A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis
Abstract
1. Introduction
2. Results
2.1. Characterization of Infiltrating and Circulating Immune Cells by Flow-Cytometry
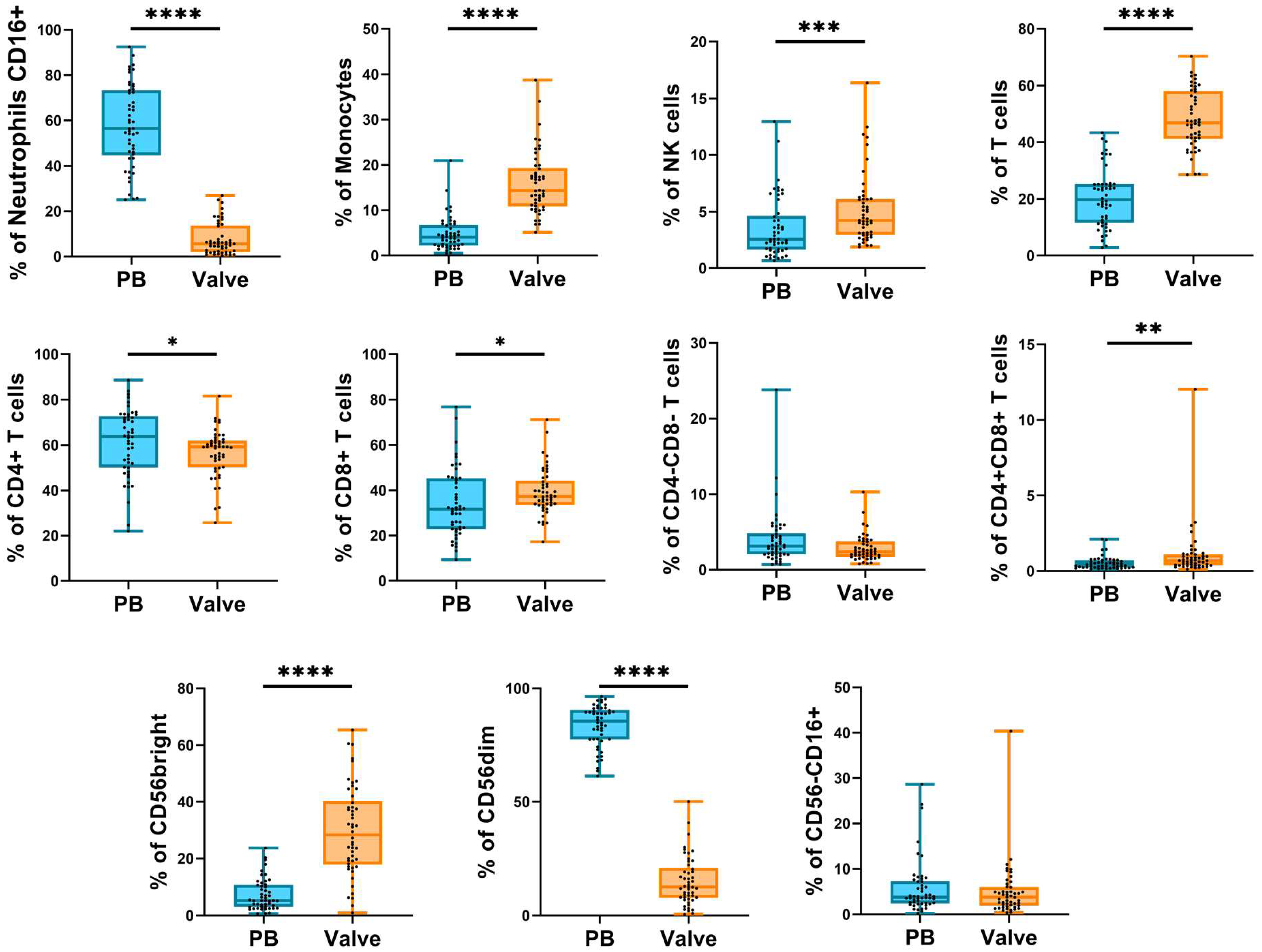
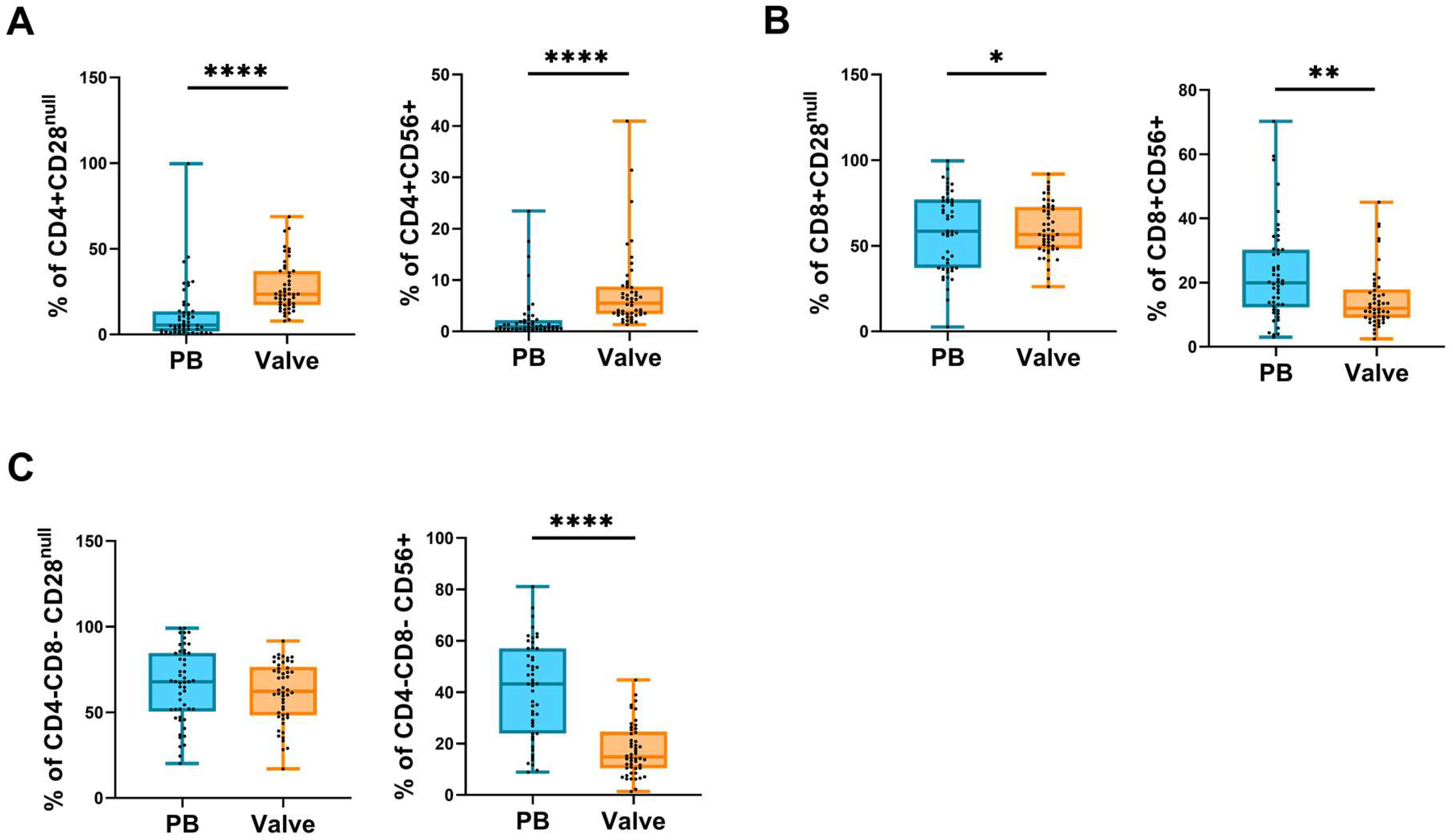
2.2. Frequency of Circulating and Infiltrating Leukocyte Subpopulations
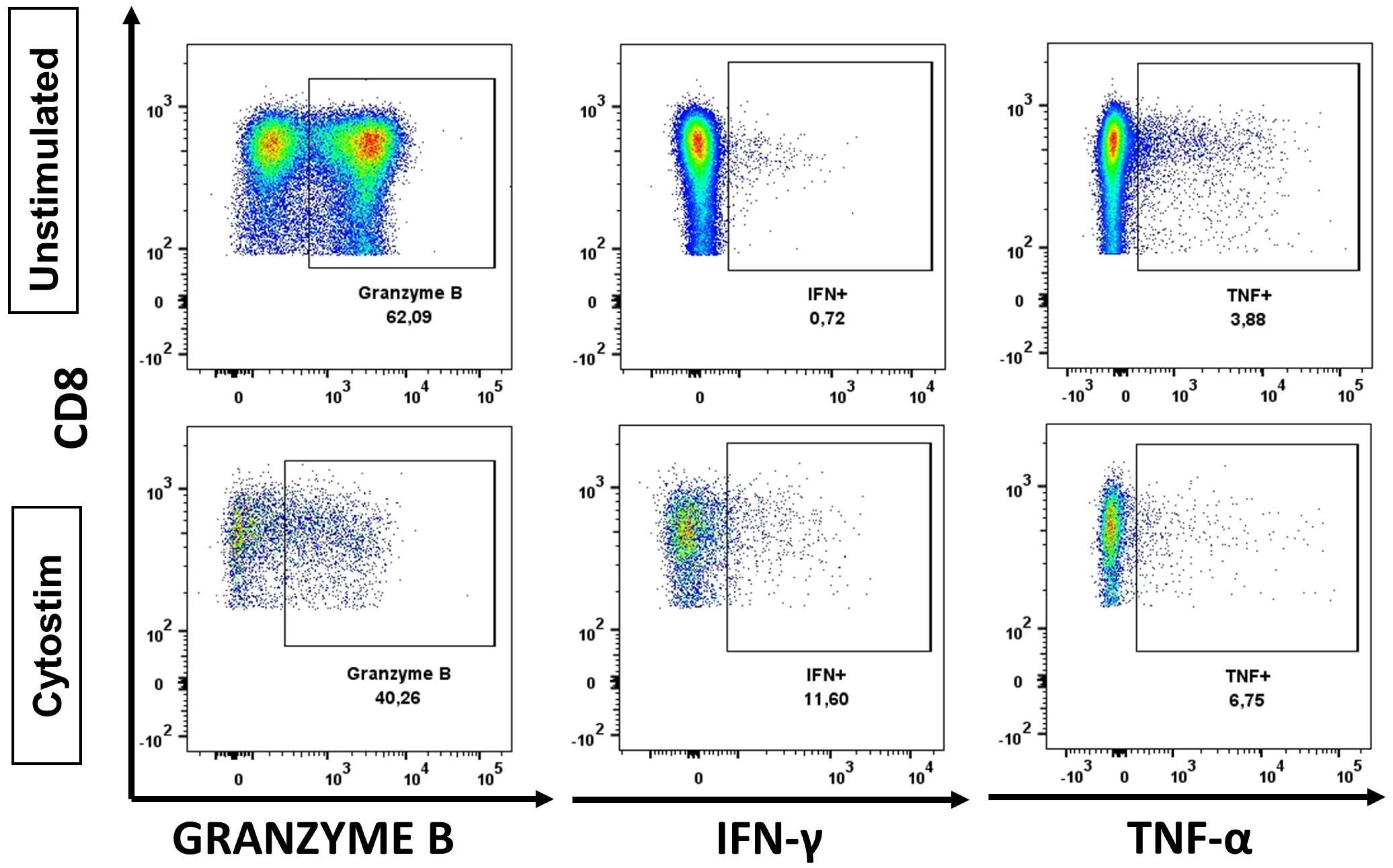
2.3. Functional Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Reagents
- RPMI 1640 medium (PanBiotech, cat. P04-17500. Aidenbach, Germany);
- Phosphate-buffered saline (PanBiotech, cat. P04-36500. Aidenbach, Germany);
- Fetal Bovine Serum (Gibco, ref. 10270106. Waltham, USA, Massachusetts);
- Collagenase D 0.24U/mg lyophilized (Roche Diagnostics, ref. 11088866001. Basel, Switzerland);
- FcBlock (BD Becton Dickinson, cat. 564220. Franklin Lakes, USA, New Jersey);
- MACSQuant Running Buffer (Miltenyi, cat. 130-092-747. Bergisch Gladbach, Germany);
- Brefeldin A/Golgi Plug (BD Becton Dickinson, cat. 555029. Franklin Lakes, USA, New Jersey);
- Cytostim human (Miltenyi, cat. 130-092-173. Bergisch Gladbach, Germany);
- EDTA (PanReac AppliChem, cat. A4892. Chicago, USA, Illinois);
- Glutamine (Biowest, cat. X0550-100. Nuaillé, France);
- Penicillin/Streptomycin (Biowest, cat. L0022-100. Nuaillé, France).
- 15 mL tubes with strew cap (NerbePlus, ref. 02-502-8001. Winsen, Germany);
- 50 mL tubes with strew cap (NerbePlus, ref. 02-572-8001. Winsen, Germany);
- 5 mL Flow Cytometry tubes (Corning, ref. 352052. Corning, USA, New York);
- 70 μm tube filter (Biologix, ref. 15-1070. Jinan, Shandong, China);
- 20 μm tube filter (Pluriselect, ref. 43-10020. Leipzig, Alemania);
- Scalpel Blades (Heinz Herenz, ref. 1110923. Hamburg, Germany);
- Needle (BD Becton Dickinson, ref. 305899. Franklin Lakes, USA, New Jersey);
- Syringe (BD Becton Dickinson, ref. 300928. Franklin Lakes, USA, New Jersey);
- Sterile Petri dish (Deltalab, ref. 200219. Barcelona, Spain);
- Sterile 5 mL Flow Cytometry tubes (Corning, ref. 352054. Corning, USA, New York,).
4.3. Reagent Preparation
4.4. Surgical Procedure for Aortic Valve Tissue Collection
4.5. Histological and Immunohistochemical (IHC) Analysis
4.6. Cell Isolation Protocol
- Wash leaflets 3–5 times with 10 mL of PBS + 1% FBS, to ensure no peripheral blood contamination.
- Inject 1 mL of digestive solution per 1 cm of valve leaflet and incubate for 5 min at room temperature.
- Cut the valve tissue using the scalpels to avoid calcification.
- Transfer the processed tissue to the digestion solution tube and incubate it at 37 °C for 1 h and 30 min under continuous stirring.
- Filter the digested tissue through a 70 μm ∅ cell restrainer to a 50 mL tube and top up with 10 mL of washing solution.
- Centrifuge the cells for 5 min at 400× g, then discard the supernatant and vortex gently.
- Add 4.5 mL of PBS + 1% FBS and filter the cells through a 20 μm ∅ cell restrainer to a sterile 5 mL FACS tube.
- Centrifuge the cells for 5 min at 400× g, then discard the supernatant and vortex gently.
- Continue with surface or intracellular staining protocol.
4.7. Surface Cell Staining for Flow Cytometry
4.8. Functionality Assay
- Add 250 μL of culture medium to the cell suspension in a sterile FACS tube. For PBMCs functionality assay, 2 × 106 cells were stimulated.
- Add 5 μL Cytostim, vortex, and incubate the cells for 2 h at 37 °C, 5% CO2.
- Add 245.5 μL of culture medium and 0.5 μL Brefeldin A. vortex and incubate the cells overnight (14 h) at 37 °C, 5% CO2.
- The following morning, stop the stimulation with 100 μL EDTA 20nM, vortex, and incubate for 10 min at room temperature.
- Add 3 mL PBS + 1% FBS (4 °C).
- Centrifuge the cells for 5 min at 400× g, then discard the supernatant and vortex gently.
- Continue with the intracellular staining protocol.
4.9. Intracellular Staining (ICS) for Flow Cytometry
4.10. Whole Blood Antibody Staining
4.11. Acquisition and Data Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, W.; Wang, Z.; Sun, C.; Zhang, D.-M. Pathogenesis and Molecular Immune Mechanism of Calcified Aortic Valve Disease. Front. Cardiovasc. Med. 2021, 8, 765419. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.-I. Recent Advances in Research on Human Aortic Valve Calcification. J. Pharmacol. Sci. 2014, 124, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic Associations with Valvular Calcification and Aortic Stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef]
- Otto, C.M.; Burwash, I.G.; Legget, M.E.; Munt, B.I.; Fujioka, M.; Healy, N.L.; Kraft, C.D.; Miyake-Hull, C.Y.; Schwaegler, R.G. Prospective Study of Asymptomatic Valvular Aortic Stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997, 95, 2262–2270. [Google Scholar] [CrossRef]
- Cho, K.I.; Sakuma, I.; Sohn, I.S.; Jo, S.-H.; Koh, K.K. Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease. Atherosclerosis 2018, 277, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M. Calcific Aortic Stenosis—Time to Look More Closely at the Valve. N. Engl. J. Med. 2008, 359, 1395–1398. [Google Scholar] [CrossRef]
- Molnár, A.; Pásztor, D.; Merkely, B. Cellular Senescence, Aging and Non-Aging Processes in Calcified Aortic Valve Stenosis: From Bench-Side to Bedside. Cells 2022, 11, 3389. [Google Scholar] [CrossRef]
- Boudoulas, K.D.; Borer, J.S.; Boudoulas, H. Etiology of Valvular Heart Disease in the 21st Century. Cardiology 2013, 126, 139–152. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef]
- Adler, Y.; Vaturi, M.; Wiser, I.; Shapira, Y.; Herz, I.; Weisenberg, D.; Sela, N.; Battler, A.; Sagie, A. Nonobstructive aortic valve calcium as a window to atherosclerosis of the aorta. Am. J. Cardiol. 2000, 86, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kraler, S.; Blaser, M.C.; Aikawa, E.; Camici, G.G.; Lüscher, T.F. Calcific aortic valve disease: From molecular and cellular mechanisms to medical therapy. Eur. Heart J. 2021, 43, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, K.D.; Wolfe, B.; Ravi, Y.; Lilly, S.; Nagaraja, H.N.; Sai-Sudhakar, C.B. The aortic stenosis complex: Aortic valve, atherosclerosis, aortopathy. J. Cardiol. 2015, 65, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.; Airhart, N.; Lopez, L.; Silberbach, M. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: Report of the international turner syndrome aortic dissection registry. Circulation 2012, 126, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical Factors Associated with Calcific Aortic Valve Disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Galli, D.; Manuguerra, R.; Monaco, R.; Manotti, L.; Goldoni, M.; Becchi, G.; Carubbi, C.; Vignali, G.; Cucurachi, N.; Gherli, T.; et al. Understanding the structural features of symptomatic calcific aortic valve stenosis: A broad-spectrum clinico-pathologic study in 236 consecutive surgical cases. Int. J. Cardiol. 2017, 228, 364–374. [Google Scholar] [CrossRef]
- Šteiner, I.; Krbal, L.; Rozkoš, T.; Harrer, J.; Laco, J. Calcific aortic valve stenosis: Immunohistochemical analysis of inflammatory infiltrate. Pathol. Res. Pract. 2012, 208, 231–234. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.-H. Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells. Immune Netw. 2016, 16, 26–32. [Google Scholar] [CrossRef]
- Winchester, R.; Wiesendanger, M.; O’Brien, W.; Zhang, H.-Z.; Maurer, M.S.; Gillam, L.D.; Schwartz, A.; Marboe, C.; Stewart, A.S. Circulating Activated and Effector Memory T Cells Are Associated with Calcification and Clonal Expansions in Bicuspid and Tricuspid Valves of Calcific Aortic Stenosis. J. Immunol. 2011, 187, 1006–1014. [Google Scholar] [CrossRef]
- Steiner, I.; Krbal, L.; Dominik, J. Blood vessels and lymphatics in calcific aortic stenosis--in support of its inflammatory pathogenesis. Ceskoslovenska Patol. 2010, 46, 33–36. [Google Scholar]
- Li, G.; Qiao, W.; Zhang, W.; Li, F.; Shi, J.; Dong, N. The shift of macrophages toward M1 phenotype promotes aortic valvular calcification. J. Thorac. Cardiovasc. Surg. 2017, 153, 1318–1327.e1. [Google Scholar] [CrossRef] [PubMed]
- Ginghină, C.; Florian, A.; Beladan, C.; Iancu, M.; Călin, A.; Popescu, B.A.; Jurcuţ, R. Calcific aortic valve disease and aortic atherosclerosis--two faces of the same disease? Rom. J. Intern. Med. 2009, 47, 319–329. [Google Scholar] [PubMed]
- Broeders, W.; Bekkering, S.; El Messaoudi, S.; Joosten, L.A.B.; van Royen, N.; Riksen, N.P. Innate immune cells in the pathophysiology of calcific aortic valve disease: Lessons to be learned from atherosclerotic cardiovascular disease? Basic Res. Cardiol. 2022, 117, 28. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.D.; Maurer, M.S.; Friedman, R.A.; Marboe, C.C.; Ruiz-Vazquez, E.M.; Ramakrishnan, R.; Schwartz, A.; Tilson, M.D.; Stewart, A.S.; Winchester, R. The Lymphocytic Infiltration in Calcific Aortic Stenosis Predominantly Consists of Clonally Expanded T Cells. J. Immunol. 2007, 178, 5329–5339. [Google Scholar] [CrossRef]
- Nagy, E.; Lei, Y.; Martínez-Martínez, E.; Body, S.C.; Schlotter, F.; Creager, M.; Assmann, A.; Khabbaz, K.; Libby, P.; Hansson, G.K.; et al. Interferon-γ Released by Activated CD8+ T Lymphocytes Impairs the Calcium Resorption Potential of Osteoclasts in Calcified Human Aortic Valves. Am. J. Pathol. 2017, 187, 1413–1425. [Google Scholar] [CrossRef]

| Group Name | Sex (Male/Female) | Age (Mean ± SD) | n |
|---|---|---|---|
| Aortic stenosis | 34/16 | 42–79 (67 ± 8.76) | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Heredia, P.; Domínguez-del-Castillo, J.J.; Reina-Alfonso, I.; Gutiérrez-González, C.; Hassouneh, F.; Batista-Duharte, A.; Trujillo-Aguilera, A.; López-Romero, R.; Muñoz, I.; Solana, R.; et al. A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis. Int. J. Mol. Sci. 2023, 24, 2194. https://doi.org/10.3390/ijms24032194
Álvarez-Heredia P, Domínguez-del-Castillo JJ, Reina-Alfonso I, Gutiérrez-González C, Hassouneh F, Batista-Duharte A, Trujillo-Aguilera A, López-Romero R, Muñoz I, Solana R, et al. A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis. International Journal of Molecular Sciences. 2023; 24(3):2194. https://doi.org/10.3390/ijms24032194
Chicago/Turabian StyleÁlvarez-Heredia, Pablo, José Joaquín Domínguez-del-Castillo, Irene Reina-Alfonso, Carmen Gutiérrez-González, Fakhri Hassouneh, Alexander Batista-Duharte, Antonio Trujillo-Aguilera, Rosalía López-Romero, Ignacio Muñoz, Rafael Solana, and et al. 2023. "A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis" International Journal of Molecular Sciences 24, no. 3: 2194. https://doi.org/10.3390/ijms24032194
APA StyleÁlvarez-Heredia, P., Domínguez-del-Castillo, J. J., Reina-Alfonso, I., Gutiérrez-González, C., Hassouneh, F., Batista-Duharte, A., Trujillo-Aguilera, A., López-Romero, R., Muñoz, I., Solana, R., & Pera, A. (2023). A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis. International Journal of Molecular Sciences, 24(3), 2194. https://doi.org/10.3390/ijms24032194









