Abstract
Hyper-reactivity to sensory inputs is a common and debilitating symptom of autism spectrum disorder (ASD), but the underlying neural abnormalities remain unclear. Two of three patients in our clinical cohort screen harboring de novo SHANK2 mutations also exhibited high sensitivity to visual, auditory, and tactile stimuli, so we examined whether shank2 deficiencies contribute to sensory abnormalities and other ASD-like phenotypes by generating a stable shank2b-deficient zebrafish model (shank2b−/−). The adult shank2b−/− zebrafish demonstrated reduced social preference and kin preference as well as enhanced behavioral stereotypy, while larvae exhibited hyper-sensitivity to auditory noise and abnormal hyperactivity during dark-to-light transitions. This model thus recapitulated the core developmental and behavioral phenotypes of many previous genetic ASD models. Expression levels of γ-aminobutyric acid (GABA) receptor subunit mRNAs and proteins were also reduced in shank2b−/− zebrafish, and these animals exhibited greater sensitivity to drug-induced seizures. Our results suggest that GABAergic dysfunction is a major contributor to the sensory hyper-reactivity in ASD, and they underscore the need for interventions that target sensory-processing disruptions during early neural development to prevent disease progression.
1. Introduction
Autism spectrum disorder (ASD) is an umbrella term for neurodevelopmental syndromes characterized by deficits in social interaction and communication, stereotyped repetitive behaviors, restricted interests, and various sensory abnormalities [1,2]. Atypical sensory processing in ASD may be manifested as hypersensitivity, hyposensitivity, or unusual interest in specific sensory aspects of the environment, and such abnormalities are observed in up to 90% of autistic individuals [2,3,4,5]. Furthermore, these abnormalities influence every sensory modality (vision, smell, taste, audition, proprioception, touch, and balance).
Atypical visual processing has been observed in human infants who eventually develop autism as early as 6 months of age [6,7], considerably earlier than core autistic phenotypes such as impairments in joint attention (14–18 months) [5,8]. Moreover, previous studies have reported associations between lower joint attention in children with autism and lack of orienting to both social and nonsocial sensory stimuli [8]. Sensory symptoms in infants not only precede but also are predictive of social deficits [9], repetitive behaviors [10], and eventual diagnosis of ASD in childhood [9]. Thus, perceptual symptoms could serve as early diagnostic biomarkers for ASD, provide clues to the underlying pathobiology, and reveal novel targets for therapeutic intervention [11,12].
Many genes have been implicated in ASDs, including those that regulate synaptic transmission [13,14]. Among these is SHANK2 [15,16], which encodes a major scaffold protein (SHANK2/ProSAP1) at glutamatergic synapses essential for the assembly and integrity of the postsynaptic density (PSD) [13,14]. Previous studies have identified eighteen patients with neurodevelopmental disorder harboring de novo variants in the human SHANK2 gene [15,16,17,18,19,20,21,22,23,24,25], and we identified three additional patients with de novo variants of SHANK2 in our clinical screening cohort. Moreover, two of these patients showed intellectual deficits and heightened sensitivity to sensory stimuli, such as exaggerated reflexive responses to sudden sounds and passive seeking of external visual stimuli. In accord with clinical observations, a line of Shank2 knockout mice also demonstrated hyposensitivity to painful stimuli [26]. Accordingly, we speculated that SHANK2 loss-of-function in autism would be associated with abnormal sensory processing, especially in the auditory and visual pathways [2]. However, the exact molecular mechanisms remain to be elucidated.
These ASD-related sensory deficits and core symptomatic behaviors have been attributed to an imbalance between excitatory and inhibitory neurotransmission [27,28,29,30,31] due to deficient inhibitory γ-aminobutyric acid (GABA) signaling. Lines of evidence for GABAergic dysfunction in ASD include underexpression of GABA receptor subunits in post-mortem brain tissue of autism patients, associations between ASD and allelic variants of GABAergic genes, and deficient GABAergic transmission in a number of otherwise distinct animal models of ASD [32,33,34,35]. Moreover, a recent human study found deficient binocular rivalry, which is dependent on cortical GABAergic signaling, in autism patients [36]. There is also evidence for the involvement of GABAergic dysfunction in the tactile and auditory processing deficits of autism [11,37,38]. Collectively, these findings indicate that altered GABAergic signaling may contribute to abnormal sensory perception in autism.
The zebrafish is a powerful tool for studying neurological diseases including ASD at the genetic, developmental, and behavioral levels [39]. Here, we report the generation of a shank2 mutant zebrafish line that provides strong support for the contribution of shank2 deficits in the sensory processing and behavioral abnormalities of ASD. Moreover, we report that these animals exhibit reduced expression of GABA receptors in the brain and enhanced sensitivity to drug-induced seizures, suggesting GABAergic signaling insufficiency.
2. Results
2.1. Generation and Morphometric Characterization of shank2-Deficient Zebrafish
Zebrafish have two shank2 paralogs that are expressed during development and continue to be enriched in the mature central nervous system (Supplementary Figure S1A,B). Shank2a and shank2b proteins show 46.63% and 60.58% identity to the human protein (Supplementary Table S2), respectively, and contain the same major functional domains (Figure 1A, Supplementary Tables S3 and S4). Moreover, a comparison of protein sequences from multiple species revealed high evolutionary conservation of SHANK2 among vertebrates (Supplementary Figure S1C).
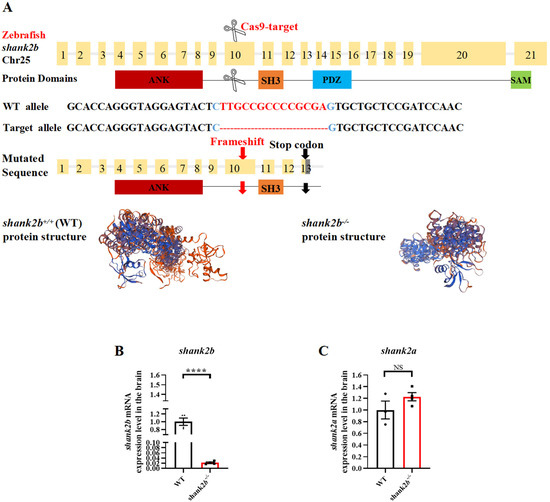
Figure 1.
Generation of a zebrafish shank2b mutant using CRISPR/Cas9. (A) Structure of zebrafish shank2b gene and protein. The protein domains (ANK, ankyrin repeat domain; SH3, Src homology 3 domain; PDZ, PSD-95/Discs large/ZO-1 domain; SAM, sterile alpha motif domain) are aligned to the corresponding exons. Diagram of target site on 10th exon of zebrafish shank2b genomic DNA and a 14 bp deletion mutation by CRISPR/Cas9 gene editing. Predicted structures of wild-type (WT) and shank2b mutant proteins in zebrafish. The 14 bp deletion resulted in a stop codon on 13th exon and premature termination before the PDZ and SAM domains. (B) Reduced expression of shank2b mRNA in the brain of shank2b−/− adult male zebrafish at 4.5 months post-fertilization (mpf) compared to WT fish, analyzed by RT-qPCR (WT n = 4, shank2b−/− n = 4, **** p < 0.0001, Student’s t test). Data are shown as mean ± SEM. (C) The expression of shank2a mRNA in the brain of WT and shank2b−/− adult (4.5 mpf) male zebrafish was not affected (WT n = 3, shank2b−/− n = 4, ns, p = 0.1957, Student’s t test). Data are shown as mean ± SEM.
To examine shank2 functions, we generated a shank2b-deficient zebrafish line using CRISPR/Cas9 to target the tenth exon of the shank2b gene, creating a 14-bp deletion (Figure 1A, Supplementary Figure S1D). The resulting frame shift produces a premature stop codon in exon 13 that disrupts the amino acid sequences of downstream PSD-95/Discs large/ZO-1 (PDZ) and sterile alpha motif (SAM) domains (Figure 1A).
To assess whether the mutant shank2 transcript is subject to nonsense-mediated decay, we examined shank2b mRNA expression in the zebrafish brain. Quantitative RT-PCR (RT-qPCR) revealed an overall reduction in shank2b mRNA among homozygous mutants compared to the wild types (WT) (Figure 1B), whereas the expression of shank2a mRNA did not differ between genotypes (Figure 1C). The relative abundance of shank2b mRNA was also significantly reduced in heart, liver–gall–spleen, skin–muscle, and gonad, compared to WTs, suggesting a loss of mutant transcript via nonsense-mediated decay (Supplementary Figure S1E).
We observed no difference in gross morphology between WT and homozygous shank2b−/− adults (Supplementary Tables S6 and S7). While shank2b−/− zebrafish larvae at 58 h post-fertilization (hpf) displayed a small head phenotype and shorter body length (Supplementary Figure S2A–D) compared to WTs, the ratios of several head measurement parameters to body length were normal (Supplementary Figure S2E,F) and all brain regions were well-preserved in adult mutant fish (Supplementary Figure S2G).
2.2. Autism-like Behaviors in Adult and Larval shank2b−/− Zebrafish
Human genetic studies have implicated SHANK2 in ASDs [15,16,18,21,22,23,24,25], and previous rodent experiments have reported autism-like behaviors in two different lines of Shank2 mutant mice as well as impaired social and cognitive behaviors in Shank2Δe24 mice [40,41,42]. We conducted a series of behavioral tests to assess whether shank2b−/− zebrafish also display ASD-like phenotypes analogous to those associated with SHANK2 mutations in humans.
We first tested social preference behaviors using three-chamber tests. As shown in Figure 2B, adult shank2b−/− mutants in the central chamber spent less time and swam a shorter distance in the conspecific sector compared to WTs. Moreover, mutants showed no preference for kin fish in one outer chamber (i.e., spent no more time swimming in the zone adjacent to the chamber) relative to non-kin fish in the other outer chamber, in contrast to the significant kin preference of WTs (Figure 2D). Furthermore, shank2b−/− zebrafish spent much less time and swam a shorter distance parallel to conspecifics than WT zebrafish (Figure 2E). These results suggest that shank2b deficiency leads to low social preference in zebrafish.
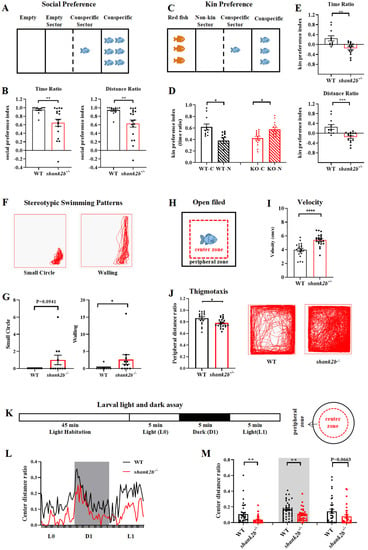
Figure 2.
The shank2b−/− deficient zebrafish displayed autism-like behaviors. (A) Schematic diagram of the social preference test of adult male zebrafish. (B) The shank2b−/− zebrafish displayed a significantly reduced preference for conspecifics compared to WT (time ratio, ** p = 0.0063; distance ratio, ** p = 0.0057; WT, n = 12, shank2b−/−, n = 18, Student’s t test). Data are shown as mean ± SEM. (C) Schematic diagram of the kin preference test of adult male zebrafish. (D) The shank2b−/− zebrafish significantly preferred spending time exploring non-kin sector to interact with red fish (n = 15, * p = 0.0278, paired t test), whereas WT zebrafish preferred conspecifics (n = 11, * p = 0.0498, paired t test). (E) In addition, the mutants displayed a significantly reduced kin preference index compared to WT zebrafish (time ratio, ** p = 0.0018; distance ratio, *** p = 0.007; Student’s t test). Data are shown as mean ± SEM. (F) The stereotyped swimming patterns in zebrafish are shown as “small circle” and “walling”. (G) The shank2b−/− zebrafish at 4 mpf exhibited a trend of higher frequency of “small circle” stereotyped behavior (p = 0.0941) and significant increase in “walling” stereotyped behavior (* p = 0.0284), Mann–Whitney test. (H) Schematic diagram of the open field test. (I) The velocity of shank2b mutant at 2 mpf, quantified by average distance moved per one second over 30 min in this test, was increased compared to that of WT zebrafish (WT n = 20, shank2b−/− = 22, **** p < 0.0001, Student’s t test). Data are shown as mean ± SEM. (J) Representative traces of individual WT and shank2b−/− zebrafish at 2 mpf in the thigmotaxis test. The shank2b−/− zebrafish tended to stay in the center area so that distance ratio of thigmotaxis was decreased significantly (WT n = 18, shank2b−/− = 22, * p = 0.0104, Student’s t test). Data are shown as mean ± SEM. (K) Light and dark experimental setup for analysis of larvae at 7 days post-fertilization (dpf). (L) The horizontal axis denotes the time period of the alternating light and dark conditions. The vertical axis shows the ratio of distance moved in the center ring. (M) Under illumination, the shank2b−/− larvae at 7 dpf exhibited a significant decrease during L0 period and a trend of decrease during L1 period. The results also showed significant decrease under dark condition (D1) compared to WT larvae. L0 (left), ** p = 0.0036; D1 (middle), ** p = 0.0033; L1, p = 0.0663. WT larvae n = 36, shank2b−/− larvae n = 34. Student’s t test. Data are shown as mean ± SEM.
Also consistent with an ASD-like phenotype, adult shank2b−/− zebrafish spent more time engaged in repetitive and stereotyped swimming behaviors in the open field, especially stereotypic wall swimming (walling) (Figure 2G). Mutants also exhibited a greater frequency of small circle swimming, although the difference from WTs did not reach statistical significance (Figure 2G). Overall motor activity in the open field was also significantly greater among adult shank2b−/− zebrafish than WTs (Figure 2I). Moreover, the proportion (%) of total distance traveled in the peripheral zones of a new tank (thigmotaxis) was significantly lower among adult shank2b−/− fish (Figure 2J), suggesting higher trait anxiety. Similarly, the proportion of swimming distance in the tank periphery relative to the center was greater among zebrafish larvae at 7 days post-fertilization (dpf) compared to age-matched WTs (Figure 2L,M). Larval shank2b−/− fish also demonstrated a significant decrease in the center distance ratio during the first dark period (D1) of the dark–light cycle compared to WT larvae, as well as a numerical reduction during the first light period (L1). Thigmotaxis was also greater among shank2b−/− larvae at 7 dpf under continuous illumination (L0). Collectively, these findings suggest that shank2b deficiency leads to an enhanced anxiety-like behavioral phenotype.
2.3. Abnormal Responses to Visual and Auditory Stimuli among shank2b−/− Larvae
Humans with SHANK2 deficiency are reported to have atypical sensory processing [20,22]. To determine if shank2b larvae exhibit similar sensory processing abnormalities, we compared visual motor responses (VMRs) to light–dark or dark–light transitions and startle responses to high-intensity sound stimuli between mutants and WT larvae.
Zebrafish larvae normally demonstrate dramatically enhanced activity in response to an abrupt light-to-dark transition (darkness stimulation) (Supplementary Figure S3B–D) and a brief period of hypoactivity when exposed to a sudden dark-to-light transition (light stimulation) (Figure 3B–D). When exposed to abrupt light stimulation, however, 13 dpf shank2b−/− larvae showed no significant change in activity, and rather than the expected hypoactivity, some animals exhibited increased activity in response to a second or third dark-to-light transition (Figure 3E–G). Overall, there was a significant difference in activity during dark-to-light transitions between WT and shank2b−/− mutant larvae (Figure 3H). In contrast, both genotypes exhibited increased activity in response to a light-to-dark transition (Supplementary Figure S3E–G) and there was no significant difference in the magnitude of this change between genotypes over three light-to-dark transition cycles (Supplementary Figure S3H).
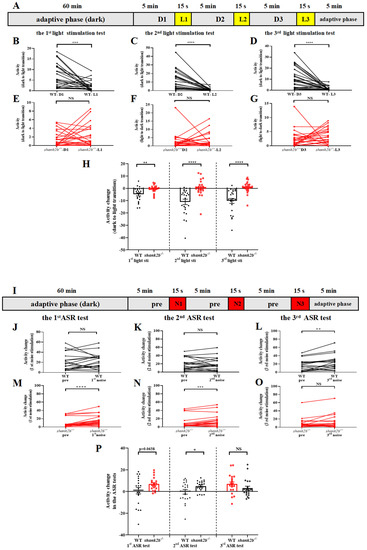
Figure 3.
Abnormal response to visual and auditory stimuli in shank2b larva. (A) Scheme and behavioral setup applied for locomotor activity tracking in VMR response to light stimulation of zebrafish larvae at 13 dpf. The experiment consisted of a 60-min adaptation period under continuous dark and a 20 min 45 s testing period consisting of three VMR. The VMR experiment consisted of 5 min of conditioning to darkness and 15 s of stimulation by a sudden light stimulus. (B–D) WT larvae at 13 dpf exhibited normal decrease in response to sudden light stimuli (1st light stimuli, *** p = 0.0006; 2nd light stimuli, **** p < 0.0001; 3rd light stimuli, **** p < 0.0001. n = 23, paired t test), (E–G) whereas shank2b−/− models showed completely different response during dark–light transitions, characterized by dynamic slight increases in activity without statistical significance (1st light stimuli, ns p = 0.3395; 2nd light stimuli, ns p = 0.7621; 3rd light stimuli, ns p = 0.3446. n = 24, paired t test). (H) Column plots compare activity detected during the 1 min before and the 15 s after each light stimulation exposure between WT and shank2b mutants. Each transition from dark to light induced significant difference in activity change between WT and shank2b larva (1st light stimuli, ** p = 0.0029; 2nd light stimuli, **** p < 0.0001; 3rd light stimuli, **** p < 0.0001. WT, n = 23, shank2b−/− n = 24, Student’s t test). Data are presented as the mean ± SEM. (I) Scheme and behavioral setup applied for locomotor activity tracking in AMR response to loud noise stimulation of zebrafish larvae at 13 dpf. The experiment consisted of a 60 min adaptation period and a 20 min 45 s testing period consisting of three AMR tests. An AMR experiment consisted of 5 min of conditioning to ambient sound and 15 s of stimulation by a sudden loud noise. (J–L) WT larvae at 13 dpf exhibited a significant and robust increase in activity induced by the third exposure to the sudden noise stimulus after twice being exposed to ASR assays (1st noise stimuli, ns p = 0.8148; 2nd noise stimuli, ns p = 0.8905; 3rd noise stimuli, ** p = 0.0028. n = 20, paired t test). (M–O) The first and second noise stimuli induced significant increase in activity of shank2b−/− larvae (1st noise stimuli, **** p < 0.0001; 2nd noise stimuli, *** p = 0.0003; 3rd noise stimuli, ns, p = 0.1257. n = 20, paired t test). (P) Column plots compare activity detected during the 1 min before and the 15 s after each loud noise stimulation exposure between WT and shank2b mutants. Similarly, in first two sudden loud noise stimulations, the velocity change of shank2b larva at 13 dpf was more dramatic than that of the WT larvae (1st noise stimuli, p = 0.0638; 2nd noise stimuli, * p = 0.0369; 3rd dark noise, ns p = 0.1645. WT n = 20, shank2b−/− n = 20, Student’s t test). Data are presented as the mean ± SEM.
Zebrafish larvae normally respond to sudden broad-band noise with a dramatic increase in motor activity (Figure 3J,K), which is analogous to the acoustic startle response (ASR) observed in rodents and humans. Furthermore, in accord with ASRs in other animals, WT larvae at 13 dpf demonstrated a degree of habituation upon repeated stimulus presentation. However, mutants exhibited both markedly enhanced activity compared to WTs in response to the first stimulus, indicating greater baseline ASR sensitivity, as well as a greater response to the second stimulus (Figure 3M,N) compared to WTs (Figure 3L), indicating reduced habituation. The ASR of mutants was also numerically greater than that of WTs in response to a third stimulus, although the difference did not reach statistical significance (Figure 3P).
2.4. GABAergic Deficits in shank2b−/− Zebrafish
Atypical sensory responses in ASD mouse models are associated with alterations in GABAergic interneuron development [31,43,44]. To examine if alterations in GABAergic neuron development or signaling also occur in shank2b mutants, we compared the mRNA levels of GABA receptor subunits between adult WT and shank2b−/− mutants. Indeed, RT-qPCR revealed significant decreases in the mRNA expression levels of gabra1, gabra2a, gabra4, gabra5, gabra6b, gabrb2a, gabrg1, and gabrd in shank2b−/− zebrafish compared to WTs (Figure 4A). Moreover, GABAA receptor α1 protein expression was also significantly reduced in mutants, suggesting GABAergic system dysfunction and a concomitant excitatory shift in the excitation/inhibition balance (Figure 4B). To provide further evidence for such a shift, we examined the susceptibility of WT and mutant larvae to seizures induced by the GABAA receptor antagonist pentylenetetrazol (PTZ), which is widely used to induce seizures in both rodent models and zebrafish. PTZ-induced seizures appear as suppression in the expected motor activity increase following a sudden transition from light to darkness. Consistent with an excitatory shift in excitation/inhibition balance due to deficient GABA receptor signaling, shank2b mutants demonstrated greater sensitivity to PTZ-induced behavior changes (Supplementary Figure S4B–D,H,I). Moreover, PTZ treatment induced significantly more activity change in shank2b−/− larva at 9 dpf compared to age-matched WT larvae during the light–dark transition (Figure 4D–G). Collectively, our findings provide strong evidence that neurodevelopmental GABAergic signaling deficits underlie the ASD-like behavioral abnormalities in shank2b mutants.
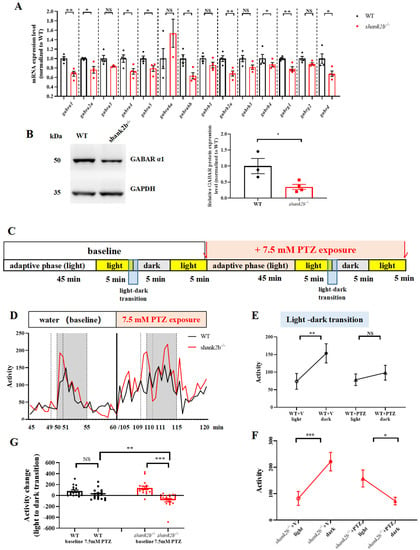
Figure 4.
The shank2b mutants display GABAergic deficits. (A) RT-qPCR showed altered expression levels of GABAR subunits in adult shank2b−/− brain tissue. gabra1, ** p = 0.003; gabra2a, * p = 0.0394; gabra3 ns p = 0.0993; gabra3 ns p = 0.0660; gabra4, * p = 0.0341; gabra5, * p = 0.0371; gabrg2 ns p = 0.0730; gabra6a ns p = 0.3488; gabra6b, * p = 0.0138; gabrb1 ns p = 0.1258; gabrb2a, ** p = 0.0083; gabrg1, ** p = 0.005; gabrd, * p = 0.0318; n = 3–4 for each group. Student’s t test. Data are presented as the mean ± SEM. (B) Western blot analysis of GABA A receptor α1 protein in adult shank2b−/− brain tissue. * p = 0.0313, WT n = 3, shank2b−/− n = 4, Student’s t test. Data are presented as the mean ± SEM. (C) Pentylenetetrazol (PTZ)-treated behavior experimental procedure. (D) The experiment consisted of basal activity change during light to dark transition and 7.5 mM PTZ-treated induced activity change exposure to the same basal experiment. (E) WT larva at 9 dpf exhibited a dynamic increase of activity during light–dark transitions (WT + v (vector) light vs. WT + v dark, ** p = 0.0022,; n = 17, paired t test). Remarkably, PTZ concentrations of 7.5 mM of WT larva at 9 dpf did not elict a decline in activity (WT + PTZ light vs. WT + PTZ dark, ns, p = 0.3869, n = 17, paired t test). (F) However, 7.5 mM PTZ elicited a decline in activity of shank2b mutants (shank2b−/− + V light vs. shank2b−/− + V dark, *** p = 0.0005; shank2b−/− + PTZ light vs. shank2b−/− + PTZ dark, * p = 0.0157; n = 16, paired t test). (G) Interestingly, the activity change of shank2b larvae at 9 dpf after 7.5 mM PTZ treatment was more than the activity change before PTZ treatment (shank2b−/− − baseline vs. shank2b−/− + PTZ, *** p = 0.0006, n = 16, paired t test), and even greater than the activity change after WT larvae treated with PTZ (WT + PTZ vs. shank2b−/− + PTZ, ** p = 0.0096, WT + PTZ n = 17, shank2b−/− + PTZ n = 16 Student’s t test). Remarkably, WT larva at 9 dpf did not have significant difference in the activity change during light–dark transition before and after 7.5 mM PTZ treatment (WT-baseline vs. WT + PTZ, ns p = 0.0961, n = 17, paired t test).
3. Discussion
Human genetic studies have implicated SHANK2 in a wide spectrum of neurodevelopmental conditions, including autism symptoms, intellectual disability, hyperactivity, and anxiety. Two out of three patients in our clinical cohort screen harboring SHANK2 mutations also exhibited hyper-sensitivity to visual, auditory, and tactile stimuli, but there have been few descriptions of the SHANK2-deficient sensory phenotype. Aberrant sensory reactivity is now regarded as a diagnostic feature of ASDs, but only one of the previous 18 patients known to harbor de novo SHANK2 variants has received detailed examinations of atypical sensory processing. The current study expands the spectrum of potential behavioral phenotypes exhibited by shank2-deficient animal models. Furthermore, this phenotypic profile is generally consistent with the spectrum of clinical features ascribed to SHANK2-related disorders in humans. These shank2b mutant zebrafish were hyperactive in the open field and displayed an anxiety-like phenotype under continuous illumination, reduced social preference and kin preference, and greater stereotypy. Further, shank2b mutants showed reduced expression levels of GABA receptor subunit mRNAs and α1 subunit protein, and increased sensitivity to drug-induced seizures. Intriguingly, several molecules involved in GABAergic signaling have been implicated in autistic sensory symptoms [11]. Taken together, our data suggest that shank2b−/− zebrafish are a valuable model for dissecting the pathophysiology of ASD in humans, and that the sensory abnormalities of ASD may stem at least in part from reduced GABA receptor activity.
Studies have shown that two lines of Shank2 knockout mice altering different regions of the protein displayed similar autism behavioral phenotypes [40,41], which were different from bipolar-associated mania-like behavior exhibited in the third line of Shank2Δex24 knockout mice [42]. Generally, all three conventional and other conditional Shank2-deficient mouse models showed distinct neuropsychiatric-like phenotypes, having no sensory abnormalities except hyposensitivity to painful stimuli. Here, we created the shank2b−/− model in zebrafish to test the common correlation of autism and sensory processing problems. Potential sensory processing deficits associated with shank2b deficiency were examined in detail using two light-stimulation-based tasks and one noise-stimulation-based task, all of which revealed enhanced stimulus sensitivity in mutant larvae compared to WT larvae. Mutants exhibited an exaggerated acoustic startle response and reduced ASR habituation as well as relative hyperactivity in response to sudden transitions from darkness to light. Our current behavioral study suggests that this visual response hyperactivity contributes to reduced kin preference. Similarly, visual hypersensitivity has been observed in several mouse models of ASD and related neurodevelopmental disorders, including lines with mutations/deficiencies in Mecp2, Fmr1, Shank3, Gabrb3, and Cntnap2 [31,43,45,46,47,48]. These abnormal sensory behaviors support the face validity of the zebrafish model for studying SHANK2-related ASD in humans.
These ASD-related sensory abnormalities and other symptomatic behaviors may arise from an imbalance between synaptic excitation and inhibition [28] due to insufficient inhibitory GABA neurotransmission [49]. In turn, GABAergic dysfunction may be a downstream consequence of mutations in genes essential for synaptic formation, signaling, and molecular organization [49], as several mouse lines harboring putative ASD mutations show disrupted inhibitory signaling. For example, deletion of the autism-risk gene contactin-associated protein 2 (Cntnap2) reduced the number of GABAergic interneurons in mouse cortex, including fast-spiking parvalbumin-immunopositive interneurons [50]. Zebrafish cntnap2ab mutants also exhibited reductions in GABAergic neuron numbers, particularly of parvalbumin-positive (PV+) interneurons [51]. In mice lacking Shank3, PV+ interneurons were decreased in the somatosensory cortex (S1) and basolateral amygdala (BLA) [45]. While Shank family proteins are enriched in the excitatory postsynaptic density, numerous studies also suggest widespread expression in GABAergic neurons, including PV-positive interneurons [52,53,54]. For instance, PV immunoreactivity was also reduced in Shank1 knockout mice [55] and GABAergic neurotransmission was impaired in Shank2 knockout mice [56].
Interneurons are critically involved in sensory processing [54], and the aforementioned associations between shank gene deficiencies and GABAergic dysfunction in the CNS suggest that shank2 and other family members act as part of a biological network regulating GABAergic signaling in multiple sensory systems, leading to the sensory processing abnormalities observed in ASD. In our study, not only did these mutant fish display sensory phenotypes consistent with humans, they also had decreased expression levels of GABA receptor as well as increased sensitivity to PTZ-induced seizures. RT-qPCR analysis revealed reduced mRNA expression levels of subunit genes gabra1, gabra2a, gabra4, gabra5, gabra6b, gabrb2a, gabrg1, and gabrd, and multiple GABAA receptor subunits, including GABRG1, GABRA2, GABRA4, and GABRA5 have been linked to autism by genetic analyses [57,58,59]. Expression levels of GABAA receptor α1 protein, which plays a key role in defining the critical period for plasticity in developing rodent visual cortex [60], was also significantly reduced in shank2b−/− zebrafish. These GABAA receptor gene and protein deficits were associated with hyperactivity under illumination as well as increased sensitivity to PTZ-induced seizures, suggesting loss of inhibitory drive in mutant zebrafish brain. GABAA receptor activity is critical for neurodevelopment by stimulating neural progenitor cell proliferation, migration, and differentiation, neurite growth, and synaptogenesis [61]. Furthermore, GABAergic interneurons help establish the receptive field organization of sensory cortex. Moreover, there is now strong convergent evidence for abnormal expression patterns of GABAA receptor genes and other genes expressed at relatively high levels by GABA interneurons in idiopathic ASD [49,62]. Thus, we suggest that shank2 contributes to GABAergic network development in zebrafish, and that loss of shank2 expression during development results in aberrant sensory system organization and sensory processing throughout life, which in turn contributes to other ASD-like behavioral phenotype.
The duplicated and conserved shank2a and shank2b are similar and share high sequence identity at the overall protein structure (Supplementary Table S5), whereas a loss of an evolutionary conserved domain of shank2a can be detected (Supplementary Figures S5 and S6). In contrast, several important protein-ANK, PDZ and SAM C-terminal region, of human SHANK2 are more highly conserved in shank2b than shank2a (Supplementary Tables S3 and S4). Therefore, shank2b is more suitable as a model for human SHANK2. However, based on the expression of shank2a and shank2b transcripts in the brain of zebrafish, we also speculate that both paralogs may be important for the formation of the nervous system in zebrafish. It would be interesting to compare the phenotypes of shank2a−/−, shank2b−/− and shank3&b double homozygous models in parallel. Another limitation of this study is that we did not directly analyze GABAergic interneuron development or examine whether GABA agonists mitigate the behavioral abnormalities of shank2b mutants. Moreover, shank3&b deficient zebrafish model should be more appropriate to fully unravel the molecular mechanisms by which shank2 regulates GABAergic network development and behavior in zebrafish.
In conclusion, we revealed ASD-like behavioral and sensory deficits associated with GABAergic system dysfunction in a novel shank2b-deficient zebrafish model (Figure 5). This model is a valuable tool for dissecting the pathophysiology of SHANK2-related disorders in humans and may assist in the development of new treatments for ASD with abnormal sensory processing.
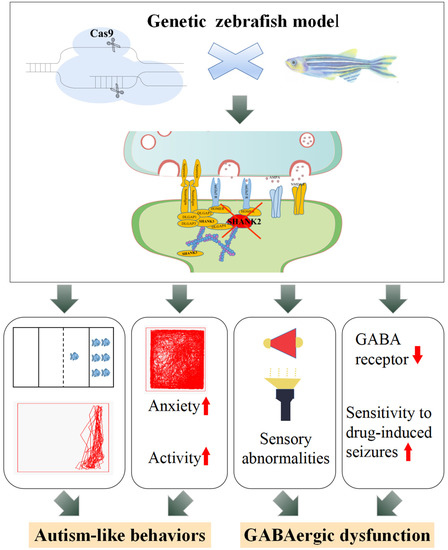
Figure 5.
Potential mechanisms for regulation of GABAergic system in the shank2-deficient zebrafish model.
4. Materials and Methods
4.1. Fish Maintenance and Husbandry
Zebrafish of the Tubingen (Tu) strain were housed at the Institute of Zebrafish, Children’s Hospital of Fudan University of China (Shanghai, China). Both larvae and adults were maintained in a recirculation system at 28.5 °C under a 14 h/10 h light/dark circadian cycle. All animal experiments were performed in compliance with the Guiding Principles for the Care and Use of Laboratory Animals and received approval from the institutional animal care committee of the Children’s Hospital of Fudan University.
4.2. Generation of shank2b-Deficient Zebrafish Using the CRISPR-Cas9 System
The zebrafish shank2b gene and exon/intron boundaries were identified by searching the NCBI database (gene ID: shank2b NC_007136.7), and a mutation in shank2b was generated using CRISPR/Cas9 editing as previously reported [63]. The site-specific single guide RNA (sgRNA) of shank2b was designed to target the 10th exon sequence 5′-GGATCGGAGCAGCACTCGCG-3′ and synthesized by in vitro transcription using the MAXIscript™ T7 kit (AM1314M, Invitrogen, Waltham, MA, USA). A micro-injector was then used to inject 150: 600 pg of sgRNA: cas9 Nuclease (En GenTM spy cas9 NLS #M0646, New England Biolabs, Ipswich, MA, USA) into one-cell-stage fertilized WT zebrafish embryos (F0). Injected embryos were raised for 72 h before preparing genomic DNA to check mutagenic efficiency using the primers listed in Supplementary Table S1 and Sanger sequencing. Identified mutations were then analyzed for unique restriction digest sites usable for genotyping. Progeny were propagated via a series of out-crossings with WT zebrafish and genotyping of each generation. Eventually, these animals were in-crossed to obtain the homozygous deficient shank2b−/− mutants.
4.3. RT-qPCR
Changes in gene expression during development were assessed by RT-qPCR analysis of embryos at 24 hpf, 48 hpf, 3 dpf, and 5 dpf. All analyses included three or four independent embryo pools with 25 embryos per pool. In addition, brains were collected from larvae at 3 weeks post-fertilization (wpf) and adults at 2 months post-fertilization (mpf) (three independent brain pools, 10 fish per pool). Brain, heart, liver–gall–spleen, skin–muscle, and spermatic cord tissues were also collected from adult male zebrafish and ovaries from adult female zebrafish at 4.5 mpf (three pools per tissue type, 5 fish per pool), and quick-frozen on dry ice. Total RNA was extracted from harvested tissues and whole embryos using the MiniBEST Universal RNA Extraction kit (No. 9767, Takara, Japan) according to the manufacturer’s protocol and reverse transcribed to cDNA using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (RR047A, Takara, Japan) following the manufacturer’s instructions. RT-qPCR was performed using an Applied Biosystems™ 7500 system (Bio-Rad, USA) and TB Green Premix Ex Taq II (Tli RNaseH Plus) (RR820A, Takara, Japan) to estimate the mRNA expression levels of shank2b, shank2a, and GABA receptor subunits according to the manufacturers’ protocols. Expression levels were normalized to that of Rpl13α as the internal control using the delta CT method. The primer sequences for RT-qPCR are listed in Supplementary Table S1. All measurements were conducted on at least three independently harvested pools of tissue or whole embryos.
4.4. Western Blot
A custom antibody-recognizing zebrafish shank2a and shank2b (RB1661) was generated by HUABIO using a peptide antigen shared by both proteins (CRSLSMPDTSEDIPP-amide, corresponding to amino acids 860 to 873 of shank2). For Western blotting, total protein was isolated from the brains of 4.5 mpf WT and shank2b−/− zebrafish, separated by SDS-PAGE (50 μg per gel lane), and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% BSA or 7% non-fat skim milk powder at room temperature for 2 h, incubated with an affinity-purified primary antibody against GABARα (Abcam, ab211131, 1:1000) overnight at 4 °C, washed in TBS containing 0.1% Tween-20 (TBST), and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000) for 1 h at room temperature. Following three washes in TBST, the blots were incubated with ECL reagent (BeyoECL Plus, P0018M) and exposed to Kodak X-ray film (Tanon 5200). The gray scale values of proteins were analyzed by ImageJ software (NIH, Bethesda, MD, USA), and normalized to GAPDH (1:5000) as the gel-loading control.
4.5. Larval Responses to Light and Darkness
Larval zebrafish at 7 dpf were placed individually into the wells of 24-well plates for video recording of activity using the Zebrabox system (ViewPoint Life Sciences, Lyons, France). Every larva was given 45 min to habituate to the illumination environment before video acquisition. Subsequently, larvae were exposed to 5 min of continuous illumination, followed by one dark/light cycle (5 min of dark and 5 min of light, Figure 2K). The light intensity was 100% in the light period and 0% in the dark period. The time and distance traveled were recorded every 30 s using the Viewpoint tracking system and custom software. The total recording time was 60 min. For analysis, a circle was drawn at the center of each well that divided the well into inner and outer regions of equal area (Figure 2K) and was thigmotaxis defined as,
4.6. Acoustic Startle Response
Wild type and shank2b−/− larvae were gathered at 13 dpf to evaluate the ASR following a previously published method [64]. Briefly, larvae were placed individually in wells of 24-well plates and exposed to broad-band noise within a ZebraBox equipped with an infrared illuminator for imaging in the dark. The broad-band noise was computer generated and played through two commercial loudspeakers placed inside but not physically connected to the chamber. Video footage was shot at 25 frames per second (fps) and binned into 1-s time windows to evaluate the ASR. After a 60 min acclimatization period, zebrafish were monitored during three individual ASR experiments which consisted of 5 min conditioning to ambient sound and 15 s of stimulation by a sudden loud noise (Figure 3I). The activity counts in groups during the 1 min before and the 15 s during each sudden sound exposure were quantified and used to calculate the ASR according to the equation
Activity change in the ASR tests = Average activity during 15 s-noise phase (post) − Average activity during 60 s-pre-noise (pre)
4.7. Visual Motor Behavior Assay
Larvae at 13 dpf were pipetted individually into the wells of 24-well plates and visual motor behavior analyzed during light-to-dark and dark-to-light transitions. Briefly, the specifications for video acquisition and tracking were the same as those in the ASR assay. For analysis of behavioral changes during light-to-dark transitions, fish were acclimated to the observation chamber under continuous maximum white light illumination for one hour (adaptive phase). Then, three illumination-off cycles (5 min illumination, followed by darkness) were delivered (Supplementary Figure S3A). For analysis of behavioral changes during dark-to-light transitions, fish were acclimated to the observation chamber under darkness for 1 h (adaptive phase). Then, three illumination-on cycles (5 min darkness, followed by 15 s light) were delivered (Figure 3A).
The activity changes were analyzed for both WT and shank2b mutants according to the equations
Activity change (light to dark transition) = Activity detected in post dark 15 s − Activity detected in pre illumination 60 s
Activity change (dark to light transition) = Activity detected in post illumination 15 s − Activity detected in pre dark 60 s
4.8. Pentylenetetrazol Activity Assay
To assess behavioral alterations and seizure susceptibility as indices of an E/I imbalance, we measured the dose–response of 9 dpf larvae to the GABAA receptor antagonist PTZ. Wild type and shank2b−/− larvae were placed individually in wells of 24-well plates containing standard embryo water plus different concentrations of PTZ (P6500, Sigma-Aldrich, Zwijndrecht, The Netherlands) pre-dissolved in distilled water. Briefly, four treatment groups were established: WT-standard water, WT-PTZ, shank2b−/−-standard water, and shank2b−/−-PTZ. The PTZ was first dissolved in distilled water and then further in methylene blue diluted system water (0.15 mg/L methylene blue, 8.01 mg/L NaCl, 5.04 mg/L KCl, 5.50 mg/L Na2HPO4, 0.44 mg/L KH2PO4, 1.30 mg/L CaCl2, 1.00 mg/L MgSO4, and 4.20 mg/L NaHCO3) to yield working solutions. Subsequently, 500 μL of PTZ working solution was quickly added to 500 μL standard egg water per well to reach a final volume of 1 mL/well containing 1, 2.5, 5, or 7.5 mM PTZ. The plates were then placed in a custom-modified Zebrabox to record video of zebrafish larval activity. After a 5 min acclimation period with illumination, spontaneous activity was recorded for 45 min. Animals were then exposed to one 10 min light–dark cycle (5 min illumination followed by 5 min dark) to examine responses to changes in lighting conditions under the influence of PTZ. Responses were analyzed by comparing the distance traveled in the one min before and after transition from light to darkness. Each experimental session lasted for 60 min in total, including the acclimation period (Supplementary Figure S4A).
We used a novel behavioral test to determine whether 7.5 mM PTZ, selected because it induced abnormal activity in shank2b mutants, elicited the greatest differential response in WT versus mutant fish at 9 dpf. Briefly, shank2b−/− and WT larvae at 9 dpf were pipetted individually into the wells of 24-well plates containing 1 mL of standard embryo water and activity recorded using the paradigm described above. Subsequently, 500 μL of egg water was removed and 500 μL of PTZ in egg water was added to the well to yield a final PTZ concentration of 7.5 mM. We used a repeated light–dark challenge assay to elicit PTZ-induced responses (Figure 4C). Responses were analyzed by normalizing the post-PTZ activity of each WT and shank2b mutant fish to baseline.
4.9. Open Field Test
The locomotor activity of adult zebrafish was tested at 2–4 mpf in an adapted open field paradigm (Figure 2H). Videos were captured in a 30 × 30 × 30 cm opaque tank filled with system water using a suspended camera. Each male zebrafish was habituated in the tank for 5 min before the 30 min recording period. Time in motion and distance traveled were analyzed every 30 s using Zebralab software (ViewPoint Life Sciences, Lissieu, Calvados, Lower Normandy Region, Lyons, France). For analysis of stereotyped behaviors, an experimenter blind to genotype and treatment history scored the swimming pattern each minute from the transformational visual route of fish trajectory, and counted the frequency of two stereotyped swimming patterns, small circling and walling (Figure 2F).
The locomotor activity of adult zebrafish was tested at 2–4 mpf in an open field paradigm (Figure 2H). Videos were captured in a 30 × 30 × 30 cm opaque tank filled with system water using a suspended camera right above. Each male zebrafish was habituated in the tank for 5 min before the 30 min test. The time and distances were collected and analyzed every 30 s from the 30 min recorded video using Zebralab software. For stereotyped behaviors, the experimenter was required, under double-blind control, to score the swimming pattern within each minute of the Zebralab-software-generated visual route of fish trajectory, and count the number of the four stereotyped swimming pattern episodes separately, specifically, small circling and walling (Figure 2F).
Thigmotaxis, a well-validated sign of anxiety in adult zebrafish, was scored as
4.10. Three-Chamber Social Preference Tests
Social preference was tested in a transparent mating tank (dimensions 21 × 11 × 7.5 cm) separated into three chambers by two transparent dividers. Six male WT conspecifics of similar size were placed into one outer chamber while the other outer chamber was left unoccupied (Figure 2A). Then, the subject male zebrafish (WT or mutant) at 3.5 mpf was placed into the middle chamber and given five minutes of free access to the entire apparatus. Videos were recorded for 30 min. Social preference behavior was quantified as the distance or time spent adjacent to the group of conspecifics. The distance ratio was the distance traveled in the conspecific sector adjacent to the conspecific chamber divided by the total distance traveled, and the time ratio was the time spent in the conspecific sector adjacent to the conspecific chamber divided by the total test time.
To further assay the sociality of the shank2−/− zebrafish, we performed a kin preference test in the same tank. Briefly, three adult kin zebrafish were placed in the left outer chamber and three adult non-kin zebrafish were placed in the right outer chamber (Figure 2C). Then, the subject male zebrafish (WT or mutant) at 3.5 mpf was placed into the middle chamber. Social preference was assessed by a social preference index (SPI) where
4.11. Statistical Analysis
All data were processed using the GraphPad Prism Software (GraphPad Software, San Diego, CA, USA). Significance of differences were determined either with one-tailed Student’s t test, Mann–Whitney test, or paired t test, as indicated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24032208/s1.
Author Contributions
X.X. conceived the project. Y.W., C.L., Q.X. and H.L. designed the experiment. Y.W. conducted experiments and analyzed the data. C.L., J.D. and Q.L. assisted the generation of zebrafish shank2b mutant. C.L. and J.L. provided technical assistance in behavioral tests. M.H. helped conduct biochemical experiments. C.H. collected and collated the phenotypic data. Y.W. and X.X. wrote the manuscript. All authors contributed to the editing of the final intellectual product. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (2021NSFC: 82171540 and 82101945), the Key Subject Construction Project of Shanghai Municipal Health Commission (No. shslczdzk02903) and the National Natural Science Foundation of China (2017NSFC: 61733011).
Institutional Review Board Statement
The authors declare that all experiments involving zebrafish were ethical. All of our experiments were conducted in compliance with the standard ethical guidelines and under the control of the Institute of Zebrafish, Children’s Hospital of Fudan University.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Yonghui Jiang (Yale School of Medicine) and Mu Yu (Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for valuable comments on this project. We would also like to warmly thank all the patients who kindly consented to participate in our study.
Conflicts of Interest
The authors declare no competing interests.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef]
- Zachor, D.A.; Ben-Itzchak, E. The Relationship between Clinical Presentation and Unusual Sensory Interests in Autism Spectrum Disorders: A Preliminary Investigation. J. Autism Dev. Disord. 2013, 44, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Molholm, S.; Del Bene, V.A.; Frey, H.-P.; Russo, N.N.; Blanco, D.; Saint-Amour, D.; Ross, L.A. Severe Multisensory Speech Integration Deficits in High-Functioning School-Aged Children with Autism Spectrum Disorder (ASD) and Their Resolution during Early Adolescence. Cereb. Cortex 2013, 25, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Toth, K.; Abbott, R.; Osterling, J.; Munson, J.; Estes, A.; Liaw, J. Early Social Attention Impairments in Autism: Social Orienting, Joint Attention, and Attention to Distress. Dev. Psychol. 2004, 40, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Watson, L.R.; Boyd, B.A.; Poe, M.D.; David, F.J.; McGuire, L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev. Psychopathol. 2013, 25, 307–320. [Google Scholar] [CrossRef]
- Estes, A.; Network, I.; Zwaigenbaum, L.; Gu, H.; John, T.S.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Meltzoff, A.N. The importance of eyes: How infants interpret adult looking behavior. Dev. Psychol. 2002, 38, 958–966. [Google Scholar] [CrossRef]
- Turner-Brown, L.M.; Baranek, G.T.; Reznick, J.S.; Watson, L.R.; Crais, E.R. The First Year Inventory: A longitudinal follow-up of 12-month-old to 3-year-old children. Autism 2012, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.A.; Baranek, G.; Sideris, J.; Poe, M.D.; Watson, L.R.; Patten, E.; Miller, H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Res. 2010, 3, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Balasco, L.; Provenzano, G.; Bozzi, Y. Sensory Abnormalities in Autism Spectrum Disorders: A Focus on the Tactile Domain, from Genetic Mouse Models to the Clinic. Front. Psychiatry 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Ehlers, M.D. Modeling Autism by SHANK Gene Mutations in Mice. Neuron 2013, 78, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef]
- Berkel, S.; Marshall, C.R.; Weiss, B.; Howe, J.; Roeth, R.; Moog, U.; Endris, V.; Roberts, W.; Szatmari, P.; Pinto, D.; et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010, 42, 489–491. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef]
- Wischmeijer, A.; Magini, P.; Giorda, R.; Gnoli, M.; Ciccone, R.; Cecconi, I.; Franzoni, E.; Mazzanti, L.; Romeo, G.; Zuffardi, O.; et al. Olfactory Receptor-Related Duplicons Mediate a Microdeletion at 11q13.2q13.4 Associated with a Syndromic Phenotype. Mol. Syndr. 2010, 1, 176–184. [Google Scholar] [CrossRef]
- Doddato, G.; Fabbiani, A.; Scandurra, V.; Canitano, R.; Mencarelli, M.A.; Renieri, A.; Ariani, F. Identification of a Novel SHANK2 Pathogenic Variant in a Patient with a Neurodevelopmental Disorder. Genes 2022, 13, 688. [Google Scholar] [CrossRef]
- Bowling, K.M.; Thompson, M.L.; Amaral, M.D.; Finnila, C.R.; Hiatt, S.M.; Engel, K.L.; Cochran, J.N.; Brothers, K.B.; East, K.M.; Gray, D.E.; et al. Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med. 2017, 9, 43. [Google Scholar] [CrossRef]
- Guo, H.; Wang, T.; Wu, H.; Long, M.; Coe, B.P.; Li, H.; Xun, G.; Ou, J.; Chen, B.; Duan, G.; et al. Inherited and multiple de novo mutations in autism/developmental delay risk genes suggest a multifactorial model. Mol. Autism 2018, 9, 64. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Li, Z.; Lin, X.; Li, J.; Wang, S.; Yang, C.; Wu, Q.; Ye, A.Y.; Wang, M.; et al. Targeted resequencing of 358 candidate genes for autism spectrum disorder in a Chinese cohort reveals diagnostic potential and genotype–phenotype correlations. Hum. Mutat. 2019, 40, 801–815. [Google Scholar] [CrossRef]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Heinrich, J.; Delorme, R.; Proepper, C.; Betancur, C.; Huguet, G.; Konyukh, M.; Chaste, P.; Ey, E.; Rastam, M.; et al. Genetic and Functional Analyses of SHANK2 Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders. PLoS Genet. 2012, 8, e1002521. [Google Scholar] [CrossRef] [PubMed]
- Caumes, R.; Smol, T.; Thuillier, C.; Balerdi, M.; Lestienne-Roche, C.; Manouvrier-Hanu, S.; Ghoumid, J. Phenotypic spectrum of SHANK2-related neurodevelopmental disorder. Eur. J. Med Genet. 2020, 63, 104072. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, H.; Huang, T.-N.; Chung, C.; Shin, W.; Kim, K.; Koh, J.-Y.; Hsueh, Y.-P.; Kim, E. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat. Commun. 2015, 6, 7168. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Foss-Feig, J.H.; Adkinson, B.D.; Ji, J.L.; Yang, G.; Srihari, V.H.; McPartland, J.C.; Krystal, J.H.; Murray, J.D.; Anticevic, A. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol. Psychiatry 2017, 81, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Pereira, A.C.; Velthuis, H.; Wong, N.M.; Ellis, C.L.; Ponteduro, F.M.; Dimitrov, M.; Kowalewski, L.; Lythgoe, D.J.; Rotaru, D.; et al. GABAB receptor modulation of visual sensory processing in adults with and without autism spectrum disorder. Sci. Transl. Med. 2022, 14, eabg7859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Deister, C.A.; Gao, X.; Guo, B.; Lynn-Jones, T.; Chen, N.; Wells, M.F.; Liu, R.; Goard, M.J.; Dimidschstein, J.; et al. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat. Neurosci. 2020, 23, 520–532. [Google Scholar] [CrossRef]
- Pereira, A.C.; Violante, I.R.; Mouga, S.; Oliveira, G.; Castelo-Branco, M. Medial Frontal Lobe Neurochemistry in Autism Spectrum Disorder is Marked by Reduced N-Acetylaspartate and Unchanged Gamma-Aminobutyric Acid and Glutamate + Glutamine Levels. J. Autism Dev. Disord. 2018, 48, 1467–1482. [Google Scholar] [CrossRef]
- Ferreira, H.; Sousa, A.C.; Sereno, J.; Martins, J.; Castelo-Branco, M.; Gonçalves, J. Sex-Dependent Social and Repetitive Behavior and Neurochemical Profile in Mouse Model of Autism Spectrum Disorder. Metabolites 2022, 12, 71. [Google Scholar] [CrossRef]
- Sousa, B.; Martins, J.; Castelo-Branco, M.; Gonçalves, J. Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder. J. Clin. Med. 2022, 11, 2839. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.; Wodka, E.L.; Harris, A.D.; Crocetti, D.; Tommerdahl, M.; Mostofsky, S.H.; Edden, R.A. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2016, 10, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Ratai, E.-M.; Kanwisher, N. Reduced GABAergic Action in the Autistic Brain. Curr. Biol. 2015, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Port, R.G.; Gaetz, W.; Bloy, L.; Wang, D.-J.; Blaskey, L.; Kuschner, E.S.; Levy, S.E.; Brodkin, E.S.; Roberts, T.P. Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Res. 2016, 10, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Singel, D.; Steinmetz, S.; Hepburn, S.; Brown, M.S. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage 2014, 86, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Nguyen, M.; Wong, K.; Poudel, M.K.; Kalueff, A.V. Developing zebrafish models of autism spectrum disorder (ASD). Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 50, 27–36. [Google Scholar] [CrossRef]
- Won, H.; Lee, H.-R.; Gee, H.Y.; Mah, W.; Kim, J.-I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.-L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.L.; Bey, A.L.; Wang, X.; Rossi, M.; Kim, Y.H.; Yan, H.; Porkka, F.; Duffney, L.J.; Phillips, S.M.; Cao, X.; et al. Deficiency of Shank2 causes mania-like behavior that responds to mood stabilizers. J. Clin. Investig. 2017, 2, e92052. [Google Scholar] [CrossRef]
- Möhrle, D.; Wang, W.; Whitehead, S.N.; Schmid, S. GABAB Receptor Agonist R-Baclofen Reverses Altered Auditory Reactivity and Filtering in the Cntnap2 Knock-Out Rat. Front. Integr. Neurosci. 2021, 15, 710593. [Google Scholar] [CrossRef]
- Razak, K.A.; Binder, D.K.; Ethell, I.M. Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome. Front. Psychiatry 2021, 12, 720752. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Mosko, J.R.; Morency, D.T.; Wells, M.F.; Tasnim, A.; Mozeika, S.M.; Ye, M.; Chirila, A.M.; Emanuel, A.J.; Rankin, G.; et al. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 2019, 178, 867–886.e24. [Google Scholar] [CrossRef] [PubMed]
- Gogolla, N.; Takesian, A.E.; Feng, G.; Fagiolini, M.; Hensch, T.K. Sensory Integration in Mouse Insular Cortex Reflects GABA Circuit Maturation. Neuron 2014, 83, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bonnan, A.; Bony, G.; Ferezou, I.; Pietropaolo, S.; Ginger, M.; Sans, N.; Rossier, J.; Oostra, B.; LeMasson, G.; et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci. 2014, 17, 1701–1709. [Google Scholar] [CrossRef]
- Orefice, L.L.; Zimmerman, A.L.; Chirila, A.M.; Sleboda, S.J.; Head, J.P.; Ginty, D.D. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 2016, 166, 299–313. [Google Scholar] [CrossRef]
- Coghlan, S.; Horder, J.; Inkster, B.; Mendez, M.A.; Murphy, D.G.; Nutt, D.J. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci. Biobehav. Rev. 2012, 36, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Peñagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Turner, K.J.; Fernandez, J.M.; Cifuentes, D.; Ghosh, M.; Ijaz, S.; Jain, R.A.; Kubo, F.; Bill, B.R.; Baier, H.; et al. Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Mao, W.; Watanabe, T.; Cho, S.; Frost, J.L.; Truong, T.; Zhao, X.; Futai, K. Shank1 regulates excitatory synaptic transmission in mouse hippocampal parvalbumin-expressing inhibitory interneurons. Eur. J. Neurosci. 2015, 41, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jr, J.L.H.; Schaaf, C.P.; Lu, H.; Chen, H.; Kang, H.; Tang, J.; Wu, Z.; Hao, S.; Cheung, S.W.; et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 2013, 503, 72–77. [Google Scholar] [CrossRef]
- Lee, S.; Lee, E.; Kim, R.; Kim, J.; Lee, S.; Park, H.; Kim, E. Shank2 Deletion in Parvalbumin Neurons Leads to Moderate Hyperactivity, Enhanced Self-Grooming and Suppressed Seizure Susceptibility in Mice. Front. Mol. Neurosci. 2018, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Filice, F.; Vörckel, K.J.; Sungur, A.; Wöhr, M.; Schwaller, B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol. Brain 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-S.; Kim, H.; Yu, N.-K.; Kang, S.J.; Kim, T.; Ko, H.-G.; Lee, J.; Yang, J.-E.; Ryu, H.-H.; Park, T.; et al. Enhancing inhibitory synaptic function reverses spatial memory deficits in Shank2 mutant mice. Neuropharmacology 2017, 112, 104–112. [Google Scholar] [CrossRef]
- Ashley-Koch, A.E.; Mei, H.; Jaworski, J.; Ma, D.Q.; Ritchie, M.D.; Menold, M.M.; Delong, G.R.; Abramson, R.K.; Wright, H.H.; Hussman, J.P.; et al. An analysis paradigm for investigating multi-locus effects in complex disease: Examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder. Ann. Hum. Genet. 2006, 70 Pt 3, 281–292. [Google Scholar] [CrossRef]
- Delong, R. GABA(A) receptor alpha5 subunit as a candidate gene for autism and bipolar disorder: A proposed endophenotype with parent-of-origin and gain-of-function features, with or without oculocutaneous albinism. Autism 2007, 11, 135–147. [Google Scholar] [CrossRef]
- Menold, M.M.; Shao, Y.; Wolpert, C.M.; Donnelly, S.L.; Raiford, K.L.; Martin, E.R.; Ravan, S.A.; Abramson, R.K.; Wright, H.H.; Delong, G.R.; et al. Association analysis of chromosome 15 GABAA receptor subunit genes in autistic disorder. J. Neurogenet. 2001, 15, 245–259. [Google Scholar] [CrossRef]
- Fagiolini, M.; Fritschy, J.M.; Low, K.; Mohler, H.; Rudolph, U.; Hensch, T.K. Specific GABAA circuits for visual cortical plasticity. Science 2004, 303, 1681–1683. [Google Scholar] [CrossRef]
- Represa, A.; Ben-Ari, Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005, 28, 278–283. [Google Scholar] [CrossRef]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Albadri, S.; De Santis, F.; Di Donato, V.; Del Bene, F. CRISPR/Cas9-Mediated Knockin and Knockout in Zebrafish. In Genome Editing in Neurosciences; Springer: Berlin/Heidelberg, Germany, 2017; pp. 41–49. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, Y.; Guo, N.; Li, Q. Sound shock response in larval zebrafish: A convenient and high-throughput assessment of auditory function. Neurotoxicol. Teratol. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).