Transcriptome Analysis and Screening of Genes Associated with Flower Size in Tomato (Solanum lycopersicum)
Abstract
1. Introduction
2. Results
2.1. Quality Analysis of RNA-Seq Data
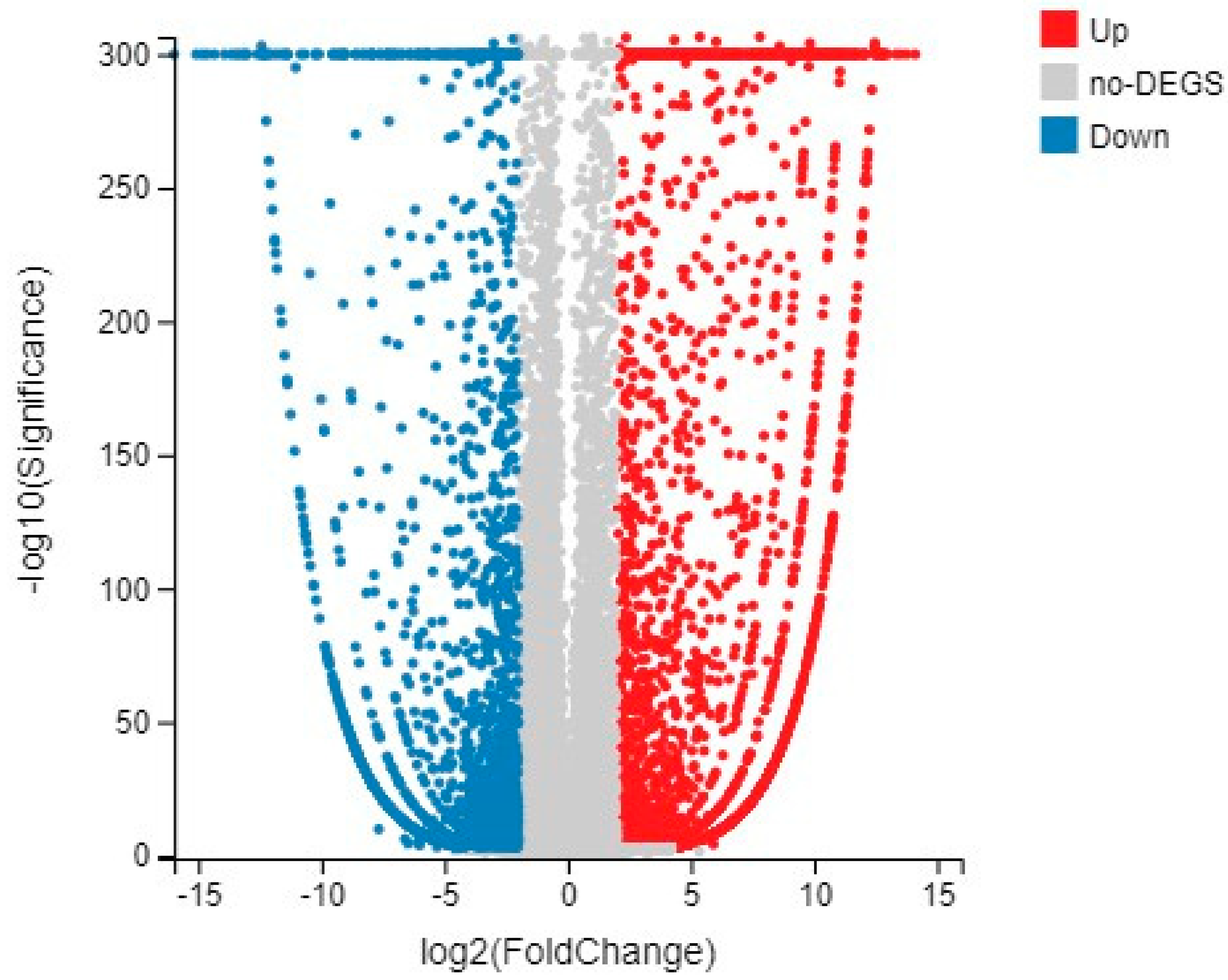
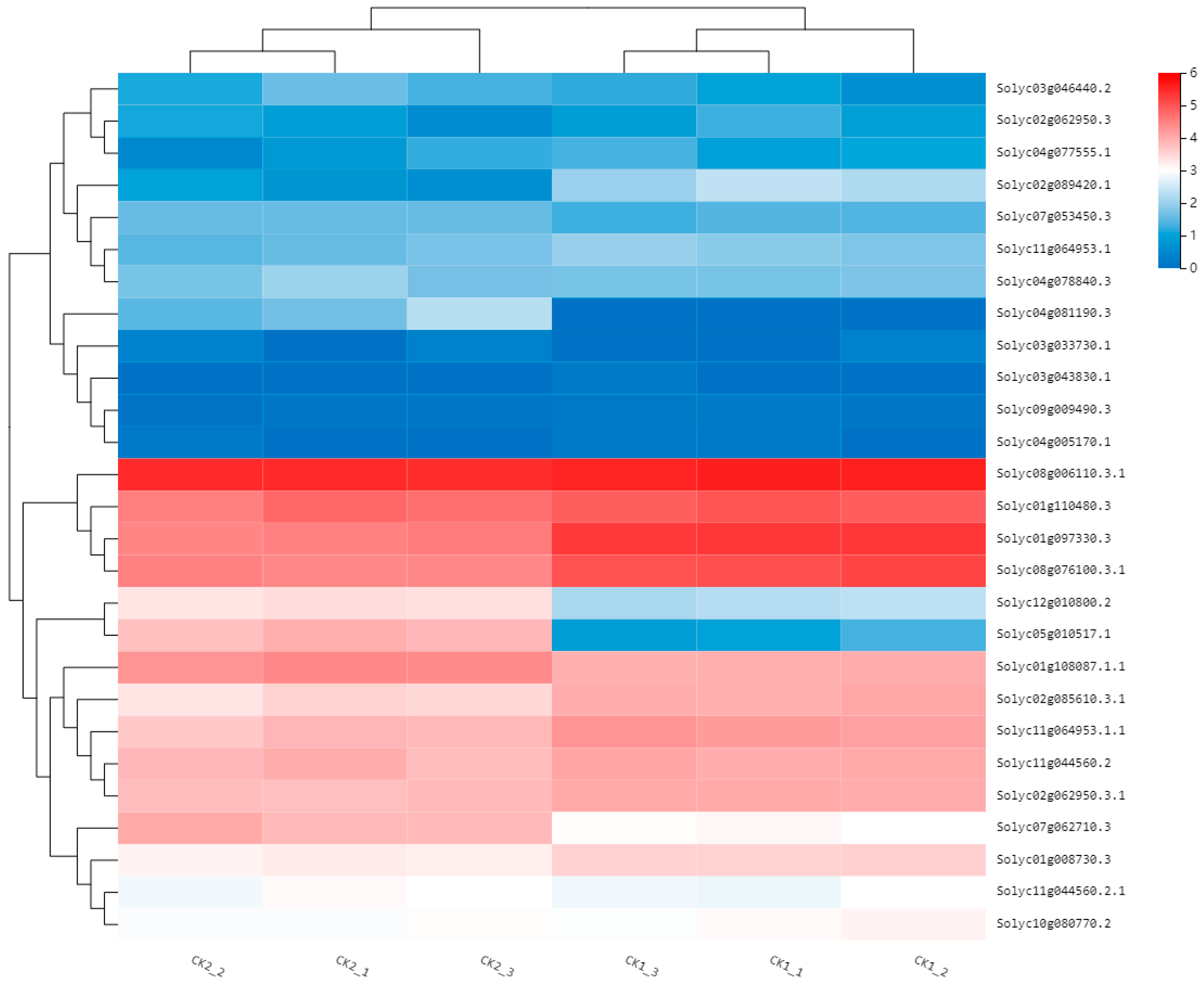
2.2. Analysis of Differentially Expressed Genes
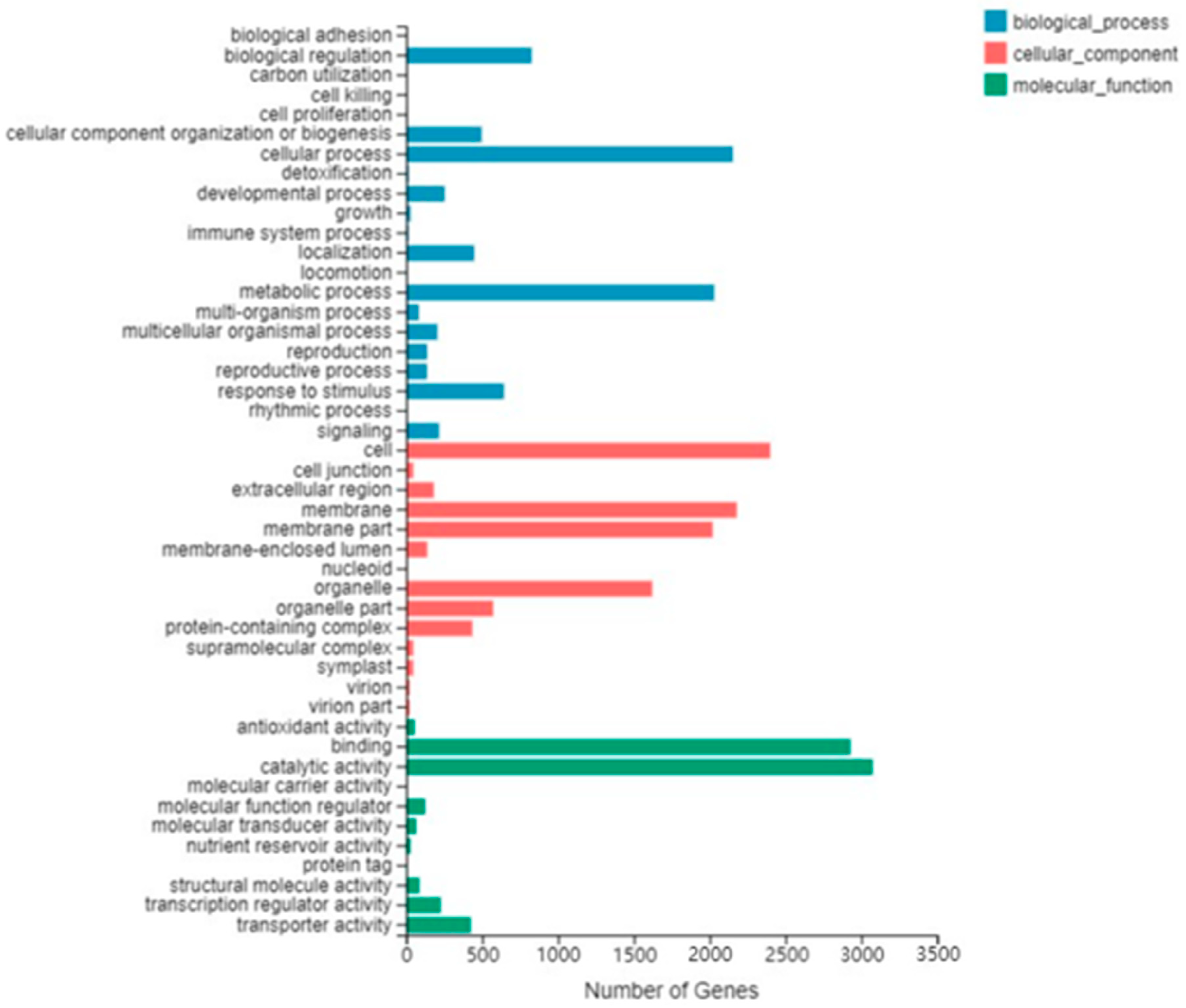
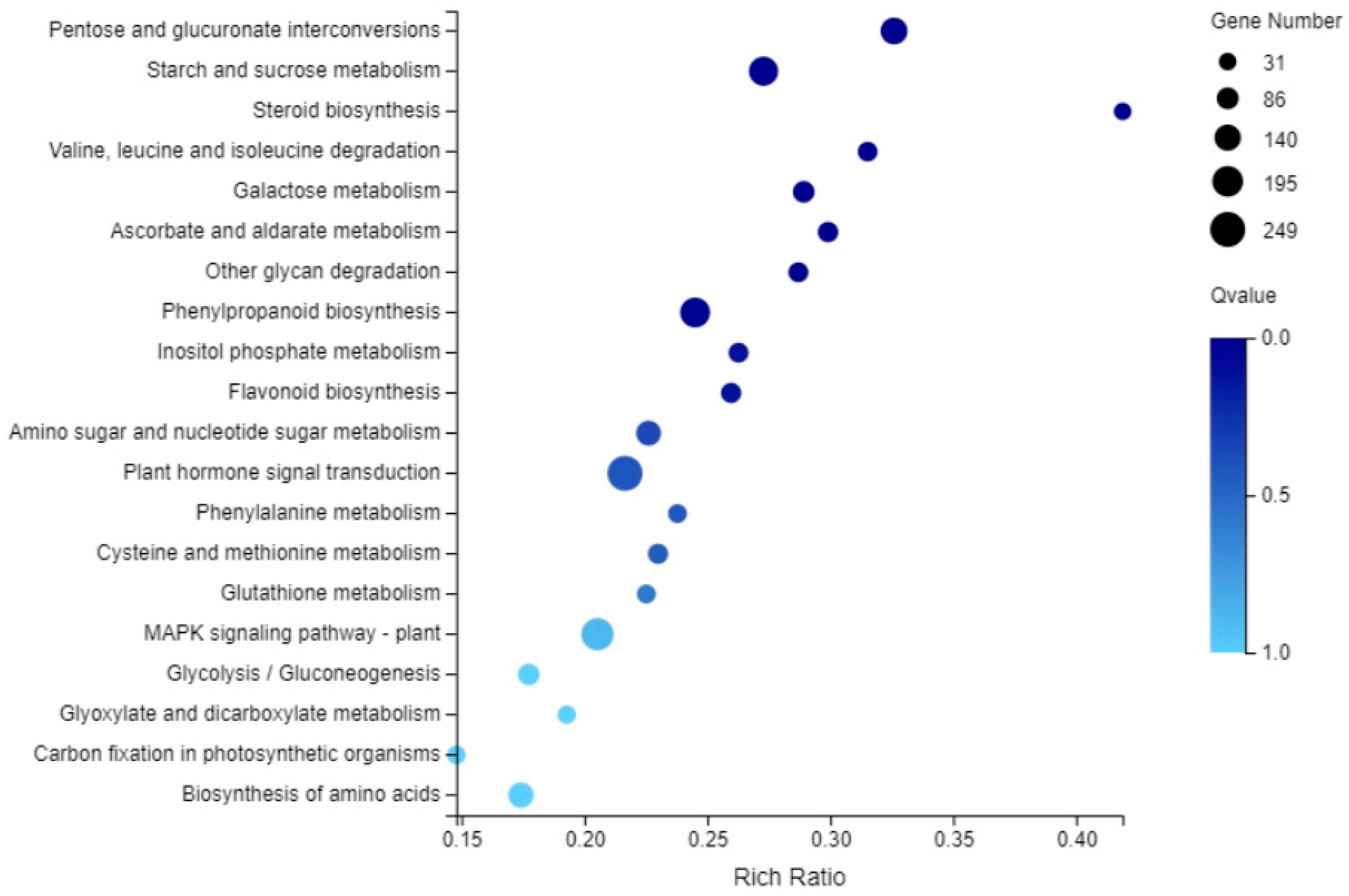
2.3. GO Analysis and KEGG Analysis of Differentially Expressed Genes
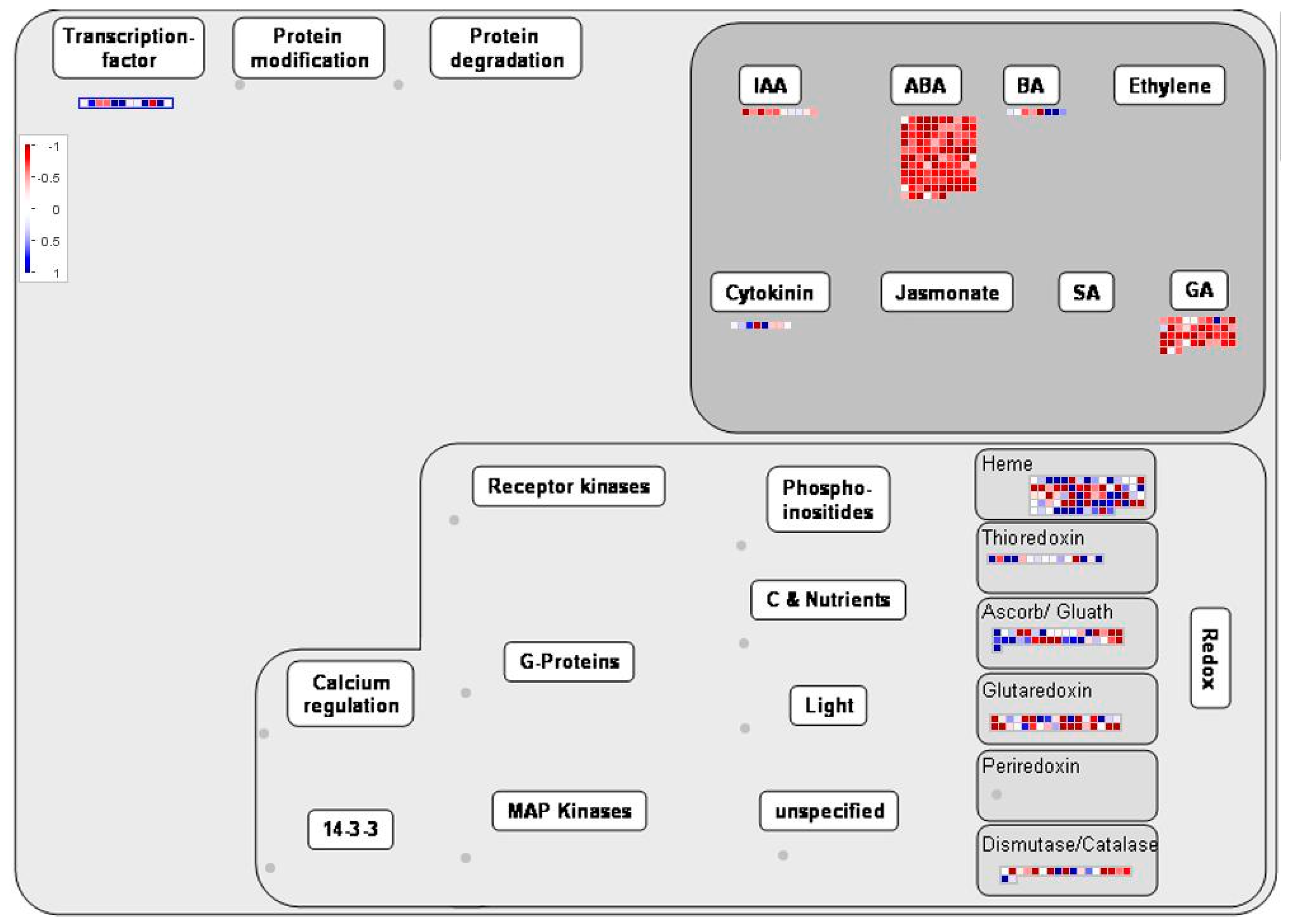
2.4. Metabolic and Regulatory Pathway Analysis of DEGs
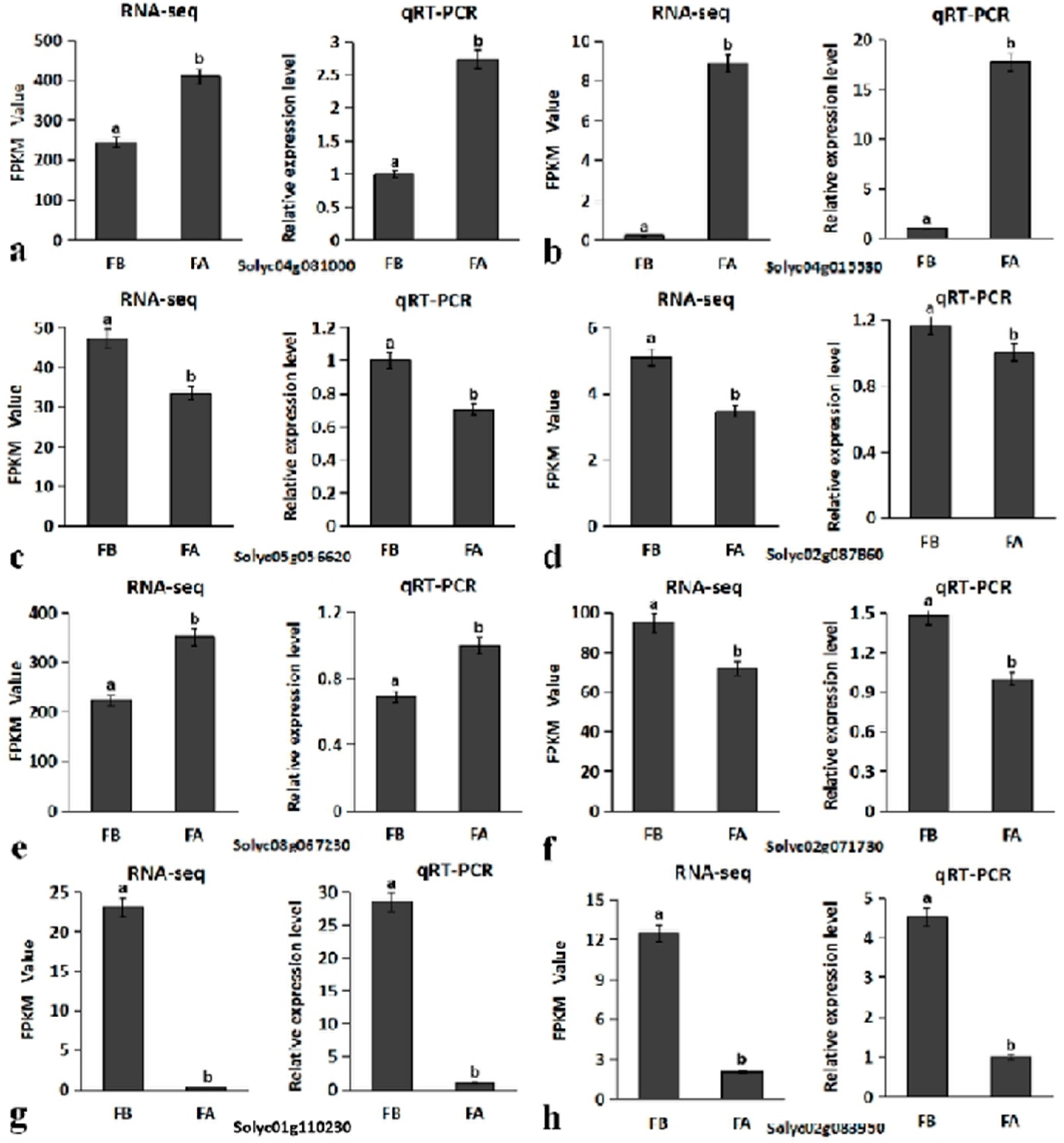
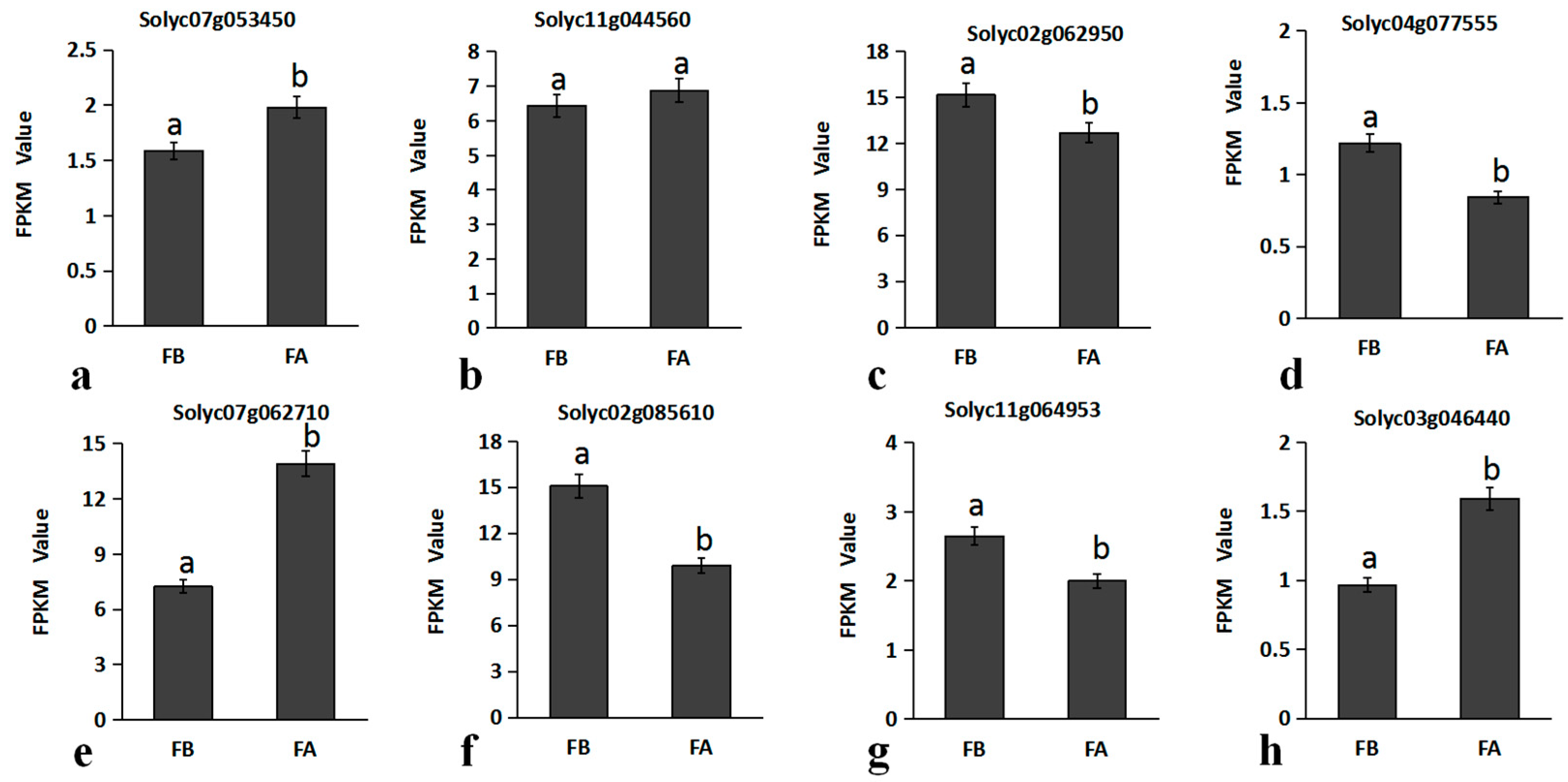
2.5. qRT-PCR Validation of RNA-Seq Data
2.6. Screening of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Sample Preparation
4.3. Construction of a Transcriptome Library
4.4. RNA-Seq Data Analysis
4.5. qRT-PCR Verification of Related Genes
4.6. Screening of Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prunet, N.; Jack, T.P. Flower development in Arabidopsis: There is more to it than learning your ABCs. Methods Mol. Biol. 2014, 1110, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The War of the Whorls: Genetic Interactions Controlling Flower Development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Angenent, G.C.; Franken, J.; Busscher, M.; van Dijken, A.; van Went, J.L.; Dons, H.J.; van Tunen, A.J. A novel class of MADS box genes is involved in ovule development in petunia. Plant Cell 1995, 7, 1569–1582. [Google Scholar] [CrossRef]
- Colombo, L.; Franken, J.; Koetje, E.; van Went, J.; Dons, H.J.; Angenent, G.C.; van Tunen, A.J. The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 1995, 7, 1859–1868. [Google Scholar] [CrossRef]
- Pinyopich, A.; Ditta, G.S.; Savidge, B.; Liljegren, S.J.; Baumann, E.; Wisman, E.; Yanofsky, M.F. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 2003, 424, 85–88. [Google Scholar] [CrossRef]
- Mizukami, Y.; Ma, H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 1992, 71, 119–131. [Google Scholar] [CrossRef]
- Theissen, G. Development of floral organ identity: Stories from the MADS house. Curr. Opin. Plant Biol. 2001, 4, 75–85. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Quinet, M.; Fernández-Lozano, A.; Pineda, B.; Moreno, V.; Angosto, T.; Lozano, R. Characterization of vegetative inflorescence (mc-vin) mutant provides new insight into the role of MACROCALYX in regulating inflorescence development of tomato. Sci. Rep. 2016, 6, 18796. [Google Scholar] [CrossRef]
- Geuten, K.; Irish, V. Hidden variability of floral homeotic B genes in Solanaceae provides a molecular basis for the evolution of novel functions. Plant Cell 2010, 22, 2562–2578. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Z.; Yin, W.; Yu, X.; Zhu, Z.; Zhang, J.; Chen, G. The tomato floral homeotic protein FBP1-like gene, SlGLO1, plays key roles in petal and stamen development. Sci. Rep. 2016, 6, 20454. [Google Scholar] [CrossRef]
- Yang, L.; Qi, S.; Touqeer, A.; Li, H.; Zhang, X.; Liu, X.; Wu, S. SlGT11 controls floral organ patterning and floral determinacy in tomato. BMC Plant Biol. 2020, 20, 562. [Google Scholar] [CrossRef] [PubMed]
- Amanda, B.S.; Luciane, G.; Juarez, P.T.; Poliana, R.C.; Sandra, C.G.; Michel, V. The Arabidopsis AtbZIP9 protein fused to the VP16 transcriptional activation domain alters leaf and vascular development. Plant Sci. 2007, 172, 1148–1156. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, H.W.; Hwang, I.S.; Choi, D.S.; Hwang, B.K. Functional roles of the pepper pathogen-induced bZIP transcription factor, CAbZIP1, in enhanced resistance to pathogen infection and environmental stresses. Planta 2006, 224, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Guan, Y.; Ren, H.; Zhang, F.; Chen, F. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol. Biol. 2008, 66, 675–683. [Google Scholar] [CrossRef]
- Chuang, C.F.; Running, M.P.; Williams, R.W.; Meyerowitz, E.M. The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana. Genes Dev. 1999, 13, 334–344. [Google Scholar] [CrossRef]
- Guan, Y.; Ren, H.; Xie, H.; Ma, Z.; Chen, F. Identification and characterization of bZIP-type transcription factors involved in carrot (Daucus carota L.) somatic embryogenesis. Plant J. 2009, 60, 207–217. [Google Scholar] [CrossRef]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Sagor, G.H.; Berberich, T.; Tanaka, S.; Nishiyama, M.; Kanayama, Y.; Kojima, S.; Muramoto, K.; Kusano, T. A novel strategy to produce sweeter tomato fruits with high sugar contents by fruit-specific expression of a single bZIP transcription factor gene. Plant Biotechnol. J. 2016, 14, 1116–1126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Yang, T.; Zhang, J.; Liu, B.; Zhan, X.; Liang, Y. The GAMYB-like gene SlMYB33 mediates flowering and pollen development in tomato. Hortic Res. 2020, 7, 133. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Hyodo, H.; Yamakawa, S.; Takeda, Y.; Tsuduki, M.; Yokota, A.; Nishitani, K.; Kohchi, T. Active gene expression of a xyloglucan endotransglucosylase/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F1000Research 2016, 5, F1000 Faculty Rev-119. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Fu, Y.; Wang, J.; Liang, B.; Li, Q.; Leng, P. Functional analysis of SlNCED1 in pistil development and fruit set in tomato (Solanum lycopersicum L.). Sci. Rep. 2019, 9, 16943. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, E.; Castañeda, L.; Pineda, B.; Pan, I.L.; Moreno, V.; Angosto, T.; Lozano, R. TOMATO AGAMOUS1 and ARLEQUIN/TOMATO AGAMOUS-LIKE1 MADS-box genes have redundant and divergent functions required for tomato reproductive development. Plant Mol. Biol. 2016, 91, 513–531. [Google Scholar] [CrossRef]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- Vrebalov, J.; Pan, I.L.; Arroyo, A.J.; McQuinn, R.; Chung, M.; Poole, M.; Rose, J.; Seymour, G.; Grandillo, S.; Giovannoni, J.; et al. Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1. Plant Cell 2009, 21, 3041–3062. [Google Scholar] [CrossRef]
- Wang, H.; Schauer, N.; Usadel, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latché, A.; Pech, J.C.; Fernie, A.R.; Bouzayen, M. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 2009, 21, 1428–1452. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Chen, S.; Fu, D.Q.; Jiang, C.Z. Metabolomic and Transcriptomic Analyses Reveal That a MADS-Box Transcription Factor TDR4 Regulates Tomato Fruit Quality. Front. Plant Sci. 2019, 10, 792. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Fitzpatrick, A.H.; Shrestha, N.; Bhandari, J.; Crowell, D.N. Roles for farnesol and ABA in Arabidopsis flower development. Plant Signal Behav. 2011, 6, 1189–1191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. Omics Approaches Toward Defining the Comprehensive Abscisic Acid Signaling Network in Plants. Plant Cell Physiol. 2015, 56, 1043–1052. [Google Scholar] [CrossRef]
- Hwang, K.; Susila, H.; Nasim, Z.; Jung, J.Y.; Ahn, J.H. Arabidopsis ABF3 and ABF4 Transcription Factors Act with the NF-YC Complex to Regulate SOC1 Expression and Mediate Drought-Accelerated Flowering. Mol. Plant 2019, 12, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Gibalová, A.; Steinbachová, L.; Hafidh, S.; Bláhová, V.; Gadiou, Z.; Michailidis, C.; Műller, K.; Pleskot, R.; Dupľáková, N.; Honys, D. Characterization of pollen-expressed bZIP protein interactions and the role of ATbZIP18 in the male gametophyte. Plant Reprod. 2017, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Cao, K.; Wang, X. A conserved proline residue in the leucine zipper region of AtbZIP34 and AtbZIP61 in Arabidopsis thaliana interferes with the formation of homodimer. Biochem Biophys Res. Commun. 2007, 362, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Q20 (%) | Q30 (%) | Clean Reads Ratio (%) |
|---|---|---|---|---|---|---|
| CK1_1 | 43.82 | 42.94 | 6.44 | 96.12 | 90.89 | 97.99 |
| CK1_2 | 43.82 | 43.08 | 6.46 | 95.91 | 90.42 | 98.3 |
| CK1_3 | 43.82 | 42.95 | 6.44 | 95.97 | 90.55 | 98.02 |
| CK2_1 | 43.82 | 42.54 | 6.38 | 96.1 | 90.86 | 97.08 |
| CK2_2 | 43.82 | 42.9 | 6.44 | 96 | 90.64 | 97.9 |
| CK2_3 | 43.82 | 42.78 | 6.42 | 96.05 | 90.73 | 97.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, A.; Yang, W.; Jia, X.; Fu, Q.; Zhao, T.; Jiang, J.; Li, J.; Yang, H.; Xu, X. Transcriptome Analysis and Screening of Genes Associated with Flower Size in Tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2022, 23, 15624. https://doi.org/10.3390/ijms232415624
Zhang Y, Zhang A, Yang W, Jia X, Fu Q, Zhao T, Jiang J, Li J, Yang H, Xu X. Transcriptome Analysis and Screening of Genes Associated with Flower Size in Tomato (Solanum lycopersicum). International Journal of Molecular Sciences. 2022; 23(24):15624. https://doi.org/10.3390/ijms232415624
Chicago/Turabian StyleZhang, Yiyao, Aining Zhang, Wenhui Yang, Xinyi Jia, Qingjun Fu, Tingting Zhao, Jingbin Jiang, Jingfu Li, Huanhuan Yang, and Xiangyang Xu. 2022. "Transcriptome Analysis and Screening of Genes Associated with Flower Size in Tomato (Solanum lycopersicum)" International Journal of Molecular Sciences 23, no. 24: 15624. https://doi.org/10.3390/ijms232415624
APA StyleZhang, Y., Zhang, A., Yang, W., Jia, X., Fu, Q., Zhao, T., Jiang, J., Li, J., Yang, H., & Xu, X. (2022). Transcriptome Analysis and Screening of Genes Associated with Flower Size in Tomato (Solanum lycopersicum). International Journal of Molecular Sciences, 23(24), 15624. https://doi.org/10.3390/ijms232415624






