An Overview of Inter-Tissue and Inter-Kingdom Communication Mediated by Extracellular Vesicles in the Regulation of Mammalian Metabolism
Abstract
1. Introduction
2. Inter-Organ Communication Mediated by EVs
2.1. EVs from the Adipose Tissues
2.1.1. White Adipocytes and Macrophages
2.1.2. Brown Adipocytes
2.1.3. Mesenchymal Stem Cells
2.2. EVs from the Muscle
2.3. EVs from the Liver
2.4. EVs from Pancreatic β-Cells
3. Inter-Kingdom Communication Mediated by EVs
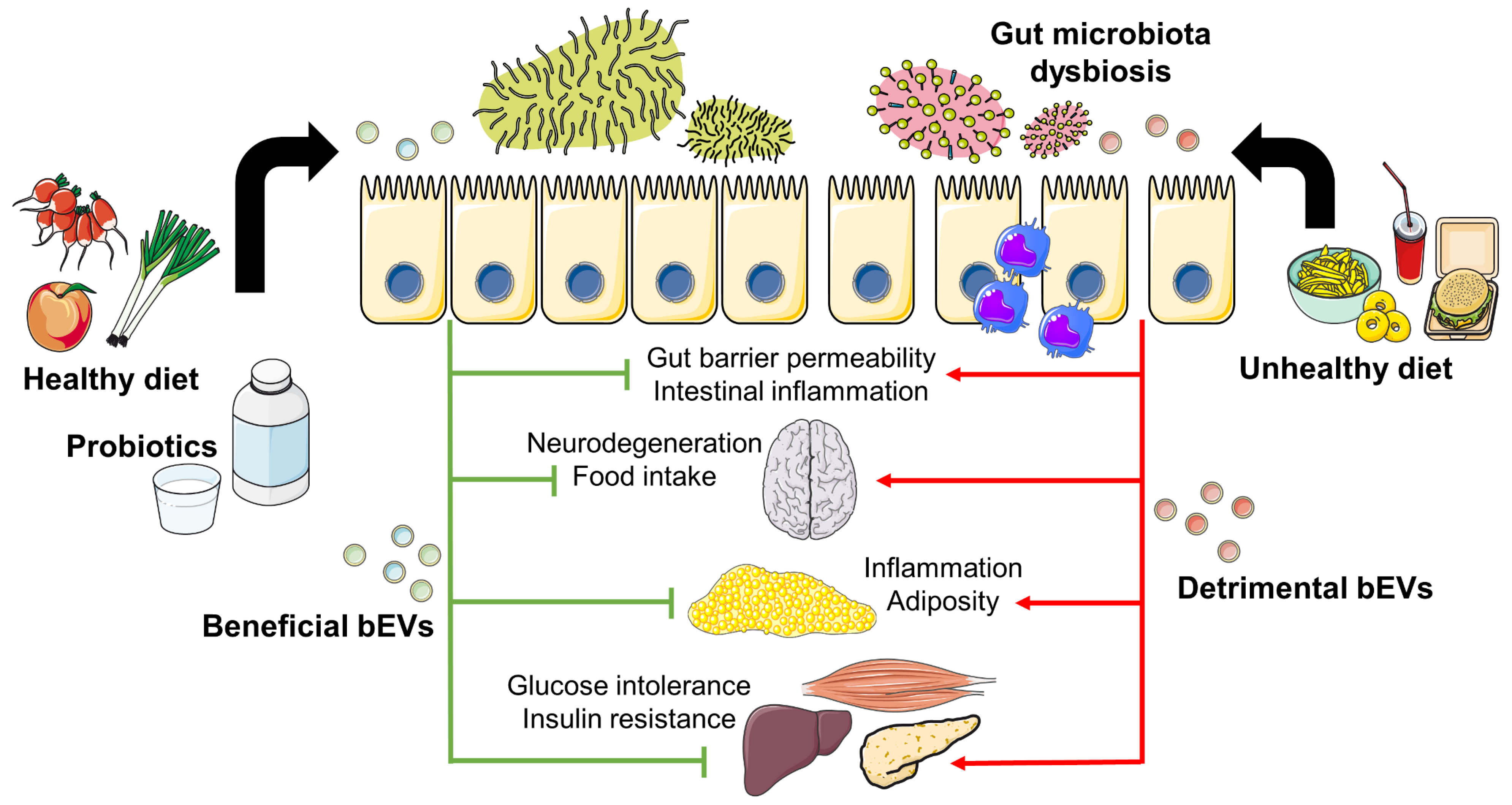
3.1. Environmental Factors Shaping Gut Microbiota and bEVs
3.2. bEVs and Their Impact on Inflammation and Metabolism
Properties of bEVs Derived from Probiotics
3.3. Food-Derived EVs as Potential Therapeutic Agents
4. Open Questions and Perspectives
4.1. Current Limitations in EV Characterization
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health. 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; Asúnsolo, Á.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Holly, J.M.P.; Biernacka, K.; Maskell, N.; Perks, C.M. Obesity, Diabetes and COVID-19: An Infectious Disease Spreading From the East Collides With the Consequences of an Unhealthy Western Lifestyle. Front. Endocrinol. 2020, 11, 582870. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Heni, M.; Tabak, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; Hrabě de Angelis, M.; Birkenfeld, A.L.; Stefan, N.; Peter, A.; et al. Pathophysiology-Based Subphenotyping of Individuals at Elevated Risk for Type 2 Diabetes. Nat. Med. 2021, 27, 49–57. [Google Scholar] [CrossRef]
- Udler, M.S.; Kim, J.; von Grotthuss, M.; Bonas-Guarch, S.; Cole, J.B.; Chiou, J.; Boehnke, M.; Laakso, M.; Atzmon, G.; Glaser, B.; et al. Type 2 Diabetes Genetic Loci Informed by Multi-Trait Associations Point to Disease Mechanisms and Subtypes: A Soft Clustering Analysis. PLoS Med. 2018, 15, e1002654. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel Subgroups of Adult-Onset Diabetes and Their Association with Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Priest, C.; Tontonoz, P. Inter-Organ Cross-Talk in Metabolic Syndrome. Nat. Metab. 2019, 1, 1177–1188. [Google Scholar] [CrossRef]
- Bays, H.E.; González-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic Potential of Adipose Tissue and Metabolic Consequences of Adipocyte Hypertrophy and Increased Visceral Adiposity. Expert Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2017, 27, 22–41. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic Communication during Exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from Diet-Induced Gut Microbiota Dysbiosis and Obesity Can Be Ameliorated by Fecal Microbiota Transplantation: A Multiomics Approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Solvsten Burgdorf, K.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The Gut Microbiota in Internal Medicine: Implications for Health and Disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar] [PubMed]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The Gut Microbiome: Relationships with Disease and Opportunities for Therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go. J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front. Cell. Infect. Microbiol. 2020, 9, 455. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Novials, A.; Párrizas, M. Exosomes and Diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3107. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived MicroRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Layton, E.; Fairhurst, A.M.; Griffiths-Jones, S.; Grencis, R.K.; Roberts, I.S. Regulatory RNAs: A Universal Language for Inter-Domain Communication. Int. J. Mol. Sci. 2020, 21, 8919. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Connolly, K.D.; Wadey, R.M.; Mathew, D.; Johnson, E.; Rees, D.A.; James, P.E. Evidence for Adipocyte-Derived Extracellular Vesicles in the Human Circulation. Endocrinology 2018, 159, 3259–3267. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Flaherty, S.E., 3rd; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W., Jr. A Lipase-Independent Pathway of Lipid Release and Immune Modulation by Adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and Stoichiometric Analysis of the MicroRNA Content of Exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Li, Q.; Lai, H.; Zhang, H.; Hu, Z.; Li, Y.; Huang, S. EV-Origin: Enumerating the Tissue-Cellular Origin of Circulating Extracellular Vesicles Using ExLR Profile. Comput. Struct. Biotechnol. J. 2020, 18, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, H.; Jin, X.; Zhang, Y.; Kang, X.; Zhang, Z.; Xu, M.; Qian, Z.; Ma, Z.; Gao, X.; et al. Adipose-Specific Knockdown of Sirt1 Results in Obesity and Insulin Resistance by Promoting Exosomes Release. Cell Cycle 2019, 18, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, R.; Brandao, B.B.; Thomou, T.; Altindis, E.; Kahn, C.R. Tissue Differences in the Exosomal/Small Extracellular Vesicle Proteome and Their Potential as Indicators of Altered Tissue Metabolism. Cell Rep. 2022, 38, 110277. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Marques Zanotto, T.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA Sequence Codes for Small Extracellular Vesicle Release and Cellular Retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Gao, H.; Castellani, F.; Dos, G.; Jin, Z.; Ly, C.; Olefsky, J.M.; Ying, W.; Gao, H.; Castellani, F.; et al. MiR-690, an Exosomal-Derived MiRNA from M2- Polarized Macrophages, Improves Insulin Sensitivity in Obese Mice Article from M2-Polarized Macrophages, Improves Insulin Sensitivity in Obese Mice. Cell Metab. 2021, 33, 781–790. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate in Vivo and in Vitro Insulin Sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef]
- Jalabert, A.; Reininger, L.; Berger, E.; Coute, Y.; Meugnier, E.; Forterre, A.; Errazuriz-Cerda, E.; Geloen, A.; Aouadi, M.; Bouzakri, K.; et al. Profiling of Ob/Ob Mice Skeletal Muscle Exosome-like Vesicles Demonstrates Combined Action of MiRNAs, Proteins and Lipids to Modulate Lipid Homeostasis in Recipient Cells. Sci. Rep. 2022, 11, 21626. [Google Scholar] [CrossRef]
- Ling, D.; Song, H.; Shuo, L.; Wang, L.; Xie, P.; Li, W.; Liu, J.; Tong, Y.; Zhang, C.-Y.; Jiang, X.; et al. Gonadal White Adipose Tissue-Derived Exosomasl MiR-222 Promotes Obesity-Associated Insulin Resistance. Aging 2020, 12, 22719–22742. [Google Scholar]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Ren, X.; Li, H.; Feng, C. SNHG9, Delivered by Adipocyte-Derived Exosomes, Alleviates Inflammation and Apoptosis of Endothelial Cells through Suppressing TRADD Expression. Eur. J. Pharmacol. 2020, 872, 172977. [Google Scholar] [CrossRef]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-Cadherin System Enhances Exosome Biogenesis and Decreases Cellular Ceramides by Exosomal Release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular Adipose Tissue-Derived Extracellular Vesicle MiR-221-3p Mediates Vascular Remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Chen, J.X.; Ren, Y.; Fan, L.C.; Xiang, W.; He, X.J. Exosomal MiR-122 Promotes Adipogenesis and Aggravates Obesity through the VDR/SREBF1 Axis. Obesity 2022, 30, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Mleczko, J.; Ortega, F.J.; Falcon-Perez, J.M.; Wabitsch, M.; Fernandez-Real, J.M.; Mora, S. Extracellular Vesicles from Hypoxic Adipocytes and Obese Subjects Reduce Insulin-Stimulated Glucose Uptake. Mol. Nutr. Food Res. 2018, 62, 1700917. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tu, H.; Tang, K.; Huang, H.; Ou, S.; Wu, J. MiR-3064 in Epicardial Adipose-Derived Exosomes Targets Neuronatin to Regulate Adipogenic Differentiation of Epicardial Adipose Stem Cells. Front. Cardiovasc. Med. 2021, 8, 709079. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hui, X.; Chong Hoo, R.L.; Ye, D.; Cheung Chan, C.Y.; Feng, T.; Wang, Y.; Ling Lam, K.S.; Xu, A. Adipocyte-Secreted Exosomal MicroRNA-34a Inhibits M2 Macrophage Polarization to Promote Obesity-Induced Adipose Inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, X.; Teng, Y.; Zhao, T.; Lin, L.; Li, Y.; Shang, H.; Jin, Y.; Jin, Q. Adipocytes-Derived Exosomal MicroRNA-1224 Inhibits M2 Macrophage Polarization in Obesity-Induced Adipose Tissue Inflammation via MSI2-Mediated Wnt/β-Catenin Axis. Mol. Nutr. Food Res. 2022, 66, e2100889. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef]
- Man, W.; Song, X.; Xiong, Z.; Gu, J.; Lin, J.; Gu, X.; Yu, D.; Li, C.; Jiang, M.; Zhang, X.; et al. Exosomes Derived from Pericardial Adipose Tissues Attenuate Cardiac Remodeling Following Myocardial Infarction by Adipsin-Regulated Iron Homeostasis. Front. Cardiovasc. Med. 2022, 9, 2408. [Google Scholar] [CrossRef]
- Itokazu, M.; Onodera, Y.; Mori, T.; Inoue, S.; Yamagishi, K.; Moritake, A.; Iwawaki, N.; Shigi, K.; Takehara, T.; Higashimoto, Y.; et al. Adipose-Derived Exosomes Block Muscular Stem Cell Proliferation in Aged Mouse by Delivering MiRNA Let-7d-3p That Targets Transcription Factor HMGA2. J. Biol. Chem. 2022, 298, 102098. [Google Scholar] [CrossRef]
- Dang, S.Y.; Leng, Y.; Wang, Z.X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.Z.; Hong, A.; Ma, Y. Exosomal Transfer of Obesity Adipose Tissue for Decreased MiR-141-3p Mediate Insulin Resistance of Hepatocytes. Int. J. Biol. Sci. 2019, 15, 351–368. [Google Scholar] [CrossRef]
- Li, Y.; Luan, Y.; Li, J.; Song, H.; Li, Y.; Qi, H.; Sun, B.; Zhang, P.; Wu, X.; Liu, X.; et al. Exosomal MiR-199a-5p Promotes Hepatic Lipid Accumulation by Modulating MST1 Expression and Fatty Acid Metabolism. Hepatol. Int. 2020, 14, 1057–1074. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular Vesicles Mediate the Communication of Adipose Tissue with Brain and Promote Cognitive Impairment Associated with Insulin Resistance. Cell Metab. 2022, 34, 1264–1279. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Dong, J.; Wu, Y.; Tian, W. Nucleophosmin3 Carried by Small Extracellular Vesicles Contribute to White Adipose Tissue Browning. J. Nanobiotechnol. 2022, 20, 165. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Zhang, H.; Zhang, J.; Zeng, Y.; Fan, C.; Zou, S.; Wu, C.; Fang, S.; Li, P.; et al. Brown Adipose Tissue Transplantation Ameliorates Diabetic Nephropathy through the MiR-30b Pathway by Targeting Runx1. Metabolism 2021, 125, 154916. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.; Kim, H.K.; Yeom, S.H.; Woo, C.H.; Jung, Y.J.; Yun, Y.E.; Park, S.Y.; Han, J.; Kim, E.; et al. Extracellular Vesicles from Adipose Tissue-Derived Stem Cells Alleviate Osteoporosis through Osteoprotegerin and MiR-21-5p. J. Extracell. Vesicles 2021, 10, e12152. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, T.; Wang, Z.; Wang, F.; Huang, D.; Sun, H.; Liu, H. Adipose-Derived Mesenchymal Stem Cell-Secreted Extracellular Vesicles Alleviate Non-Alcoholic Fatty Liver Disease via Delivering MiR-223-3p. Adipocyte 2022, 11, 572–587. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Liu, D. Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-MTOR-HIF-1α Signaling Pathway. Tissue. Eng. Regen. Med. 2021, 18, 1035–1044. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia Adipose Stem Cell-Derived Exosomes Promote High-Quality Healing of Diabetic Wound Involves Activation of PI3K/Akt Pathways. J. Nanobiotechnol. 2021, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, Y.-C.; Cheng, P.; Shao, H.-G. Adipose Tissue Macrophage-Derived Exosomal MiR-29a Regulates Obesity-Associated Insulin Resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Yang, Y.; Tang, N.; Wang, J.; Sun, P.; Yang, N.; Chen, F.; Wu, T.; Sun, T.; Li, Y.; et al. M1 Macrophage-Derived Exosomes Impair Beta Cell Insulin Secretion via MiR-212-5p by Targeting SIRT2 and Inhibiting Akt/GSK-3β/β-Catenin Pathway in Mice. Diabetologia 2021, 64, 2037–2051. [Google Scholar] [CrossRef]
- Gao, H.; Luo, Z.; Jin, Z.; Ji, Y.; Ying, W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations through Secreted MiRNA-Containing Extracellular Vesicles. Cells 2021, 10, 2451. [Google Scholar] [CrossRef]
- Castaño, C.; Mirasierra, M.; Vallejo, M.; Novials, A.; Parrizas, M. Delivery of Muscle-Derived Exosomal MiRNAs Induced by HIIT Improves Insulin Sensitivity through down-Regulation of Hepatic FoxO1 in Mice. Proc. Natl. Acad. Sci. USA 2020, 117, 30335–30343. [Google Scholar] [CrossRef]
- Vechetti, I.J.; Peck, B.D.; Wen, Y.; Walton, R.G.; Valentino, T.R.; Alimov, A.P.; Dungan, C.M.; van Pelt, D.W.; von Walden, F.; Alkner, B.; et al. Mechanical Overload-Induced Muscle-Derived Extracellular Vesicles Promote Adipose Tissue Lipolysis. FASEB J. 2021, 35, e21644. [Google Scholar] [CrossRef]
- Di, W.; Amdanee, N.; Zhang, W.; Zhou, Y. Long-Term Exercise-Secreted Extracellular Vesicles Promote Browning of White Adipocytes by Suppressing MiR-191a-5p. Life Sci. 2020, 263, 118464. [Google Scholar] [CrossRef]
- Shao, X.; Gong, W.; Wang, Q.; Wang, P.; Shi, T.; Mahmut, A.; Qin, J.; Yao, Y.; Yan, W.; Chen, D.; et al. Atrophic Skeletal Muscle Fibre-Derived Small Extracellular Vesicle MiR-690 Inhibits Satellite Cell Differentiation during Ageing. J. Cachex. Sarcopenia Muscle 2022, 13, 3163–3180. [Google Scholar] [CrossRef]
- Jalabert, A.; Vial, G.; Guay, C.; Wiklander, O.P.B.; Nordin, J.Z.; Aswad, H.; Forterre, A.; Meugnier, E.; Pesenti, S.; Regazzi, R.; et al. Exosome-like Vesicles Released from Lipid-Induced Insulin-Resistant Muscles Modulate Gene Expression and Proliferation of Beta Recipient Cells in Mice. Diabetologia 2016, 59, 1049–1058. [Google Scholar] [CrossRef]
- Kim, K.; Kang, J.K.; Jung, Y.H.; Lee, S.B.; Rametta, R.; Dongiovanni, P.; Valenti, L.; Pajvani, U.B. Adipocyte PHLPP2 Inhibition Prevents Obesity-Induced Fatty Liver. Nat. Commun. 2021, 12, 1822. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, J.E.; Kim, E.; Lee, H.; Lee, H.; Shin, E.A.; Lee, J.W. Liver-Originated Small Extracellular Vesicles with TM4SF5 Target Brown Adipose Tissue for Homeostatic Glucose Clearance. J. Extracell. Vesicles 2022, 11, e12262. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Luo, Z.; Gao, H.; dos Reis, F.C.G.; Bandyopadhyay, G.; Jin, Z.; Manda, K.A.; Isaac, R.; Yang, M.; Fu, W.; et al. Hepatocyte-Derived Exosomes from Early Onset Obese Mice Promote Insulin Sensitivity through MiR-3075. Nat. Metab. 2021, 3, 1163–1174. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, P.; Liu, J.; Xie, X. Exosomal MicroRNA-122 Mediates Obesity-Related Cardiomyopathy through Suppressing Mitochondrial ADP-Ribosylation Factor-like 2. Clin. Sci. 2019, 133, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wu, J.; Feng, M.; Dang, X.; Wu, G.; Yang, H.; Wang, Y.; Li, J.; Zhao, Y.; Shi, C.; et al. Exercise Promotes Angiogenesis by Enhancing Endothelial Cell Fatty Acid Utilization via Liver-Derived Extracellular Vesicle MiR-122-5p. J. Sport Health Sci. 2022, 11, 495–508. [Google Scholar] [CrossRef]
- Castaño, C.; Kalko, S.; Novials, A.; Párrizas, M. Obesity-Associated Exosomal MiRNAs Modulate Glucose and Lipid Metabolism in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12158–12163. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, L.; Gui, W.; Xiao, L.; Wang, W.; Xia, J.; Fan, H.; Li, Z.; Zhu, Q.; Hou, X.; et al. Hepatocyte TGF-β Signaling Inhibiting WAT Browning to Promote NAFLD and Obesity Is Associated With Let-7b-5p. Hepatol. Commun. 2022, 6, 1301–1321. [Google Scholar] [CrossRef]
- He, Y.; Rodrigues, R.M.; Wang, X.; Seo, W.; Ma, J.; Hwang, S.; Fu, Y.; Trojnár, E.; Mátyás, C.; Zhao, S.; et al. Neutrophil-to-Hepatocyte Communication via LDLR-Dependent MiR-223–Enriched Extracellular Vesicle Transfer Ameliorates Nonalcoholic Steatohepatitis. J. Clin. Invest. 2021, 131, e141513. [Google Scholar] [CrossRef]
- Xu, H.; Du, X.; Xu, J.; Zhang, Y.; Tian, Y.; Liu, G.; Wang, X.; Ma, M.; Duid, W.; Liu, Y.; et al. Pancreatic β Cell MicroRNA-26a Alleviates Type 2 Diabetes by Improving Peripheral Insulin Sensitivity and Preserving β Cell Function. PLoS Biol. 2020, 18, e3000603. [Google Scholar] [CrossRef]
- Burillo, J.; Fernández-Rhodes, M.; Piquero, M.; López-Alvarado, P.; Menéndez, J.C.; Jiménez, B.; González-Blanco, C.; Marqués, P.; Guillén, C.; Benito, M. Human Amylin Aggregates Release within Exosomes as a Protective Mechanism in Pancreatic β Cells: Pancreatic β-Hippocampal Cell Communication. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118971. [Google Scholar] [CrossRef]
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic β Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019, 29, 348–361.e6. [Google Scholar] [CrossRef] [PubMed]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 Polarized Human Macrophage Exosomes Control Cardiometabolic Inflammation and Diabetes in Obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef] [PubMed]
- de Silva, N.; Samblas, M.; Martínez, J.A.; Milagro, F.I. Effects of Exosomes from LPS-Activated Macrophages on Adipocyte Gene Expression, Differentiation, and Insulin-Dependent Glucose Uptake. J. Physiol. Biochem. 2018, 74, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Gesmundo, I.; Pardini, B.; Gargantini, E.; Gamba, G.; Birolo, G.; Fanciulli, A.; Banfi, D.; Congiusta, N.; Favaro, E.; Deregibus, M.C.; et al. Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight 2021, 6, e141962. [Google Scholar] [CrossRef]
- Ge, Q.; Xie, X.; Chen, X.; Huang, R.; Rui, C.X.; Zhen, Q.; Hu, R.; Wu, M.; Xiao, X.; Li, X. Circulating Exosome-like Vesicles of Humans with Nondiabetic Obesity Impaired Islet β-Cell Proliferation, Which Was Associated with Decreased Omentin-1 Protein Cargo. Genes Dis. 2022, 9, 1099–1113. [Google Scholar] [CrossRef]
- Gu, H.; Yang, K.; Shen, Z.; Jia, K.; Liu, P.; Pan, M.; Sun, C. ER Stress-Induced Adipocytes Secrete-Aldo-Keto Reductase 1B7-Containing Exosomes That Cause Nonalcoholic Steatohepatitis in Mice. Free Radic. Biol. Med. 2021, 163, 220–233. [Google Scholar] [CrossRef]
- Fuchs, A.; Samovski, D.; Smith, G.I.; Cifarelli, V.; Farabi, S.S.; Yoshino, J.; Pietka, T.; Chang, S.W.; Ghosh, S.; Myckatyn, T.M.; et al. Associations Among Adipose Tissue Immunology, Inflammation, Exosomes and Insulin Sensitivity in People With Obesity and Nonalcoholic Fatty Liver Disease. Gastroenterology 2021, 161, 968–981.e12. [Google Scholar] [CrossRef]
- Gan, L.; Xie, D.; Liu, J.; Bond Lau, W.; Christopher, T.A.; Lopez, B.; Zhang, L.; Gao, E.; Koch, W.; Ma, X.L.; et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020, 141, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Wang, Y.; Cao, Y.; Yao, D.; Sun, L.; Qin, L.; Qiu, H.; Zhan, X. Adipocyte-Derived Extracellular Vesicles Modulate Appetite and Weight through MTOR Signalling in the Hypothalamus. Acta Physiol. 2020, 228, e13339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Wang, C.; Shi, R.; Zhou, X.; Li, Z.; Sun, W.; Zhao, L.; Yuan, L. Maternal Obesity Increases the Risk of Fetal Cardiac Dysfunction via Visceral Adipose Tissue Derived Exosomes. Placenta 2021, 105, 85–93. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-Derived Exosomes Promote Lung Cancer Metastasis by Increasing MMP9 Activity via Transferring MMP3 to Lung Cancer Cells. Oncotarget 2017, 8, 81880–81891. [Google Scholar] [CrossRef] [PubMed]
- Clement, E.; Lazar, I.; Attané, C.; Carrié, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; le Gonidec, S.; et al. Adipocyte Extracellular Vesicles Carry Enzymes and Fatty Acids That Stimulate Mitochondrial Metabolism and Remodeling in Tumor Cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Anselmi, M.; Carollo, E.; Sartori, P.; Procacci, P.; Carter, D.; Limonta, P. Adipocyte-Derived Extracellular Vesicles Promote Prostate Cancer Cell Aggressiveness by Enabling Multiple Phenotypic and Metabolic Changes. Cells 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- La Camera, G.; Gelsomino, L.; Malivindi, R.; Barone, I.; Panza, S.; de Rose, D.; Giordano, F.; D’Esposito, V.; Formisano, P.; Bonofiglio, D.; et al. Adipocyte-Derived Extracellular Vesicles Promote Breast Cancer Cell Malignancy through HIF-1α Activity. Cancer Lett. 2021, 521, 155–168. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Carrié, L.; Esteve, D.; Dauvillier, S.; Moutahir, M.; Dalle, S.; Delmas, V.; Andrieu-Abadie, N.; Larue, L.; et al. Adipocyte Extracellular Vesicles Decrease P16INK4A in Melanoma: An Additional Link between Obesity and Cancer. J. Invest. Dermatol. 2022, 142, 2488–2498.e8. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- An, Y.; Wang, G.; Diao, Y.; Sun, H.; Wang, H.; Wu, Z. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev. Cell 2017, 41, 382–391. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New Role of Bone Morphogenetic Protein 7 in Brown Adipogenesis and Energy Expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown Adipose Tissue as a Secretory Organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef]
- Hong, P.; Wu, Y.; Zhang, Q.; Liu, P.; Zhang, S.; Yu, M.; Tian, W. Identification of Thermogenesis-Related LncRNAs in Small Extracellular Vesicles Derived from Adipose Tissue. BMC Genom. 2022, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Qi, M.; Zhao, P.; Duan, Y.; Yang, G.; Yuan, L. Brown Adipose Tissue-Derived Exosomes Mitigate the Metabolic Syndrome in High Fat Diet Mice. Theranostics 2020, 10, 8197–8210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Feng, J.; Guo, J.; Wang, J.; Xiu, G.; Xu, J.; Ning, K.; Ling, B.; Fu, Q.; Xu, J. ADSCs-Derived Exosomes Ameliorate Hepatic Fibrosis by Suppressing Stellate Cell Activation and Remodeling Hepatocellular Glutamine Synthetase-Mediated Glutamine and Ammonia Homeostasis. Stem Cell Res. Ther. 2022, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, P.; Li, F.; Jiang, X.; Jiao, X.; Shen, Y.; Lai, X. Human Umbilical Cord-Derived Mesenchymal Stem Cell-Exosomal MiR-627-5p Ameliorates Non-Alcoholic Fatty Liver Disease by Repressing FTO Expression. Hum. Cell 2021, 34, 1697–1708. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome Secreted from Adipose-Derived Stem Cells Attenuates Diabetic Nephropathy by Promoting Autophagy Flux and Inhibiting Apoptosis in Podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing MicroRNA-26a-5p Target TLR4 and Protect against Diabetic Nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef]
- Kang, Y.; Song, Y.; Luo, Y.; Song, J.; Li, C.; Yang, S.; Guo, J.; Yu, J.; Zhang, X. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Experimental Non-Alcoholic Steatohepatitis via Nrf2/NQO-1 Pathway. Free Radic. Biol. Med. 2022, 192, 25–36. [Google Scholar] [CrossRef]
- Chen, M.-T.; Zhao, Y.-T.; Zhou, L.-Y.; Li, M.; Zhang, Q.; Han, Q.; Xiao, X.-H. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhance Insulin Sensitivity in Insulin Resistant Human Adipocytes. Curr. Med. Sci. 2021, 41, 87–93. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus through Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2017, 12, 7613–7628. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Okada, Y.; Turudome, Y.; Ushijima, K. Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 10906. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xiao, Y.; Xiao, Y.; Guo, Q.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.H. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef]
- Trovato, E.; di Felice, V.; Barone, R. Extracellular Vesicles: Delivery Vehicles of Myokines. Front. Physiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Karstoft, K.; Pedersen, B.K. Skeletal Muscle as a Gene Regulatory Endocrine Organ. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.P.; Nie, Y.; Evans, S.; Kargl, C.K.; Hettinger, Z.R.; Garner, R.T.; Hubal, M.J.; Kuang, S.; Stout, J.; Gavin, T.P. Obesity and Exercise Training Alter Inflammatory Pathway Skeletal Muscle Small Extracellular Vesicle MicroRNAs. Exp. Physiol. 2022, 107, 462–475. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef]
- Mullen, M.; Williams, K.; LaRocca, T.; Duke, V.; Hambright, W.S.; Ravuri, S.K.; Bahney, C.S.; Ehrhart, N.; Huard, J. Mechanical Strain Drives Exosome Production, Function, and MiRNA Cargo in C2C12 Muscle Progenitor Cells. J. Orthop. Res. 2022. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Li, Y.; Jia, L.; Zhai, L.; Wei, W.; Zhang, L.; Jiang, H.; Bai, Y. Exercise-Induced Browning of White Adipose Tissue and Improving Skeletal Muscle Insulin Sensitivity in Obese/Non-Obese Growing Mice: Do Not Neglect Exosomal MiR-27a. Front. Nutr. 2022, 9, 1276. [Google Scholar] [CrossRef]
- Takafuji, Y.; Kawao, N.; Ohira, T.; Mizukami, Y.; Okada, K.; Jo, J.-I.; Tabata, Y.; Kaji, H. Extracellular Vesicles Secreted from Mouse Muscle Cells Improve Delayed Bone Repair in Diabetic Mice. Endocr. J. 2022, 56, 0918–8959. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Kawao, N.; Okada, K.; Muratani, M.; Kaji, H. MicroRNA-196a-5p in Extracellular Vesicles Secreted from Myoblasts Suppresses Osteoclast-like Cell Formation in Mouse Cells. Calcif. Tissue Int. 2021, 108, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Sato, Y.; Garner, R.T.; Kargl, C.; Wang, C.; Kuang, S.; Gilpin, C.J.; Gavin, T.P. Skeletal Muscle-Derived Exosomes Regulate Endothelial Cell Functions via Reactive Oxygen Species-Activated Nuclear Factor-ΚB Signalling. Exp. Physiol. 2019, 104, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, T.; Chen, T.; Sun, J.; Luo, J.; He, J.; Wei, L.; Zeng, B.; Zhang, H.; Li, W.; et al. Hepatic Exosome-Derived MiR-130a-3p Attenuates Glucose Intolerance via Suppressing PHLPP2 Gene in Adipocyte. Metabolism 2020, 103, 154006. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGF-β Signalling and Liver Disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Ogino, N.; Takahashi, H.; Nagaoka, K.; Harada, Y.; Kubo, M.; Miyagawa, K.; Kusanaga, M.; Oe, S.; Honma, Y.; Harada, M.; et al. Possible Contribution of Hepatocyte Secretion to the Elevation of Plasma Exosomal Arginase-1 in High-Fat Diet-Fed Mice. Life Sci. 2021, 278, 119588. [Google Scholar] [CrossRef]
- Royo, F.; Moreno, L.; Mleczko, J.; Palomo, L.; Gonzalez, E.; Cabrera, D.; Cogolludo, A.; Perez Vizcaino, F.; Van-Liempd, S.; Falcon-Perez, J.M. Hepatocyte-Secreted Extracellular Vesicles Modify Blood Metabolome and Endothelial Function by an Arginase-Dependent Mechanism. Sci. Rep. 2017, 7, 42798. [Google Scholar] [CrossRef]
- Shah, R.; Murthy, V.; Pacold, M.; Danielson, K.; Tanriverdi, K.; Larson, M.G.; Hanspers, K.; Pico, A.; Mick, E.; Reis, J.; et al. Extracellular RNAs Are Associated with Insulin Resistance and Metabolic Phenotypes. Diabetes Care 2017, 40, 546–553. [Google Scholar] [CrossRef]
- Das, A.; Basu, S.; Bandyopadhyay, D.; Mukherjee, K.; Datta, D.; Chakraborty, S.; Jana, S.; Adak, M.; Bose, S.; Chakrabarti, S.; et al. Inhibition of Extracellular Vesicle-Associated MMP2 Abrogates Intercellular Hepatic MiR-122 Transfer to Liver Macrophages and Curtails Inflammation. iScience 2021, 24, 103428. [Google Scholar] [CrossRef]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Guan, F.; Wu, J.; Wang, X.; Shi, C.; et al. Exosomes from Activated Hepatic Stellate Cells Contain GLUT1 and PKM2: A Role for Exosomes in Metabolic Switch of Liver Nonparenchymal Cells. FASEB J. 2019, 33, 8530–8542. [Google Scholar] [CrossRef]
- Chidester, S.; Livinski, A.A.; Fish, A.F.; Joseph, P.V. The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review. Front. Endocrinol. 2020, 11, 375. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, Q.; Shen, C.; Wang, C.; Jia, W. Exosomes from β-Cells Alleviated Hyperglycemia and Enhanced Angiogenesis in Islets of Streptozotocin-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. 2019, 12, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Horvath, I.; Heath, N.; Hicks, R.; Forslöw, A.; Wittung-Stafshede, P. Extracellular Vesicles from Human Pancreatic Islets Suppress Human Islet Amyloid Polypeptide Amyloid Formation. Proc. Natl. Acad. Sci. USA 2017, 114, 11127–11132. [Google Scholar] [CrossRef] [PubMed]
- Lakhter, A.J.; Pratt, R.E.; Moore, R.E.; Doucette, K.K.; Maier, B.F.; Dimeglio, L.A.; Sims, E.K. Beta Cell Extracellular Vesicle MiR-21-5p Cargo Is Increased in Response to Inflammatory Cytokines and Serves as a Biomarker of Type 1 Diabetes. Diabetologia 2018, 61, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Javeed, N.; Her, T.K.; Brown, M.R.; Vanderboom, P.; Rakshit, K.; Egan, A.M.; Vella, A.; Lanza, I.; Matveyenko, A.v. Pro-Inflammatory β Cell Small Extracellular Vesicles Induce β Cell Failure through Activation of the CXCL10/CXCR3 Axis in Diabetes. Cell Rep. 2021, 36, 109613. [Google Scholar] [CrossRef]
- Cao, M.; Isaac, R.; Yan, W.; Ruan, X.; Jiang, L.; Wan, Y.; Wang, J.; Wang, E.; Caron, C.; Neben, S.; et al. Cancer-Cell-Secreted Extracellular Vesicles Suppress Insulin Secretion through MiR-122 to Impair Systemic Glucose Homeostasis and Contribute to Tumour Growth. Nat. Cell Biol. 2022, 24, 954–967. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The Gut Microbiota Regulates White Adipose Tissue Inflammation and Obesity via a Family of MicroRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H.N.; Yim, J.E. Effect of Diet on the Gut Microbiota Associated with Obesity. J. Obes. Metab. Syndr. 2019, 28, 216–224. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics Improve Gut Microbiota Dysbiosis in Obese Mice Fed a High-Fat or High-Sucrose Diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict Vegetarian Diet Improves the Risk Factors Associated with Metabolic Diseases by Modulating Gut Microbiota and Reducing Intestinal Inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Alfredo Martínez, J.; Milagro, F.I.; Barceló, A. Gut Microbiota Bacterial Species Associated with Mediterranean Diet-Related Food Groups in a Northern Spanish Population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut Microbiome Diversity and High-Fibre Intake Are Related to Lower Long-Term Weight Gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Lv, M.; Hu, Q.; Guo, L.; Xiong, D. Correlation between Alterations of Gut Microbiota and MiR-122-5p Expression in Patients with Type 2 Diabetes Mellitus. Ann. Transl. Med. 2020, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western Diet Induces a Shift in Microbiota Composition Enhancing Susceptibility to Adherent-Invasive, E. Coli Infection and Intestinal Inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia Cinnamomea Reduces Obesity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Maldonado, J.; Dente, B.; Krajmalnik-Brown, R.; Bruening, M. Diet, Physical Activity and Screen Time but Not Body Mass Index Are Associated with the Gut Microbiome of a Diverse Cohort of College Students Living in University Housing: A Cross-Sectional Study. BMC Microbiol. 2018, 18, 210. [Google Scholar] [CrossRef]
- Maniar, K.; Moideen, A.; Bhattacharyya, R.; Banerjee, D. Metformin Exerts Anti-Obesity Effect via Gut Microbiome Modulation in Prediabetics: A Hypothesis. Med. Hypotheses 2017, 104, 117–120. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Kim, J.; An, J.; Lee, S.; Kong, H.; Song, Y.; Lee, C.K.; Kim, K. Modulation of the Gut Microbiota by Metformin Improves Metabolic Profiles in Aged Obese Mice. Gut Microbes 2018, 9, 155–165. [Google Scholar] [CrossRef]
- Myronovych, A.; Peck, B.C.E.; An, M.; Zhu, J.; Warm, A.; Kupe, A.; Lubman, D.M.; Seeley, R.J. Intestinal Extracellular Vesicles Are Altered by Vertical Sleeve Gastrectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G153–G165. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.J.; Seo, J.Y.; Nam, J.; Yang, J.; McDowell, A.; Kim, Y.K.; Lee, J.H. Bariatric/Metabolic Surgery Induces Noticeable Changes of Microbiota and Their Secreting Extracellular Vesicle Composition in the Gut. Obes. Surg. 2019, 29, 2470–2484. [Google Scholar] [CrossRef] [PubMed]
- Stark, C.M.; Susi, A.; Emerick, J.; Nylund, C.M. Antibiotic and Acid-Suppression Medications during Early Childhood Are Associated with Obesity. Gut 2019, 68, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.M.; Guerri, C.; Urena, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ni, D.; Taitz, J.; Pinget, G.V.; Read, M.; Senior, A.; Wali, J.A.; Elnour, R.; Shanahan, E.; Wu, H.; et al. Dietary Protein Increases T-Cell-Independent SIgA Production through Changes in Gut Microbiota-Derived Extracellular Vesicles. Nat. Commun. 2022, 13, 4336. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kwon, Y.; Kim, D.K.; Jeon, J.; Jang, S.C.; Wang, T.; Ban, M.; Kim, M.H.; Jeon, S.G.; Kim, M.S.; et al. Gut Microbe-Derived Extracellular Vesicles Induce Insulin Resistance, Thereby Impairing Glucose Metabolism in Skeletal Muscle. Sci. Rep. 2015, 5, 15878. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; de Scheerder, M.A.; Miinalainen, I.; Rappu, P.; de Geest, B.G.; et al. Increased Levels of Systemic LPS-Positive Bacterial Extracellular Vesicles in Patients with Intestinal Barrier Dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef]
- Ashrafian, F.; Behrouzi, A.; Shahriary, A.; Badi, S.A.; Davari, M.; Khatami, S.; Jamnani, F.R.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative Study of Effect of Akkermansia Muciniphila and Its Extracellular Vesicles on Toll-like Receptors and Tight Junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter Splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in Vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef]
- Alvarez, C.S.; Gimenez, R.; Canas, M.A.; Vera, R.; Diaz-Garrido, N.; Badia, J.; Baldoma, L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia Coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. Coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia Muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-Inflammatory Effects of Outer Membrane Vesicles from Escherichia Coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Torchia, M.L.G.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Alpdundar Bulut, E.; Bayyurt Kocabas, B.; Yazar, V.; Aykut, G.; Guler, U.; Salih, B.; Surucu Yilmaz, N.; Ayanoglu, I.C.; Polat, M.M.; Akcali, K.C.; et al. Human Gut Commensal Membrane Vesicles Modulate Inflammation by Generating M2-like Macrophages and Myeloid-Derived Suppressor Cells. J. Immunol. 2020, 205, 2707–2718. [Google Scholar] [CrossRef]
- Patten, D.A.; Hussein, E.; Davies, S.P.; Humphreys, P.N.; Collett, A. Commensal-Derived OMVs Elicit a Mild Proinflammatory Response in Intestinal Epithelial Cells. Microbiology 2017, 163, 702–711. [Google Scholar] [CrossRef]
- Cañas, M.A.; Fábrega, M.J.; Giménez, R.; Badia, J.; Baldomà, L. Outer Membrane Vesicles From Probiotic and Commensal Escherichia Coli Activate NOD1-Mediated Immune Responses in Intestinal Epithelial Cells. Front. Microbiol. 2018, 9, 498. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Akhavan Sepahi, A.; Mousavi, S.F.; Vaziri, F.; Siadat, S.D. The Effect of Faecalibacterium Prausnitzii and Its Extracellular Vesicles on the Permeability of Intestinal Epithelial Cells and Expression of PPARs and ANGPTL4 in the Caco-2 Cell Culture Model. J. Diabetes Metab. Disord. 2020, 19, 1061–1069. [Google Scholar] [CrossRef]
- Hickey, C.A.; Kuhn, K.A.; Donermeyer, D.L.; Porter, N.T.; Jin, C.; Cameron, E.A.; Jung, H.; Kaiko, G.E.; Wegorzewska, M.; Malvin, N.P.; et al. Colitogenic Bacteroides Thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host Microbe 2015, 17, 672–680. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Lari, A.; Shahryari, A.; Abdollahiyan, S.; Moradi, H.R.; Masoumi, M.; Davari, M.; Khatami, S.; Omrani, M.D.; et al. Extracellular Vesicles and Pasteurized Cells Derived from Akkermansia Muciniphila Protect against High-Fat Induced Obesity in Mice. Microb. Cell Fact. 2021, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Kim, J.K.; Han, S.K.; Lee, D.Y.; Lee, H.J.; Yim, S.V.; Kim, D.H. The Extracellular Vesicle of Gut Microbial Paenalcaligenes Hominis Is a Risk Factor for Vagus Nerve-Mediated Cognitive Impairment. Microbiome 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Seyama, M.; Yoshida, K.; Yoshida, K.; Fujiwara, N.; Ono, K.; Eguchi, T.; Kawai, H.; Guo, J.; Weng, Y.; Haoze, Y.; et al. Outer Membrane Vesicles of Porphyromonas Gingivalis Attenuate Insulin Sensitivity by Delivering Gingipains to the Liver. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165731. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, S.C.; Hong, S.H.; Lee, H.J. Secretable Small RNAs via Outer Membrane Vesicles in Periodontal Pathogens. J. Dent. Res. 2017, 96, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-C.; Choi, S.-Y.; Lee, Y.; Park, J.-W.; Hong, S.-H.; Lee, H.-J. Extracellular RNAs in Periodontopathogenic Outer Membrane Vesicles Promote TNF-a Production in Human Macrophages and Cross the Blood-Brain Barrier in Mice. FASEB J. 2019, 33, 13412–13422. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jin, Z.; Tang, K.; Ji, Y.; Suarez, J.; Suarez, J.A.; e Rocha, K.C.; Zhang, D.; Dillmann, W.H.; Mahata, S.K.; et al. Microbial DNA Enrichment Promotes Adrenomedullary Inflammation, Catecholamine Secretion, and Hypertension in Obese Mice. J. Am. Heart Assoc. 2022, 11, e024561. [Google Scholar] [CrossRef]
- Luo, Z.; Ji, Y.; Zhang, D.; Gao, H.; Jin, Z.; Yang, M.; Ying, W. Microbial DNA Enrichment Promotes Liver Steatosis and Fibrosis in the Course of Non-Alcoholic Steatohepatitis. Acta Physiol. 2022, 235, e13827. [Google Scholar] [CrossRef]
- Luo, Z.; Ji, Y.; Gao, H.; Gomes Dos Reis, F.C.; Bandyopadhyay, G.; Jin, Z.; Ly, C.; Chang, Y.J.; Zhang, D.; Kumar, D.; et al. CRIg + Macrophages Prevent Gut Microbial DNA-Containing Extracellular Vesicle-Induced Tissue Inflammation and Insulin Resistance. Gastroenterololy 2021, 160, 863–874. [Google Scholar] [CrossRef]
- Gao, H.; Luo, Z.; Ji, Y.; Tang, K.; Jin, Z.; Ly, C.; Sears, D.D.; Mahata, S.; Ying, W. Accumulation of Microbial DNAs Promotes to Islet Inflammation and β Cell Abnormalities in Obesity in Mice. Nat. Commun. 2022, 13, 565. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus Paracasei-Derived Extracellular Vesicles Attenuate the Intestinal Inflammatory Response by Augmenting the Endoplasmic Reticulum Stress Pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Vargoorani, M.E.; Modarressi, M.H.; Vaziri, F.; Motevaseli, E.; Siadat, S.D. Stimulatory Effects of Lactobacillus Casei Derived Extracellular Vesicles on Toll-like Receptor 9 Gene Expression and Cytokine Profile in Human Intestinal Epithelial Cells. J. Diabetes Metab. Disord. 2020, 19, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of Active, Inactive, and Derivatives of Akkermansia Muciniphila on the Expression of the Endocannabinoid System and PPARs Genes. Sci. Rep. 2022, 12, 12301. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Park, S.C.; Kim, N.E.; Shin, C.; Lee, S.K.; Jung, Y.; Yoon, D.; Kim, H.; Kim, S.; et al. Role of an Unclassified Lachnospiraceae in the Pathogenesis of Type 2 Diabetes: A Longitudinal Study of the Urine Microbiome and Metabolites. Exp. Mol. Med. 2022, 54, 1125–1132. [Google Scholar] [CrossRef]
- Nah, G.; Park, S.C.; Kim, K.; Kim, S.; Park, J.; Lee, S.; Won, S. Type-2 Diabetics Reduces Spatial Variation of Microbiome Based on Extracellur Vesicles from Gut Microbes across Human Body. Sci. Rep. 2019, 9, 20136. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus Plantarum HAC01 Regulates Gut Microbiota and Adipose Tissue Accumulation in a Diet-Induced Obesity Murine Model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodríguez-Peña, M.-M.; Ceperuelo-Mallafré, V.; Cedó, L.; Núñez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodríguez, M.; Balvay, A.; et al. Orally Administered Odoribacter Laneus Improves Glucose Control and Inflammatory Profile in Obese Mice by Depleting Circulating Succinate. Microbiome 2021, 10, 135. [Google Scholar] [CrossRef]
- Çelik, M.N.; Ünlü Söğüt, M. Probiotics Improve Chemerin Levels and Metabolic Syndrome Parameters in Obese Rats. Balkan Med. J. 2019, 36, 270–275. [Google Scholar]
- Rodovalho, V.D.R.; Luz, B.S.R.D.; Rabah, H.; do Carmo, F.L.R.; Folador, E.L.; Nicolas, A.; Jardin, J.; Briard-Bion, V.; Blottière, H.; Lapaque, N.; et al. Extracellular Vesicles Produced by the Probiotic Propionibacterium Freudenreichii CIRM-BIA 129 Mitigate Inflammation by Modulating the NF-ΚB Pathway. Front. Microbiol. 2020, 11, 1544. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus Plantarum-Derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus Aureus-Derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef]
- Müller, L.; Kuhn, T.; Koch, M.; Fuhrmann, G. Stimulation of Probiotic Bacteria Induces Release of Membrane Vesicles with Augmented Anti-Inflammatory Activity. ACS Appl. Bio. Mater. 2021, 4, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Haque, M.M.; Gote, M.; Jain, M.; Bhaduri, A.; Dubey, A.K.; Mande, S.S. A Prospective Randomized, Double-Blind, Placebo-Controlled, Dose-Response Relationship Study to Investigate Efficacy of Fructo-Oligosaccharides (FOS) on Human Gut Microflora. Sci. Rep. 2019, 9, 5473. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, Ł.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. Biomed. Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermúdez, P.; Blesa, J.; Soriano, J.M.; Marcilla, A. Extracellular Vesicles in Food: Experimental Evidence of Their Secretion in Grape Fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Rome, S. Biological Properties of Plant-Derived Extracellular Vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Fernández-Alonso, N.; Tomás-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Dávalos, A. Breast Milk MicroRNAs Harsh Journey towards Potential Effects in Infant Development and Maturation. Lipid Encapsulation Can Help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Zheng, X.; Zhu, H.; Qi, Q.; Liu, S.; Zhang, H.; Che, J. Latest Trend of Milk Derived Exosomes: Cargos, Functions, and Applications. Front. Nutr. 2021, 8, 855. [Google Scholar] [CrossRef]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a Promising Anti-Cancer Therapeutic: A Review of Its Chemical Properties, Bioactivity and Approaches to Cancer Cell Delivery. RSC Adv. 2014, 4, 10815. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Munir, J.; Lee, M.; Ryu, S. Exosomes in Food: Health Benefits and Clinical Relevance in Diseases. Adv. Nutr. 2020, 11, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, 1901251. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk Exosomal MiRNAs: Potential Drivers of AMPK-to-MTORC1 Switching in β-Cell de-Differentiation of Type 2 Diabetes Mellitus. Nutr. Metab. 2019, 16, 85. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of Pasteurized Milk: Potential Pathogens of Western Diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes From Mothers With Type 1 Diabetes Contain Aberrant Levels of MicroRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Shah, K.B.; Chernausek, S.D.; Garman, L.D.; Pezant, N.P.; Plows, J.F.; Kharoud, H.K.; Demerath, E.W.; Fields, D.A. Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients 2021, 13, 1091. [Google Scholar] [CrossRef]
- Shah, K.B.; Fields, D.A.; Pezant, N.P.; Kharoud, H.K.; Gulati, S.; Jacobs, K.; Gale, C.A.; Kharbanda, E.O.; Nagel, E.M.; Demerath, E.W.; et al. Gestational Diabetes Mellitus Is Associated with Altered Abundance of Exosomal MicroRNAs in Human Milk. Clin. Ther. 2022, 44, 172–185. [Google Scholar] [CrossRef]
- Yan, C.; Chen, J.; Wang, C.; Yuan, M.; Kang, Y.; Wu, Z.; Li, W.; Zhang, G.; Machens, H.G.; Rinkevich, Y.; et al. Milk Exosomes-Mediated MiR-31-5p Delivery Accelerates Diabetic Wound Healing through Promoting Angiogenesis. Drug Deliv. 2022, 29, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, G.; Dong, Y.; Yang, J.; Liu, Y.; Song, H.; Song, H.; Wang, Y. Liraglutide-Loaded Milk Exosomes Lower Blood Glucose When Given by Sublingual Route. Chem. Med. Chem. 2022, 17, e202100758. [Google Scholar] [CrossRef] [PubMed]
- Timms, K.; Holder, B.; Day, A.; Mclaughlin, J.; Forbes, K.A.; Westwood, M. Watermelon-Derived Extracellular Vesicles Influence Human Ex Vivo Placental Cell Behavior by Altering Intestinal Secretions. Mol. Nutr. Food Res. 2022, 66, 2200013. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sainz, E.; Lorente-Cebrián, S.; Aranaz, P.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Kumar, A.; Ren, Y.; Sundaram, K.; Mu, J.; Sriwastva, M.K.; Dryden, G.W.; Lei, C.; Zhang, L.; Yan, J.; Zhang, X.; et al. MiR-375 Prevents High-Fat Diet-Induced Insulin Resistance and Obesity by Targeting the Aryl Hydrocarbon Receptor and Bacterial Tryptophanase (TnaA) Gene. Theranostics 2021, 11, 4061–4077. [Google Scholar] [CrossRef]
- Taşlı, P.N. Usage of Celery Root Exosome as an Immune Suppressant; Lipidomic Characterization of Apium Graveolens Originated Exosomes and Its Suppressive Effect on PMA/Ionomycin Mediated CD4+ T Lymphocyte Activation. J. Food Biochem. 2022, 46, e14393. [Google Scholar] [CrossRef]
- Aquilano, K.; Ceci, V.; Gismondi, A.; de Stefano, S.; Iacovelli, F.; Faraonio, R.; di Marco, G.; Poerio, N.; Minutolo, A.; Minopoli, G.; et al. Adipocyte Metabolism Is Improved by TNF Receptor-Targeting Small RNAs Identified from Dried Nuts. Commun. Biol. 2019, 2, 317. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharm. 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering Exosomes for Targeted Drug Delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, J.; de Wever, O.; Hendrix, A. Analyzing Bacterial Extracellular Vesicles in Human Body Fluids by Orthogonal Biophysical Separation and Biochemical Characterization. Nat. Protoc. 2020, 15, 40–67. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Iliuk, A.; Petrilli, A.M.; Glass, C.; Casu, A.; Pratley, R.E. Proteomics and Phosphoproteomics of Circulating Extracellular Vesicles Provide New Insights into Diabetes Pathobiology. Int. J. Mol. Sci. 2022, 23, 5779. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Meza-Ramos, A.; Batlle, M.; Guasch, E.; Novials, A.; Párrizas, M. Treatment with EV-MiRNAs Alleviates Obesity-Associated Metabolic Dysfunction in Mice. Int. J. Mol. Sci. 2022, 23, 14920. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem. Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Thakral, S.; Ghoshal, K. MiR-122 Is a Unique Molecule with Great Potential in Diagnosis, Prognosis of Liver Disease, and Therapy Both as MiRNA Mimic and Antimir. Curr. Gene Ther. 2015, 15, 142–150. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs in Vivo with Antagomirs. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Yuan, X.; Berg, N.; Lee, J.W.; Le, T.-T.; Neudecker, V.; Jing, N.; Eltzschig, H. MicroRNA MiR-223 as Regulator of Innate Immunity. J. Leukoc. Biol. 2018, 104, 515–524. [Google Scholar] [CrossRef]
- Zhang, M.W.; Shen, Y.J.; Shi, J.; Yu, J.G. MiR-223-3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Front. Cardiovasc. Med. 2021, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of MiR-223 in the Pathophysiology of Liver Diseases. Exp. Mol. Med. 2018, 50, 128. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F. The Emerging Role of MiR-223 as Novel Potential Diagnostic and Therapeutic Target for Inflammatory Disorders. Cell Immunol. 2016, 303, 1–6. [Google Scholar] [CrossRef]
- Parrizas, M.; Mundet, X.; Castano, C.; Canivell, S.; Cos, X.; Brugnara, L.; Giraldez-Garcia, C.; Regidor, E.; Mata-Cases, M.; Franch-Nadal, J.; et al. MiR-10b and MiR-223-3p in Serum Microvesicles Signal Progression from Prediabetes to Type 2 Diabetes. J. Endocrinol. Invest. 2020, 43, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted Exosome-Mediated Delivery of Opioid Receptor Mu SiRNA for the Treatment of Morphine Relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef]
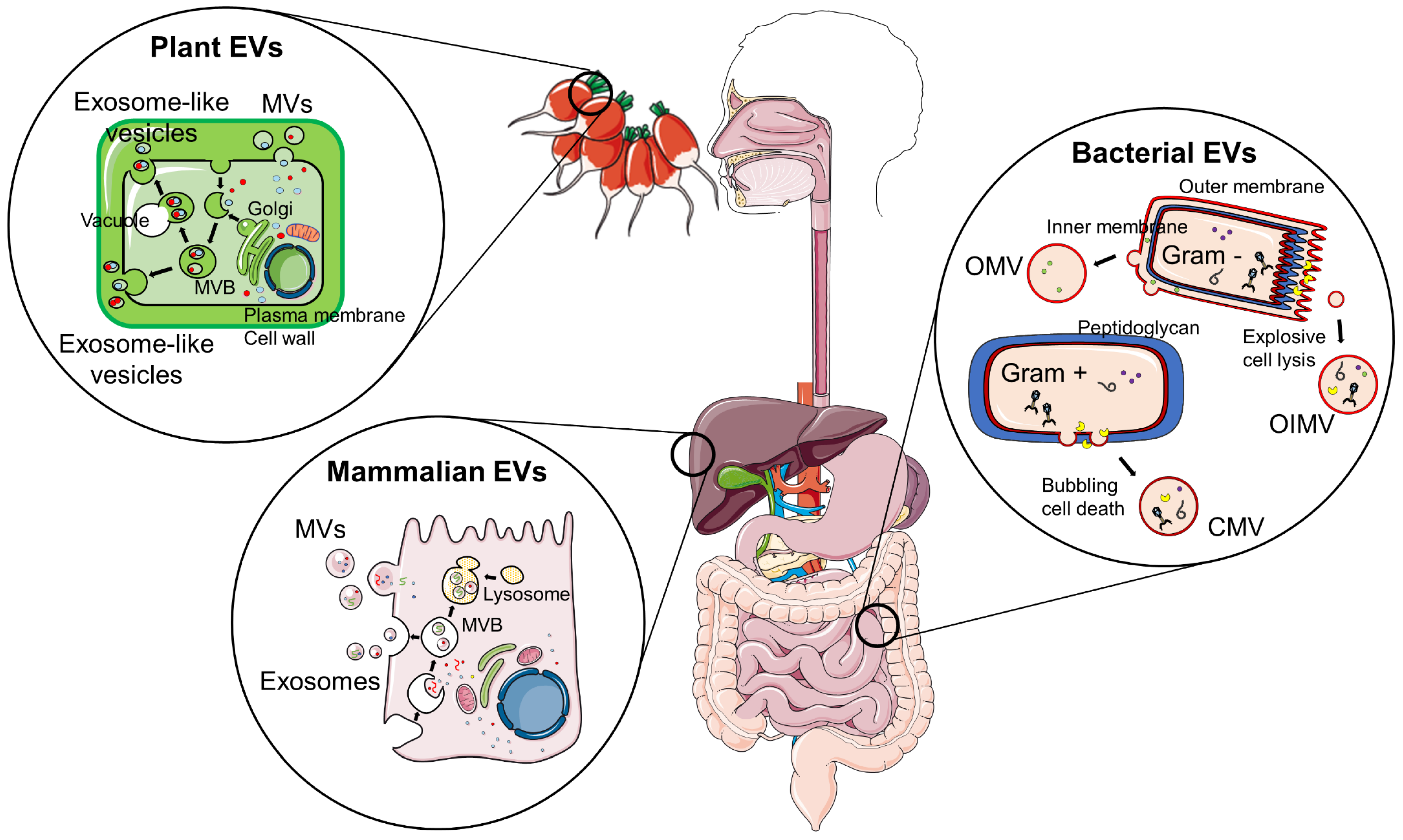
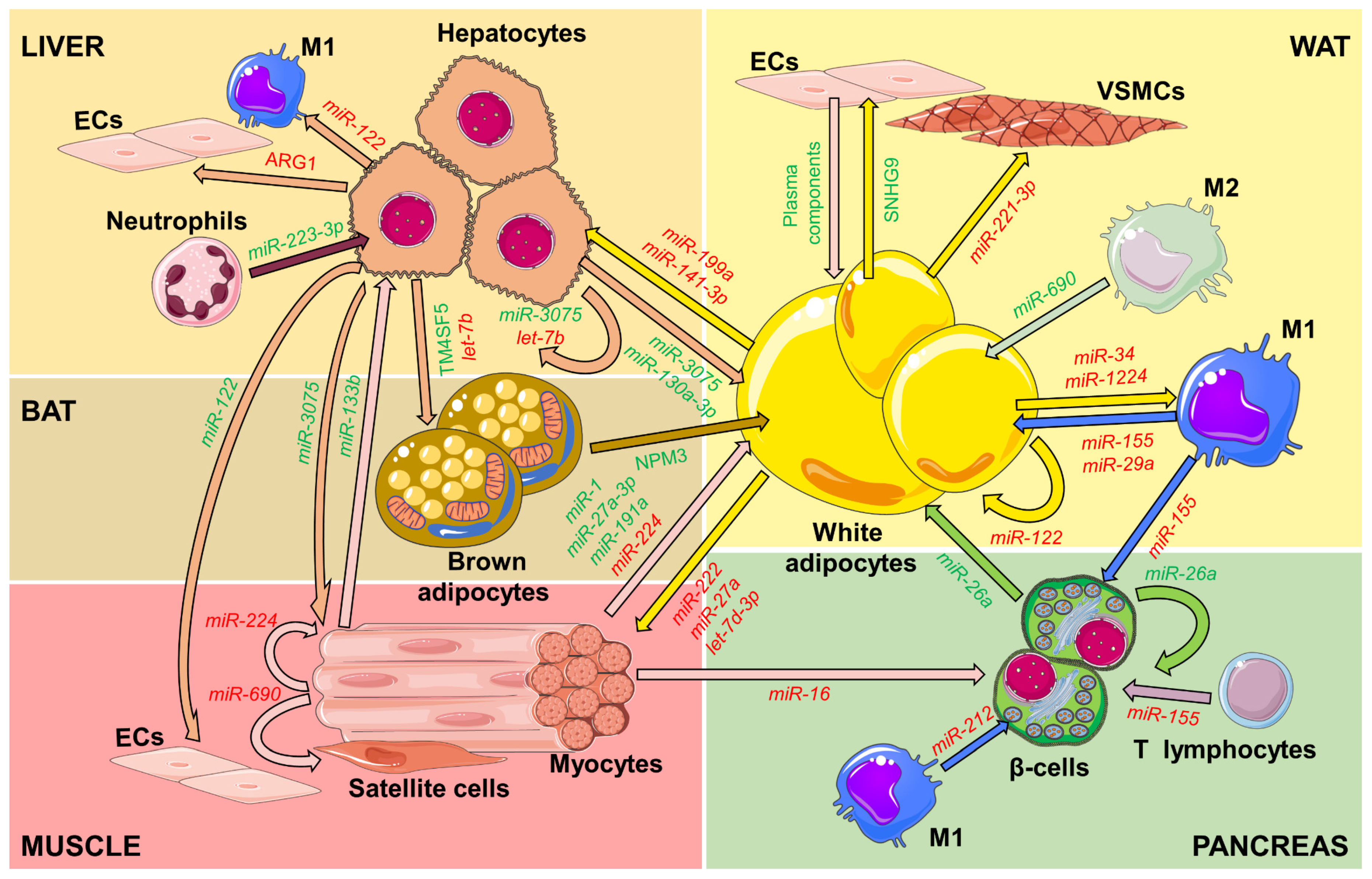

| Source of EVs | Relevant Cargo | Target Cell/Tissue | Effect | Ref |
|---|---|---|---|---|
| ECs | Plasma components | Adipocytes | Signaling | [39] |
| Adipocytes | SNHG9 | ECs | Anti-inflammation | [40] |
| Adipocytes | miR-221-3p | VSMCs | Vascular remodeling | [42] |
| Adipocytes | miR-122 | Preadipocytes | Adipogenesis | [43] |
| Adipocytes | miR-3604 | Preadipocytes | Reduced adipogenesis | [45] |
| Adipocytes | miR-34, miR-1224 | Macrophages | M2 polarization inhibition | [46,47] |
| Adipocytes | ND | Macrophages | Foam cell formation | [48] |
| Adipocytes | miR-222, miR-27a | Myocytes | Lower insulin sensitivity | [38,49] |
| Adipocytes | Mitochondria | Cardiomyocytes | Antioxidant | [50] |
| Adipocytes | Adipsin | Cardiomyocytes | Attenuated remodeling | [51] |
| Adipocytes | let-7d-3p | Muscle stem cells | Decreased proliferation | [52] |
| Adipocytes | miR-199a, miR-141-3p | Hepatocytes | Hepatic steatosis and lower insulin sensitivity | [53,54] |
| Adipocytes | miR-9-3p | Brain | Cognitive impairment | [55] |
| Brown adipocytes | NPM3 | WAT | Browning | [56] |
| Brown adipocytes | miR-30b | Kidney | Reduced fibrosis | [57] |
| Adipose MSCs | STAT3 | Macrophages | M2 polarization | [58] |
| Adipose MSCs | OPG | Macrophages | Decreased osteoclast differentiation | [59] |
| Adipose MSCs | miR-627, miR-223-3p | Hepatocytes | Decreased steatosis | [60] |
| Adipose MSCs | miR-21-3p, miR-126 | Fibroblasts | Accelerated wound healing | [61,62] |
| M1 macrophages | miR-155, miR-29a | Adipocytes, hepatocytes, myocytes | Lower insulin sensitivity | [36,63] |
| M1 macrophages | miR-212, miR-155 | Pancreatic β-cells | Decreased insulin secretion | [64,65] |
| M2 macrophages | miR-690 | Adipocytes, hepatocytes, myocytes | Higher insulin sensitivity | [35] |
| Muscle | miR-133b | Liver | Higher insulin sensitivity | [66] |
| Muscle | miR-1 | WAT | Increased lipolysis | [67] |
| Muscle | miR-191a, miR-27a-3p | WAT | Browning | [68] |
| Muscle | miR-690 | Muscle stem cells | Inhibition of differentiation | [69] |
| Muscle | miR-16 | Pancreatic β-cells | Increased proliferation | [70] |
| Liver | miR-130a-3p | Adipocytes | Higher insulin sensitivity | [71] |
| Liver | TM4SF5 | Brown adipocytes | Higher insulin sensitivity | [72] |
| Liver | miR-3075 | Adipocytes, hepatocytes, myocytes | Higher insulin sensitivity | [73] |
| Liver | miR-122 | Cardiomyocytes | Impaired mitochondrial function | [74] |
| Liver | miR-122 | ECs | Angiogenesis | [75] |
| Hepatocytes | miR-122 | Macrophages | Increased inflammation | [76] |
| Hepatocytes | let−7b | Brown adipocytes | Decreased thermogenesis | [77] |
| Neutrophil | miR-223-3p | Hepatocytes | Decreased inflammation and hepatic steatosis | [78] |
| Pancreatic β-cells | miR-26a | Pancreatic β-cells | Improved insulin secretion | [79] |
| Pancreatic β-cells | IAPP | Hippocampus | Mitochondrial fission | [80] |
| T lymphocytes | miR-155 | Pancreatic β-cells | Apoptosis | [81] |
| Source of EVs | Relevant Cargo | Target Cell/Tissue | Effect | Ref |
|---|---|---|---|---|
| A. muciniphila | ND | IECs | Anti-inflammatory | [159] |
| A. muciniphila | ND | IECs | Gut fortification | [170] |
| A. muciniphila | ND | Liver, AT | Anti-obesity | [171,172] |
| A. muciniphila, EcN1917 | ND | Gut | Colitis protection | [162,163] |
| O. splanchnicus | ND | IECs | Anti-inflammatory | [160] |
| B. Fragilis | PSA | DCs | Colitis protection | [164] |
| B. thetaiotaomicron | anSME | Gut | Colitis | [169] |
| P. hominis | LPS | Hippocampus | Cognitive impairment | [173] |
| P. panacis | LPS | Adipose, muscle | Insulin resistance | [157] |
| P. pentosaceus | ND | Macrophages | Immuno-suppression | [165] |
| P. gingivalis | Proteases | Liver | Impaired glucose metabolism | [174] |
| P. gingivalis, T. denticola, A. actinomycetemcomitans | miRNAs | T cells | Anti-inflammatory | [175] |
| A. actinomycetemcomitans | miRNA-like | Macrophages | Neuro-inflammatory | [176] |
| E. coliC25 | ND | IECs | Pro-inflammatory | [166] |
| EcN1917, ECOR12 | ND | IECs | Pro-inflammatory | [167] |
| EcN1917, ECOR63 | ND | IECs | Anti-inflammatory | [161] |
| F. prausnitzii | ND | IECs | Pro-inflammatory | [168] |
| Gut | mbDNA | Macrophages, Adrenal cells | Hypertension | [177] |
| Gut | mbDNA | Hepatocytes | Liver fibrosis | [178] |
| Gut | mbDNA | Liver, adipose, muscle | Pro-inflammatory | [179] |
| Gut | DNA | β-cell | Insulin resistance | [180] |
| L. paracasei | ND | IECs | Colitis protection | [181] |
| L. casei | ND | IECs | Anti-inflammatory | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaño, C.; Novials, A.; Párrizas, M. An Overview of Inter-Tissue and Inter-Kingdom Communication Mediated by Extracellular Vesicles in the Regulation of Mammalian Metabolism. Int. J. Mol. Sci. 2023, 24, 2071. https://doi.org/10.3390/ijms24032071
Castaño C, Novials A, Párrizas M. An Overview of Inter-Tissue and Inter-Kingdom Communication Mediated by Extracellular Vesicles in the Regulation of Mammalian Metabolism. International Journal of Molecular Sciences. 2023; 24(3):2071. https://doi.org/10.3390/ijms24032071
Chicago/Turabian StyleCastaño, Carlos, Anna Novials, and Marcelina Párrizas. 2023. "An Overview of Inter-Tissue and Inter-Kingdom Communication Mediated by Extracellular Vesicles in the Regulation of Mammalian Metabolism" International Journal of Molecular Sciences 24, no. 3: 2071. https://doi.org/10.3390/ijms24032071
APA StyleCastaño, C., Novials, A., & Párrizas, M. (2023). An Overview of Inter-Tissue and Inter-Kingdom Communication Mediated by Extracellular Vesicles in the Regulation of Mammalian Metabolism. International Journal of Molecular Sciences, 24(3), 2071. https://doi.org/10.3390/ijms24032071






