Evaluation of CRISPR/Cas9 Constructs in Wheat Cell Suspension Cultures
Abstract
1. Introduction
2. Results
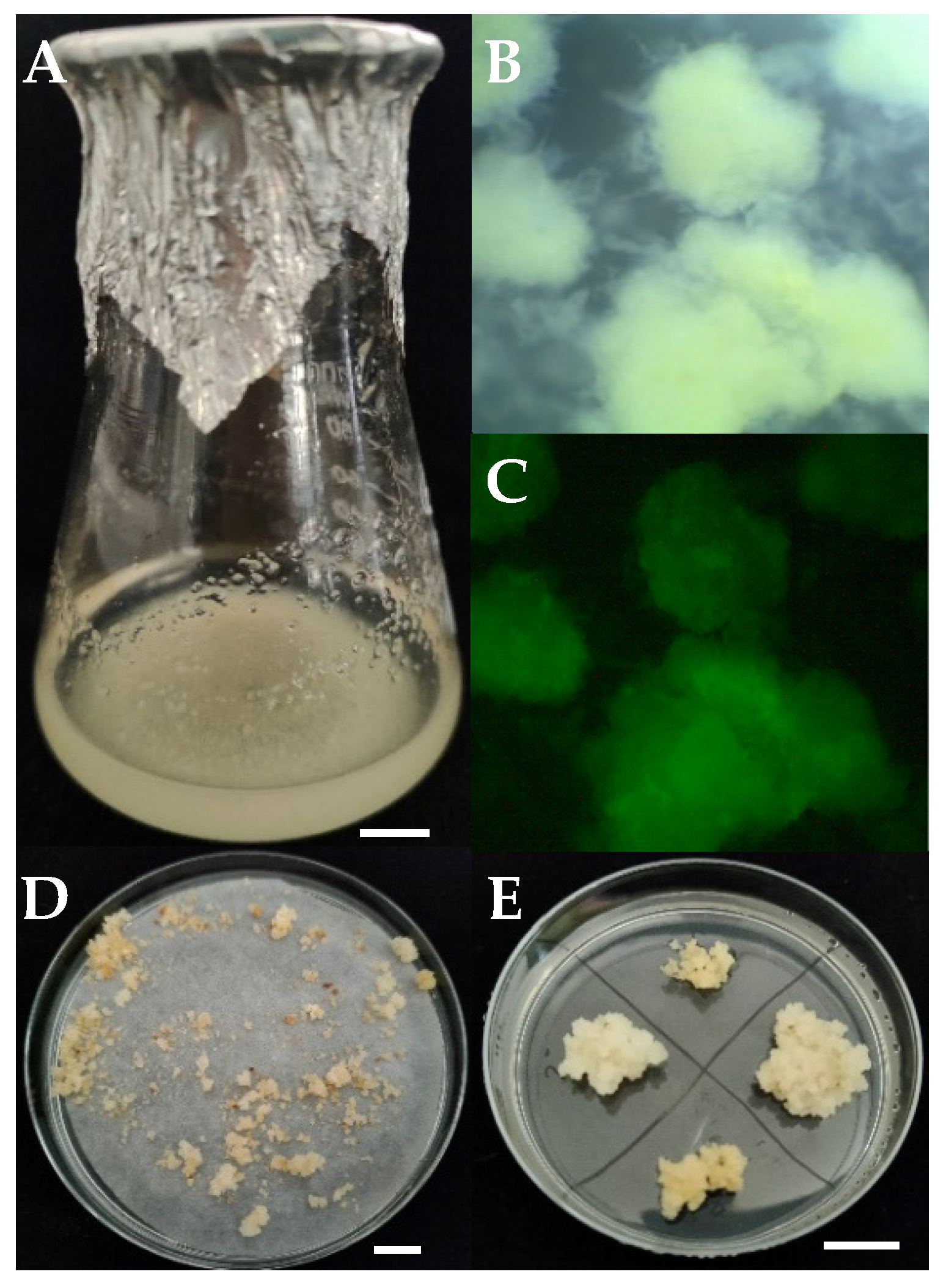
2.1. Wheat Cell Suspension Production and Transformation
2.2. Evaluation of On- and Off-Target Editing Efficiency
3. Discussion
4. Materials and Methods
4.1. Wheat Cell Suspension Culture
4.2. gRNA/Cas9 Constructs and Transformation
4.3. Evaluation of On- and Off-Target Editing Efficiency
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhunov, E.; Nicolet, C.; Dvorak, J. Single Nucleotide Polymorphism Genotyping in Polyploid Wheat with the Illumina GoldenGate Assay. Theor. Appl. Genet. 2009, 119, 507–517. [Google Scholar] [CrossRef]
- Mikami, M.; Toki, S.; Endo, M. Comparison of CRISPR/Cas9 Expression Constructs for Efficient Targeted Mutagenesis in Rice. Plant Mol. Biol. 2015, 88, 561–572. [Google Scholar] [CrossRef]
- Jacobs, T.B.; Zhang, N.; Patel, D.; Martin, G.B. Generation of a Collection of Mutant Tomato Lines Using Pooled CRISPR Libraries. Plant Physiol. 2017, 174, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome Editing with CRISPR–Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Michalski, K.; Hertig, C.; Mańkowski, D.R.; Kumlehn, J.; Zimny, J.; Linkiewicz, A.M. Functional Validation of Cas9/GuideRNA Constructs for Site-Directed Mutagenesis of Triticale ABA8′OH1 Loci. IJMS 2021, 22, 7038. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Zhi, S.; Chen, Y.; Wu, G.; Wen, J.; Wu, J.; Liu, Q.; Li, Y.; Kang, R.; Hu, S.; Wang, J.; et al. Dual-AAV Delivering Split Prime Editor System for in Vivo Genome Editing. Mol. Ther. 2022, 30, 283–294. [Google Scholar] [CrossRef]
- Che, P.; Wu, E.; Simon, M.K.; Anand, A.; Lowe, K.; Gao, H.; Sigmund, A.L.; Yang, M.; Albertsen, M.C.; Gordon-Kamm, W.; et al. Wuschel2 Enables Highly Efficient CRISPR/Cas-Targeted Genome Editing during Rapid de Novo Shoot Regeneration in Sorghum. Commun. Biol. 2022, 5, 344. [Google Scholar] [CrossRef]
- Qiu, F.; Xing, S.; Xue, C.; Liu, J.; Chen, K.; Chai, T.; Gao, C. Transient Expression of a TaGRF4-TaGIF1 Complex Stimulates Wheat Regeneration and Improves Genome Editing. Sci. China Life Sci. 2022, 65, 731–738. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Chen, Y.; Guo, H.; Tian, W.; Chen, Y.; Li, L. Fertile Plants Regenerated from Suspension Culture-Derived Protoplasts of an Indica Type Rice (Oryza Sativa L.). Plant Cell Tissue Organ Cult. 1993, 32, 61–68. [Google Scholar] [CrossRef]
- Biswas, G.C.G.; Zapata, F.J. High-Frequency Plant Regeneration from Protoplasts of Indica Rice (Oryza Sativa L.) Using Maltose. J. Plant. Physiol. 1993, 141, 470–475. [Google Scholar] [CrossRef]
- Wang, X.-H.; Lörz, H. Plant Regeneration from Protoplasts of Wild Barley (Hordeum Murinum L.). Plant Cell Rep. 1994, 13, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Guiderdoni, E.; Chair, H. Plant Regeneration from Haploid Cell Suspension-Derived Protoplasts of Mediterranean Rice (Oryza Sativa L. Cv. Miara). Plant Cell Rep. 1992, 11, 618–622. [Google Scholar] [CrossRef]
- Jähne, A.; Lazzeri, P.A.; Jäger-Gussen, M.; Lörz, H. Plant Regeneration from Embryogenic Cell Suspensions Derived from Anther Cultures of Barley (Hordeum Vulgare L.). Theoret. Appl. Genet. 1991, 82, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lührs, R.; Nielsen, K. Microspore Cultures as Donor Tissue for the Initiation of Embryogenic Cell Suspensions in Barley. Plant Cell Tissue Organ Cult. 1992, 31, 169–178. [Google Scholar] [CrossRef]
- Denchev, P.D.; Songstad, D.D.; McDaniel, J.K.; Conger, B.V. Transgenic Orchardgrass (Dactylis Glomerata) Plants by Direct Embryogenesis from Microprojecticle Bombarded Leaf Cells. Plant Cell Rep. 1997, 16, 813–819. [Google Scholar] [CrossRef]
- Haliloglu, K.; Aydin, M. Efficient Regeneration System from Rye Leaf Base Segments. SpringerPlus 2016, 5, 2005. [Google Scholar] [CrossRef]
- Biesaga-Kościelniak, J.; Kościelniak, J.; Filek, M.; Janeczko, A. Rapid Production of Wheat Cell Suspension Cultures Directly from Immature Embryos. Plant Cell Tissue Organ Cult. 2008, 94, 139–147. [Google Scholar] [CrossRef]
- Han, Y.; Broughton, S.; Liu, L.; Zhang, X.-Q.; Zeng, J.; He, X.; Li, C. Highly Efficient and Genotype-Independent Barley Gene Editing Based on Anther Culture. Plant Commun. 2021, 2, 100082. [Google Scholar] [CrossRef]
- Poddar, S.; Tanaka, J.; Cate, J.H.D.; Staskawicz, B.; Cho, M.-J. Efficient Isolation of Protoplasts from Rice Calli with Pause Points and Its Application in Transient Gene Expression and Genome Editing Assays. Plant Methods 2020, 16, 151. [Google Scholar] [CrossRef]
- Lin, C.; Hsu, C.; Yang, L.; Lee, L.; Fu, J.; Cheng, Q.; Wu, F.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R.; et al. Application of Protoplast Technology to CRISPR/Cas9 Mutagenesis: From Single-cell Mutation Detection to Mutant Plant Regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Kumar, J.; Alok, A.; Tuli, R. RNA-Guided Genome Editing for Target Gene Mutations in Wheat. G3 Genes Genomes Genet. 2013, 3, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Mercx, S.; Smargiasso, N.; Chaumont, F.; De Pauw, E.; Boutry, M.; Navarre, C. Inactivation of the β(1,2)-Xylosyltransferase and the α(1,3)-Fucosyltransferase Genes in Nicotiana Tabacum BY-2 Cells by a Multiplex CRISPR/Cas9 Strategy Results in Glycoproteins without Plant-Specific Glycans. Front. Plant Sci. 2017, 8, 403. [Google Scholar] [CrossRef]
- Permyakova, N.V.; Sidorchuk, Y.V.; Marenkova, T.V.; Khozeeva, S.A.; Kuznetsov, V.V.; Zagorskaya, A.A.; Rozov, S.M.; Deineko, E.V. CRISPR/Cas9-Mediated Gfp Gene Inactivation in Arabidopsis Suspension Cells. Mol. Biol. Rep. 2019, 46, 5735–5743. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Chardonnay (Vitis Vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, D.; De Smet, I. From Early Farmers to Norman Borlaug—the Making of Modern Wheat. Curr. Biol. 2017, 27, R858–R862. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, H.; Berner, T.; Lang, D.; Beier, S.; Stein, N.; Himmelbach, A.; Kilian, B.; Keilwagen, J. Insights into Breeding History, Hotspot Regions of Selection, and Untapped Allelic Diversity for Bread Wheat Breeding. Plant J. 2022, 112, 897–918. [Google Scholar] [CrossRef]
- Saeed, S.; Usman, B.; Shim, S.-H.; Khan, S.U.; Nizamuddin, S.; Saeed, S.; Shoaib, Y.; Jeon, J.-S.; Jung, K.-H. CRISPR/Cas-Mediated Editing of Cis-Regulatory Elements for Crop Improvement. Plant Sci. 2022, 324, 111435. [Google Scholar] [CrossRef]
- Arndell, T.; Sharma, N.; Langridge, P.; Baumann, U.; Watson-Haigh, N.S.; Whitford, R. GRNA Validation for Wheat Genome Editing with the CRISPR-Cas9 System. BMC Biotechnol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Kim, D.; Hager, M.; Brant, E.; Budak, H. Efficient Genome Editing in Wheat Using Cas9 and Cpf1 (AsCpf1 and LbCpf1) Nucleases. Funct. Integr. Genom. 2021, 21, 355–366. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 Genome Editing in Wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Eudes, F.; Acharya, S.; Laroche, A.; Selinger, L.B. A Novel Method to Induce Direct Somatic Embryogenesis, Secondary Embryogenesis and Regeneration of Fertile Green Cereal Plants. Plant Cell Tissue Organ Cult. 2003, 73, 147–157. [Google Scholar] [CrossRef]
- Targońska, M.; Hromada-Judycka, A.; Bolibok-Brągoszewska, H.; Rakoczy-Trojanowska, M. The Specificity and Genetic Background of the Rye (Secale Cereale L.) Tissue Culture Response. Plant Cell Rep. 2013, 32, 1–9. [Google Scholar] [CrossRef]
- Zimny, J.; Michalski, K. Development of in Vitro Culture Techniques for Advancement of Rye (Secale Cereale L.) Breeding. Acta Biol. Crac. S. Bot. 2019, 61, 7–15. [Google Scholar]
- Xu, M.; Du, Q.; Tian, C.; Wang, Y.; Jiao, Y. Stochastic Gene Expression Drives Mesophyll Protoplast Regeneration. Sci. Adv. 2021, 7, eabg8466. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, M.H.; Jin, D.M.; Ju, S.J.; Ahn, W.S.; Jie, E.Y.; Lee, J.M.; Lee, J.; Kim, C.Y.; Kim, S.W. TSA Promotes CRISPR/Cas9 Editing Efficiency and Expression of Cell Division-Related Genes from Plant Protoplasts. Int. J. Mol. Sci. 2021, 22, 7817. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yin, K.; Zhang, Q.; Gao, C.; Qiu, J.-L. Modulating Chromatin Accessibility by Transactivation and Targeting Proximal DsgRNAs Enhances Cas9 Editing Efficiency in Vivo. Genome Biol. 2019, 20, 145. [Google Scholar] [CrossRef]
- Pauk, J.; Manninen, O.; Mattila, I.; Salo, Y.; Pulli, S. Androgenesis in Hexaploid Spring Wheat F2 Populations and Their Parents Using a Multiple-Step Regeneration System. Plant Breed. 1991, 107, 18–27. [Google Scholar] [CrossRef]
- Kumlehn, J.; Serazetdinova, L.; Hensel, G.; Becker, D.; Loerz, H. Genetic Transformation of Barley (Hordeum Vulgare L.) via Infection of Androgenetic Pollen Cultures with Agrobacterium Tumefaciens. Plant Biotechnol. J. 2006, 4, 251–261. [Google Scholar] [CrossRef]
- Chomczynski, P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy Quantitative Assessment of Genome Editing by Sequence Trace Decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Subgenome | ||
|---|---|---|---|
| A | B | D | |
| gABA/1/364 Phos | |||
| g1P1 | 64.7% | 62.8% | 27.8% |
| g1P2 | 84.2% | 75.4% | 89.4% |
| g1P3 | 77.5% | ND | 97.7% |
| mean ± SD | 75.5 ± 9.9% | 69.1 ± 8.9% | 71.6 ± 38.2% |
| gABA/1/364 Hyg | |||
| g1H1 | 48.1% | ND | 6.9% |
| g1H2 | 40.1% | ND | 7.7% |
| g1H3 | 11% | ND | ND |
| mean ± SD | 33.1 ± 19.5% | 0% | 7.3 ± 0.6% |
| gABA/2/323 Phos | |||
| g2P1 | 76.1% | ND | 63.5% |
| g2P2 | 96.7% | ND | 19.5% |
| g2P3 | 90.9% | ND | 33% |
| mean ± SD | 87.9 ± 10.6% | 0% | 38.7 ± 22.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalski, K.; Ziąbska, P.; Sowa, S.; Zimny, J.; Linkiewicz, A.M. Evaluation of CRISPR/Cas9 Constructs in Wheat Cell Suspension Cultures. Int. J. Mol. Sci. 2023, 24, 2162. https://doi.org/10.3390/ijms24032162
Michalski K, Ziąbska P, Sowa S, Zimny J, Linkiewicz AM. Evaluation of CRISPR/Cas9 Constructs in Wheat Cell Suspension Cultures. International Journal of Molecular Sciences. 2023; 24(3):2162. https://doi.org/10.3390/ijms24032162
Chicago/Turabian StyleMichalski, Krzysztof, Paulina Ziąbska, Sławomir Sowa, Janusz Zimny, and Anna M. Linkiewicz. 2023. "Evaluation of CRISPR/Cas9 Constructs in Wheat Cell Suspension Cultures" International Journal of Molecular Sciences 24, no. 3: 2162. https://doi.org/10.3390/ijms24032162
APA StyleMichalski, K., Ziąbska, P., Sowa, S., Zimny, J., & Linkiewicz, A. M. (2023). Evaluation of CRISPR/Cas9 Constructs in Wheat Cell Suspension Cultures. International Journal of Molecular Sciences, 24(3), 2162. https://doi.org/10.3390/ijms24032162






