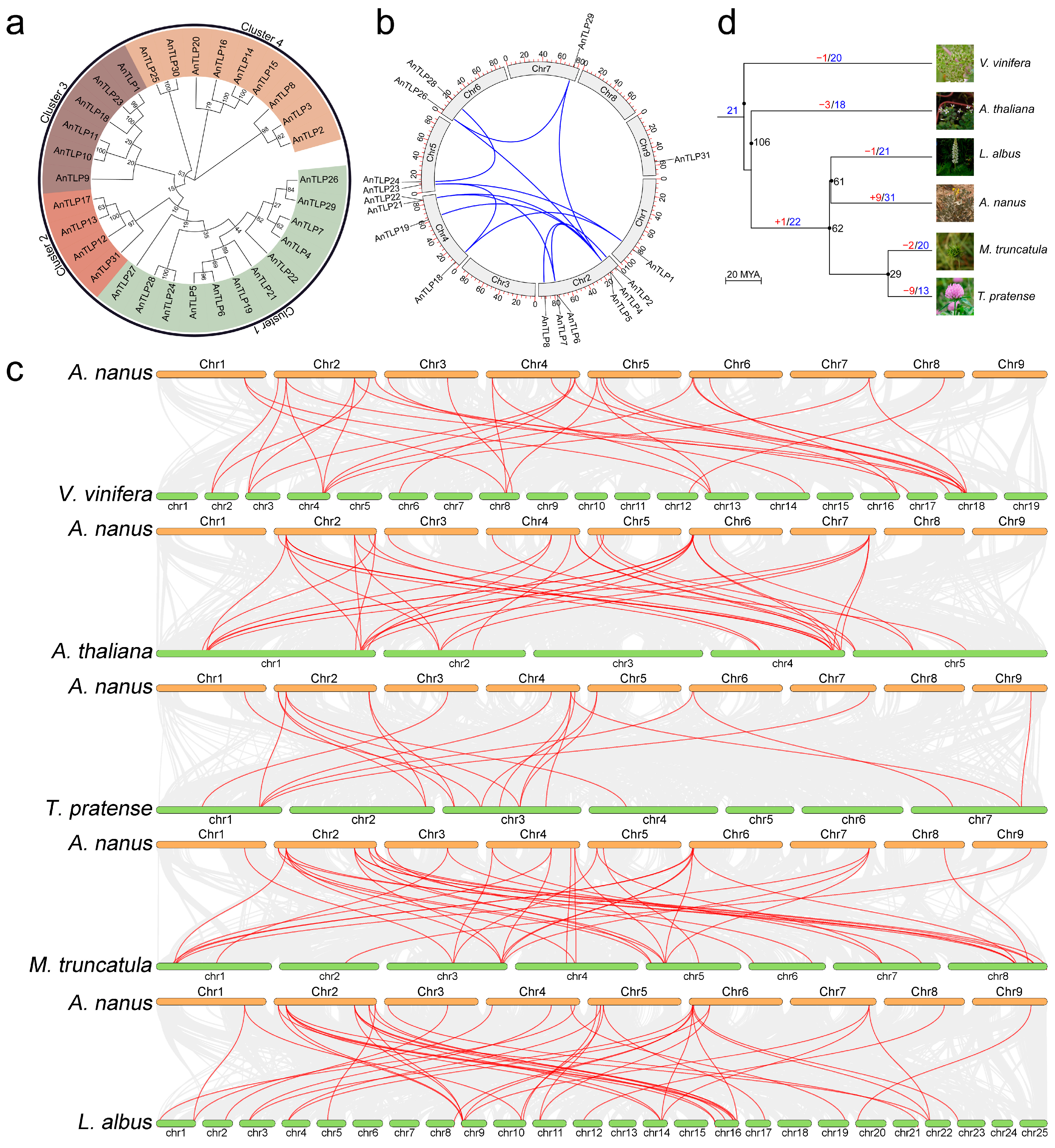
2.3. Phylogenetics, Gene Duplication, and Divergence of TLP Family in A. nanus
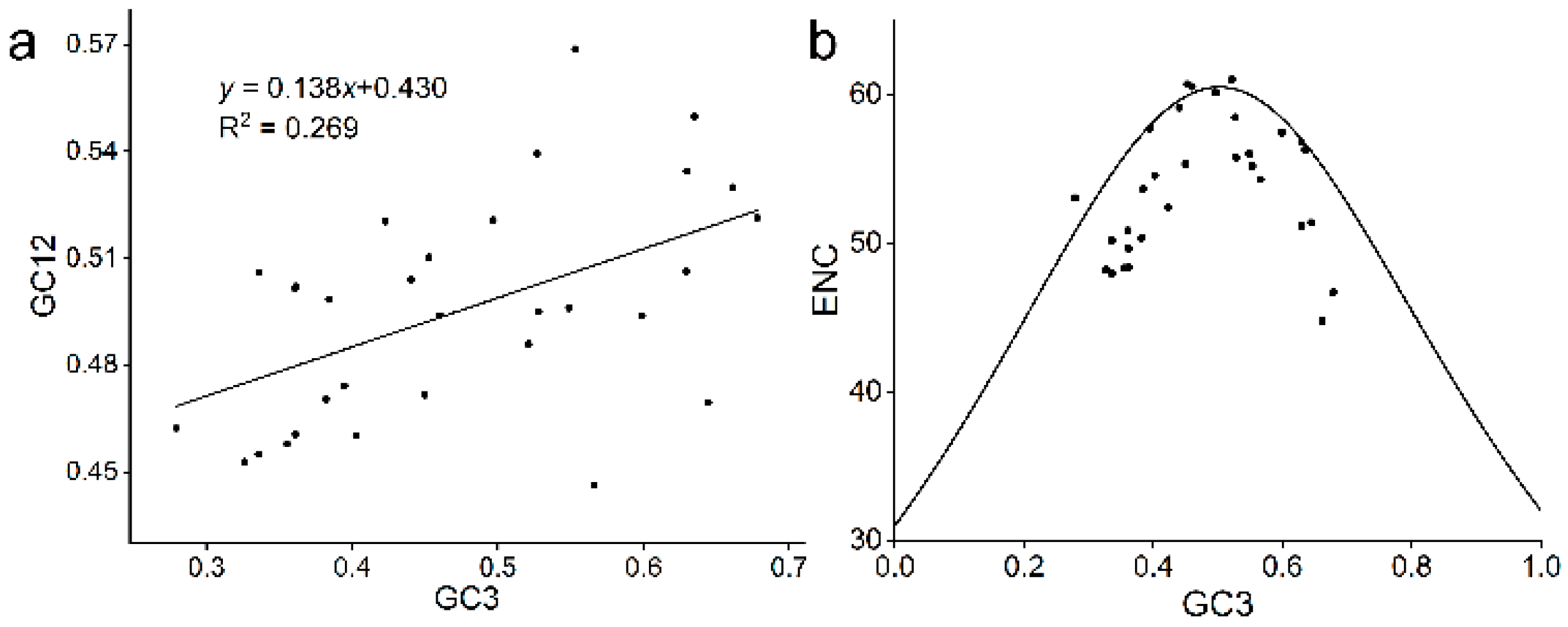
2.4. Positive Selection and Codon Usage Bias Analysis of TLP Gene Family in A. nanus
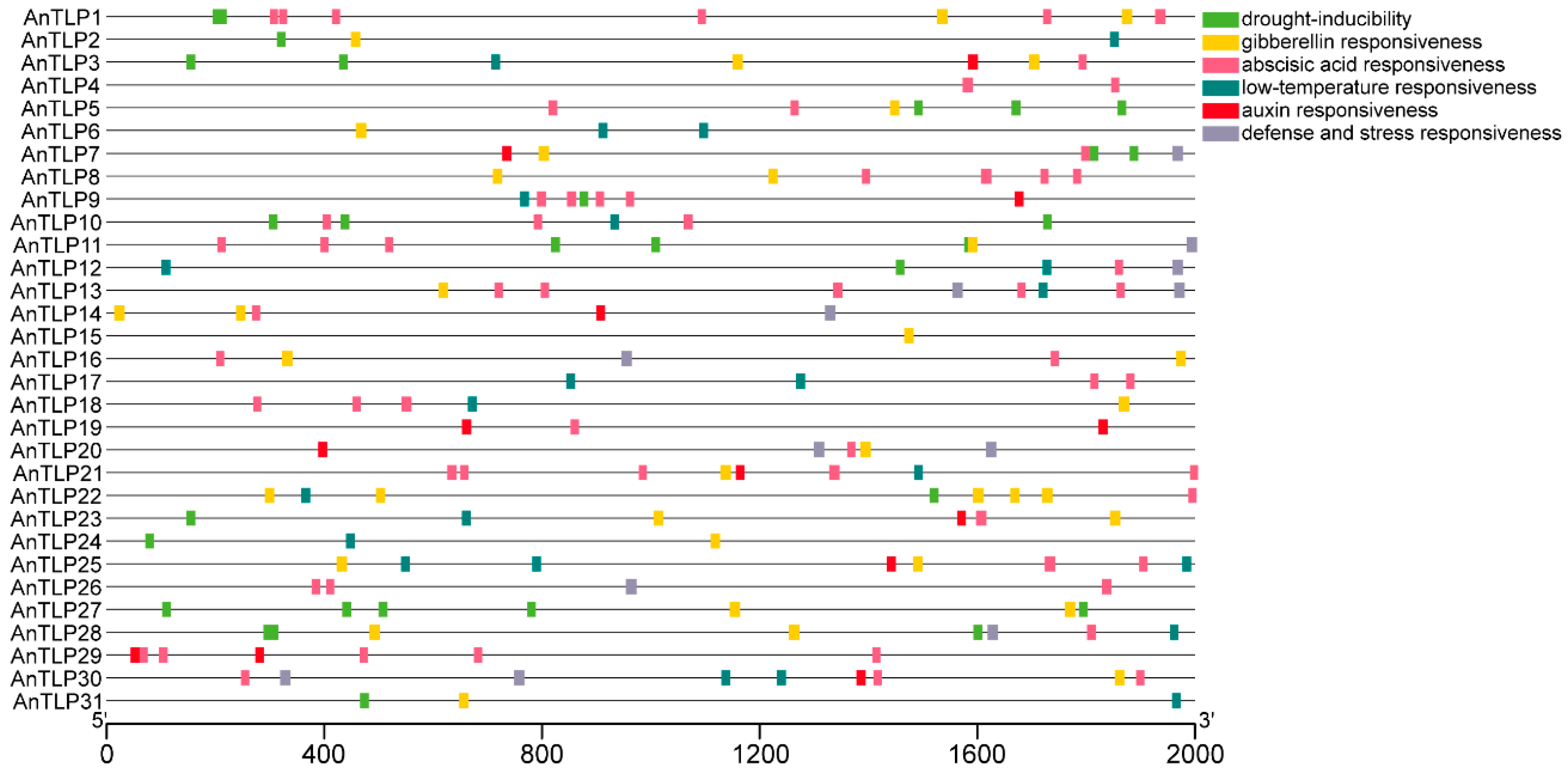
2.5. Prediction of the Cis-Acting Elements in Promoter Regions of the TLP Genes in A. nanus
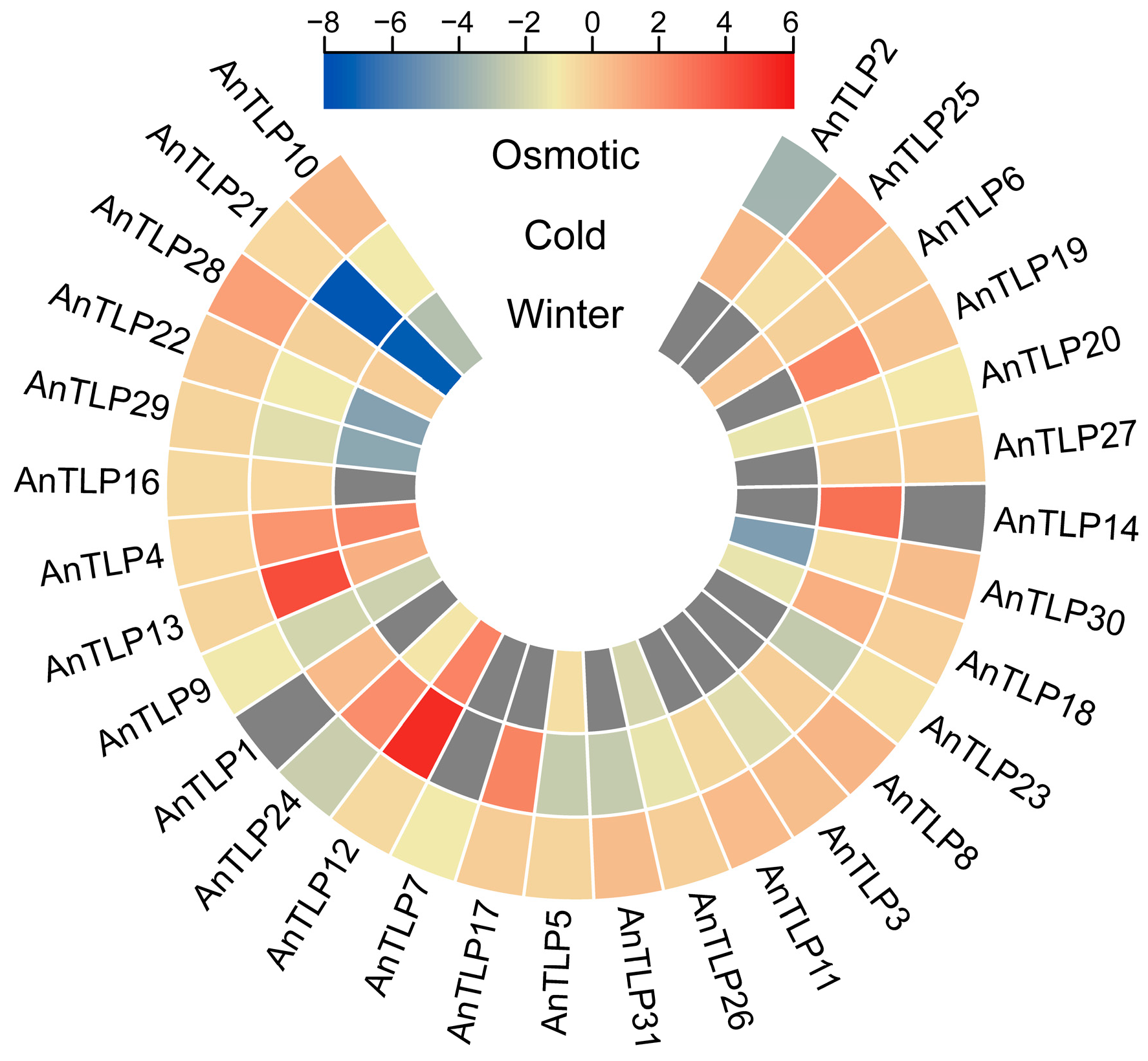
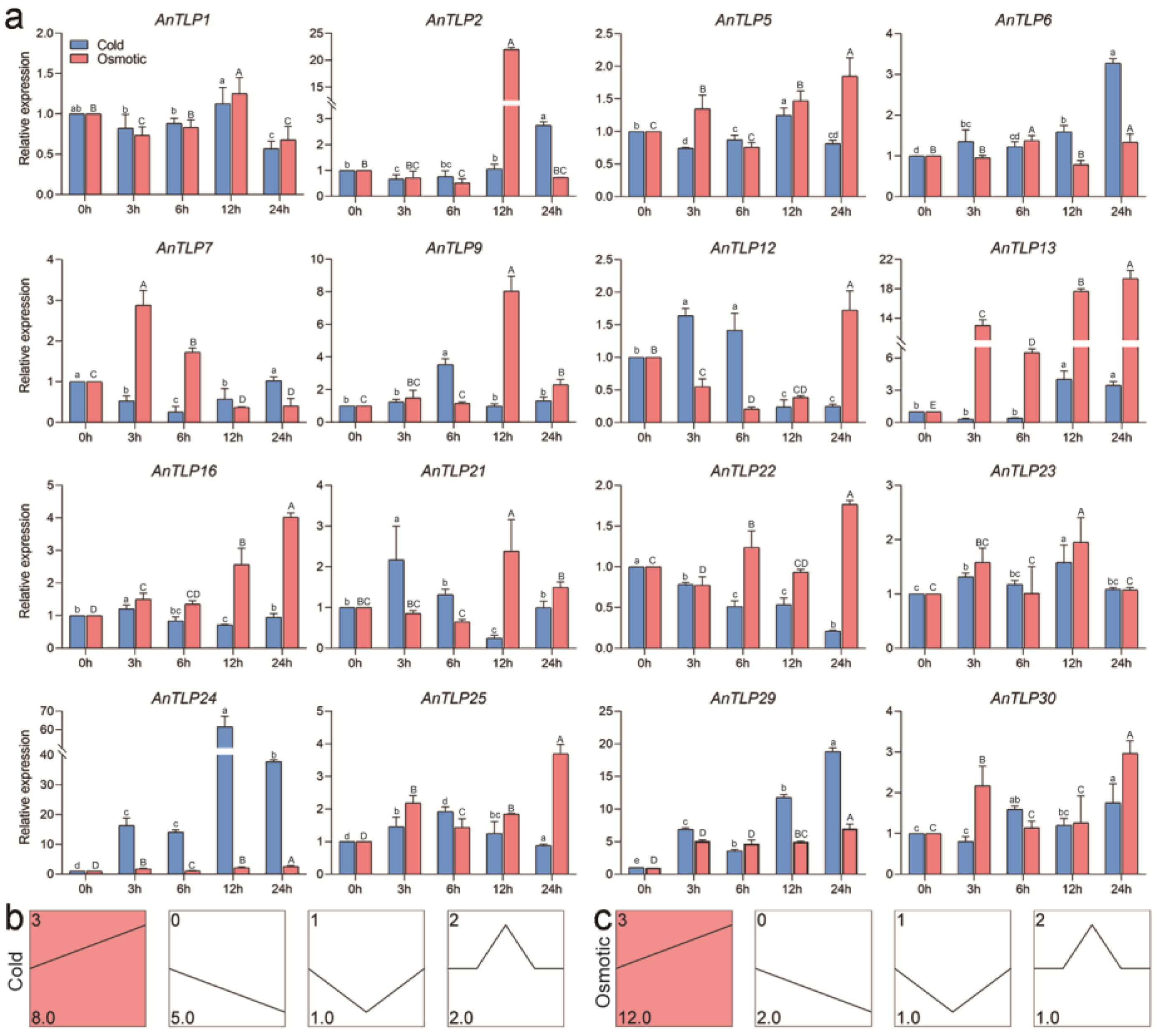
2.6. Expression Patterns of A. nanus TLP Genes under Osmotic and Cold Stresses
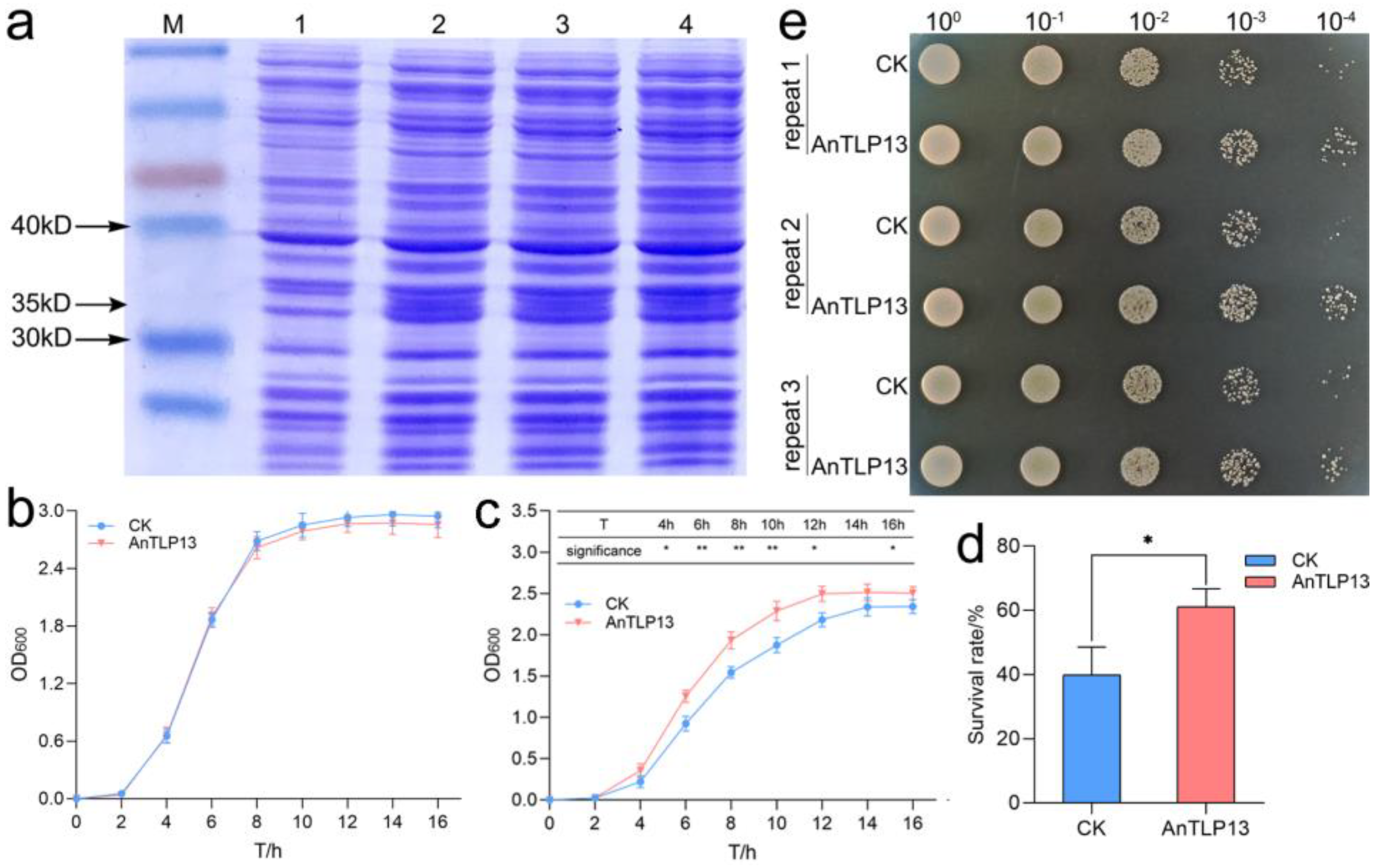
2.7. Overexpression of AnTLP13 Gene Enhanced the Tolerance to Low-Temperature Stress in E. coli and Yeast
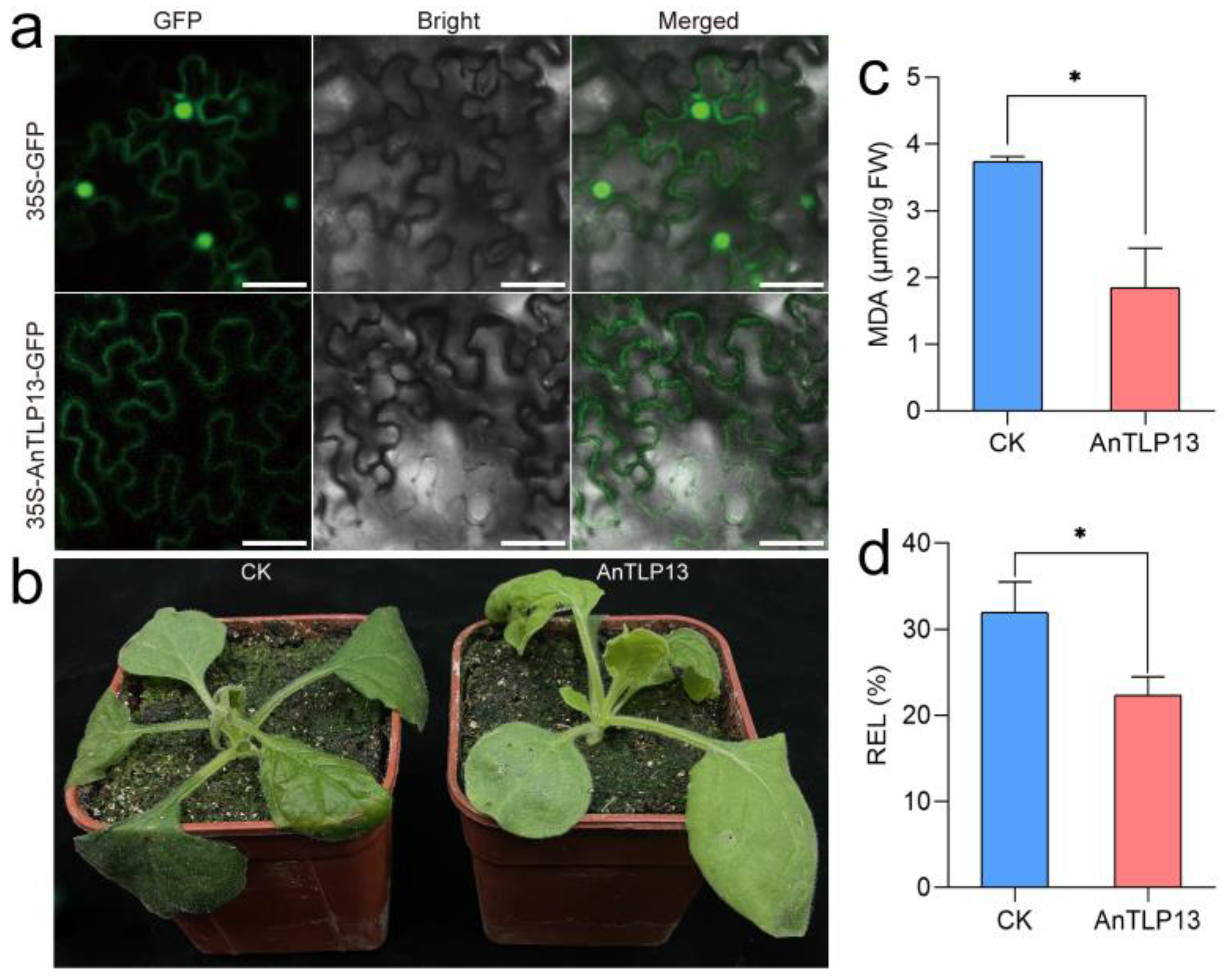
2.8. Overexpression of AnTLP13 Gene Enhanced the Tolerance of Tobacco to Freezing Stress
3. Discussion
4. Materials and Methods
4.1. Identification of the TLP Proteins in A. nanus
4.2. Chromosomal Location and Gene Structure Analysis of A. nanus TLP Family Genes
4.3. Multiple Sequence Alignment and Phylogenetic Analysis
4.4. Prediction of Cis-Acting Elements in Promoter Regions of TLP Genes
4.5. Gene Expression Analysis Based on the Transcriptome Data
4.6. Plant Materials and Stress Treatment
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. Vector Construction and Expression in E. coli
4.9. Vector Construction and Expression in Yeast
4.10. Transient Transformation of AnTLP13 in Tobacco
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Zhou, Y.; Movahedi, A.; Wei, H.; Zhuge, Q. Thaumatin-like protein (Pe-TLP) acts as a positive factor in transgenic poplars enhanced resistance to spots disease. Physiol. Mol. Plant Pathol. 2020, 112, 101512. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Brandazza, A.; Angeli, S.; Tegoni, M.; Cambillau, C.; Pelosi, P. Plant stress proteins of the thaumatin-like family discovered in animals. FEBS Lett. 2004, 572, 3–7. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Cui, Y.; Qiao, K.; Zhu, L.; Fan, S.; Ma, Q. Comprehensive genome-wide analysis of thaumatin-like gene family in four cotton species and functional identification of GhTLP19 involved in regulating tolerance to verticillium dahlia and drought. Front. Plant Sci. 2020, 11, 575015. [Google Scholar] [CrossRef]
- Iqbal, I.; Tripathi, R.K.; Wilkins, O.; Singh, J. Thaumatin-like protein (TLP) gene family in barley: Genome-wide exploration and expression analysis during germination. Genes 2020, 11, 1080. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Zhou, X.; Luan, Y.; Luan, F. Genome-wide identification, characterization and expression analysis of the TLP gene family in melon (Cucumis melo L.). Genomics 2020, 112, 2499–2509. [Google Scholar] [CrossRef]
- Ram, C.; Danish, S.; Kesawat, M.S.; Panwar, B.S.; Verma, M.; Arya, L.; Yadav, S.; Sharma, V. Genome-wide comprehensive characterization and expression analysis of TLP gene family revealed its responses to hormonal and abiotic stresses in watermelon (Citrullus lanatus). Gene 2022, 844, 146818. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, H.; Zhang, X.; Guo, C.; Wang, M.; Wang, Y.; Wang, X. Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 2017, 7, 4269. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, H.; Rajput, R.; Pandey, A.; Upadhyay, S.K. Molecular characterization revealed the role of thaumatin-like proteins of bread wheat in stress response. Front. Plant Sci. 2021, 12, 807448. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant thaumatin-like proteins: Function, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef]
- Kalpana, K.; Maruthasalam, S.; Rajesh, T.; Poovannan, K.; Kumar, K.K.; Kokiladevi, E.; Raja, J.A.J.; Sudhakar, D.; Velazhahan, R.; Samiyappan, R.; et al. Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci. 2006, 170, 203–215. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Zhao, H.; Cao, J.; Jiang, W. Antifungal activity of an abundant thaumatin-like protein from banana against Penicillium expansum, and its possible mechanisms of action. Molecules 2018, 23, 1442. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumar, K.R.; Kumar, D.; Shukla, P.; Kirti, P.B. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lin, K.H.; Ho, S.L.; Chiang, C.M.; Yang, C.M. Enhancing the abiotic stress tolerance of plants: From chemical treatment to biotechnological approaches. Physiol. Plant 2018, 164, 452–466. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Yu, H.Q.; Yong, T.M.; Li, H.J.; Liu, Y.P.; Zhou, S.F.; Fu, F.L.; Li, W.C. Overexpression of a phospholipase Dalpha gene from Ammopiptanthus nanus enhances salt tolerance of phospholipase Dalpha1-deficient Arabidopsis mutant. Planta 2015, 242, 1495–1509. [Google Scholar] [CrossRef]
- Ding, L.; Guo, X.; Wang, K.; Pang, H.; Liu, Y.; Yang, Q.; Fu, F.; Li, W.; Yu, H. Genome-wide analysis of BES1/BZR1 transcription factors and their responses to osmotic stress in Ammopiptanthus nanus. J. For. Res. 2020, 26, 127–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Meng, S.; Liu, Y.; Zhao, X.; Pang, C.; Zhang, H.; Xu, T.; He, Y.; Qi, M.; et al. Expression of galactinol synthase from Ammopiptanthus nanus in tomato improves tolerance to cold stress. J. Exp. Bot. 2020, 71, 435–449. [Google Scholar] [CrossRef]
- Yu, H.Q.; Han, N.; Zhang, Y.Y.; Tao, Y.; Chen, L.; Liu, Y.P.; Zhou, S.F.; Fu, F.L.; Li, W.C. Cloning and characterization of vacuolar H+-pyrophosphatase gene (AnVP1) from Ammopiptanthus nanus and its heterologous expression enhances osmotic tolerance in yeast and Arabidopsis thaliana. Plant Growth Regul. 2017, 81, 385–397. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Li, Z.; Shi, W.; Gao, F.; Zhou, Y.; Zhang, G.; Feng, J. Genome-wide identification and expression analyses of the chitinases under cold and osmotic stress in Ammopiptanthus nanus. Genes 2019, 10, 472. [Google Scholar] [CrossRef]
- Zhao, J.P.; Su, X.H. Patterns of molecular evolution and predicted function in thaumatin-like proteins of Populus trichocarpa. Planta 2010, 232, 949–962. [Google Scholar] [CrossRef]
- Cao, J.; Yueqing, L.; Hou, Z.; Li, X.; Ding, L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regul. 2016, 79, 299–307. [Google Scholar] [CrossRef]
- Liu, J.J.; Zamani, A.; Ekramoddoullah, A.K. Expression profiling of a complex thaumatin-like protein family in western white pine. Planta 2010, 231, 637–651. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. SHOOT: Phylogenetic gene search and ortholog inference. Genome Biol. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Koonin, E.V. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 2005, 39, 309–338. [Google Scholar] [CrossRef]
- Pimpat, Y.; Saralamba, N.; Boonyuen, U.; Pukrittayakamee, S.; Nosten, F.; Smithuis, F.; Day, N.P.J.; Dondorp, A.M.; Imwong, M. Genetic analysis of the orthologous CRT and MDR1 genes in Plasmodium malariae from Thailand and Myanmar. Malar J. 2020, 19, 315. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–1561. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Elmer, K.R.; Meyer, A. Genomics of adaptation and speciation in cichlid fishes: Recent advances and analyses in African and Neotropical lineages. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; You, F.M.; Datla, R.; Ravichandran, S.; Jia, B.; Cloutier, S. Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.). BMC Genom. 2020, 21, 722. [Google Scholar] [CrossRef]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon usage bias. Mol. Biol. Rep. 2022, 49, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, A.; Lamolle, G.; Musto, H. Codon Usage Bias: An endless tale. J. Mol. Evol. 2021, 89, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Himani, S.; Sonia, S.; Sneh, N.; Rekha, M.; Indu, S.; Ravish, C. Computational analysis of cis-acting regulatory elements in 5’ regulatory regions of sucrose transporter gene families in wheat and Arabidopsis. J. Biotechnol. 2014, 9, 75–81. [Google Scholar]
- Chow, C.-N.; Chiang-Hsieh, Y.-F.; Chien, C.-H.; Zheng, H.-Q.; Lee, T.-Y.; Wu, N.-Y.; Tseng, K.-C.; Hou, P.-F.; Chang, W.-C. Delineation of condition specific cis-and trans-acting elements in plant promoters under various endo-and exogenous stimuli. BMC Genom. 2018, 19, 109–121. [Google Scholar] [CrossRef]
- Kakei, Y.; Ogo, Y.; Itai, R.N.; Kobayashi, T.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. Development of a novel prediction method of cis-elements to hypothesize collaborative functions of cis-element pairs in iron-deficient rice. Rice 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Xiao, Z.; Wei, C.; Feng, J.; Zhou, Y.J.T. De novo transcriptome analysis of Ammopiptanthus nanus and its comparative analysis with A. mongolicus. Trees 2018, 32, 287–300. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Li, X.; Xu, M.; Li, H.; Abla, M.; Sun, H.; Wei, S.; Feng, J.; Zhou, Y.J.G. Long-read sequencing and de novo genome assembly of Ammopiptanthus nanus, a desert shrub. GigaScience 2018, 7, giy074. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, 597–603. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, S.; Sui, X.; Wang, J.; Geng, Y.; Gao, F.; Zhou, Y. Genome-wide characterization, evolution, and expression analysis of the ascorbate peroxidase and glutathione peroxidase gene families in response to cold and osmotic stress in Ammopiptanthus nanus. J. Plant Growth Regul. 2023, 42, 502–522. [Google Scholar] [CrossRef]
- Abla, M.; Sun, H.; Li, Z.; Wei, C.; Gao, F.; Zhou, Y.; Feng, J. Identification of miRNAs and their response to cold stress in Astragalus Membranaceus. Biomolecules 2019, 9, 182. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef]
- Dhanyalakshmi, K.H.; Nataraja, K.N. Universal stress protein-like gene from mulberry enhances abiotic stress tolerance in Escherichia coli and transgenic tobacco cells. Plant Biol. 2021, 23, 1190–1194. [Google Scholar] [CrossRef]
- Yin, J.; Sun, L.; Li, Y.; Xiao, J.; Wang, S.; Yang, J.; Qu, Z.; Zhan, Y. Functional identification of BpMYB21 and BpMYB61 transcription factors responding to MeJA and SA in birch triterpenoid synthesis. BMC Plant Biol. 2020, 20, 374. [Google Scholar] [CrossRef]
- Wydro, M.; Kozubek, E.; Lehmann, P. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta Biochim. Pol. 2006, 53, 289–298. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, W.; Chen, Q.; Zhang, G.; Gao, F.; Zhou, Y. Integrated transcriptomics, proteomics, and metabolomics identified biological processes and metabolic pathways involved in heat stress response in jojoba. Ind. Crops Prod. 2022, 183, 114946. [Google Scholar] [CrossRef]


| Sequence ID | Gene Name | Genome Location | Amino Acid Length | Molecular Weight/kD | Theoretical pI | GRAVY | Signal Peptide | TMHs | Subcellular Localization * |
|---|---|---|---|---|---|---|---|---|---|
| EVM0028587 | AnTLP1 | chr1: 84629737-84630810 | 237 | 25.36 | 7.43 | 0.157 | 1-23 | 0 | extr |
| EVM0000288 | AnTLP2 | chr2: 3943782-3944871 | 245 | 26.88 | 6.76 | −0.371 | 1-25 | 0 | extr |
| EVM0012162 | AnTLP3 | chr2: 3949219-3950299 | 241 | 26.33 | 5.33 | −0.068 | 1-39 | 1 | extr |
| EVM0031681 | AnTLP4 | chr2: 11425283-11428175 | 304 | 31.34 | 4.45 | 0.062 | 1-26 | 1 | chlo |
| EVM0023773 | AnTLP5 | chr2: 11445664-11447849 | 316 | 32.84 | 5.22 | −0.075 | 1-28 | 1 | extr |
| EVM0003940 | AnTLP6 | chr2: 77169927-77172678 | 313 | 32.80 | 6.19 | −0.147 | 1-35 | 0 | extr |
| EVM0027347 | AnTLP7 | chr2: 77240207-77241420 | 196 | 20.87 | 5.00 | 0.343 | 1-23 | 1 | extr |
| EVM0011680 | AnTLP8 | chr2: 91149625-91150307 | 212 | 22.51 | 5.85 | 0.31 | 1-24 | 1 | golg |
| EVM0028885 | AnTLP9 | chr2: 97035730-97037285 | 250 | 27.01 | 9.25 | 0.037 | 1-28 | 1 | extr |
| EVM0036902 | AnTLP10 | chr3: 3630861-3632795 | 272 | 29.75 | 6.89 | −0.059 | 1-36 | 1 | extr |
| EVM0012276 | AnTLP11 | chr3: 3636405-3640791 | 225 | 24.81 | 8.57 | −0.145 | NO | 1 | extr |
| EVM0027709 | AnTLP12 | chr3: 60728536-60730716 | 242 | 25.54 | 4.78 | −0.167 | 1-21 | 0 | extr |
| EVM0030154 | AnTLP13 | chr3: 60822641-60824723 | 242 | 25.62 | 4.78 | −0.236 | 1-21 | 0 | extr |
| EVM0006019 | AnTLP14 | chr3: 60829095-60830741 | 240 | 26.04 | 4.97 | −0.138 | 1-22 | 0 | extr |
| EVM0035676 | AnTLP15 | chr3: 60870728-60872244 | 232 | 25.08 | 4.92 | −0.074 | NO | 0 | extr |
| EVM0033132 | AnTLP16 | chr3: 60875649-60877597 | 270 | 29.52 | 4.7 | −0.25 | NO | 0 | chlo |
| EVM0026680 | AnTLP17 | chr3: 66587898-66589488 | 228 | 24.26 | 4.37 | −0.196 | NO | 0 | extr |
| EVM0006621 | AnTLP18 | chr4: 5755011-5756575 | 249 | 26.13 | 7.38 | 0.105 | 1-26 | 1 | extr |
| EVM0005233 | AnTLP19 | chr4: 62429388-62432876 | 300 | 31.55 | 5.39 | −0.168 | NO | 1 | extr |
| EVM0005658 | AnTLP20 | chr4: 81197773-81199137 | 257 | 27.41 | 7.17 | −0.023 | 1-31 | 1 | chlo |
| EVM0036386 | AnTLP21 | chr4: 85077259-85079741 | 348 | 35.13 | 4.24 | 0.02 | 1-22 | 1 | extr |
| EVM0034723 | AnTLP22 | chr4: 85093701-85097072 | 315 | 33.52 | 8.75 | 0.151 | 1-24 | 0 | extr |
| EVM0007971 | AnTLP23 | chr5: 8628389-8630328 | 245 | 25.71 | 6.07 | 0.164 | 1-22 | 0 | extr |
| EVM0028324 | AnTLP24 | chr5: 12202458-12204306 | 307 | 32.80 | 5.02 | −0.006 | 1-25 | 2 | extr |
| EVM0001585 | AnTLP25 | chr5: 14996048-14997745 | 287 | 30.79 | 8.65 | 0.08 | 1-27 | 1 | plas |
| EVM0014534 | AnTLP26 | chr6: 3060387-3064918 | 246 | 26.66 | 4.87 | −0.243 | 1-21 | 0 | chlo |
| EVM0005703 | AnTLP27 | chr6: 4178850-4180284 | 240 | 24.7 | 5.37 | −0.075 | NO | 0 | chlo |
| EVM0035081 | AnTLP28 | chr6: 19546129-19547818 | 309 | 33.29 | 9.02 | −0.002 | 1-27 | 2 | extr |
| EVM0034418 | AnTLP29 | chr7: 75592532-75595722 | 325 | 34.15 | 4.82 | 0.051 | 1-22 | 0 | extr |
| EVM0006518 | AnTLP30 | chr8: 57726394-57727987 | 288 | 31.30 | 6.29 | −0.111 | 1-27 | 0 | extr |
| EVM0016538 | AnTLP31 | chr9: 56159846-56162293 | 264 | 28.23 | 5.48 | −0.059 | NO | 0 | extr |
| Segment Pairs | Ka | Ks | Ka/Ks | T(MYA) | Selection Pressure |
|---|---|---|---|---|---|
| AnTLP1-AnTLP18 | 0.203 | 2.191 | 0.093 | 534.39 | Purifying selection |
| AnTLP4-AnTLP22 | 0.381 | 1.170 | 0.325 | 285.37 | Purifying selection |
| AnTLP5-AnTLP21 | 0.274 | 1.805 | 0.152 | 440.24 | Purifying selection |
| AnTLP6-AnTLP21 | 0.266 | 1.202 | 0.221 | 293.17 | Purifying selection |
| AnTLP6-AnTLP5 | 0.152 | 0.724 | 0.210 | 176.59 | Purifying selection |
| AnTLP7-AnTLP22 | 0.491 | 1.521 | 0.323 | 370.98 | Purifying selection |
| AnTLP7-AnTLP4 | 0.325 | 0.827 | 0.392 | 201.71 | Purifying selection |
| AnTLP8-AnTLP2 | 0.463 | 3.322 | 0.139 | 810.24 | Purifying selection |
| AnTLP23-AnTLP1 | 0.058 | 0.402 | 0.144 | 98.05 | Purifying selection |
| AnTLP24-AnTLP28 | 0.139 | 0.331 | 0.422 | 80.73 | Purifying selection |
| AnTLP26-AnTLP29 | 0.187 | 0.827 | 0.227 | 201.71 | Purifying selection |
| AnTLP26-AnTLP4 | 0.274 | 1.313 | 0.209 | 320.24 | Purifying selection |
| AnTLP29-AnTLP4 | 0.290 | 2.994 | 0.097 | 730.24 | Purifying selection |
| Gene Name | CAI | Fop | ENC | Gene Name | CAI | Fop | ENC |
|---|---|---|---|---|---|---|---|
| AnTLP25 | 0.19 | 0.37 | 50.35 | AnTLP9 | 0.24 | 0.48 | 44.78 |
| AnTLP24 | 0.21 | 0.37 | 50.83 | AnTLP31 | 0.25 | 0.42 | 53.03 |
| AnTLP23 | 0.23 | 0.38 | 52.40 | AnTLP20 | 0.30 | 0.55 | 46.70 |
| AnTLP30 | 0.19 | 0.36 | 50.18 | AnTLP19 | 0.20 | 0.44 | 55.75 |
| AnTLP1 | 0.20 | 0.30 | 48.39 | AnTLP21 | 0.20 | 0.44 | 55.19 |
| AnTLP28 | 0.17 | 0.35 | 53.65 | AnTLP22 | 0.25 | 0.50 | 56.81 |
| AnTLP26 | 0.31 | 0.55 | 57.45 | AnTLP18 | 0.18 | 0.34 | 47.94 |
| AnTLP27 | 0.29 | 0.55 | 56.25 | AnTLP14 | 0.22 | 0.35 | 49.64 |
| AnTLP29 | 0.26 | 0.46 | 60.52 | AnTLP10 | 0.24 | 0.38 | 48.33 |
| AnTLP3 | 0.29 | 0.54 | 54.28 | AnTLP16 | 0.24 | 0.39 | 57.74 |
| AnTLP8 | 0.24 | 0.47 | 61.00 | AnTLP11 | 0.22 | 0.42 | 54.54 |
| AnTLP6 | 0.23 | 0.46 | 60.14 | AnTLP15 | 0.23 | 0.37 | 48.21 |
| AnTLP2 | 0.27 | 0.52 | 56.00 | AnTLP12 | 0.27 | 0.46 | 60.68 |
| AnTLP5 | 0.23 | 0.45 | 58.46 | AnTLP13 | 0.28 | 0.45 | 59.12 |
| AnTLP7 | 0.21 | 0.43 | 51.37 | AnTLP17 | 0.28 | 0.46 | 55.33 |
| AnTLP4 | 0.25 | 0.48 | 51.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Sui, X.; Wang, Y.; Zhu, M.; Zhou, Y.; Gao, F. Genome-Wide Analyses of Thaumatin-like Protein Family Genes Reveal the Involvement in the Response to Low-Temperature Stress in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 2209. https://doi.org/10.3390/ijms24032209
Liu Q, Sui X, Wang Y, Zhu M, Zhou Y, Gao F. Genome-Wide Analyses of Thaumatin-like Protein Family Genes Reveal the Involvement in the Response to Low-Temperature Stress in Ammopiptanthus nanus. International Journal of Molecular Sciences. 2023; 24(3):2209. https://doi.org/10.3390/ijms24032209
Chicago/Turabian StyleLiu, Qi, Xiangyu Sui, Ying Wang, Ming Zhu, Yijun Zhou, and Fei Gao. 2023. "Genome-Wide Analyses of Thaumatin-like Protein Family Genes Reveal the Involvement in the Response to Low-Temperature Stress in Ammopiptanthus nanus" International Journal of Molecular Sciences 24, no. 3: 2209. https://doi.org/10.3390/ijms24032209
APA StyleLiu, Q., Sui, X., Wang, Y., Zhu, M., Zhou, Y., & Gao, F. (2023). Genome-Wide Analyses of Thaumatin-like Protein Family Genes Reveal the Involvement in the Response to Low-Temperature Stress in Ammopiptanthus nanus. International Journal of Molecular Sciences, 24(3), 2209. https://doi.org/10.3390/ijms24032209






