Inhibition Effect of Adipogenesis and Lipogenesis via Activation of AMPK in Preadipocytes Treated with Canavalia gladiata Extract
Abstract
1. Introduction
2. Results and Discussion
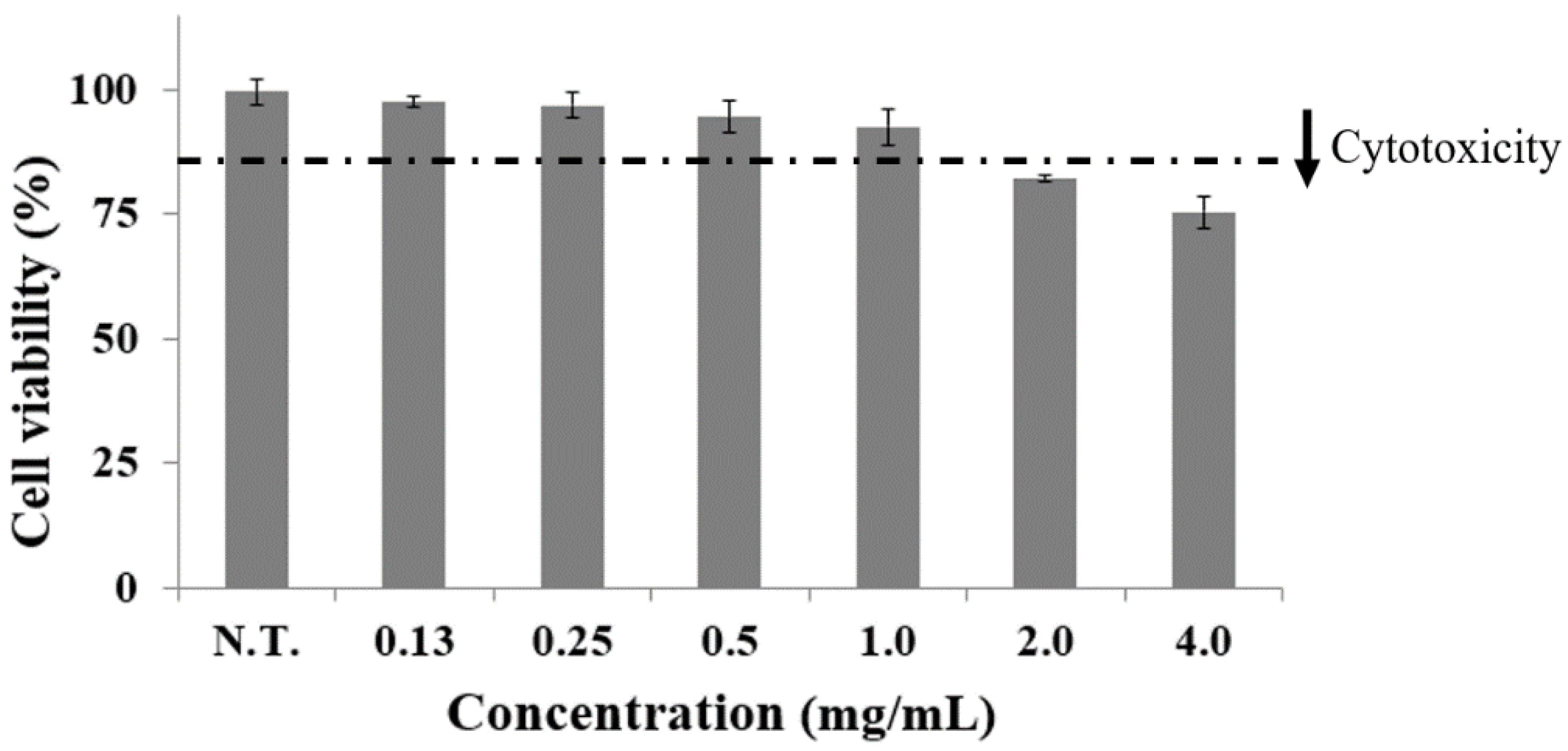
2.1. Effect of CGE on Cell Viability
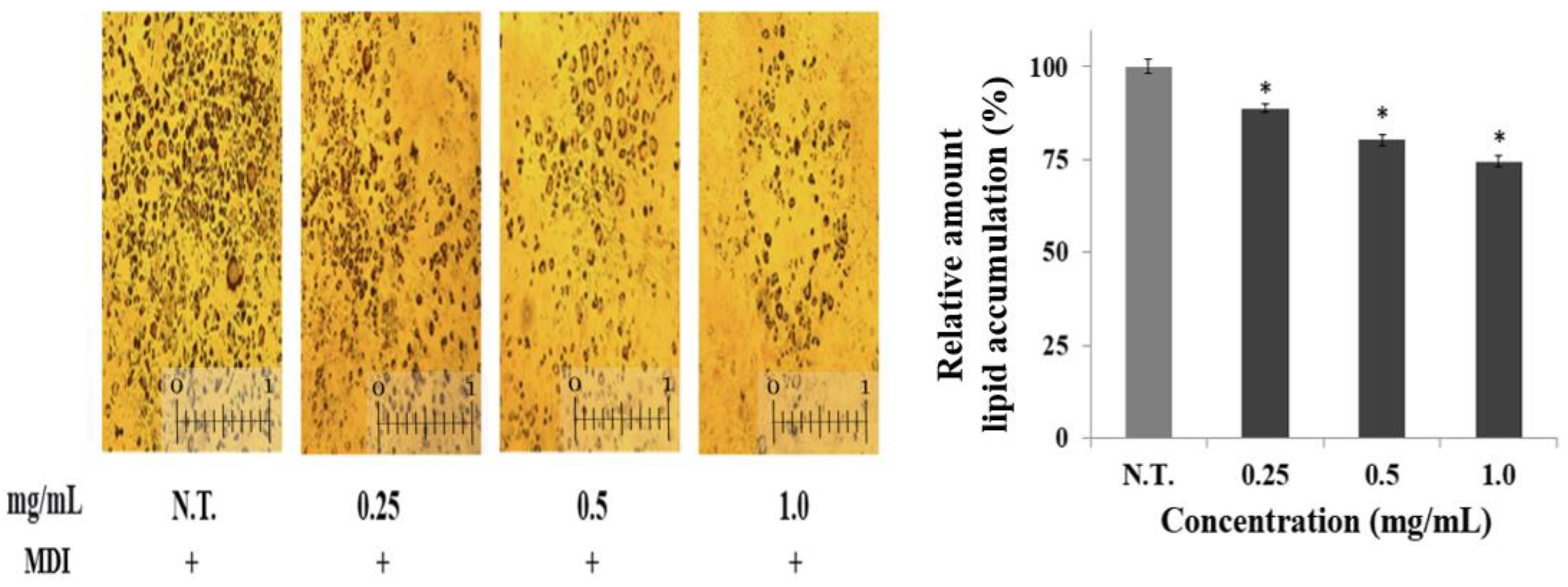
2.2. Effect of CGE on Lipid Accumulation
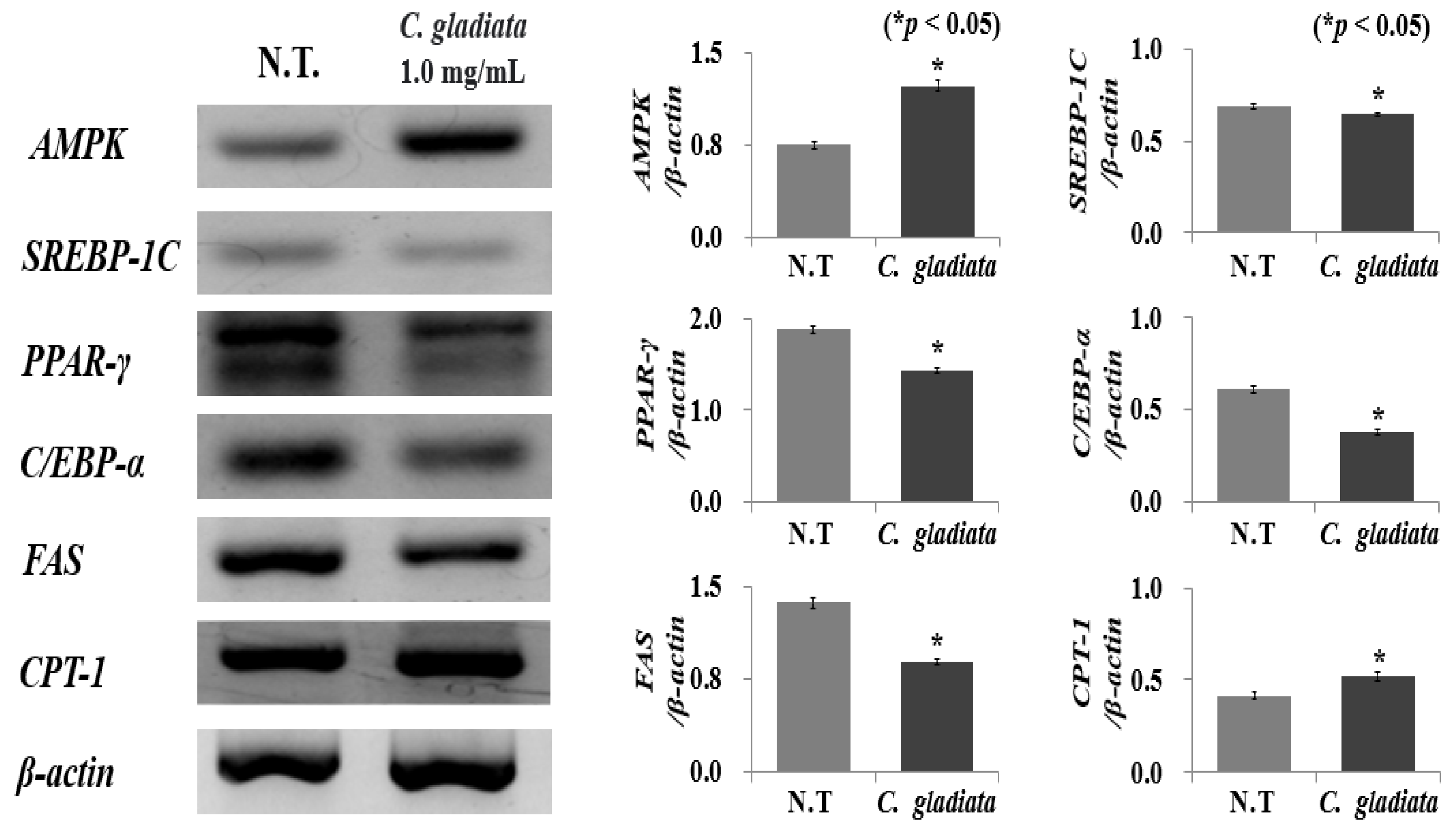
2.3. Effect of CGE on Expression of Adipogenesis and Lipogenesis Genes
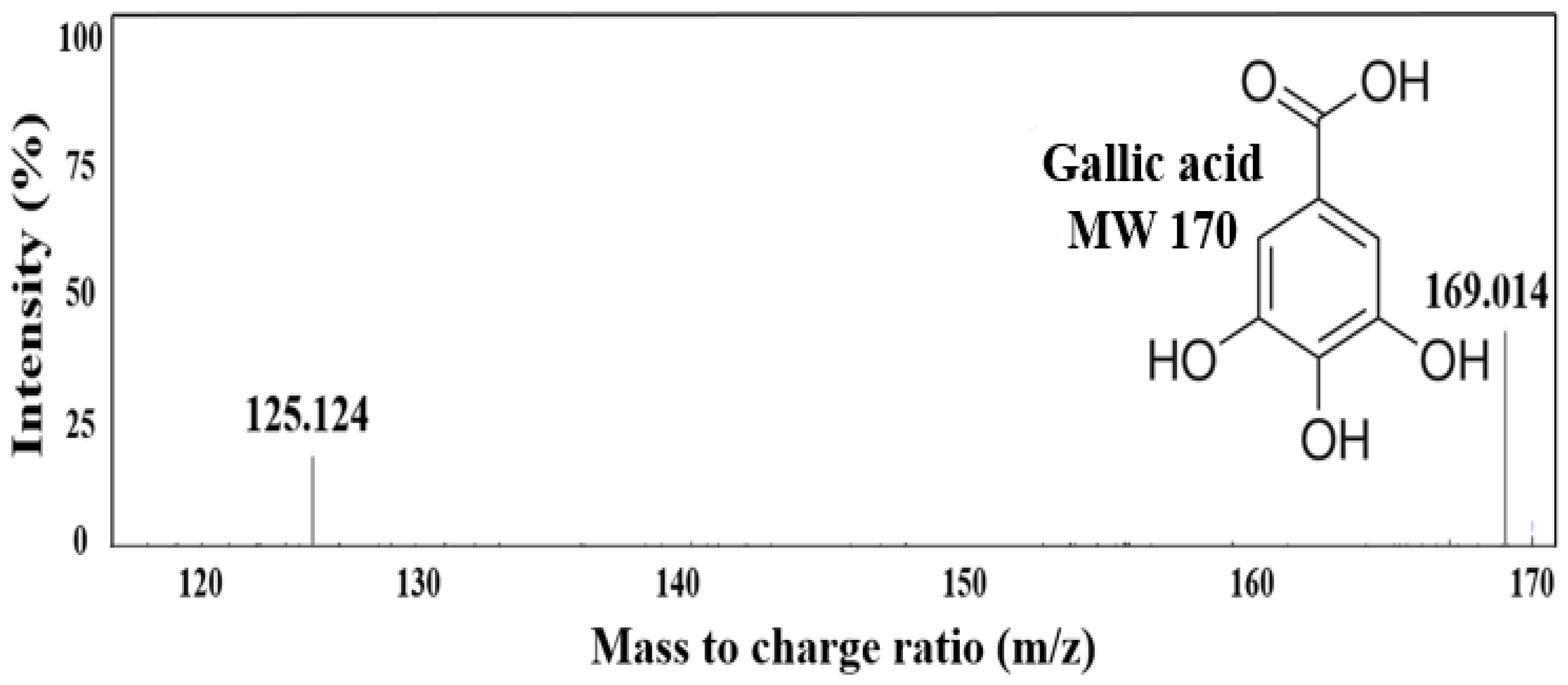
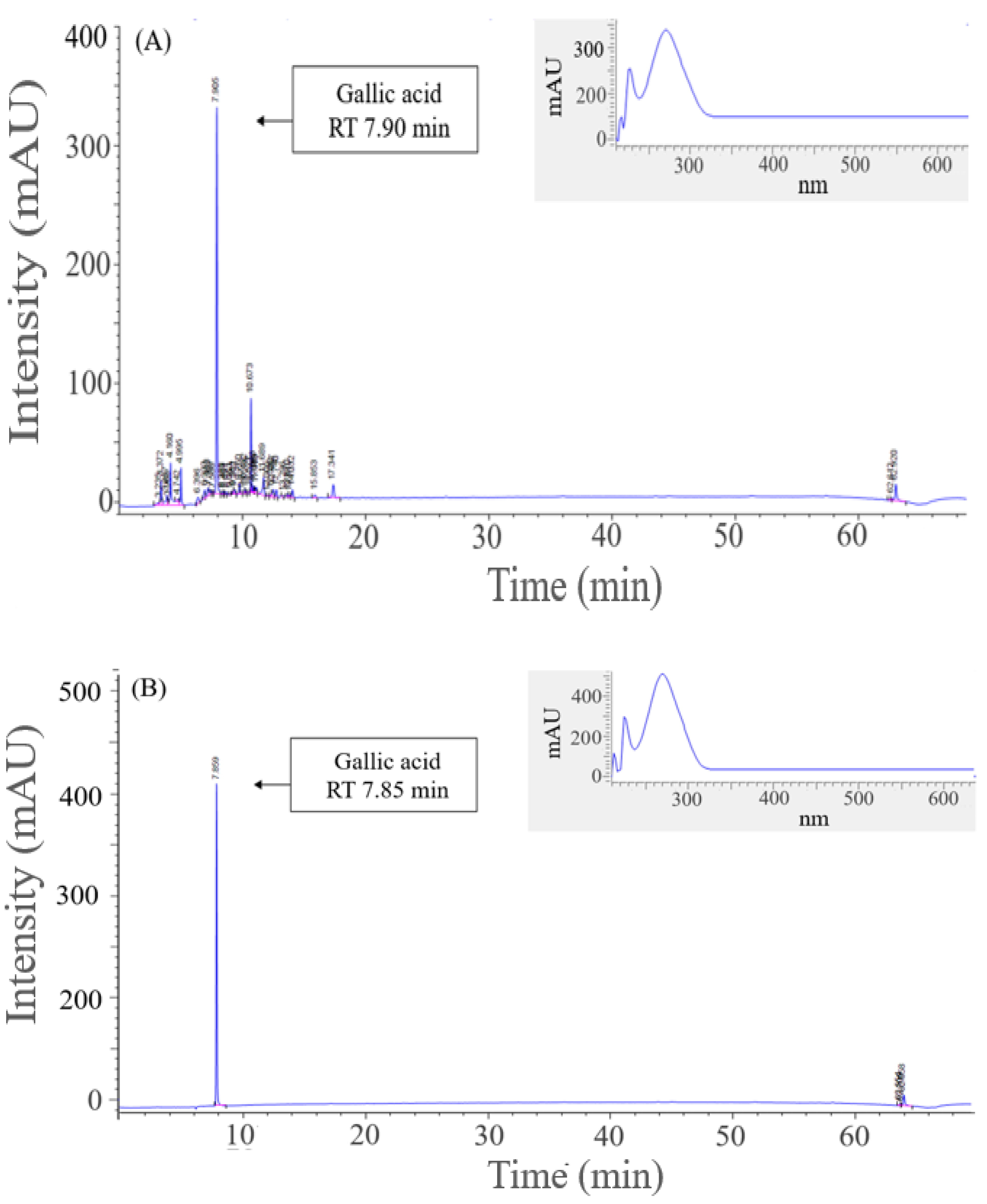
2.4. Major Substance Analysis Using LC-MS/MS
2.5. Quantification of Major Substance Using HPLC
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of CGE
3.3. Measurement of Preadipocyte Differentiation
3.4. Cell Viability Assay
3.5. Lipid Accumulation Assay
3.6. Measurement of Adipogenesis and Lipogenesis Genes Expression
3.7. LC-MS/MS Analysis
3.8. HPLC Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, H.J.; Jang, Y.S.; Ha, J.W.; Ra, M.-J.; Jung, S.-M.; Yu, J.-N.; Kim, K.; Kim, K.H.; Um, S.H. Bioactive Phytochemicals from Salix pseudolasiogyne Twigs: Anti-Adipogenic Effect of 2′-O-Acetylsalicortin in 3T3-L1 Cells. Int. J. Mol. Sci. 2022, 23, 12006. [Google Scholar] [CrossRef]
- Elebeedy, D.; Ghanem, A.; Saleh, A.; Ibrahim, M.H.; Al Kamaly, O.; Abourehab, M.A.S.; Ali, M.A.; Abd El Maksoud, A.I.; El Hassab, M.A.; Eldehna, W.M. In Vivo and In Silico Investigation of the Anti-Obesity Effects of Lactiplantibacillus plantarum Combined with Chia Seeds, Green Tea, and Chitosan in Alleviating Hyperlipidemia and Inflammation. Int. J. Mol. Sci. 2022, 23, 12200. [Google Scholar] [CrossRef] [PubMed]
- Gam, D.H.; Hong, J.W.; Yeom, S.H.; Kim, J.W. Polyphenols in peanut shells and their antioxidant activity: Optimal extraction conditions and the evaluation of anti-obesity effects. J. Nutr. Health 2021, 54, 116–128. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, K.; Guo, X.; Tu, J.; Zhang, D.; Sun, W.; Kong, X. Low-intensity pulsed ultrasound inhibits adipogenic differentiation via HDAC1 signalling in rat visceral preadipocytes. Adipocyte 2019, 8, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Cho, Y.-W.; Deng, C.-X.; Ge, K. MLL3/MLL4-associated PAGR1 regulates adipogenesis by controlling induction of C/EBPβ and C/EBPδ. Mol. Cell. Biol. 2020, 40, e00209-20. [Google Scholar] [CrossRef]
- Fu, C.-N.; Wei, H.; Gao, W.-S.; Song, S.-S.; Yue, S.-W.; Qu, Y.-J. Obesity increases neuropathic pain via the AMPK-ERK-NOX4 pathway in rats. Aging 2021, 13, 18606. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Chen, Y.-L.; Huang, W.-C.; Liou, C.-J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. The inhibitory effects of djulis (Chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef]
- Lee, H.B.; Kang, S.-S. Inhibitory Effect of Bacterial Lysates Extracted from Pediococcus acidilactici on the Differentiation of 3T3-L1 Pre-Adipocytes. Int. J. Mol. Sci. 2022, 23, 11614. [Google Scholar] [CrossRef]
- van Weeghel, M.; Abdurrachim, D.; Nederlof, R.; Argmann, C.A.; Houtkooper, R.H.; Hagen, J.; Nabben, M.; Denis, S.; Ciapaite, J.; Kolwicz, S.C., Jr. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc. Res. 2018, 114, 1324–1334. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Hwang, Y.J.; Kim, Y.J.; Kim, M.; Hwang, K.-A. Immature sword bean pods (Canavalia gladiata) inhibit adipogenesis in C3H10T1/2 cells and mice with high-fat diet–induced obesity. J. Chinese Med. Assoc. 2022, 85, 67–76. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Rhee, J.-K. Roasting and Cryogenic Grinding Enhance the Antioxidant Property of Sword Beans (Canavalia gladiata). J. Microbiol. Biotechnol. 2020, 30, 1706–1719. [Google Scholar] [CrossRef]
- Kaur, A.; Verma, P.; Kaur, S.; Kumar, V. In vitro anti-inflammatory and antioxidant potential of leaves of Canavalia gladiata. Pharmaspire 2019, 11, 76–78. [Google Scholar]
- Jeon, S.Y.; Park, J.Y.; Shin, I.; Kim, S.O.; An, H.D.; Kim, M.R. Ethanol extract of Plantago asiatica L. controls intracellular fat accumulation and lipid metabolism in 3T3-L1 Adipocytes. Korea J. Herbol. 2014, 29, 77–82. [Google Scholar] [CrossRef]
- Hong, J.W.; Park, H.Y.; Park, J.H.; Kim, S.H.; Kim, H.A.; Kim, J.-W. Inhibition of Lipase Activity and Preadipocyte Differentiation in 3T3-L1 Cells Treated with Sargassum horneri Extract. Ocean Polar Res. 2022, 44, 61–67. [Google Scholar]
- Blanco, J.; Guardia-Escote, L.; Mulero, M.; Basaure, P.; Biosca-Brull, J.; Cabré, M.; Colomina, M.T.; Domingo, J.L.; Sánchez, D.J. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food Chem. Toxicol. 2020, 137, 111171. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, K.K.; Kim, T.W.; Yang, C.S.; Choe, M. Evaluation of the anti-obesity activity of Platycodon grandiflorum root and Curcuma longa root fermented with Aspergillus oryzae. Korean J. Food Sci. Technol. 2015, 47, 111–118. [Google Scholar] [CrossRef]
- van der Vaart, J.I.; Boon, M.R.; Houtkooper, R.H. The role of AMPK signaling in brown adipose tissue activation. Cells 2021, 10, 1122. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Niu, R.; Gu, X.; Hao, E.; Hou, X.; Deng, J.; Bai, G. The combination of cinnamaldehyde and kaempferol ameliorates glucose and lipid metabolism disorders by enhancing lipid metabolism via AMPK activation. J. Funct. Foods 2021, 83, 104556. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xu, H.; Fang, J.; Zhang, C.; Song, J.; Zhang, X.; Hao, B.; Yin, B.; Xia, G. miR-15a Targets ABAT Gene to Regulate the Differentiation of Preadipocytes of Yanbian Yellow Cattle; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Jia, D.; Li, Z.; Gao, Y.; Feng, Y.; Li, W. A novel triazine ring compound (MD568) exerts in vivo and in vitro effects on lipid metabolism. Biomed. Pharmacother. 2018, 103, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Kong, K.-W.; Li, H.-B.; Wu, K.; Ge, Y.-Y.; Chan, C.-L.; Shi, X.-M.; Corke, H. Separation, identification, and bioactivities of the main gallotannins of red sword bean (Canavalia gladiata) coats. Front. Chem. 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Q.; Meng, X.; Zhang, Y. Natural Polyphenols: A Potential prevention and treatment strategy for Metabolic Syndrome. Food Funct. 2022, 13, 9734–9753. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic acid regulates adipocyte hypertrophy and suppresses inflammatory gene expression induced by the paracrine interaction between adipocytes and macrophages in vitro and in vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef]
- Marwicka, J.; Zięba, A. Antioxidants as a defence against reactive oxygen species. Aesthetic Cosmetol. Med 2021, 10, 271–276. [Google Scholar] [CrossRef]
- Gam, D.H.; Park, J.H.; Kim, J.H.; Beak, D.H.; Kim, J.W. Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules 2021, 27, 21. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-J.; Jang, S.-H.; Ha, J.H.; Song, Y.M.; Ko, Y.-G.; Kim, H.-D.; Min, W.; Kang, S.N.; Cho, J.-H. Sasa borealis stem. extract attenuates hepatic steatosis in high-fat diet-induced obese rats. Nutrients 2014, 6, 2179–2195. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
| Genes | Forward (5’-3’) | Reverse (5’-3’) | Size (bp) |
|---|---|---|---|
| AMPK(1) | CCATGCTGGAACTGATGGAG | CTGAACTGTGTGACCCAGCC | 275 |
| CPT-1(2) | CGACATGCTCGGCCTCATAG | GCCAGAAGCCCCCAAGAAAC | 813 |
| SREBP-1c(3) | GGTTTAGGGATGTTTGGGTTTT | AAGCCCACTTCATTTCATTGGT | 122 |
| PPAR-γ(4) | AGCCGAGATAAAGCCAAACAAC | GAATCTCCTAGTCCTGGCTTGC | 325 |
| C/EBP-α(5) | CCCTGAAATCCCAGCACTTC | GGCATGGCTGCTGTAGGGGT | 137 |
| FAS(6) | CGACATGCTCGGCCTCATAG | GCCAGAAGCCCCCAAGAAAC | 416 |
| β-actin | AGCACAGAGCCTCGCCTTT | CTTAATGTCACGCACGATTTCC | 295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.W.; Park, H.Y.; Kim, H.A.; Hwang, Y.S.; Lee, E.J.; Kim, J.W. Inhibition Effect of Adipogenesis and Lipogenesis via Activation of AMPK in Preadipocytes Treated with Canavalia gladiata Extract. Int. J. Mol. Sci. 2023, 24, 2108. https://doi.org/10.3390/ijms24032108
Hong JW, Park HY, Kim HA, Hwang YS, Lee EJ, Kim JW. Inhibition Effect of Adipogenesis and Lipogenesis via Activation of AMPK in Preadipocytes Treated with Canavalia gladiata Extract. International Journal of Molecular Sciences. 2023; 24(3):2108. https://doi.org/10.3390/ijms24032108
Chicago/Turabian StyleHong, Ji Woo, Ha Young Park, Han A. Kim, Yun Seon Hwang, Eun Jae Lee, and Jin Woo Kim. 2023. "Inhibition Effect of Adipogenesis and Lipogenesis via Activation of AMPK in Preadipocytes Treated with Canavalia gladiata Extract" International Journal of Molecular Sciences 24, no. 3: 2108. https://doi.org/10.3390/ijms24032108
APA StyleHong, J. W., Park, H. Y., Kim, H. A., Hwang, Y. S., Lee, E. J., & Kim, J. W. (2023). Inhibition Effect of Adipogenesis and Lipogenesis via Activation of AMPK in Preadipocytes Treated with Canavalia gladiata Extract. International Journal of Molecular Sciences, 24(3), 2108. https://doi.org/10.3390/ijms24032108






