Ipconazole Disrupts Mitochondrial Homeostasis and Alters GABAergic Neuronal Development in Zebrafish
Abstract
1. Introduction
2. Results
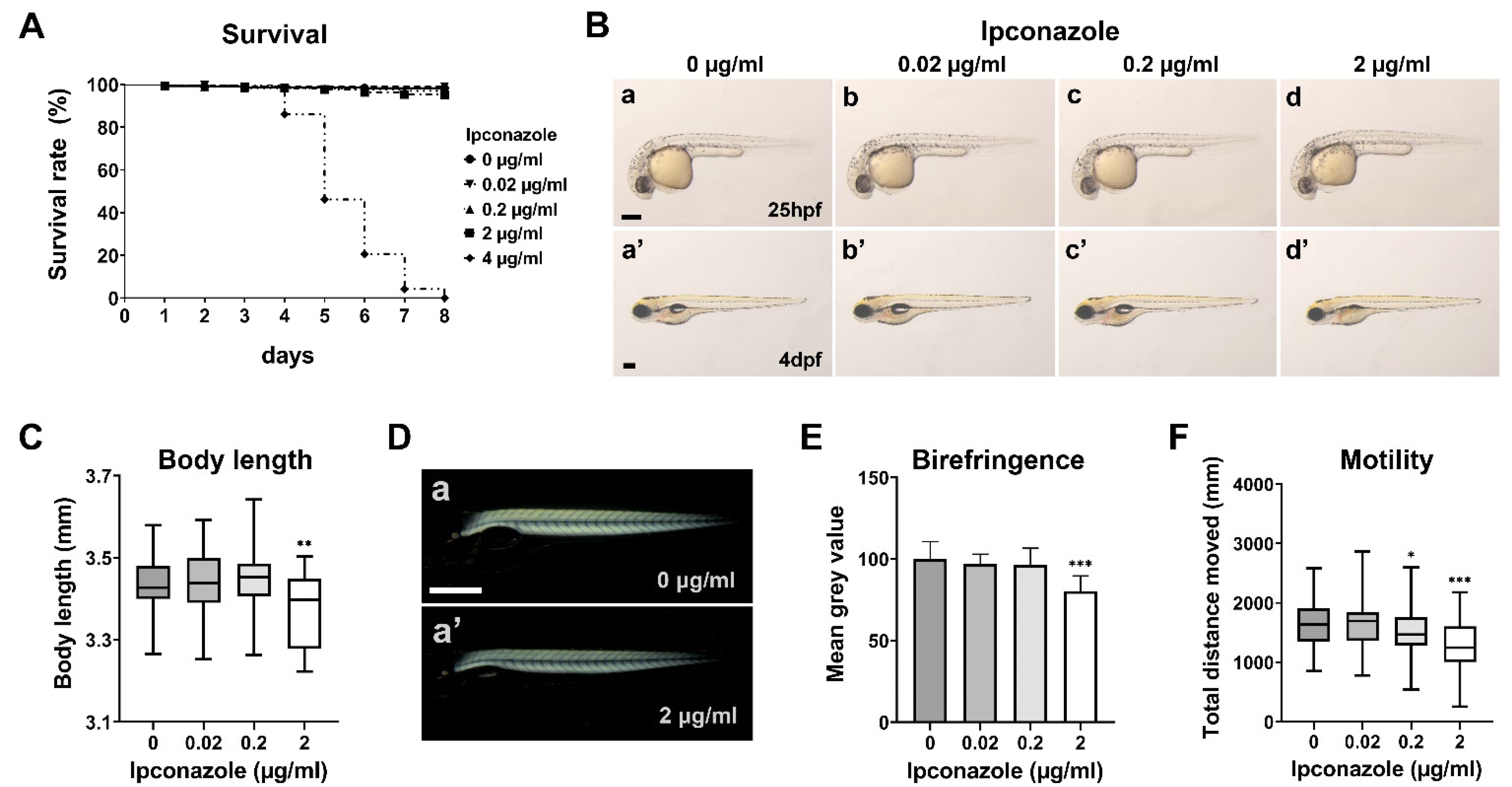
2.1. Ipconazole Pesticide Alters Larval Locomotive Behavior in Zebrafish Development
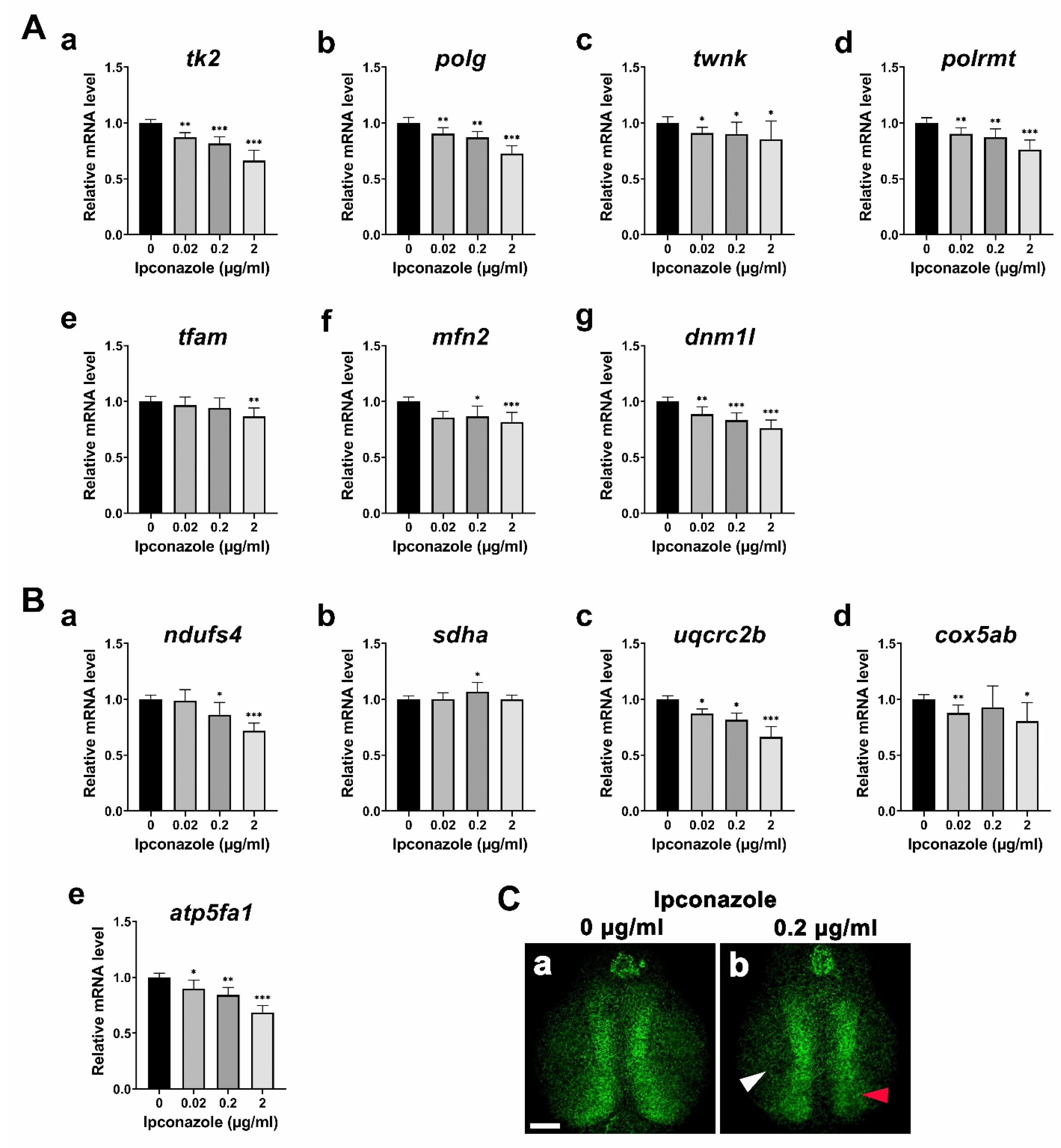
2.2. Ipconazole Disrupts Mitochondrial Genome Maintenance and Functions
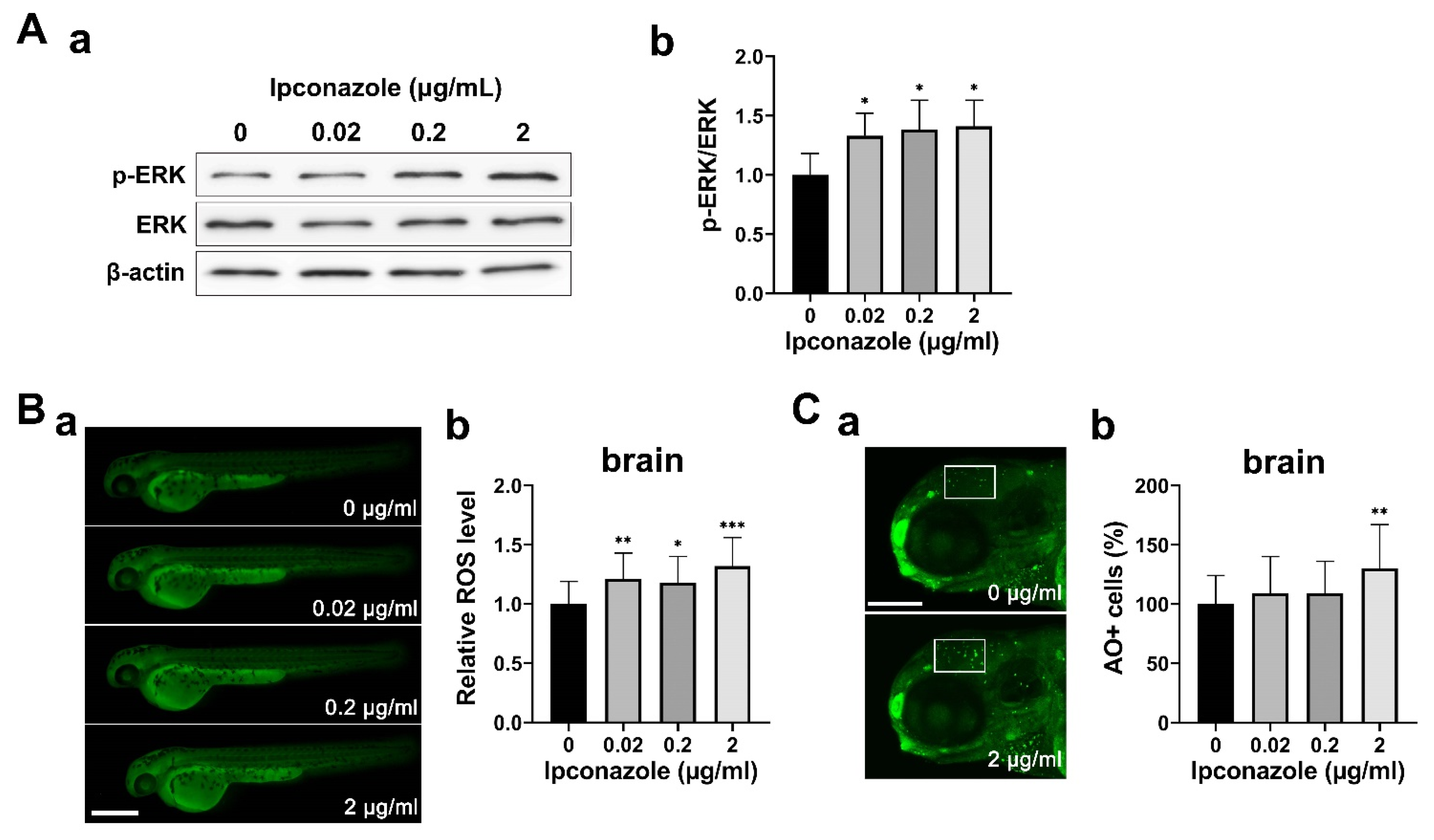
2.3. Ipconazole Increases Oxidative Stress and ERK1/2 Activation
2.4. GABAergic Neuron Is Susceptible to Ipconazole Toxicity
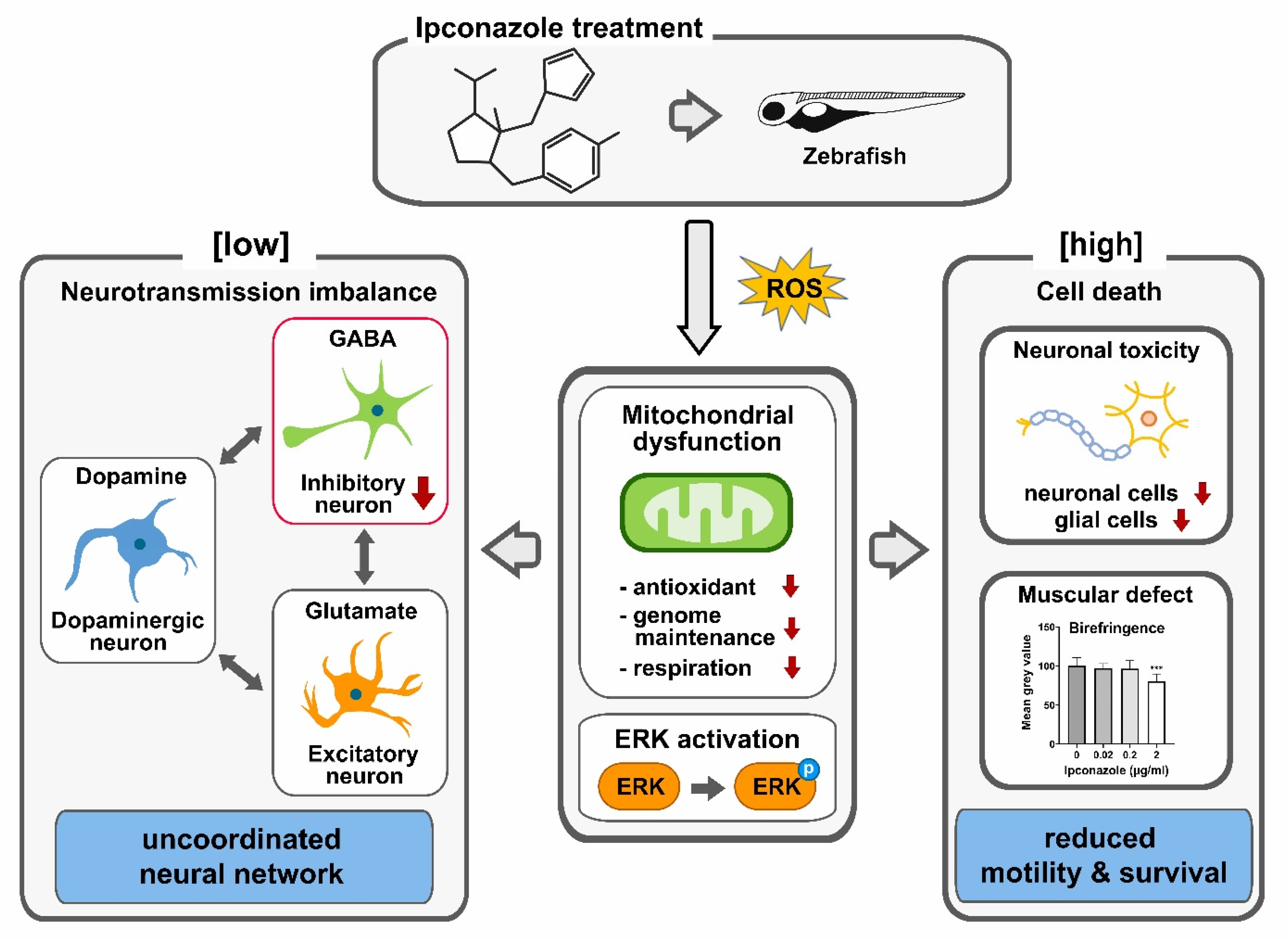
3. Discussion
4. Materials and Methods
4.1. Zebrafish Genetic Background and Maintenance
4.2. Chemical Treatment
4.3. Bright-Field Imaging
4.4. Birefringence Measurement
4.5. Locomotive Behavior Test
4.6. Real-Time PCR (RT-PCR)
4.7. Western Blot Analysis
4.8. Measurement of Reactive Oxygen Species (ROS)
4.9. Acridine Orange Staining
4.10. Confocal Microscopy of Transgenic Larvae
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saishoji, T.; Ito, A.; Kumazawa, S.; Chuman, H. Structure-activity relationships of enantiomers of the azole fungicide ipconazole and its related compounds: Fungicidal and plant growth inhibitory activities. J. Pestic. Sci. 1998, 23, 129–136. [Google Scholar] [CrossRef][Green Version]
- OECD. Guidance document on residues in livestock. In Series on Pesticides No 73. ENV/JM/MONO (2013) 8, 04 September 2013; OECD: Paris, France, 2013. [Google Scholar]
- Authority, E.F.S. Statement concerning the review of the approval of the active substance ipconazole. EFSA J. 2022, 20, e07133. [Google Scholar]
- Authority, E.F.S.A.M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Review of the existing maximum residue levels for ipconazole according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, 5961. [Google Scholar]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Ingram, S.L.; Scimemi, A. Regulation of glutamate, GABA and dopamine transporter uptake, surface mobility and expression. Front. Cell. Neurosci. 2021, 15, 670346. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, Z.M.; Reddy, V.; Pillarisetty, L.S. Physiology, neurotransmitters. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Sotolongo, K.; Ghiso, J.; Rostagno, A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage. Alzheimer’s Res. Ther. 2020, 12, 1–22. [Google Scholar] [CrossRef]
- He, K.; Aizenman, E. ERK signaling leads to mitochondrial dysfunction in extracellular zinc-induced neurotoxicity. J. Neurochem. 2010, 114, 452–461. [Google Scholar] [CrossRef]
- Lebovitz, R.M.; Zhang, H.; Vogel, H.; Cartwright, J., Jr.; Dionne, L.; Lu, N.; Huang, S.; Matzuk, M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 9782–9787. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Unsicker, K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010, 277, 22–29. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Reis, H.; Guatimosim, C.; Paquet, M.; Santos, M.; Ribeiro, F.; Kummer, A.; Schenatto, G.; Salgado, J.; Vieira, L.; Teixeira, A.; et al. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr. Med. Chem. 2009, 16, 796–840. [Google Scholar] [CrossRef]
- Padilla, S.; Cowden, J.; Hinton, D.E.; Yuen, B.; Law, S.; Kullman, S.W.; Johnson, R.; Hardman, R.C.; Flynn, K.; Au, D.W. Use of medaka in toxicity testing. Curr. Protoc. Toxicol. 2009, 39, 1.10.1–1.10.36. [Google Scholar] [CrossRef]
- Tran, C.M.; Lee, H.; Lee, B.; Ra, J.-S.; Kim, K.-T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard. Mater. 2020, 401, 123389. [Google Scholar] [CrossRef]
- Blader, P.; Plessy, C.; Strähle, U. Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech. Dev. 2003, 120, 211–218. [Google Scholar] [CrossRef]
- Kim, M.J.; Kang, K.H.; Kim, C.-H.; Choi, S.-Y. Real-time imaging of mitochondria in transgenic zebrafish expressing mitochondrially targeted GFP. BioTechniques 2008, 45, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Bianco, I.H.; Hamaoka, T.; Miyashita, T.; Uemura, O.; Concha, M.L.; Russell, C.; Wilson, S.W.; Okamoto, H. Laterotopic Representation of Left-Right Information onto the Dorso-Ventral Axis of a Zebrafish Midbrain Target Nucleus. Curr. Biol. 2005, 15, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.P.; Choi, S.-Y.; Kee, Y.; Kim, M.J.; Kim, S.-H.; Lee, Y.; Park, H.-C.; Ro, H. Transgenic fluorescent zebrafish lines that have revolutionized biomedical research. Lab. Anim. Res. 2021, 37, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Ki, S.; Kwon, S.-H.; Eum, J.; Raslan, A.A.; Kim, K.-N.; Hwang, B.J.; Kee, Y. 3D light-sheet assay assessing novel valproate-associated cardiotoxicity and folic acid relief in zebrafish embryogenesis. Chemosphere 2019, 227, 551–560. [Google Scholar] [CrossRef]
- Berger, J.; Sztal, T.; Currie, P.D. Quantification of birefringence readily measures the level of muscle damage in zebrafish. Biochem. Biophys. Res. Commun. 2012, 423, 785–788. [Google Scholar] [CrossRef]
- Lee, S.; Eum, J.; Park, S.; Ki, S.; Hwang, B.J.; Kee, Y.; Chae, J.H. TNNT1 myopathy with novel compound heterozygous mutations. Neuromuscul. Disord. 2021, 32, 176–184. [Google Scholar] [CrossRef]
- Chowdhury, A.U.; Raslan, A.A.; Lee, E.; Eum, J.; Hwang, B.J.; Kwon, S.-H.; Kee, Y. Histopathological assessment of laterality defects in zebrafish development. Anim. Cells Syst. 2021, 25, 136–145. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Banik, A.; Eum, J.; Hwang, B.J.; Kwon, S.-H.; Kee, Y. Ipconazole Disrupts Mitochondrial Homeostasis and Alters GABAergic Neuronal Development in Zebrafish. Int. J. Mol. Sci. 2023, 24, 496. https://doi.org/10.3390/ijms24010496
Lee G, Banik A, Eum J, Hwang BJ, Kwon S-H, Kee Y. Ipconazole Disrupts Mitochondrial Homeostasis and Alters GABAergic Neuronal Development in Zebrafish. International Journal of Molecular Sciences. 2023; 24(1):496. https://doi.org/10.3390/ijms24010496
Chicago/Turabian StyleLee, Giyoung, Amit Banik, Juneyong Eum, Byung Joon Hwang, Seung-Hae Kwon, and Yun Kee. 2023. "Ipconazole Disrupts Mitochondrial Homeostasis and Alters GABAergic Neuronal Development in Zebrafish" International Journal of Molecular Sciences 24, no. 1: 496. https://doi.org/10.3390/ijms24010496
APA StyleLee, G., Banik, A., Eum, J., Hwang, B. J., Kwon, S.-H., & Kee, Y. (2023). Ipconazole Disrupts Mitochondrial Homeostasis and Alters GABAergic Neuronal Development in Zebrafish. International Journal of Molecular Sciences, 24(1), 496. https://doi.org/10.3390/ijms24010496





