A Combination of Caffeine Supplementation and Enriched Environment in an Alzheimer’s Disease Mouse Model
Abstract
1. Introduction
2. Results
2.1. Weight Assessment in Tg4-42 Mice
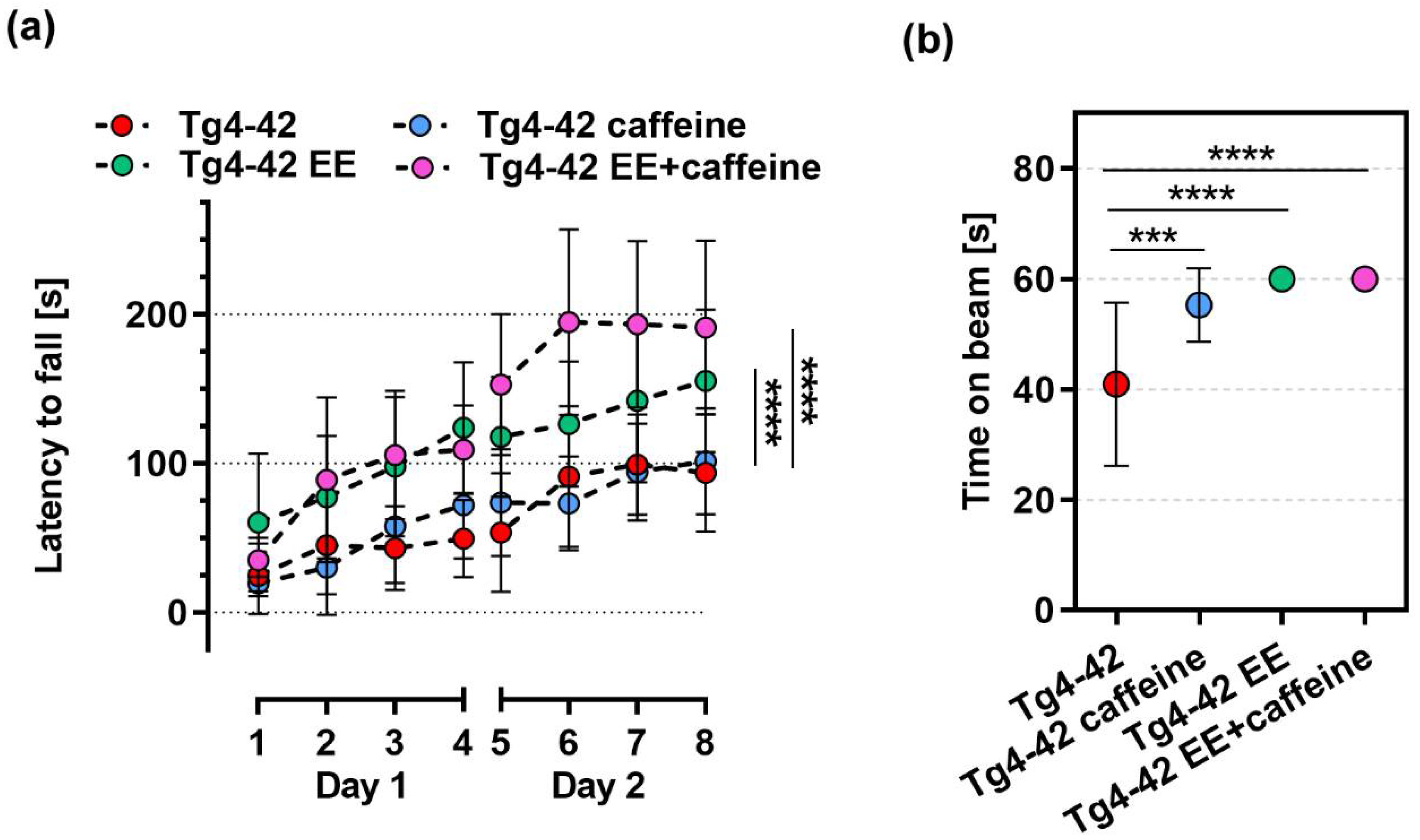
2.2. Motor Performance in Tg4-42 Mice upon Enriched Environment Housing and Caffeine Treatment
2.3. Caffeine Treatment and/or Enriched Housing Improved Recognition Memory
2.4. Spatial Memory in Tg4-42 Mice upon Caffeine Treatment and Enriched Housing Conditions
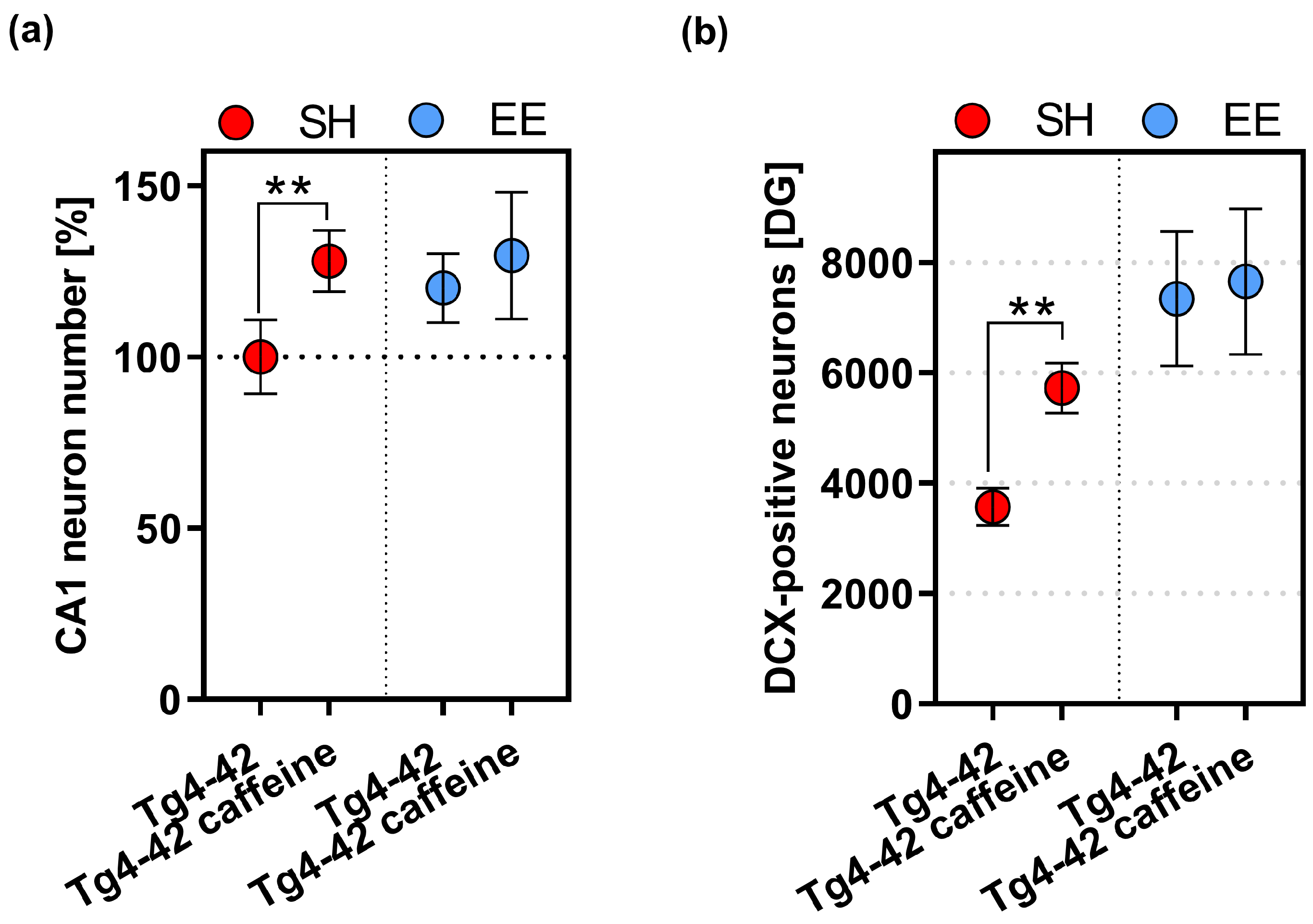
2.5. CA1 Neuron Numbers in Caffeine and EE-Treated Tg4-42 Mice
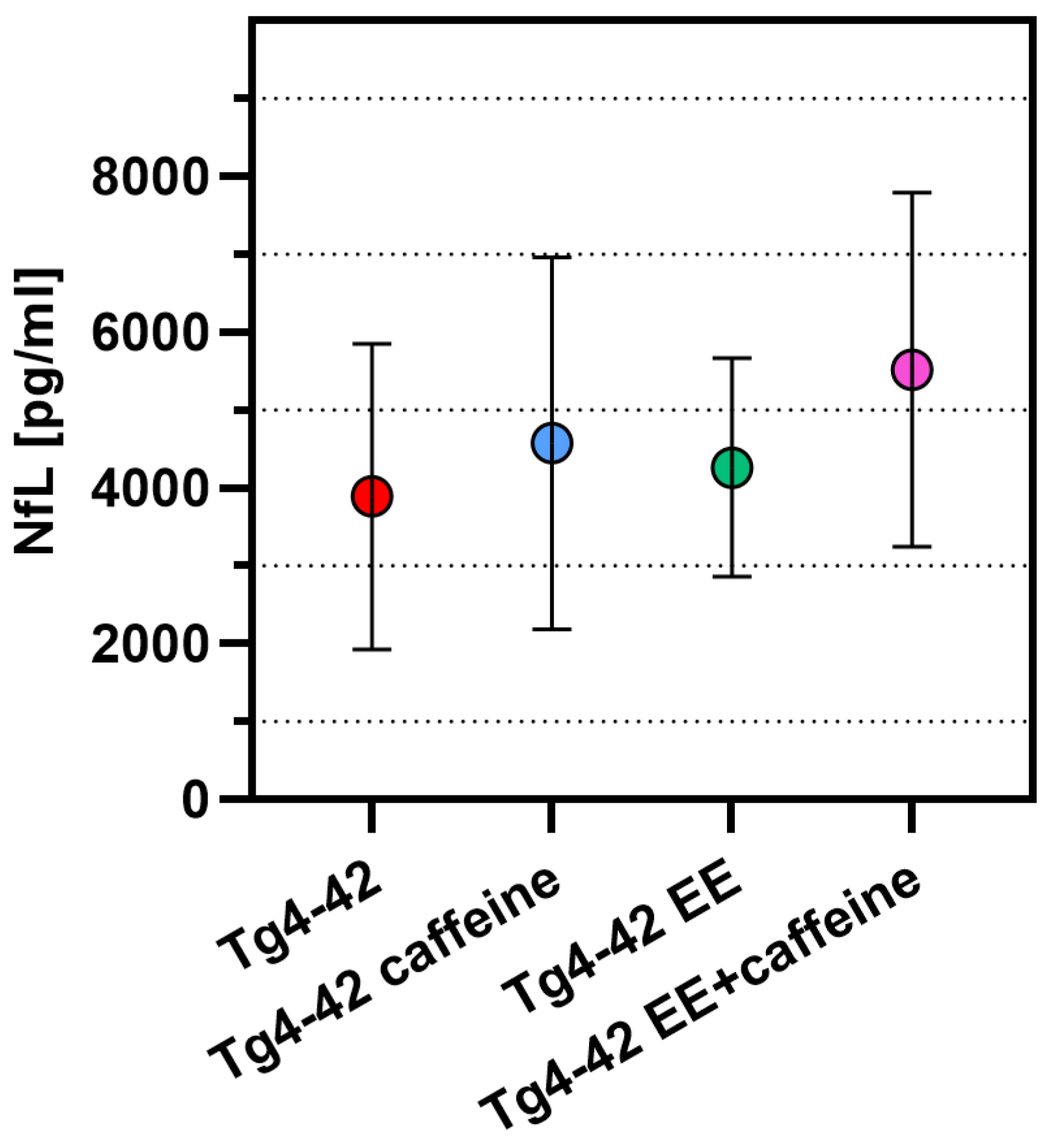
2.6. Unchanged Neurofilament Light Chain Levels in the Plasma of Tg4-42 Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Behavioral Tasks
4.2.1. Balance Beam
4.2.2. Accelerating Rotarod
4.2.3. Open Field and Novel Object Recognition
4.2.4. Morris Water Maze
4.3. Tissue Collection and Preservation
4.4. Quantification of CA1 Neuron Number
4.5. Analysis of Adult Neurogenesis
4.6. Neurofilament Light Chain Levels in Murine Plasma
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferré, S. Mechanisms of the psychostimulant effects of caffeine: Implications for substance use disorders. Psychopharmacology 2016, 233, 1963–1979. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of Coffee Consumption With Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Londzin, P.; Zamora, M.; Kąkol, B.; Taborek, A.; Folwarczna, J. Potential of Caffeine in Alzheimer’s Disease-A Review of Experimental Studies. Nutrients 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef] [PubMed]
- Stazi, M.; Lehmann, S.; Sakib, M.S.; Pena-Centeno, T.; Büschgens, L.; Fischer, A.; Weggen, S.; Wirths, O. Long-term caffeine treatment of Alzheimer mouse models ameliorates behavioural deficits and neuron loss and promotes cellular and molecular markers of neurogenesis. Cell. Mol. Life Sci. 2022, 79, 55. [Google Scholar] [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Müller, C.E.; Buée, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging 2014, 35, 2079–2090. [Google Scholar] [CrossRef]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef]
- Cao, C.; Cirrito, J.R.; Lin, X.; Wang, L.; Verges, D.K.; Dickson, A.; Mamcarz, M.; Zhang, C.; Mori, T.; Arendash, G.W.; et al. Caffeine Suppresses Amyloid-β Levels in Plasma and Brain of Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2009, 17, 681–697. [Google Scholar] [CrossRef]
- Ritchie, K.; Carrière, I.; de Mendonça, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 2007, 69, 536–545. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, B.M.; Buijsse, B.; Tijhuis, M.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur. J. Clin. Nutr. 2007, 61, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, L. The Neuroprotective Effects of Moderate and Regular Caffeine Consumption in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimers Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-d.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Cao, C.; Loewenstein, D.A.; Lin, X.; Zhang, C.; Wang, L.; Duara, R.; Wu, Y.; Giannini, A.; Bai, G.; Cai, J.; et al. High Blood caffeine levels in MCI linked to lack of progression to dementia. J. Alzheimers Dis. 2012, 30, 559–572. [Google Scholar]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Jeon, S.Y.; Jung, G.; Lee, H.N.; Sohn, B.K.; Lee, J.-Y.; Kim, Y.K.; et al. Coffee intake and decreased amyloid pathology in human brain. Transl. Psychiatry 2019, 9, 270. [Google Scholar] [CrossRef]
- Kivimäki, M.; Walker, K.A.; Pentti, J.; Nyberg, S.T.; Mars, N.; Vahtera, J.; Suominen, S.B.; Lallukka, T.; Rahkonen, O.; Pietiläinen, O.; et al. Cognitive stimulation in the workplace, plasma proteins, and risk of dementia: Three analyses of population cohort studies. BMJ 2021, 374, n1804. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.F.; Wan, Y.; Tan, C.-C.; Yu, J.-T.; Tan, L. Leisure time physical activity and dementia risk: A dose-response meta-analysis of prospective studies. BMJ Open 2017, 7, e014706. [Google Scholar] [CrossRef]
- del Pozo Cruz, B.; Ahmadi, M.; Naismith, S.L.; Stamatakis, E. Association of Daily Step Count and Intensity With Incident Dementia in 78 430 Adults Living in the UK. JAMA Neurol. 2022, 79, 1059–1063. [Google Scholar] [CrossRef]
- Smith, K.J.; Ainslie, P.N. Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 2017, 102, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Geda, Y.E.; Graff-Radford, N.R.; Petersen, R.C. Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging. Mayo Clin. Proc. 2011, 86, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Iso-Markku, P.; Waller, K.; Kujala, U.M.; Kaprio, J. Physical activity and dementia: Long-term follow-up study of adult twins. Ann. Med. 2015, 47, 81–87. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kobilo, T.; Liu, Q.-R.; Gandhi, K.; Mughal, M.; Shaham, Y.; van Praag, H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Memory 2011, 18, 605–609. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.-J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 2011, 43, 486–494. [Google Scholar] [CrossRef]
- Costa, D.A.; Cracchiolo, J.R.; Bachstetter, A.D.; Hughes, T.F.; Bales, K.R.; Paul, S.M.; Mervis, R.F.; Arendash, G.W.; Potter, H. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol. Aging 2007, 28, 831–844. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Salinas, G.; Wirths, O. Effects of long-term environmental enrichment on anxiety, memory, hippocampal plasticity and overall brain gene expression in C57BL6 mice. Front. Mol. Neurosci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Kazlauckas, V.; Pagnussat, N.; Mioranzza, S.; Kalinine, E.; Nunes, F.; Pettenuzzo, L.; Souza, D.O.; Portela, L.V.; Porciúncula, L.O.; Lara, D.R. Enriched environment effects on behavior, memory and BDNF in low and high exploratory mice. Physiol. Behav. 2011, 102, 475–480. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Robinson, J.; Tang, Y.P.; Hairston, I.S.; Korade-Mirnics, Z.; Lee, V.M.; Hersh, L.B.; Sapolsky, R.M.; Mirnics, K.; Sisodia, S.S. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005, 120, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Cracchiolo, J.R.; Mori, T.; Nazian, S.J.; Tan, J.; Potter, H.; Arendash, G.W. Enhanced cognitive activity--over and above social or physical activity--is required to protect Alzheimer’s mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol. Learn. Mem. 2007, 88, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Garcia, M.F.; Costa, D.A.; Cracchiolo, J.R.; Wefes, I.M.; Potter, H. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable beta-amyloid deposition. Neuroreport 2004, 15, 1751–1754. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Walter, S.; Kaufmann, M.; Weggen, S.; Wirths, O. Limited Effects of Prolonged Environmental Enrichment on the Pathology of 5XFAD Mice. Mol. Neurobiol. 2017, 54, 6542–6555. [Google Scholar] [CrossRef]
- Cotel, M.C.; Jawhar, S.; Christensen, D.Z.; Bayer, T.A.; Wirths, O. Environmental enrichment fails to rescue working memory deficits, neuron loss, and neurogenesis in APP/PS1KI mice. Neurobiol. Aging 2012, 33, 96–107. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Xu, G.; Fromholt, D.; Gonzales, V.; Borchelt, D.R. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2003, 62, 1220–1227. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Brauss, A.; Kurdakova, A.; Borgers, H.; Klinker, F.; Liebetanz, D.; Salinas-Riester, G.; Wiltfang, J.; Klafki, H.W.; Wirths, O. Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Transl. Psychiatry 2016, 6, e800. [Google Scholar] [CrossRef]
- Stazi, M.; Wirths, O. Physical activity and cognitive stimulation ameliorate learning and motor deficits in a transgenic mouse model of Alzheimer’s disease. Behav. Brain Res. 2021, 397, 112951. [Google Scholar] [CrossRef]
- Portelius, E.; Bogdanovic, N.; Gustavsson, M.K.; Volkmann, I.; Brinkmalm, G.; Zetterberg, H.; Winblad, B.; Blennow, K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010, 120, 185–193. [Google Scholar] [CrossRef]
- Bouter, Y.; Dietrich, K.; Wittnam, J.L.; Rezaei-Ghaleh, N.; Pillot, T.; Papot-Couturier, S.; Lefebvre, T.; Sprenger, F.; Wirths, O.; Zweckstetter, M.; et al. N-truncated amyloid β (Aβ) 4-42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol. 2013, 126, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Maugard, M.; Doux, C.; Bonvento, G. A new statistical method to analyze Morris Water Maze data using Dirichlet distribution. F1000Res 2019, 8, 1601. [Google Scholar] [CrossRef] [PubMed]
- Stazi, M.; Wirths, O. Chronic Memantine Treatment Ameliorates Behavioral Deficits, Neuron Loss, and Impaired Neurogenesis in a Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 204–216. [Google Scholar] [CrossRef]
- Antonios, G.; Borgers, H.; Richard, B.C.; Brauß, A.; Meißner, J.; Weggen, S.; Pena, V.; Pillot, T.; Davies, S.L.; Bakrania, P.; et al. Alzheimer therapy with an antibody against N-terminal Abeta 4-X and pyroglutamate Abeta 3-X. Sci. Rep. 2015, 5, 17338. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimers Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Melnikova, T.; Fadale, D.J.; Xu, G.M.; Slunt, H.H.; Gonzales, V.; Younkin, L.H.; Younkin, S.G.; Borchelt, D.R.; Savonenko, A.V. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci. 2005, 25, 5217–5224. [Google Scholar] [CrossRef]
- Stazi, M.; Zampar, S.; Nadolny, M.; Büschgens, L.; Meyer, T.; Wirths, O. Combined long-term enriched environment and caffeine supplementation improve memory function in C57Bl6 mice. Eur. Arch. Psychiatry Clin. Neurosci. 2022. [Google Scholar] [CrossRef]
- Wagner, J.M.; Sichler, M.E.; Schleicher, E.M.; Franke, T.N.; Irwin, C.; Löw, M.J.; Beindorff, N.; Bouter, C.; Bayer, T.A.; Bouter, Y. Analysis of Motor Function in the Tg4-42 Mouse Model of Alzheimer’s Disease. Front. Behav. Neurosci. 2019, 13, 107. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.Q. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2013, 8, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Memory 2010, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Morava, A.; Fagan, M.J.; Prapavessis, H. Effects of Caffeine and Acute Aerobic Exercise on Working Memory and Caffeine Withdrawal. Sci. Rep. 2019, 9, 19644. [Google Scholar] [CrossRef]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance—an umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.L.; Guilherme, J.P.L.F.; Ferreira, L.H.B.; de Souza-Junior, T.P.; Lancha, A.H. Caffeine and Exercise Performance: Possible Directions for Definitive Findings. Front. Sports Act. Living 2020, 2, 574854. [Google Scholar] [CrossRef] [PubMed]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta–Analysis. Sports Med. 2018, 48, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Delleli, S.; Ouergui, I.; Messaoudi, H.; Trabelsi, K.; Ammar, A.; Glenn, J.M.; Chtourou, H. Acute Effects of Caffeine Supplementation on Physical Performance, Physiological Responses, Perceived Exertion, and Technical-Tactical Skills in Combat Sports: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2996. [Google Scholar] [CrossRef]
- Hogervorst, E.; Riedel, W.J.; Kovacs, E.; Brouns, F.; Jolles, J. Caffeine improves cognitive performance after strenuous physical exercise. Int. J. Sports Med. 1999, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Bandelow, S.; Schmitt, J.; Jentjens, R.; Oliveira, M.; Allgrove, J.; Carter, T.; Gleeson, M. Caffeine Improves Physical and Cognitive Performance during Exhaustive Exercise. Med. Sci. Sports Exerc. 2008, 40, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Kauser, H.; Ray, K.; Kishore, K.; Kumar, S.; Panjwani, U. Caffeine and modafinil promote adult neuronal cell proliferation during 48h of total sleep deprivation in rat dentate gyrus. Exp. Neurol. 2013, 248, 470–481. [Google Scholar] [CrossRef]
- Mao, Z.-F.; Ouyang, S.-H.; Zhang, Q.-Y.; Wu, Y.-P.; Wang, G.-E.; Tu, L.-F.; Luo, Z.; Li, W.-X.; Kurihara, H.; Li, Y.-F.; et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J. 2020, 34, 10998–11014. [Google Scholar] [CrossRef]
- Ma, C.-L.; Ma, X.-T.; Wang, J.-J.; Liu, H.; Chen, Y.-F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Houghton, V.; Du Preez, A.; Lefèvre-Arbogast, S.; de Lucia, C.; Low, D.Y.; Urpi-Sarda, M.; Ruigrok, S.R.; Altendorfer, B.; González-Domínguez, R.; Andres-Lacueva, C.; et al. Caffeine Compromises Proliferation of Human Hippocampal Progenitor Cells. Front. Cell Dev. Biol. 2020, 8, 806. [Google Scholar] [CrossRef]
- Wentz, C.T.; Magavi, S.S.P. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology 2009, 56, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Park, K.-H.; Baek, S.-Y.; Kim, B.-S.; Kim, J.-B.; Kim, H.-J.; Oh, S.-O. Inhibitory effects of caffeine on hippocampal neurogenesis and function. Biochem. Biophys. Res. Commun. 2007, 356, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Klein, B.; Mrowetz, H.; Kreutzer, C.; Rotheneichner, P.; Zaunmair, P.; Lange, S.; Coras, R.; Couillard-Despres, S.; Rivera, F.J.; Aigner, L. DCX+ neuronal progenitors contribute to new oligodendrocytes during remyelination in the hippocampus. Sci. Rep. 2020, 10, 20095. [Google Scholar] [CrossRef]
- Lewczuk, P.; Ermann, N.; Andreasson, U.; Schultheis, C.; Podhorna, J.; Spitzer, P.; Maler, J.M.; Kornhuber, J.; Blennow, K.; Zetterberg, H. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Hinteregger, B.; Loeffler, T.; Flunkert, S.; Neddens, J.; Bayer, T.A.; Madl, T.; Hutter-Paier, B. Metabolic, Phenotypic, and Neuropathological Characterization of the Tg4-42 Mouse Model for Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 1151–1168. [Google Scholar] [CrossRef]
- Shahim, P.; Zetterberg, H.; Tegner, Y.; Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017, 88, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stazi, M.; Zampar, S.; Klafki, H.-W.; Meyer, T.; Wirths, O. A Combination of Caffeine Supplementation and Enriched Environment in an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2023, 24, 2155. https://doi.org/10.3390/ijms24032155
Stazi M, Zampar S, Klafki H-W, Meyer T, Wirths O. A Combination of Caffeine Supplementation and Enriched Environment in an Alzheimer’s Disease Mouse Model. International Journal of Molecular Sciences. 2023; 24(3):2155. https://doi.org/10.3390/ijms24032155
Chicago/Turabian StyleStazi, Martina, Silvia Zampar, Hans-Wolfgang Klafki, Thomas Meyer, and Oliver Wirths. 2023. "A Combination of Caffeine Supplementation and Enriched Environment in an Alzheimer’s Disease Mouse Model" International Journal of Molecular Sciences 24, no. 3: 2155. https://doi.org/10.3390/ijms24032155
APA StyleStazi, M., Zampar, S., Klafki, H.-W., Meyer, T., & Wirths, O. (2023). A Combination of Caffeine Supplementation and Enriched Environment in an Alzheimer’s Disease Mouse Model. International Journal of Molecular Sciences, 24(3), 2155. https://doi.org/10.3390/ijms24032155





