GxxxG Motif Stabilize Ion-Channel like Pores through Cα―H···O Interaction in Aβ (1-40)
Abstract
1. Introduction
2. Results
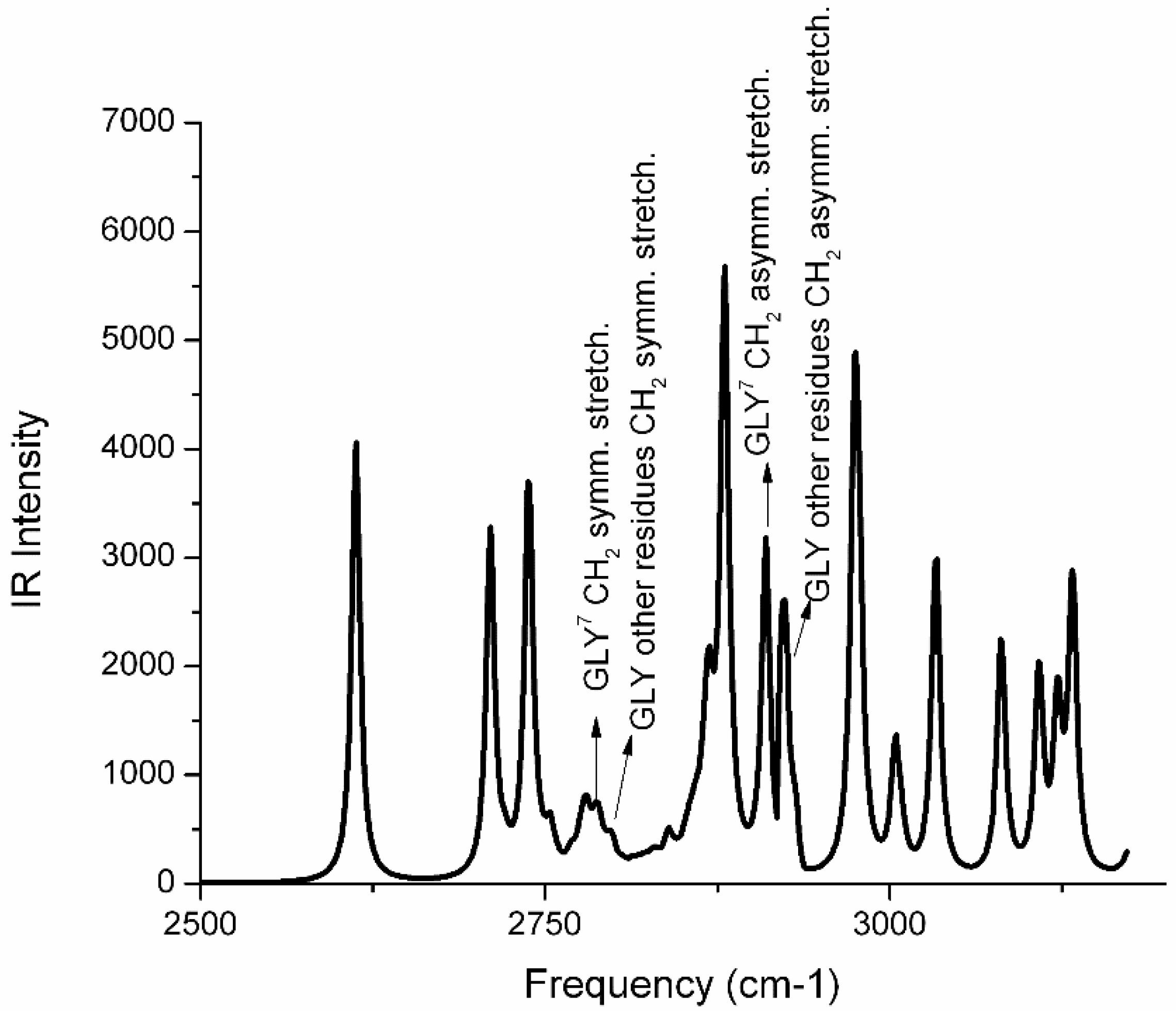
2.1. Molecular Dynamics and Quantum Mechanics Reveal Cα―H···O Interaction in Aβ(1-40) Ion-Channel like Pores
2.2. Penta-Peptide GXXXG in Water Does Not Form the Cα―H···O Hydrogen Bond
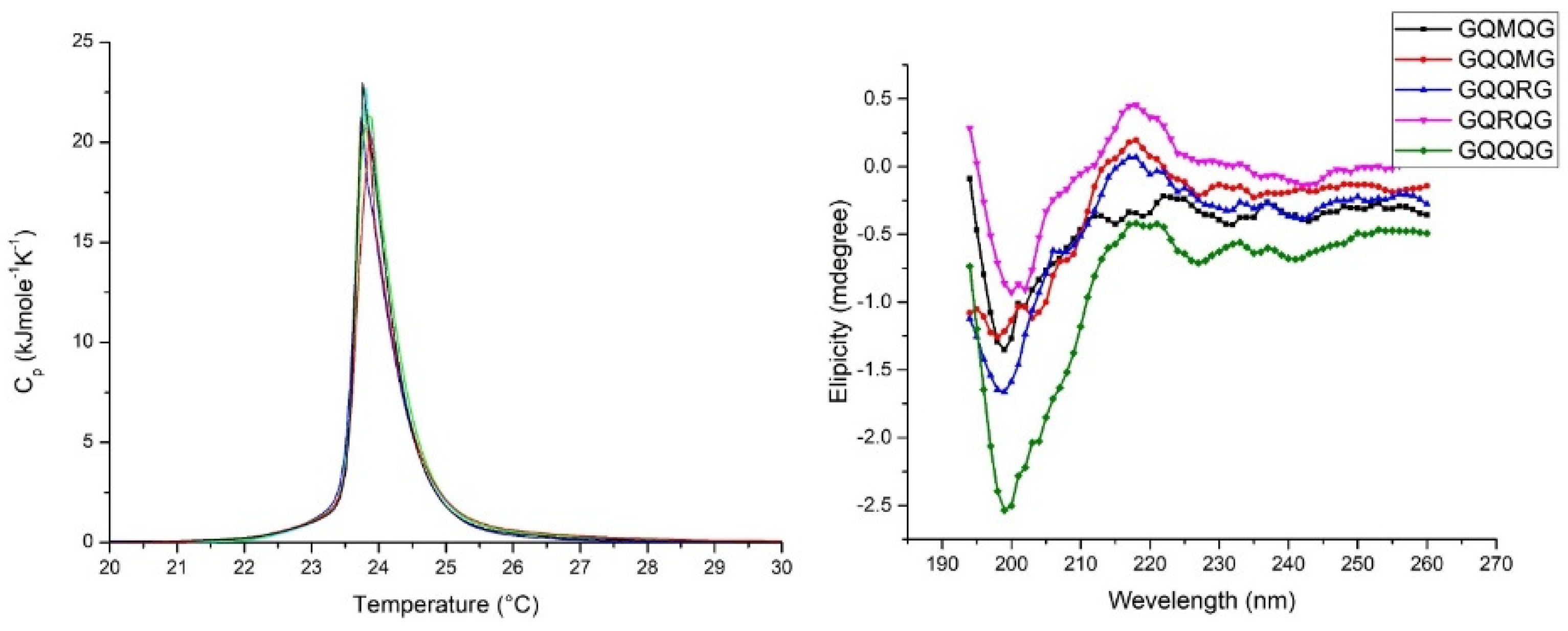
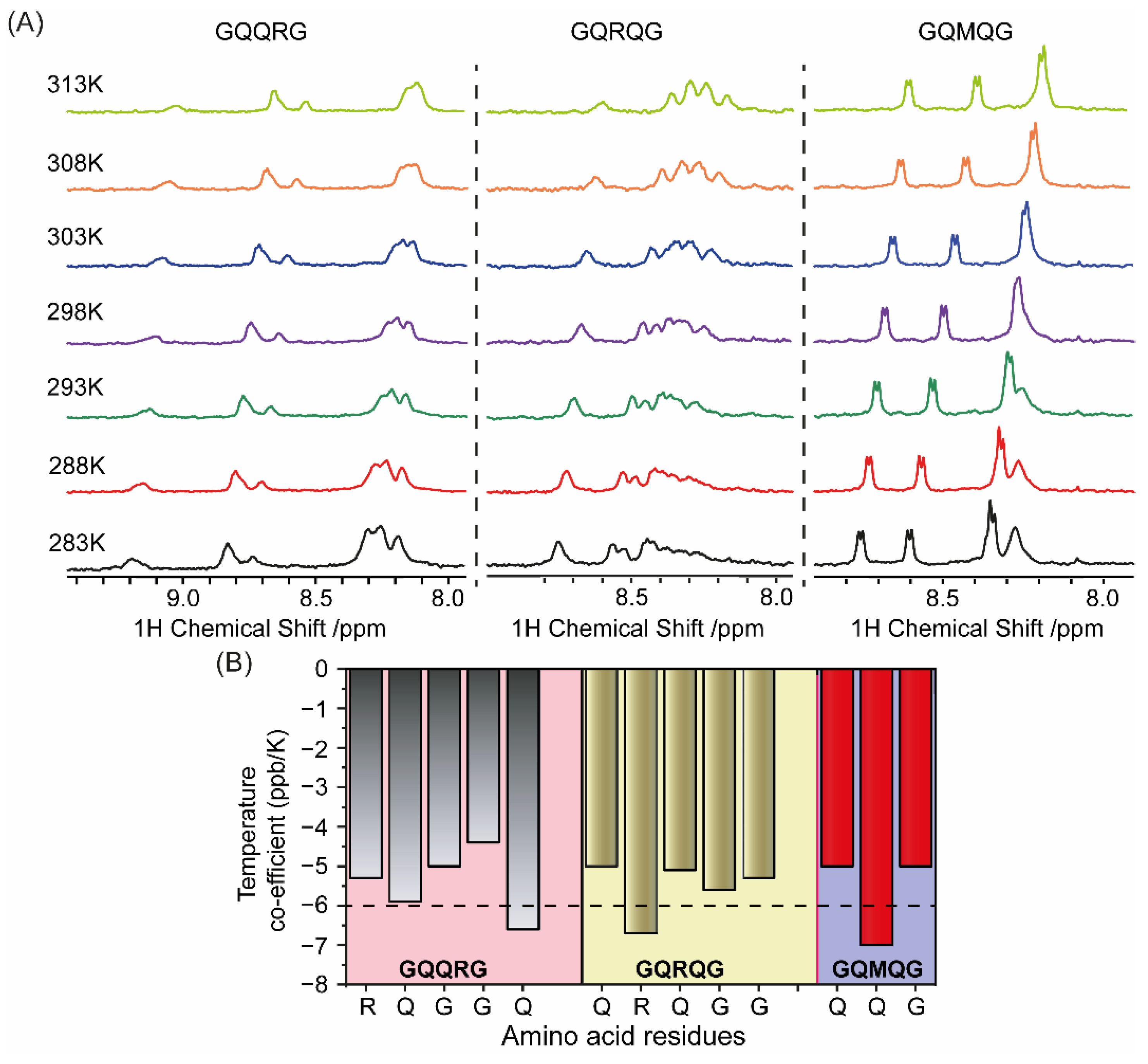
2.3. Penta-Peptide GXXXG Interacts with Model Membrane Surface
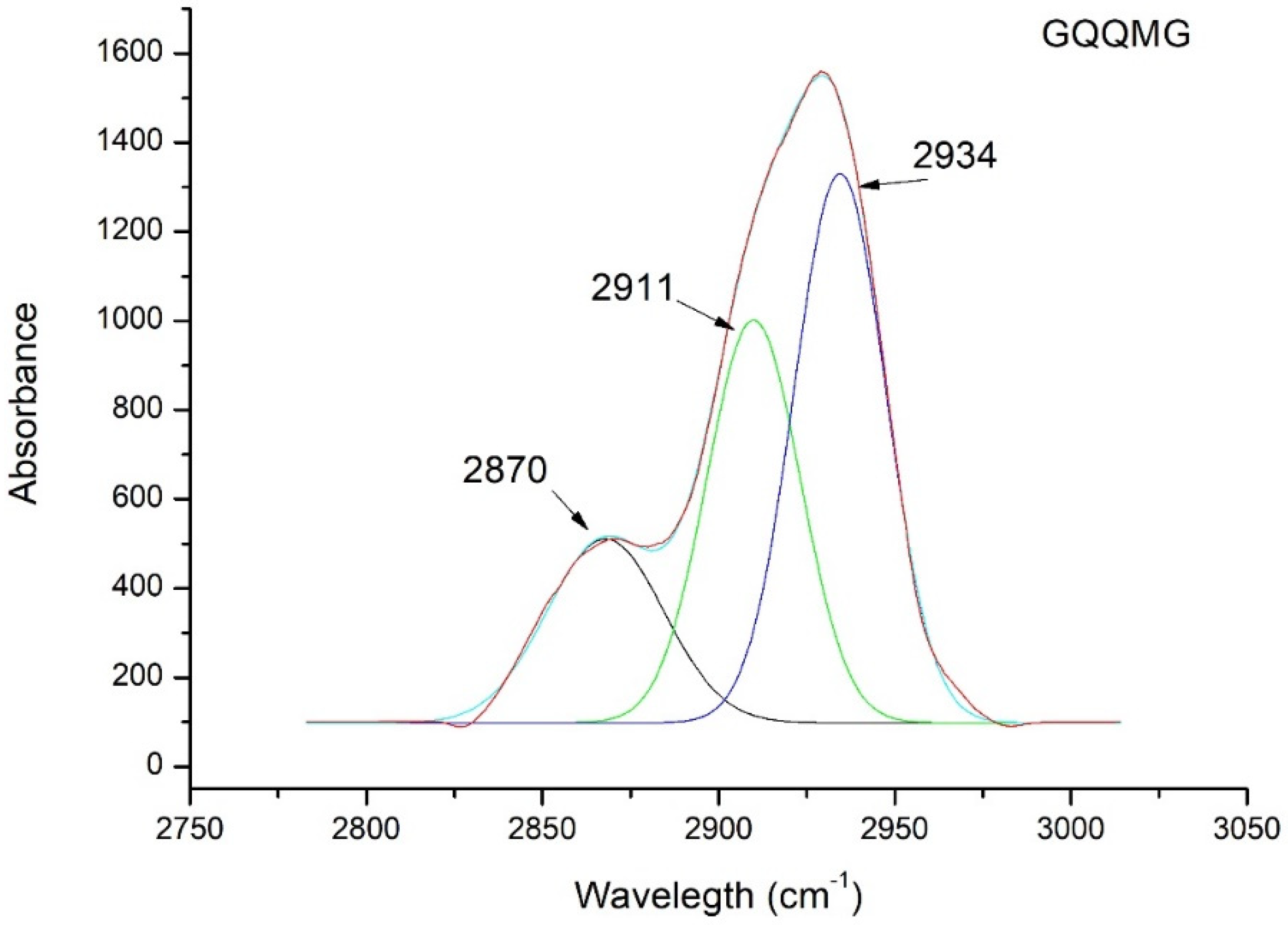
2.4. Penta-Peptide GXXXG in n-Octanol Mimic Hydrophobic Core of Bilayer Reveals Cα―H···O Interaction
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Penta-Peptide Synthesis
4.3. Quantum Mechanics
4.4. NMR Measurements
4.5. AFM Measurements
4.6. ThT Assay
4.7. Circular Dichroism
4.8. LUV Preparation
4.9. DSC Experiments
4.10. ATR-FTIR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of Islet Amyloid Polypeptide: A Molecular Perspective of Risk Factors and Protective Strategies for Type II Diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef] [PubMed]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Parkinsons Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble Protein Oligomers in Neurodegeneration: Lessons from the Alzheimer’s Amyloid β-Peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Unusual Biophysics of Intrinsically Disordered Proteins. Biochim. Biophys. Acta 2013, 1834, 932–951. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. Eur. Mol. Biol. Organ. Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.; Ramamoorthy, A. Two-Step Mechanism of Membrane Disruption by Aβ through Membrane Fragmentation and Pore Formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef]
- La Rosa, C.; Scalisi, S.; Lolicato, F.; Pannuzzo, M.; Raudino, A. Lipid-Assisted Protein Transport: A Diffusion-Reaction Model Supported by Kinetic Experiments and Molecular Dynamics Simulations. J. Chem. Phys. 2016, 144, 184901. [Google Scholar] [CrossRef] [PubMed]
- Scollo, F.; Tempra, C.; Lolicato, F.; Sciacca, M.; Raudino, A.; Milardi, D.; La Rosa, C. Phospholipids Critical Micellar Concentrations Trigger Different Mechanisms of Intrinsically Disordered Proteins Interaction with Model Membranes. J. Phys. Chem. Lett. 2018, 9, 5125–5129. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.; Lolicato, F.; Tempra, C.; Scollo, F.; Sahoo, B.; Watson, M.; García-Viñuales, S.; Milardi, D.; Raudino, A.; Lee, J.; et al. Lipid-Chaperone Hypothesis: A Common Molecular Mechanism of Membrane Disruption by Intrinsically Disordered Proteins. ACS Chem. Neurosci. 2020, 11, 4336–4350. [Google Scholar] [CrossRef]
- Scollo, F.; Rosa, C.L. Amyloidogenic Intrinsically Disordered Proteins: New Insights into Their Self-Assembly and Their Interaction with Membranes. Life 2020, 10, 144. [Google Scholar] [CrossRef]
- La Rosa, C.; Condorelli, M.; Compagnini, G.; Lolicato, F.; Milardi, D.; Do, T.N.; Karttunen, M.; Pannuzzo, M.; Ramamoorthy, A.; Fraternali, F.; et al. Symmetry-Breaking Transitions in the Early Steps of Protein Self-Assembly. Eur. Biophys. J. 2020, 49, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Arbely, E.; Arkin, I.T. Experimental Measurement of the Strength of a Cα-H···O Bond in a Lipid Bilayer. J. Am. Chem. Soc. 2004, 126, 5362–5363. [Google Scholar] [CrossRef] [PubMed]
- Yohannan, S.; Faham, S.; Yang, D.; Grosfeld, D.; Chamberlain, A.K.; Bowie, J.U. A Cα-H···O Hydrogen Bond in a Membrane Protein Is Not Stabilizing. J. Am. Chem. Soc. 2004, 126, 2284–2285. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, P.W.; Günther, S.; Goede, A.; Forrest, L.; Frömmel, C.; Preissner, R. Hydrogen-Bonding and Packing Features of Membrane Proteins: Functional Implications. Biophys. J. 2008, 94, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, G.; Eisenberg, D. GXXXG and GXXXA Motifs Stabilize FAD and NAD(P)-Binding Rossmann Folds through Cα-H···O Hydrogen Bonds and van Der Waals Interactions. J. Mol. Biol. 2002, 323, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Russ, W.P.; Engelman, D.M. The GxxxG Motif: A Framework for Transmembrane Helix-Helix Association. J. Mol. Biol. 2000, 296, 911–919. [Google Scholar] [CrossRef]
- Mueller, B.K.; Subramaniam, S.; Senes, A. A Frequent, GxxxG-Mediated, Transmembrane Association Motif Is Optimized for the Formation of Interhelical Cα-H Hydrogen Bonds. Proc. Natl. Acad. Sci. USA 2014, 111, E888–E895. [Google Scholar] [CrossRef]
- Sarkar, D.; Chakraborty, I.; Condorelli, M.; Ghosh, B.; Mass, T.; Weingarth, M.; Mandal, A.K.; La Rosa, C.; Subramanian, V.; Bhunia, A. Self-Assembly and Neurotoxicity of β-Amyloid (21–40) Peptide Fragment: The Regulatory Role of GxxxG Motifs. ChemMedChem 2020, 15, 293–301. [Google Scholar] [CrossRef]
- Pannuzzo, M.; Milardi, D.; Raudino, A.; Karttunen, M.; La Rosa, C. Analytical Model and Multiscale Simulations of a Beta Peptide Aggregation in Lipid Membranes: Towards a Unifying Description of Conformational Transitions, Oligomerization and Membrane Damage. Phys. Chem. Chem. Phys. 2013, 15, 8940–8951. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Baxter, N.J.; Williamson, M.P. Temperature Dependence of 1H Chemical Shifts in Proteins. J. Biomol. NMR 1997, 9, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K.B. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. Wires Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Pappalardo, M.; Attanasio, F.; Milardi, D.; La Rosa, C.; Grasso, D.M. Are Fibril Growth and Membrane Damage Linked Processes? An Experimental and Computational Study of IAPP12–18 and IAPP21–27 Peptides. New J. Chem. 2010, 34, 200–207. [Google Scholar] [CrossRef]
- Puglisi, G.; Fresta, M.; La Rosa, C.; Ventura, C.A.; Panico, A.M.; Mazzone, G. Liposomes as a Potential Drug Carrier for Citicoline (CDP-Choline) and the Effect of Formulation Conditions on Encapsulation Efficiency. Pharmazie 1992, 47, 211–215. [Google Scholar]


| Penta-Peptide |
|---|
| GQQQG-NH2 |
| GQQRG-NH2 |
| GQRQG-NH2 |
| GQQMG-NH2 |
| GQMQG-NH2 |
| Peptide | AssignmentAssignment | Frequency (cm−1) |
|---|---|---|
| GQQRG-NH2 | β-sheet | 1624 |
| GQRQG-NH2 | α-helix | 1656 |
| GQQMG-NH2 | β-sheet | 1633 |
| GQMQG-NH2 | β-sheet | 1627 |
| GQQQG-NH2 | β-sheet | 1624 |
| Peptide | Frequency (cm−1) |
|---|---|
| GQQQG | 2908 |
| GQQRG | 2910 |
| GQRQG | 2907 |
| GQQMG | 2910 |
| GQMQG | 2907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rando, C.; Grasso, G.; Sarkar, D.; Sciacca, M.F.M.; Cucci, L.M.; Cosentino, A.; Forte, G.; Pannuzzo, M.; Satriano, C.; Bhunia, A.; et al. GxxxG Motif Stabilize Ion-Channel like Pores through Cα―H···O Interaction in Aβ (1-40). Int. J. Mol. Sci. 2023, 24, 2192. https://doi.org/10.3390/ijms24032192
Rando C, Grasso G, Sarkar D, Sciacca MFM, Cucci LM, Cosentino A, Forte G, Pannuzzo M, Satriano C, Bhunia A, et al. GxxxG Motif Stabilize Ion-Channel like Pores through Cα―H···O Interaction in Aβ (1-40). International Journal of Molecular Sciences. 2023; 24(3):2192. https://doi.org/10.3390/ijms24032192
Chicago/Turabian StyleRando, Carola, Giuseppe Grasso, Dibakar Sarkar, Michele Francesco Maria Sciacca, Lorena Maria Cucci, Alessia Cosentino, Giuseppe Forte, Martina Pannuzzo, Cristina Satriano, Anirban Bhunia, and et al. 2023. "GxxxG Motif Stabilize Ion-Channel like Pores through Cα―H···O Interaction in Aβ (1-40)" International Journal of Molecular Sciences 24, no. 3: 2192. https://doi.org/10.3390/ijms24032192
APA StyleRando, C., Grasso, G., Sarkar, D., Sciacca, M. F. M., Cucci, L. M., Cosentino, A., Forte, G., Pannuzzo, M., Satriano, C., Bhunia, A., & La Rosa, C. (2023). GxxxG Motif Stabilize Ion-Channel like Pores through Cα―H···O Interaction in Aβ (1-40). International Journal of Molecular Sciences, 24(3), 2192. https://doi.org/10.3390/ijms24032192









