Abstract
Although a number of mood-stabilising atypical antipsychotics and antidepressants modulate serotonin type 7 receptor (5-HT7), the detailed contributions of 5-HT7 function to clinical efficacy and pathophysiology have not been fully understood. The mood-stabilising antipsychotic agent, lurasidone, and the serotonin partial agonist reuptake inhibitor, vortioxetine, exhibit higher binding affinity to 5-HT7 than other conventional antipsychotics and antidepressants. To date, the initially expected rapid onset of antidepressant effects—in comparison with conventional antidepressants or mood-stabilising antipsychotics—due to 5-HT7 inhibition has not been observed with lurasidone and vortioxetine; however, several clinical studies suggest that 5-HT7 inhibition likely contributes to quality of life of patients with schizophrenia and mood disorders via the improvement of cognition. Furthermore, recent preclinical studies reported that 5-HT7 inhibition might mitigate antipsychotic-induced weight gain and metabolic complication by blocking other monoamine receptors. Further preclinical studies for the development of 5-HT7 modulation against neurodevelopmental disorders and neurodegenerative diseases have been ongoing. To date, various findings from various preclinical studies indicate the possibility that 5-HT7 modifications can provide two independent strategies. The first is that 5-HT7 inhibition ameliorates the dysfunction of inter-neuronal transmission in mature networks. The other is that activation of 5-HT7 can improve transmission dysfunction due to microstructure abnormality in the neurotransmission network—which could be unaffected by conventional therapeutic agents—via modulating intracellular signalling during the neurodevelopmental stage or via loss of neural networks with aging. This review attempts to describe the current and novel clinical applications of 5-HT7 modulation based on preclinical findings.
1. Introduction
Serotonin (5-HT) receptor type 7 (5-HT7) is one of the most recently (1993) identified members of the 5-HT receptor family [1,2,3,4,5]. It has been demonstrated that 5-HT7 is highly expressed in functionally relevant regions of the brain [6,7]. Indeed, in the central nervous system, 5-HT7 is most predominantly expressed in the thalamus, hypothalamus, hippocampus, prefrontal cortex, basal ganglia, amygdala and dorsal raphe nucleus [8,9,10,11,12,13,14]. The predominant expression of 5-HT7 in the limbic regions provides a candidate hypothesis that 5-HT7 contributes to the regulation of memory processing, cognition and emotional perception [9,10,11,12,15,16]. The expression of 5-HT7 has been also observed in the kidney, liver, pancreas, spleen, stomach and smooth muscle cells of the arteries and gastrointestinal tract [17]. Based on these findings, 5-HT7 modulation is also considered to be a possible therapeutic target for the treatment of peripheral organs [18,19,20,21].
A number of preclinical studies have reported that 5-HT7 plays important roles in the regulation of mood, memory processing, cognition and emotional perception by following various experiments using selective 5-HT7 modulators and 5-HT7 knockout mice models [15,22,23,24,25,26]. Although modulating 5-HT7 is one of the targets for the treatment of schizophrenia and mood and anxiety disorders in current psychopharmacology, unfortunately, the clinical application of selective 5-HT7 receptor modulators has not yet been achieved [16]. However, several conventional mood-stabilising atypical antipsychotics, such as aripiprazole, brexpiprazole, clozapine, lurasidone, olanzapine, quetiapine, risperidone and zotepine are known to be inhibitors of 5-HT7 [12,27,28,29,30,31,32,33,34,35,36,37,38,39,40] (Table 1). Lurasidone is an antipsychotic agent with the highest binding affinity to 5-HT7 among mood-stabilising atypical antipsychotics [16,27] (Table 1). Furthermore, a novel antidepressant, vortioxetine, which is categorized as a 5-HT partial agonist reuptake inhibitor (SPARI), exhibits distinct pharmacodynamic profiles compared to other monoamine transporter-inhibiting antidepressants, since vortioxetine acutely and chronically suppresses the function of 5-HT7 [38,39,41,42]. Therefore, in order to clarify crucial clinical targets of 5-HT7 modulation for the treatment of several neuropsychiatric disorders, in the first half of this review we discuss the psychopharmacological perspectives of 5-HT7 modulation, based on the preclinical and clinical findings regarding 5-HT7 modulators and the clinical evaluation of lurasidone and vortioxetine to date.
Recent pharmacodynamic studies demonstrated that 5-HT7 modulation possibly contributes to a part of the characteristics of the clinical effects of both lurasidone and vortioxetine via inter-neuronal transmission [12,16,39,40,41,42,43]. Furthermore, recent preclinical findings suggest several possibilities in which 5-HT7 modulation can contribute to novel therapeutic targets for the treatment of several neuropsychiatric disorders, such as neurodevelopmental disorder, neurodegenerative diseases and metabolic complication associated with antipsychotics, through modulating intracellular signalling [5,42,44,45]. These candidate therapeutic potentials of 5-HT7 modulation are based on the findings that activated 5-HT7 enhances intracellular signalling, leading to synaptic remodelling and dendritic spine elongation [46]. In the second half of this review, we outline the recent pathophysiological hypotheses and their limitations (challenges to clarify in the future) regarding neurodevelopmental disorder and neurodegenerative diseases, based on the effects of 5-HT7 on intracellular signalling. In the final section, we introduce the possibility that inhibition of 5-HT7 is involved in the suppression of weight gain or metabolic complication induced by atypical antipsychotics via modulation of intracellular signalling [12,16,39,40,41,42,43,47,48].

Table 1.
Receptor-binding profiles of antipsychotics and antidepressants.
Table 1.
Receptor-binding profiles of antipsychotics and antidepressants.
| Receptor | LUR | APZ | Brex | CLZ | OLZ | PMZ | QTP | RIS | ZTP | VTX |
|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1A | 6.8 | 5.6 | 0.12 | 124 | >1000 | 650 | 432 | 423 | 471 | 15.0 |
| 5-HT2A | 2.0 | 8.7 | 0.47 | 5.4 | 2.3 | 48.4 | 100 | 0.2 | 2.7 | |
| 5-HT3 | >1000 | 630 | 241 | 57 | >1000 | >1000 | >1000 | 472 | 3.7 | |
| 5-HT7 | 0.5 | 10.3 | 3.7 | 18.0 | 365 | 0.5 | 307 | 6.6 | 12.0 | 19.0 |
| H1 | >1000 | 27.6 | 19 | 1.13 | 1.2 | 692 | 11 | 20.1 | 3.21 | |
| D1 | 262 | >1000 | 160 | 266 | 100 | >1000 | 712 | 244 | 71.0 | |
| D2 | 1.7 | 3.3 | 0.3 | 157 | 52.3 | 0.3 | 245 | 3.6 | 25.0 | |
| Reference | [27] | [28,29] | [30] | [31,32] | [33,49] | [34] | [35] | [29,36] | [37] | [38] |
Notes: lurasidone (LUR), aripiprazole (APZ), brexpiprazole (Brex), clozapine (CLZ), olanzapine (OLZ), pimozide (PMZ), quetiapine (QTP), risperidone (RIS), zotepine (ZTP) and antidepressant vortioxetine (VTX) against serotonin (5-HT) type 1A (5-HT1A), type 2A (5-HT2A), type 3 (5-HT3), and type 7 (5-HT7) receptor, histamine H1 (H1) receptor, dopamine receptors type 1 (D1) and 2 (D2). Data are equilibrium-constant (Ki) values (nM).
2. Preclinical Findings about Therapeutic Potential of 5-HT7 Modulation
2.1. Depression
The earliest identified physiological function of 5-HT7 was regulation of circadian rhythms [2]. In this context, 5-HT7 was found to be expressed in the suprachiasmatic nucleus [50], which is a major regulatory region of circadian rhythms [51]. The influence of 5-HT7 on sleep regulation is complicated because the 5-HT7 inverse agonist SB269970 [40,42,43,52] increased latency in the onset but decreased the total amount of time spent in rapid eye movement sleep [53]. A number of antidepressants also increased latency in the onset but decreased the total amount of time spent in rapid eye movement sleep, similar to SB269970 [54]. Based on these similar effects of SB269970 and antidepressants on sleep, exploration for antidepressant-like effects of 5-HT7 inhibitor was conducted.
The 5-HT7 knockout mice displayed a reduction of immobility times in both a forced swim test and a tail suspension test, common pharmacological behaviour tests for the screening of antidepressants (decreasing immobility time is considered to correlate to antidepressant action in humans) [22,55]. Based on this finding, 5-HT7 inhibitors/antagonists were actively explored and developed as antidepressants in the early years of this century. SB269970, an inverse agonist of 5-HT7, has been shown to reduce immobility time in forced swim and tail suspension tests; as a result, it became a candidate for use as an antidepressant [22,23,56]. Additionally, the administration of subeffective concentration of SB269970 enhanced the anti-immobility action of subeffective doses of of desipramine, citalopram and imipramine in the forced swim and tail suspension tests [56,57,58]. Most notably, SB269970 generated significantly rapid onset of antidepressant-like effects in olfactory bulbectomised models, when compared to fluoxetine [59]. As a result, 5-HT7 inhibitors were anticipated to join the rapid onset antidepressant class, since it is recognised that one of the major problems of monoamine transporter-inhibiting antidepressants, a currently/commonly prescribed antidepressant class, is that they require 2-4 weeks for the onset of antidepressive effects. The target regions for antidepressant effects of 5-HT7 inhibitors remain controversial. Injection of SB269970 into the hippocampus generated antidepressant-like activity in the forced swim test [60]. Injection of SB269970 into the lateral habenular nucleus lesion model using 6-hydroxydoapmine also exhibited antidepressant-like activity, whereas injection into the medial prefrontal cortex lesion of a model, conversely, displayed the enhancement of depressive-like activity [61,62]. AS19, a 5-HT7 agonist, demonstrated the opposite region-dependent effect against SB269970 [61,62] (Table 2).
2.2. Anxiety
The potential function of 5-HT7 on anxiety demonstrated the inconsistent results between pharmacological and molecular biological studies, similar to the case of depression. Expression of 5-HT7 mRNA increased by acute restraint stress but not by chronic variable stress in the rat hippocampus, indicating that 5-HT7 contributes to the regulation of response to stress [63]; however, the anxiety-like behaviour in the light/dark transfer or elevated plus maze tests did not affect both 5-HT7 knockdown or knockout models [55,64,65]. On the other hand, the anxiolytic-like activity of SB269970 (both systemic and intra-hippocampal local administrations) was demonstrated by the Vogel drinking test, the elevated plus maze test and the four plates test [23,60]. Both SB269970 and 5-HT7 knockout mice demonstrated anxiolytic-like activity in the marble-burying test, which is a model of anxiety and obsessive–compulsive disorder [66]. These inconsistent results among molecular biological and pharmacological experiments suggest that the level of 5-HT7 inactivation required for anxiolytic effects is probably dependent on the model utilized. In other words, appropriate 5-HT7 inhibition may be beneficial for anxiolytic effects (Table 2).
2.3. Schizophrenia
A number of pharmacological studies reported the therapeutic potential in components of positive and negative symptoms and cognitive dysfunction of schizophrenia using chemical-induced schizophrenia models. Compared with wild-type mice, 5-HT7 knockout mice were less susceptible to prepulse inhibition deficits of phencyclidine [67]. SB-269970 suppressed hyperactivity induced by ketamine, phencyclidine and amphetamine [68,69], whereas the effects of 5-HT7 on prepulse inhibition deficits were inconsistent. SB-269970 improved amphetamine-induced prepulse inhibition deficits [69], but did not affect those induced by an NMDA/glutamate inhibitor, phencyclidine or ketamine [67,70]; however, SB-258741, a 5-HT7 antagonist, did not affect the amphetamine-induced prepulse inhibition deficits, but suppressed phencyclidine-induced prepulse inhibition deficits [71]. Ketamine-induced negative symptoms associated with social withdrawal were attenuated by SB269970, but not affected by SB-258741 [70,71]. The effects of SB269970 on positive symptoms are possibly involved in dopaminergic transmission but not in glutamatergic transmission, whereas, conversely, the effects of SB-258741 on positive symptoms are possibly involved in glutamatergic transmission but not in dopaminergic transmission. Furthermore, social withdrawal induced by NMDA/glutamate receptor inhibition is prevented by SB269970 but not by SB258741. These discrepancies between SB269970 and SB258741 could not be explained by their receptor-binding profiles alone, since these compounds displayed binding affinity to 5-HT7, D2 and D3 receptors [71]. These findings suggest that the effects between SB269970 and SB258741 are probably dependent upon the materials (pharmacological and molecular biological models) and compounds employed (Table 2).
On the other hand, the effects of 5-HT7 inhibition on neurocognitive dysfunction (procognitive effects) demonstrated its promise. SB269970 attenuates amnesia in short-term memory induced by ketamine and MK801 [72,73], and this effect was suppressed by AS19 [74]. The new valuable tool for exploring the neurobiological bases of cognitive dysfunction in schizophrenia, five-choice serial reaction time task (including attention, response inhibition, cognitive flexibility and processing speed), demonstrated that SB269970 improved the impairment of working memory and impulsivity, without affecting premature responding induced by MK801 [75] (Table 2).

Table 2.
Preclinical data on the role of 5-HT7 in depression, anxiety and schizophrenia.
Table 2.
Preclinical data on the role of 5-HT7 in depression, anxiety and schizophrenia.
| Model | Agent Manipulation | Effects | Ref. |
|---|---|---|---|
| Depression | |||
| Forced swim | Knockout | Decreased immobility | [22,55] |
| SB269970 | Decreased immobility (systemic administration) | [22,23] | |
| Decreased immobility (hippocampus) | [60] | ||
| 6-OH-dopamine lesions (medial forebrain bundle) | AS-19 SB269970 | Decreased immobility (into PrL) Increased immobility (into PrL) (Prl: the prelimbic subregion of the ventral medial prefrontal cortex) | [61] |
| Tail suspension | Knockout | Decreased immobility | [22] |
| SB269970 | Decreased immobility | [22,23,56] | |
| Olfactory bulbectomy | SB269970 | Decreased hyperactivity | [59] |
| Anxiety | |||
| Elevated plus maze | Knockout Knockdown | No difference between models and wild-type | [55,64,65] |
| SB269970 | Increased time in open arms and entries into open arms | [23,60] | |
| Light/dark transfer | Knockout Knockdown | No difference between models and wild-type | [55,64,65] |
| Vogel drinking Four plates test | SB269970 | Increased the number of the accepted shocks Increased the number of punished crossings | [23,60] |
| Marble-burying | Knockout SB269970 | Reduced stereotypic behaviour | [66] |
| Schizophrenia | |||
| Prepulse inhibition | Knockout | Less susceptible to prepulse inhibition deficits by phencyclidine | [67] |
| SB269970 | Improved amphetamine-induced prepulse inhibition deficits | [69] | |
| No effects on prepulse inhibition deficits induced by phencyclidine/ketamine | [67,70] | ||
| SB258741 | No effect on amphetamine-induced prepulse inhibition deficits | [71] | |
| Suppressed phencyclidine-induced prepulse-inhibition deficits | [71] | ||
| SB269970 | Suppressed hyperactivity induced by ketamine, phencyclidine and amphetamine | [68,69] | |
| Social withdrawal | SB269970 | Improved | [70,71] |
| SB258741 | No effect | [70,71] | |
| Amnesia induced by ketamine/MK801 | SB269970 | Improved | [72,73] |
| 5-choice serial reaction time task | SB269970 | Improved working memory impairment and impulsivity | [75] |
3. Clinical Evaluation of 5-HT7 Modulators
3.1. Vortioxetine
Vortioxetine is an antidepressant belonging to the family of monoamine transporter-inhibiting antidepressants; its antidepressant effect is thought to arise from not only its monoamine transporter inhibition but also 5-HT7 inhibition. The binding affinity of vortioxetine to 5-HT7 is relatively low compared to serotonin transporter, 5-HT1A and 5-HT3 [38], but the relevant therapeutic concentration of vortioxetine functionally suppresses 5-HT7 [39,41] (Table 1).
The delay of the onset of the antidepressive effects of conventional monoaminergic antidepressant medication has been established as one of the major drawbacks of current treatments for depressive disorder, since all conventional monoamine transporter-inhibiting antidepressants require more than several weeks for the onset of beneficial antidepressive effects [76,77]. Based on the fast-acting antidepressant-like effects of 5-HT7 inhibitor SB269970 [59], a number of psychiatrists initially expected the rapid-onset antidepressant class to be prescribed in clinical settings in place of conventional monoamine transporter-inhibiting antidepressants; however, unfortunately, the onset duration of the antidepressant effect of lurasidone and vortioxetine is equivalent to that of conventional antidepressants [78,79,80,81,82]. The T1/2 value of vortioxetine is long (66 hrs), with steady-state plasma concentration levels reached after approximately 2 weeks, which may delay the onset of beneficial pharmacological effects of vortioxetine [83].
It has been recognized that comorbidity with anxiety (disorder) is the most significant factor affecting poor prognostic outcomes, such as increasing risk of recurrence, chronic course and suicidal behaviour [84,85]. Several meta-analysis studies have reported inconsistent results for the efficacy of vortioxetine on anxiety symptoms. A meta-analysis supported the efficacy of vortioxetine for generalised anxiety disorder [86]. Initially, vortioxetine was shown to be efficacious in reducing depressive and anxiety symptoms in patients with a major depressive disorder and high levels of anxiety (baseline total score of Hamilton Anxiety Rating Scale ≥ 20) [87], whereas two other meta-analyses showed that vortioxetine was not efficacious for anxiety [88,89]. However, following network meta-analysis of randomised controlled trials, vortioxetine was shown to be effective but was listed as the antidepressant with the lowest remission rates for generalised anxiety disorder [90]. Recent systematic review and meta-analysis suggested that there was uncertainty regarding the effectiveness of vortioxetine for anxiety symptom due to existing evidence arising from very low-quality studies [91].
In three placebo-controlled studies in adults with recurrent major depressive disorder, vortioxetine displayed clinically meaningful improvements in performance on two objective measures (the Digit symbol substitution test and the Rey auditory verbal learning test) that together cover a broad range of cognitive domains, including executive function, attention, processing speed, learning and memory [92,93,94]. In another meta-analysis, vortioxetine demonstrated the greatest improvements in a neurocognitive test that integrated several domains affected in major depressive disorder [95]. Notably, vortioxetine was the only antidepressant that provoked significant changes on task cognition among all antidepressant classes investigated [95]. A post hoc analysis demonstrated that vortioxetine showed general and multidomain benefits on cognitive performance, including executive function, attention/speed of processing and memory domains [96]. Therefore, the anxiolytic effects of vortioxetine did not reach expectations from preclinical findings, but vortioxetine is probably more useful in improving impairments of cognition or emotional perception than other monoamine transporter-inhibiting antidepressants.
In particular, enhancement of cognition with vortioxetine, in both subjective and objective functioning measures, was more pronounced in working patients, particularly in those whose job placed higher demands on executive functioning [94]. Indeed, patients prescribed vortioxetine recognised cognitive improvements that enhanced their workplace productivity [97]. Among the early onset of the antidepressive effect, anxiolytic effect and cognition-enhancing effect expected from the 5-HT7 inhibitory effect of vortioxetine (based on preclinical findings), the cognition-enhancing effect seems to be a characteristic effect of vortioxetine.
3.2. Lurasidone
A mood-stabilising antipsychotic agent, lurasidone possesses the highest binding affinity to 5-HT7 among antipsychotics [27] (Table 1). Several meta-analyses reported that lurasidone significantly improves positive and negative depressive symptoms [98,99,100,101]. Therefore, the general efficacy of lurasidone for the treatment of schizophrenia is considered to be comparable to that of other atypical antipsychotics [16]. Meta-analyses also demonstrated that lurasidone has antidepressant effects comparable to those of other mood-stabilising antipsychotics against major depressive disorder and bipolar depression [81,102]. The efficacy of lurasidone in the acute treatment of bipolar depression, as both monotherapy and adjunctive therapy to lithium/valproate, has been reported in clinical trials [103,104,105]. Like vortioxetine, the rapid onset of the antidepressive effects of lurasidone have not been demonstrated [78,79,80,81,82].
Several randomised control studies demonstrated that lurasidone improved comorbid anxiety symptom in schizophrenia, bipolar I and major depressive disorders. Lurasidone monotherapy (20~60 mg/day) improved depressive mood and concomitant anxiety in patients with bipolar I disorder [106]. Lurasidone monotherapy (20~80 mg/day) also improved depressive mood and anxiety in children and adolescents with bipolar depression [107]. Lurasidone (20–60 mg/day) was effective for mild and moderate-to-severe levels of anxieties for patients with major depressive disorder, suggesting the effectiveness of lurasidone on major depressive disorder with mixed features [80]. A clinical study suggested that lurasidone improved treatment-resistant schizophrenia (including clozapine-resistant schizophrenia) [108]. Notably, lurasidone improved speed of processing and executive function, which is a critical atypical antipsychotic-resistant cognitive domain [108]. In spite of limited evidence, the cognitive benefits of lurasidone in bipolar disorder and major depressive disorder was reported [109]. These demonstrations indicated the possibility that the effectiveness of lurasidone’s selective improvement of cognitive impairment is possibly modulated by specific transmission systems required for specific cognitive domains.
Considering the clinical findings regarding vortioxetine, both lurasidone and vortioxetine, at the minimum, might be classified in a distinct category of treatments that can be promised to improve cognitive function compared to conventional mood-stabilising antipsychotics and antidepressants. In contrast, the anxiolytic effects of lurasidone and vortioxetine displayed discrepancy, since vortioxetine has been evaluated to possess one of the lowest anxiolytic effects among monoamine transporter-inhibiting antidepressants [90], whereas lurasidone has demonstrated effectiveness treating anxiety symptoms in mood disorders and major depressive disorders with mixed features [80,106,107]. However, these evaluations of current meta-analyses probably could not measure the accurate efficacy of lurasidone in the treatment of schizophrenia, since dose–response meta-analyses demonstrated that 95% of the effective dose (ED95) of lurasidone for schizophrenia and depressive symptoms reached more than 160 mg/day, which was higher than the approved dose of lurasidone [101,110,111,112]. In other words, evaluation of the efficacy of lurasidone for schizophrenia needs to be supplemented with clinical data of higher doses than approval doses. The clinical findings of vortioxetine and lurasidone show that among the clinical effects expected in preclinical findings regarding 5-HT7 inhibition, only the improvement of cognitive impairment was demonstrated by both agents.
4. Intracellular Signalling Associated with 5-HT7
Four splice variants of 5-HT7 were identified. These were distinct in their carboxyl terminals due to introns in the 5-HT7 gene, including 5-HT7a, 5-HT7b and 5-HT7c in rodents, and 5-HT7a, 5-HT7b and 5-HT7d in humans [6,113,114,115,116,117]. All of these four splicing variants directly affect three intracellular signalling pathways via activations of Gαs, Gα12 and metalloproteinase-9 [4,46,118]. Significant differences among 5-HT7 splicing variants in localisation, ligand-binding affinity and adenylate cyclase activity have not been observed [6], whereas 5-HT7a isoform specifically activates the abovementioned cAMP-dependent signalling through the activation of type 1 and 8 adenylyl cyclase via Ca2+/calmodulin-dependent and Gs-independent signalling [119].
Activation of 5-HT7 enhances synthesis of cyclic adenosine monophosphate (cAMP) via activation of adenylyl cyclase [4]. Increasing cAMP activates signalling of both protein kinase A (PKA) and the exchange protein directly activated by cAMP (EPAC) [10,118,120]. These two signalling pathways affect various signalling transductions via phosphorylation of target proteins, leading to the propagation of the signalling to the next biochemical events. Subsequently, enhanced PKA stimulates cyclin-dependent kinase 5 (Cdk5) [10,118] and Ras [121,122], resulting in serine/threonine extracellular signal-regulated kinases (Erk) signalling activation [121,123]. Activation of EPAC also indirectly enhances Erk signalling [121,123]. The second signalling pathway regulates adenosine monophosphate-dependent protein kinase (AMPK) [42,47,48] via inhibitory PKA and stimulatory EPAC [124]. Furthermore, 5-HT7-induced Ras and EPAC signalling promote the activation of the mammalian target of rapamycin (mTOR) [125], but activated AMPK supresses mTOR signalling [126]. Activated signalling of Erk and mTOR by 5-HT7 enhances neurite outgrowth in embryonic brains [122,127].
Two other types of signalling associated with 5-HT7 were also identified: Gα12 [118] and metalloproteinase-9 (MMP9) [46]. It has been shown that 5-HT7/Gα12 activates cell division cycle protein 42 (Cdc42) [118,128] and activates signalling pathways associated with Gα12 [118]. In addition, it is recognized that 5-HT7/Gα12 activates both Ras homolog gene family member A (RhoA) and cell division cycle protein 42 (Cdc42) [118,128]. Another pathway associated with 5-HT7 activates MMP-9, which cleaves the extracellular domain of the hyaluronic acid receptor (CD44) resulting in subsequent detachment from the extracellular matrix (ECM) [46]. These physical interactions among extracellular matrices such as 5-HT7, CD44 and ECM, play fundamental roles in synaptic remodelling and dendritic spine elongation [46]. The detachment from ECM via CD44/MMP9 plays an initial role in both dendritic spine remodelling and synaptic pruning, followed by neurite retraction by RhoA and neurite extension/branching by mTOR, Erk and Cdc42 [122,127] (Figure 1).
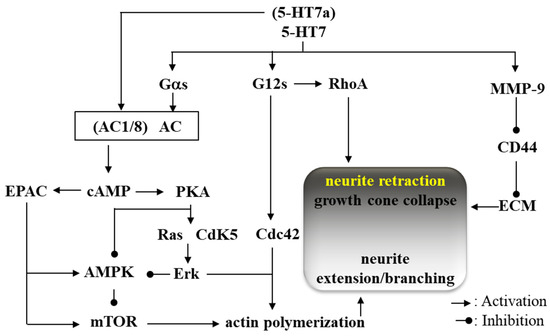
Figure 1.
Intracellular signalling associated with 5-HT7. AC, adenylyl cyclase; AMPK, adenosine monophosphate-dependent protein kinase; cAMP, cyclic adenosine monophosphate; Cdc42, cell division cycle protein 42; Cdk5, cyclin-dependent kinase 5; EPAC, exchange protein directly activated by cAMP; Erk, extracellular-regulated kinase; mTOR, mammalian target of rapamycin; PKA, protein kinase A; RhoA, Ras homolog gene family member A; CD44, hyaluronic acid receptor; MMP-9, metalloproteinase 9; ECM, extracellular matrix.
It has been established that the serotonergic system plays crucial roles in the organisation of the neural system, such as generation of neurogenesis, cell migration, axon guidance, dendritogenesis, synaptogenesis and brain wiring during the development of the mature brain [129]. During the embryonic stage, 5-HT7 leads neurite outgrowth through activations of Cdk5 with mTOR [122,127]. Therefore, 5-HT7 contributes to the establishment and maintenance of neural connectivity and synaptic plasticity in early developmental stages [130]. In other words, the reorganization of dendritic morphology induced by 5-HT7 plays important roles in new synapse growth and initial neuronal network formation, which is the target of event-related structural and functional plasticity in the early developmental stage [131,132]. Chronic 5-HT7 activation promotes dendritic spine formation and increases the number of structurally intact synapses and the expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/glutamate receptor [118]. Neural circuits can be remodelled, induced by reactions to physiological and pathological inputs well into adulthood and continuing to exhibit robust plasticity throughout the entire lifespan of individuals [133]. It is likely that 5-HT7 contributes to the modulation of synaptic plasticity and neuronal connectivity in the developing and mature brain. However, the 5-HT7 associated remodelling transforms their composition age-dependently. The impacts of 5-HT7/Gα12 signalling predominantly occur during the postnatal stages, but are reduced in older neurons, suggesting that neurite extension/branching induced by 5-HT7 is attenuated age-dependently [134]. Considered with the regulation system of neural remodelling associated with 5-HT7—e.g., neurite retraction (growth cone collapse) and neurite extension/branching—in the elderly brain, over-activation of 5-HT provides neurite retraction predominantly via 5-HT7/G12s and MMP-9 rather than through neurite extension/branching via 5-HT7/Gαs.
5. Effects of 5-HT7 on Neuronal Transmission
Behavioural study demonstrated that 5-HT7 knockout mice displayed impairments of contextual learning, seeking behaviour and allocentric spatial memory [135]. Electrophysiological study also demonstrated that 5-HT7 knockout mice exhibited impaired hippocampal long-term potentiation [64]. In addition, 5-HT7-induced activation of PKA signalling enhanced N-methyl-D-aspartate (NMDA)-evoked currents, resulting in the enhancement of population spike amplitude and bursting frequency in hippocampal CA1 and CA3 regions, respectively [136,137,138]. Furthermore, 5-HT7 activates hippocampal transmission postsynaptically due to enhanced phosphorylation of the AMPA/glutamate receptor induced by cAMP/cAMP response element-binding protein (CREB) signalling [139,140]. Additionally, 5-HT7 reversed long-term depression associated with metabotropic glutamate receptors (mGluR) [26]. These stimulatory effects of 5-HT7 on neuronal plasticity and excitability were observed in juvenile wild-type rodents, but not in elderly rodents [134]. These findings suggest that the molecular mechanisms underlying the positive action of 5-HT7 on cognition and memory are mediated by age-dependent regulation systems.
Activation of 5-HT7 during adolescence induced persistent upregulation of 5-HT7 [13]. Chronic exposure to methylphenidate during postnatal life and adolescence probably provides persistent structural rearrangements of the brain’s reward pathways associated with 5-HT7 [141]. During the pre- and postnatal periods, exposure to selective serotonin reuptake inhibitors generates long-term anxiety in adulthood without affecting the morphological alterations of the brain [142,143]. The molecular mechanism underlying 5-HT7-induced remodelling increases neurites and dendritic spine elongation via MMP-9/CD44 with Cdc42 in reversal learning and neuronal regeneration [46]. Therefore, the impacts of 5-HT7 on neuronal plasticity during early development are not limited to embryonic and early postnatal development but can also persist in adolescence and adulthood. Pathologically, activation of 5-HT7 during adolescence leads to increased dendritic arborisation in the nucleus accumbens, one of the most impactful neural circuits associated with the pathophysiology of schizophrenia [144,145,146]. Therefore, reorganization of dendritic morphology induced by 5-HT7 signalling provides new synapse growth and initial neuronal network formation in the hippocampus, which is the target of event-related structural and functional plasticity (memory) in the early developmental stage [131,132].
Although activation of hippocampal 5-HT7 contributes to the formation of learning and memory, suppression of 5-HT7, conversely, improved other cognitive components, such as executive function, which play important roles in quality of life. Traditionally, enhanced mesocortical dopaminergic transmission or dopamine release in the frontal cortex induced by blockade of D2 in the ventral tegmental area are considered to be key players in the improvement of positive and negative symptoms of schizophrenia [32]. Additionally, thalamocortical glutamatergic transmission are considered to play important roles in neurocognitive function [32,147,148,149]. 5-HT2A inhibition and/or 5-HT1A activation contributes to augmented dopamine release in the frontal cortex induced by D2 inhibition [150,151,152,153,154]. GABAergic disinhibition in the frontal cortex also contributes to enhanced dopamine release induced by some atypical antipsychotics, such as aripiprazole and clozapine [154,155]; however, lurasidone-induced dopamine release is not modulated by regional GABAergic disinhibition, similar to blonanserin, quetiapine, risperidone and zotepine [151,152,153,154]. These discrepancies in the frontal transmitter release profiles among 5-HT7 antagonistic antipsychotics (lurasidone, aripiprazole, clozapine, quetiapine, risperidone and zotepine) suggest that 5-HT7 antagonism probably does not play important roles in the enhanced dopamine release in the frontal cortex of these antipsychotics. Therefore, 5-HT7 antagonism is possibly not involved in effectiveness of the core (positive and negative) symptoms of schizophrenia.
Tonic hyperactivation of thalamocortical glutamatergic transmission was observed in schizophrenia, ADHD and autism [12,39,148,149,156,157,158,159,160,161,162,163,164,165]. Other 5-HT7 molecules, such as group II and III mGluRs and α2 adrenoceptor, which compensate thalamocortical glutamatergic transmission, have also been identified [149,156,157,159,164]. The behavioural importance of neuronal networks is affirmed by lesion studies demonstrating that lesions to hub regions are associated with task impairments across multiple functional domains [166,167,168]. Notably, the prediction of the thalamocortical pathway on task-specific cortical activity patterns was outperformed compared to the prediction of the cortical and hippocampal pathway [166,167,168]. The mediodorsal thalamic nucleus is reciprocally connected with the medial prefrontal cortex and receives glutamatergic inputs from the hippocampus [40,41,163,169,170]. The glutamatergic neurons in the mediodorsal thalamic nucleus were mainly suppressed but were activated by propagation of ripple burst during the non-rapid eye movement sleep phase [169,170,171]. Therefore, the mediodorsal thalamic nucleus plays important roles in memory processing during sleep and sensory integration during wakefulness [16,172,173,174,175]. Post-synaptic 5-HT7 on the glutamatergic neurons in the thalamus received serotonergic transmission from the dorsal raphe nucleus, resulting in attenuation of tonic activation of thalamocortical glutamatergic transmission [160,165]. Both lurasidone and vortioxetine suppress the 5-HT7-mediated hyperactivation of thalamocortical glutamatergic transmission via 5-HT7 blockade [12,39,40]. These findings indicate that inhibition of 5-HT7 in the mediodorsal thalamic nucleus contributes to sensory integration during wakefulness [16,173,174,175].
6. Therapeutic Potential in Other Disease and Disorders Based on the Preclinical Findings
6.1. Neurodevelopmental Disorders
Fragile X syndrome, which is caused by mutation of Fragile X mental retardation 1 (FMR1) gene, is a common neurodevelopmental disorder characterized by intellectual disability with autism [176]. Fragile X mental retardation protein (FMRP) is an mRNA-binding protein that plays important roles in the negative regulation of protein synthesis [176]. Loss of function of FMR1 leads to abnormal protein synthesis and overactivation of signalling via mGluR5 receptors, resulting in enhancement of long-term depression which, subsequently, induces aberrant synaptic plasticity [177,178]. FMR1 knockout mice exhibit sustained upregulation of mGluR-mediated long-term depression and decreasing density of the AMPA/glutamate receptor, whereas activation of 5-HT7 by selective 5-HT7 agonists, LP-211 and BA-10, reversed the pathological abnormalities to a normal level [26,123]. Therefore, it has been speculated that impaired cAMP-mediated signalling, which was observed in patients with Fragile X syndrome, is probably involved in the exaggerated generation of long-term depression [179].
Activation of 5-HT7 was a potential candidate target for relieving symptoms in patients with Rett syndrome. Rett syndrome is the second most common cause of mental retardation in females and plays a role in severe neurodevelopmental disorders such as breathing dysfunction, loss of coordination, abnormal eye and hand movements, epilepsy, aberrant sleeping behaviour and cognitive impairment [180]. The prime pathogenesis of Rett syndrome is known to be various genetic mutations in methyl CpG-binding protein 2 gene (MeCP2) on the X chromosome, cyclin-dependent kinase-like 5 (CDKL5), forkhead box G1 (FOXG1), WD repeat domain 45 (WDR45) or syntaxin binding protein 1 (STXBP1) [181,182]. Restoring MeCP2 function can normalise functional abnormalities of MeCP2 knockout mice, whereas overexpression of the MeCP2 gene led to neurological defects [183]. Therefore, recent preclinical studies explored the targets in MeCP2 downstream effectors and other signalling, including 5-HT7. Based on the reduced 5-HT7 expression in Rett syndrome models, the systemic administration of 5-HT7 agonist relieved the related symptoms, anxiety, environment-related exploratory behaviour and motor learning ability of Rett syndrome mice models [184,185]. Administration of 5-HT7 agonist also restored the inactivation of Rho GTPases’ downstream effectors, such as cofilin and the p21-activated kinase family, which regulate actin cytoskeleton polymerisation in Rett syndrome mice models [184,185]. Furthermore, subchronic administration of 5-HT7 agonist improved refined phenotypic alterations, locomotor response and synapse potentiation compared to non-treated model mice [185]. These studies, using genetic animal models of Rett syndrome, Mecp2-308 mice [184,185], demonstrated that subchronic administration of 5-HT7 agonist displayed persistent effects related to Rett syndrome, a result of increasing motivation regarding the development of 5-HT7 agonists for the treatment of Rett syndrome.
Association between 5-HT receptor gene polymorphism and autism spectrum disorder could not be detected by a transmission disequilibrium study [186], whereas it has been recognized that several antipsychotics used to alleviate aggressive behaviours in autism spectrum disorders, such as aripiprazole, lurasidone, pimozide and risperidone, are 5-HT7 inhibitors [16,187,188,189]. Considered alongside preclinical findings, these discrepancies suggest that any current knowledge regarding pathophysiology and pathophysiology of autism spectrum disorder is inadequate.
6.2. Neurodegenerative Diseases
Non-selectively activation of 5-HT receptors using 5-HT transporter inhibitors has shown little clinical benefit in neurodegenerative disorders [190,191]; however, several preclinical studies reported the attractive findings that 5-HT7 is a therapeutic candidate target for neurodegenerative disorders. Administration of 5-HT7 agonist also suppressed impairment of long-term potentiation and apoptosis in hippocampal streptozotocin-mediated neurodegeneration in murine models [192]. Amyloid-β induces neurotoxicity through several mechanisms including apoptosis, excitotoxicity and oxidative stress [193]; these were reverted by selective 5-HT7 agonist [194]. Amyloid-β is a key player in pathomechanisms of various neurogenerative diseases, such as Alzheimer’s disease, frontotemporal dementia, cerebral amyloid angiopathy, and cerebral amyloidosis, through multiple pathways [193,195]; however, the detailed roles of 5-HT7 in pathomechanisms of neurodegenerative disease associated with amyloid-β remain to be clarified [196,197].
6.3. Epilepsy
Various studies have reported on antiseizure activities—using audiogenic seizures in DBA/2J mice, or the absence of seizures in WAG/Rij rats—following the administration of selective and non-selective antagonists [198,199]. These studies demonstrated that SB269970 and AS-19 decreased and increased the frequency of pilocarpine-induced temporal lobe epilepsy seizures in rat models, respectively [200]. The same report also demonstrated that expression of 5-HT7 in brain tissue extracted from patients with temporal lobe epilepsy and its expression in pilocarpine-induced temporal lobe epilepsy rat models increased in comparison to normal subjects [200]. These findings suggested the possibility that pharmacologically, 5-HT7 inhibitors possess a therapeutic potential for epilepsy. Contrary to pharmacological findings, 5-HT7 knockout mice were more sensitive to pentylenetetrazole, cocaine and NMDA-induced seizures compared to wild-types [201].
6.4. Cartinoma
There are some findings indicating that 5-HT7 modulation may be therapeutic targets for the treatment of cancer. Notably, it has been anticipated that 5-HT7 antagonists would be useful for the treatment of hepatocellular cancer and small-intestinal neuroendocrine neoplasms, since 5-HT7 contributes to hepatocyte proliferation [18,19,20]. Serotonergic function activates proliferation of hepatocellular cancer and the expression of β-catenin, which plays an important role in the pathomechanisms of hepatocellular cancer via the activation of 5-HT7. The expression of 5-HT7 increased in hepatocellular cancer cell lines, but the 5-HT7 antagonist SB258719 suppressed 5-HT-induced increase in β-catenin levels and cell viability. Furthermore, SB258719 also suppressed proliferation and tumour growth. When small-intestinal neuroendocrine neoplasms, which are tumours derived from enterochromaffin cells, metastasize to the liver, the liver produces 5-HT, resulting in hyperactivation of 5-HT7 in the liver [20]. Hyperactivation of 5-HT7 in the liver leads to secretion of the growth factor IGF-1, which accelerates proliferation of metastatic tumour cells. Based on these findings, 5-HT7 inhibition is considered to be a candidate target for the treatment of hepatic metastasis cancers. It has been reported that 5-HT7 expression increased in certain types of breast cancers; this increase was amplified with tumour grade [202]. Indeed, SB269970 suppressed tumour formation in an MDA-MB-231 cancer cell [21].
7. Impacts of 5-HT7 Inhibition on Metabolic Complication in Patients with Intake of Atypical Antipsychotics
The mortality gap between patients with schizophrenia and the general population has been growing [203]. Indeed, the life expectancy of patients with schizophrenia is 16 years shorter than the general population, with more than 30% of excess deaths attributable to metabolic complications [203]. The prevalence of obesity in patients with psychiatric disorders (up to 60%) has been reported to be twice that in the general population [204]. It has been established that the hyperactivation of AMPK in the hypothalamus, induced by H1 and 5-HT2A blockade, is involved in the pathophysiology of antipsychotic-induced weight gain and metabolic complication [205]. Conversely, activation of AMPK activity in the peripheral organs is one of the most established targets for treating insulin-resistant diabetes [206,207], whereas hypothalamic AMPK seems to play fundamental roles in regulating both sides of the energy balance equation (feeding and energy expenditure) in the entire body [206]. Blockade of several monoamine receptors, such as H1 and 5-HT2A, decreases inositol trisphosphate (IP3) synthesis, which enhances calcium-induced calcium release (CICR) via the activation of the IP3 receptor [208,209,210], leading to suppressed adenosine triphosphate (ATP) synthesis [208,211]. The relatively increasing AMP or decreasing ATP intracellular levels activate AMPK activity [211]. Indeed, antipsychotics with high-risk for weight gain, such as clozapine, olanzapine, quetiapine and zotepine (possessing high binding affinity to H1 and 5-HT2A), enhance hypothalamic AMPK signalling, whereas lower-risk antipsychotics, such as lurasidone and brexpiprazole (possessing high binding affinity to 5-HT2A but lower affinity to H1) suppress AMPK signalling [42,47,48,205,212,213]. Additionally, both lurasidone and brexpiprazole possess high binding affinity with 5-HT7 inhibitors and suppress cAMP synthesis and AMPK signalling [42,48].
The detailed mechanisms of the suppression of AMPK signalling induced by 5-HT7 inhibition remained to be clarified, but decreased EPAC signalling is predicted to be one of the key players. Considering the binding profile of clozapine, which also posssesses relatively high affinity to 5-HT7, 5-HT7 inhibition cannot fully suppress the weight gain induced by H1 inhibition, but, at the minimum, it may suppress the activation of AMPK activity induced by 5-HT2A inhibition.
8. Conclusions
The present review introduced the neuropharmacological findings of 5-HT7 modulation demonstrated by preclinical and clinical studies. The expected clinical effects of 5-HT7 inhibition on schizophrenia, mood and anxiety disorders by preclinical studies probably have not been able to contribute to the improvement of the core symptoms of these disorders; however, 5-HT7 inhibition possibly improves cognitive impairments in patients with schizophrenia and mood disorders compared to predictions from preclinical studies. Furthermore, it is suggested that 5-HT7 inhibition seems to have a mitigating effect on antipsychotic-induced weight gain and metabolic complication. Preclinical studies for the clinical application of 5-HT7 agonists for neurodevelopmental disorders and neurodegenerative diseases are currently underway. Until recently, several findings of these preclinical studies suggest that 5-HT7 modifications can serve two independent targets. The first is that 5-HT7 inhibition ameliorates the dysfunction of inter-neuronal transmission in mature networks. The other is that activation of 5-HT7 can improve transmission dysfunction due to microstructure abnormality in the neurotransmission network, which could not be affected by conventional therapeutic agents, via modulating intracellular signalling during the neurodevelopmental stage or through the loss of neural networks with aging. Therefore, the 5-HT7-modulating medications may be able to acquire a more practical treatment option via selectively using enhancement and suppression in an age- or pathological condition-dependent manner, rather than a binary choice.
Author Contributions
Conceptualization, M.O.; data curation, E.M., K.F. and M.O.; funding acquisition, M.O.; methodology, M.O.; project administration; M.O., validation, E.M. and M.O.; writing—original draft, M.O.; writing—review & editing, E.M., K.F. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Science (19K08073).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bard, J.A.; Zgombick, J.; Adham, N.; Vaysse, P.; Branchek, T.A.; Weinshank, R.L. Cloning of a novel human serotonin receptor (5-ht7) positively linked to adenylate cyclase. J. Biol. Chem. 1993, 268, 23422–23426. [Google Scholar] [CrossRef] [PubMed]
- Lovenberg, T.W.; Baron, B.M.; de Lecea, L.; Miller, J.D.; Prosser, R.A.; Rea, M.A.; Foye, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W. A novel adenylyl cyclase-activating serotonin receptor (5-ht7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993, 11, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ruat, M.; Traiffort, E.; Leurs, R.; Tardivel-Lacombe, J.; Diaz, J.; Arrang, J.-M.; Schwartz, J.-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-ht7) activating camp formation. Proc. Natl. Acad. Sci. USA 1993, 90, 8547–8551. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Monsma, F.; Metcalf, M.; Jose, P.; Hamblin, M.; Sibley, D. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993, 268, 18200–18204. [Google Scholar] [CrossRef]
- Blattner, K.M.; Canney, D.J.; Pippin, D.A.; Blass, B.E. Pharmacology and therapeutic potential of the 5-ht7 receptor. ACS Chem. Neurosci. 2018, 10, 89–119. [Google Scholar] [CrossRef]
- Gellynck, E.; Heyninck, K.; Andressen, K.W.; Haegeman, G.; Levy, F.O.; Vanhoenacker, P.; Van Craenenbroeck, K. The serotonin 5-ht 7 receptors: Two decades of research. Exp. Brain Res. 2013, 230, 555–568. [Google Scholar] [CrossRef]
- Matthys, A.; Haegeman, G.; Van Craenenbroeck, K.; Vanhoenacker, P. Role of the 5-ht7 receptor in the central nervous system: From current status to future perspectives. Mol. Neurobiol. 2011, 43, 228–253. [Google Scholar] [CrossRef]
- L’Estrade, E.T.; Erlandsson, M.; Edgar, F.G.; Ohlsson, T.; Knudsen, G.M.; Herth, M.M. Towards selective cns pet imaging of the 5-ht7 receptor system: Past, present and future. Neuropharmacology 2020, 172, 107830. [Google Scholar] [CrossRef]
- Lippiello, P.; Hoxha, E.; Speranza, L.; Volpicelli, F.; Ferraro, A.; Leopoldo, M.; Lacivita, E.; Perrone-Capano, C.; Tempia, F.; Miniaci, M.C. The 5-ht7 receptor triggers cerebellar long-term synaptic depression via pkc-mapk. Neuropharmacology 2016, 101, 426–438. [Google Scholar] [CrossRef]
- Volpicelli, F.; Speranza, L.; di Porzio, U.; Crispino, M.; Perrone-Capano, C. The serotonin receptor 7 and the structural plasticity of brain circuits. Front. Behav. Neurosci. 2014, 8, 318. [Google Scholar] [CrossRef]
- Gocho, Y.; Sakai, A.; Yanagawa, Y.; Suzuki, H.; Saitow, F. Electrophysiological and pharmacological properties of gabaergic cells in the dorsal raphe nucleus. J. Physiol. Sci. 2013, 63, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Okubo, R.; Shiroyama, T.; Ueda, Y. Lurasidone sub-chronically activates serotonergic transmission via desensitization of 5-ht1a and 5-ht7 receptors in dorsal raphe nucleus. Pharmaceuticals 2019, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Nativio, P.; Zoratto, F.; Romano, E.; Lacivita, E.; Leopoldo, M.; Pascale, E.; Passarelli, F.; Laviola, G.; Adriani, W. Stimulation of 5-ht 7 receptor during adolescence determines its persistent upregulation in adult rat forebrain areas. Synapse 2015, 69, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Aubert, Y.; Allers, K.A.; Sommer, B.; de Kloet, E.R.; Abbott, D.H.; Datson, N.A. Brain regionspecific transcriptomic markers of serotonin1a receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. J. Sex. Med. 2013, 10, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Zareifopoulos, N.; Papatheodoropoulos, C. Effects of 5-ht-7 receptor ligands on memory and cognition. Neurobiol. Learn. Mem. 2016, 136, 204–209. [Google Scholar] [CrossRef]
- Okubo, R.; Hasegawa, T.; Fukuyama, K.; Shiroyama, T.; Okada, M. Current limitations and candidate potential of 5-ht7 receptor antagonism in psychiatric pharmacotherapy. Front. Psychiatry 2021, 12, 623684. [Google Scholar] [CrossRef]
- Bonaventure, P.; Nepomuceno, D.; Hein, L.; Sutcliffe, J.G.; Lovenberg, T.; Hedlund, P.B. Radioligand binding analysis of knockout mice reveals 5-hydroxytryptamine(7) receptor distribution and uncovers 8-hydroxy-2-(di-n-propylamino)tetralin interaction with alpha(2) adrenergic receptors. Neuroscience 2004, 124, 901–911. [Google Scholar] [CrossRef]
- Fatima, S.; Shi, X.; Lin, Z.; Chen, G.Q.; Pan, X.H.; Wu, J.C.; Ho, J.W.; Lee, N.P.; Gao, H.; Zhang, G.; et al. 5-hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing beta-catenin. Mol. Oncol. 2016, 10, 195–212. [Google Scholar] [CrossRef]
- Tzirogiannis, K.N.; Kourentzi, K.T.; Zyga, S.; Papalimneou, V.; Tsironi, M.; Grypioti, A.D.; Protopsaltis, I.; Panidis, D.; Panoutsopoulos, G.I. Effect of 5-ht7 receptor blockade on liver regeneration after 60–70% partial hepatectomy. BMC Gastroenterol. 2014, 14, 201. [Google Scholar] [CrossRef]
- Svejda, B.; Kidd, M.; Timberlake, A.; Harry, K.; Kazberouk, A.; Schimmack, S.; Lawrence, B.; Pfragner, R.; Modlin, I.M. Serotonin and the 5-ht7 receptor: The link between hepatocytes, igf-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013, 104, 844–855. [Google Scholar] [CrossRef]
- Gautam, J.; Banskota, S.; Regmi, S.C.; Ahn, S.; Jeon, Y.H.; Jeong, H.; Kim, S.J.; Nam, T.G.; Jeong, B.S.; Kim, J.A. Tryptophan hydroxylase 1 and 5-ht(7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol. Cancer 2016, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.B.; Huitron-Resendiz, S.; Henriksen, S.J.; Sutcliffe, J.G. 5-ht7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 2005, 58, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Nikiforuk, A.; Stachowicz, K.; Tatarczynska, E. Effect of the selective 5-ht7 receptor antagonist sb 269970 in animal models of anxiety and depression. Neuropharmacology 2006, 51, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Furini, C.; Zinn, C.; Cavalcante, L.; Ferreira, F.; Behling, J.; Myskiw, J.; Izquierdo, I. Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiol. Learn. Mem. 2017, 142, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Huang, M.; Meltzer, H.Y. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J. Pharmacol. Exp. Ther. 2011, 338, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Spatuzza, M.; D’Antoni, S.; Bonaccorso, C.M.; Trovato, C.; Musumeci, S.A.; Leopoldo, M.; Lacivita, E.; Catania, M.V.; Ciranna, L. Activation of 5-ht7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and fmr1 knockout mice, a model of fragile x syndrome. Biol. Psychiatry 2012, 72, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Horisawa, T.; Tokuda, K.; Ishiyama, T.; Ogasa, M.; Tagashira, R.; Matsumoto, K.; Nishikawa, H.; Ueda, Y.; Toma, S.; et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-ht7) and 5-ht1a receptor activity. J. Pharmacol. Exp. Ther. 2010, 334, 171–181. [Google Scholar] [CrossRef]
- Shapiro, D.A.; Renock, S.; Arrington, E.; Chiodo, L.A.; Liu, L.X.; Sibley, D.R.; Roth, B.L.; Mailman, R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003, 28, 1400–1411. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Sahraeizadeh, A.; Berk, M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 2014, 45, 185–192. [Google Scholar] [CrossRef]
- Maeda, K.; Sugino, H.; Akazawa, H.; Amada, N.; Shimada, J.; Futamura, T.; Yamashita, H.; Ito, N.; McQuade, R.D.; Mork, A.; et al. Brexpiprazole i: In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014, 350, 589–604. [Google Scholar] [CrossRef]
- Su, T.P.; Malhotra, A.K.; Hadd, K.; Breier, A.; Pickar, D. D2 dopamine receptor occupancy: A crossover comparison of risperidone with clozapine therapy in schizophrenic patients. Arch. Gen. Psychiatry 1997, 54, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. The mechanism of action of novel antipsychotic drugs. Schizophr. Bull. 1991, 17, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Alonso, J.M.; Andres, J.I.; Cid, J.M.; Diaz, A.; Iturrino, L.; Gil, P.; Megens, A.; Sipido, V.K.; Trabanco, A.A. Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J. Med. Chem. 2005, 48, 1709–1712. [Google Scholar] [CrossRef]
- Elmaci, I.; Altinoz, M.A. Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit. Rev. Oncol./Hematol. 2018, 128, 96–109. [Google Scholar] [CrossRef]
- Lopez-Munoz, F.; Alamo, C. Active metabolites as antidepressant drugs: The role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front. Psychiatry 2013, 4, 102. [Google Scholar] [CrossRef]
- Smith, C.; Rahman, T.; Toohey, N.; Mazurkiewicz, J.; Herrick-Davis, K.; Teitler, M. Risperidone irreversibly binds to and inactivates the h5-ht7 serotonin receptor. Mol. Pharmacol. 2006, 70, 1264–1270. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.; Gommeren, W.; Luyten, W.H.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Bang-Andersen, B.; Ruhland, T.; Jorgensen, M.; Smith, G.; Frederiksen, K.; Jensen, K.G.; Zhong, H.; Nielsen, S.M.; Hogg, S.; Mork, A.; et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (lu aa21004): A novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011, 54, 3206–3221. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Okubo, R.; Fukuyama, K. Vortioxetine subchronically activates serotonergic transmission via desensitization of serotonin 5-ht1a receptor with 5-ht3 receptor inhibition in rats. Int. J. Mol. Sci. 2019, 20, 6235. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Ueda, Y. Lurasidone inhibits nmda/glutamate antagonist-induced functional abnormality of thalamocortical glutamatergic transmission via 5-ht7 receptor blockade. Br. J. Pharmacol. 2019, 176, 4002–4018. [Google Scholar] [CrossRef]
- Okada, M.; Matsumoto, R.; Yamamoto, Y.; Fukuyama, K. Effects of subchronic administrations of vortioxetine, lurasidone and escitalopram on thalamocortical glutamatergic transmission associated with serotonin 5-ht7 receptor. Int. J. Mol. Sci. 2021, 22, 1351. [Google Scholar] [CrossRef]
- Fukuyama, K.; Motomura, E.; Shiroyama, T.; Okada, M. Impact of 5-ht7 receptor inverse agonism of lurasidone on monoaminergic tripartite synaptic transmission and pathophysiology of lower risk of weight gain. Biomed. Pharmacother. 2022, 148, 112750. [Google Scholar] [CrossRef] [PubMed]
- Shiroyama, T.; Fukuyama, K.; Okada, M. Distinct effects of escitalopram and vortioxetine on astroglial l-glutamate release associated with connexin43. Int. J. Mol. Sci. 2021, 22, 10013. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Avramets, D.; Jeon, B.; Choo, H. Modulation of serotonin receptors in neurodevelopmental disorders: Focus on 5-ht7 receptor. Molecules 2021, 26, 3348. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Villegas, A.; Valdes-Ferrer, S.I. Central nervous system effects of 5-ht(7) receptors: A potential target for neurodegenerative diseases. Mol. Med. 2022, 28, 70. [Google Scholar] [CrossRef] [PubMed]
- Bijata, M.; Labus, J.; Guseva, D.; Stawarski, M.; Butzlaff, M.; Dzwonek, J.; Schneeberg, J.; Bohm, K.; Michaluk, P.; Rusakov, D.A.; et al. Synaptic remodeling depends on signaling between serotonin receptors and the extracellular matrix. Cell Rep. 2017, 19, 1767–1782. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Motomura, E. Dose-dependent biphasic action of quetiapine on ampk signalling via 5-ht7 receptor: Exploring pathophysiology of clinical and adverse effects of quetiapine. Int. J. Mol. Sci. 2022, 23, 9103. [Google Scholar] [CrossRef]
- Fukuyama, K.; Motomura, E.; Okada, M. Brexpiprazole reduces 5-ht7 receptor function on astroglial transmission systems. Int. J. Mol. Sci. 2022, 23, 6571. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.J. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996, 14, 87–96. [Google Scholar] [CrossRef]
- Belenky, M.A.; Pickard, G.E. Subcellular distribution of 5-ht1b and 5-ht7 receptors in the mouse suprachiasmatic nucleus. J. Comp. Neurol. 2001, 432, 371–388. [Google Scholar] [CrossRef]
- Rusak, B.; Zucker, I. Neural regulation of circadian rhythms. Physiol. Rev. 1979, 59, 449–526. [Google Scholar] [CrossRef] [PubMed]
- Mahe, C.; Loetscher, E.; Feuerbach, D.; Muller, W.; Seiler, M.P.; Schoeffter, P. Differential inverse agonist efficacies of sb-258719, sb-258741 and sb-269970 at human recombinant serotonin 5-ht7 receptors. Eur. J. Pharmacol. 2004, 495, 97–102. [Google Scholar] [CrossRef]
- Hagan, J.J.; Price, G.W.; Jeffrey, P.; Deeks, N.J.; Stean, T.; Piper, D.; Smith, M.I.; Upton, N.; Medhurst, A.D.; Middlemiss, D.N. Characterization of sb-269970-a, a selective 5-ht7 receptor antagonist. Br. J. Pharmacol. 2000, 130, 539–548. [Google Scholar] [CrossRef]
- Winokur, A.; Gary, K.A.; Rodner, S.; Rae-Red, C.; Fernando, A.T.; Szuba, M.P. Depression, sleep physiology, and antidepressant drugs. Depress. Anxiety 2001, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Guscott, M.; Bristow, L.J.; Hadingham, K.; Rosahl, T.W.; Beer, M.S.; Stanton, J.A.; Bromidge, F.; Owens, A.P.; Huscroft, I.; Myers, J.; et al. Genetic knockout and pharmacological blockade studies of the 5-ht7 receptor suggest therapeutic potential in depression. Neuropharmacology 2005, 48, 492–502. [Google Scholar] [CrossRef]
- Bonaventure, P.; Kelly, L.; Aluisio, L.; Shelton, J.; Lord, B.; Galici, R.; Miller, K.; Atack, J.; Lovenberg, T.W.; Dugovic, C. Selective blockade of 5-hydroxytryptamine (5-ht)7 receptors enhances 5-ht transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 2007, 321, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Tatarczynska, E.; Nikiforuk, A.; Chojnacka-Wojcik, E. Enhancement of the anti-immobility action of antidepressants by a selective 5-ht7 receptor antagonist in the forced swimming test in mice. Eur. J. Pharmacol. 2007, 555, 43–47. [Google Scholar] [CrossRef]
- Wesolowska, A.; Kowalska, M. Influence of serotonin 5-ht(7) receptor blockade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol. Rep. PR 2008, 60, 464–474. [Google Scholar]
- Mnie-Filali, O.; Faure, C.; Lambas-Senas, L.; El Mansari, M.; Belblidia, H.; Gondard, E.; Etievant, A.; Scarna, H.; Didier, A.; Berod, A.; et al. Pharmacological blockade of 5-ht7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 2011, 36, 1275–1288. [Google Scholar] [CrossRef]
- Wesolowska, A.; Nikiforuk, A.; Stachowicz, K. Potential anxiolytic and antidepressant effects of the selective 5-ht7 receptor antagonist sb 269970 after intrahippocampal administration to rats. Eur. J. Pharmacol. 2006, 553, 185–190. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Du, C.X.; Tan, H.H.; Zhang, L.; Li, L.B.; Zhang, J.; Niu, X.L.; Liu, J. Activation and blockade of serotonin7 receptors in the prelimbic cortex regulate depressive-like behaviors in a 6-hydroxydopamine-induced parkinson’s disease rat model. Neuroscience 2015, 311, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Han, L.N.; Zhang, L.; Sun, Y.N.; Du, C.X.; Zhang, Y.M.; Wang, T.; Zhang, J.; Liu, J. Serotonin7 receptors in the lateral habenular nucleus regulate depressive-like behaviors in the hemiparkinsonian rats. Brain Res. 2016, 1644, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.L.; Noble, J.; Seckl, J.R. Acute restraint stress increases 5-ht7 receptor mrna expression in the rat hippocampus. Neurosci. Lett. 2001, 309, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Krucker, T.; Levy, C.L.; Slanina, K.A.; Sutcliffe, J.G.; Hedlund, P.B. Mice lacking 5-ht receptors show specific impairments in contextual learning. Eur. J. Neurosci. 2004, 19, 1913–1922. [Google Scholar] [CrossRef]
- Clemett, D.A.; Cockett, M.I.; Marsden, C.A.; Fone, K.C. Antisense oligonucleotide-induced reduction in 5-hydroxytryptamine7 receptors in the rat hypothalamus without alteration in exploratory behaviour or neuroendocrine function. J. Neurochem. 1998, 71, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.B.; Sutcliffe, J.G. The 5-ht7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci. Lett. 2007, 414, 247–251. [Google Scholar] [CrossRef]
- Semenova, S.; Geyer, M.A.; Sutcliffe, J.G.; Markou, A.; Hedlund, P.B. Inactivation of the 5-ht(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol. Psychiatry 2008, 63, 98–105. [Google Scholar] [CrossRef]
- Waters, K.A.; Stean, T.O.; Hammond, B.; Virley, D.J.; Upton, N.; Kew, J.N.; Hussain, I. Effects of the selective 5-ht(7) receptor antagonist sb-269970 in animal models of psychosis and cognition. Behav. Brain Res. 2012, 228, 211–218. [Google Scholar] [CrossRef]
- Galici, R.; Boggs, J.D.; Miller, K.L.; Bonaventure, P.; Atack, J.R. Effects of sb-269970, a 5-ht7 receptor antagonist, in mouse models predictive of antipsychotic-like activity. Behav. Pharmacol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kos, T.; Fijal, K.; Holuj, M.; Rafa, D.; Popik, P. Effects of the selective 5-ht7 receptor antagonist sb-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS ONE 2013, 8, e66695. [Google Scholar] [CrossRef]
- Pouzet, B. Sb-258741: A 5-ht7 receptor antagonist of potential clinical interest. CNS Drug Rev. 2002, 8, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liy-Salmeron, G.; Meneses, A. Effects of 5-ht drugs in prefrontal cortex during memory formation and the ketamine amnesia-model. Hippocampus 2008, 18, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A. Effects of the 5-ht7 receptor antagonists sb-269970 and dr 4004 in autoshaping pavlovian/instrumental learning task. Behav. Brain Res. 2004, 155, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, G.S.; Meneses, A. Effects of the potential 5-ht7 receptor agonist as 19 in an autoshaping learning task. Behav. Brain Res. 2005, 163, 136–140. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Holuj, M.; Potasiewicz, A.; Popik, P. Effects of the selective 5-ht7 receptor antagonist sb-269970 on premature responding in the five-choice serial reaction time test in rats. Behav. Brain Res. 2015, 289, 149–156. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Warden, D.; Trivedi, M.H.; Wisniewski, S.R.; Fava, M.; Rush, A.J. What did star*d teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr. Serv. 2009, 60, 1439–1445. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. Nmda receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Sajatovic, M.; Forester, B.P.; Tsai, J.; Kroger, H.; Pikalov, A.; Cucchiaro, J.; Loebel, A. Efficacy of lurasidone in adults aged 55 years and older with bipolar depression: Post hoc analysis of 2 double-blind, placebo-controlled studies. J. Clin. Psychiatry 2016, 77, e1324–e1331. [Google Scholar] [CrossRef]
- Suppes, T.; Silva, R.; Cucchiaro, J.; Mao, Y.; Targum, S.; Streicher, C.; Pikalov, A.; Loebel, A. Lurasidone for the treatment of major depressive disorder with mixed features: A randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 2016, 173, 400–407. [Google Scholar] [CrossRef]
- Tsai, J.; Thase, M.E.; Mao, Y.; Ng-Mak, D.; Pikalov, A.; Loebel, A. Lurasidone for major depressive disorder with mixed features and anxiety: A post-hoc analysis of a randomized, placebo-controlled study. CNS Spectr. 2017, 22, 236–245. [Google Scholar] [CrossRef]
- Ostacher, M.; Ng-Mak, D.; Patel, P.; Ntais, D.; Schlueter, M.; Loebel, A. Lurasidone compared to other atypical antipsychotic monotherapies for bipolar depression: A systematic review and network meta-analysis. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2018, 19, 586–601. [Google Scholar] [CrossRef]
- Vieta, E.; Florea, I.; Schmidt, S.N.; Areberg, J.; Ettrup, A. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: A 2-week, randomized, double-blind, placebo-controlled study. Int. Clin. Psychopharmacol. 2019, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Areberg, J.; Petersen, K.B.; Chen, G.; Naik, H. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin. Pharmacol. Toxicol. 2014, 115, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Souery, D.; Oswald, P.; Massat, I.; Bailer, U.; Bollen, J.; Demyttenaere, K.; Kasper, S.; Lecrubier, Y.; Montgomery, S.; Serretti, A.; et al. Clinical factors associated with treatment resistance in major depressive disorder: Results from a european multicenter study. J. Clin. Psychiatry 2007, 68, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Rush, A.J.; Alpert, J.E.; Balasubramani, G.K.; Wisniewski, S.R.; Carmin, C.N.; Biggs, M.M.; Zisook, S.; Leuchter, A.; Howland, R.; et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A star*d report. Am. J. Psychiatry 2008, 165, 342–351. [Google Scholar] [CrossRef]
- Yee, A.; Ng, C.G.; Seng, L.H. Vortioxetine treatment for anxiety disorder: A meta-analysis study. Curr. Drug Targets 2018, 19, 1412–1423. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Florea, I.; Jacobsen, P.L.; Zhong, W.; Nomikos, G.G. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (mdd) and high levels of anxiety symptoms. J. Affect. Disord. 2016, 206, 140–150. [Google Scholar] [CrossRef]
- Fu, J.; Peng, L.; Li, X. The efficacy and safety of multiple doses of vortioxetine for generalized anxiety disorder: A meta-analysis. Neuropsychiatr. Dis. Treat. 2016, 12, 951–959. [Google Scholar] [CrossRef]
- Qin, B.; Huang, G.; Yang, Q.; Zhao, M.; Chen, H.; Gao, W.; Yang, M. Vortioxetine treatment for generalised anxiety disorder: A meta-analysis of anxiety, quality of life and safety outcomes. BMJ Open 2019, 9, e033161. [Google Scholar] [CrossRef]
- Kong, W.; Deng, H.; Wan, J.; Zhou, Y.; Zhou, Y.; Song, B.; Wang, X. Comparative remission rates and tolerability of drugs for generalised anxiety disorder: A systematic review and network meta-analysis of double-blind randomized controlled trials. Front. Pharmacol. 2020, 11, 580858. [Google Scholar] [CrossRef]
- Meza, N.; Leyton, F. Vortioxetine for generalised anxiety disorder in adults. Medwave 2021, 21, e8172. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Lophaven, S.; Olsen, C.K. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int. J. Neuropsychopharmacol. 2014, 17, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Mahableshwarkar, A.R.; Zajecka, J.; Jacobson, W.; Chen, Y.; Keefe, R.S. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 2015, 40, 2025–2037. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Florea, I.; Tonnoir, B.; Loft, H.; Lam, R.W.; Christensen, M.C. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J. Clin. Psychiatry 2017, 78, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Brignone, M.; Larsen, K.G. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (dsst) as a measure of cognitive dysfunction in patients with major depressive disorder. Int. J. Neuropsychopharmacol. 2018, 21, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Lophaven, S.; Olsen, C.K. Which cognitive domains are improved by treatment with vortioxetine? Int. J. Neuropsychopharmacol. 2016, 19, pyw054. [Google Scholar] [CrossRef]
- Chokka, P.; Bougie, J.; Proulx, J.; Tvistholm, A.H.; Ettrup, A. Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: Results from the atworc study. CNS Spectr. 2019, 24, 616–627. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Backers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Krause, M.; Zhu, Y.; Huhn, M.; Schneider-Thoma, J.; Bighelli, I.; Chaimani, A.; Leucht, S. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: A network meta-analysis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2018, 28, 659–674. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Iwata, N. Efficacy and safety of antipsychotic treatments for schizophrenia: A systematic review and network meta-analysis of randomized trials in japan. J. Psychiatr. Res. 2021, 138, 444–452. [Google Scholar] [CrossRef]
- Phalguni, A.; McCool, R.; Wood, H.; Sanderson, A.; Rydevik, G.; Franklin, B.; James, D. Systematic literature review and network meta-analysis of lurasidone, brexpiprazole and cariprazine for schizophrenia. Int. Clin. Psychopharmacol. 2023, 38, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Ermacora, D.; Stephenson, C.; Hawken, E.R.; Vazquez, G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: A systematic review and network meta-analysis. J. Affect. Disord. 2020, 269, 154–184. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Mishima, K.; Iwata, N. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: A systematic review and network meta-analysis of randomized controlled trials. Mol. Psychiatry 2021, 26, 4146–4157. [Google Scholar] [CrossRef]
- Loebel, A.; Cucchiaro, J.; Silva, R.; Kroger, H.; Hsu, J.; Sarma, K.; Sachs, G. Lurasidone monotherapy in the treatment of bipolar i depression: A randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 2014, 171, 160–168. [Google Scholar] [CrossRef]
- Loebel, A.; Cucchiaro, J.; Silva, R.; Kroger, H.; Sarma, K.; Xu, J.; Calabrese, J.R. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar i depression: A randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 2014, 171, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ishigooka, J.; Miyajima, M.; Watabe, K.; Fujimori, T.; Masuda, T.; Higuchi, T.; Vieta, E. Double-blind, placebo-controlled study of lurasidone monotherapy for the treatment of bipolar i depression. Psychiatry Clin. Neurosci. 2020, 74, 635–644. [Google Scholar] [CrossRef] [PubMed]
- DelBello, M.P.; Goldman, R.; Phillips, D.; Deng, L.; Cucchiaro, J.; Loebel, A. Efficacy and safety of lurasidone in children and adolescents with bipolar i depression: A double-blind, placebo-controlled study. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Share, D.B.; Jayathilake, K.; Salomon, R.M.; Lee, M.A. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J. Clin. Psychopharmacol. 2020, 40, 240–249. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Seeberg, I.; Jensen, M.B.; Balanza-Martinez, V.; Del Mar Bonnin, C.; Bowie, C.R.; Carvalho, A.F.; Dols, A.; Douglas, K.; Gallagher, P.; et al. Randomised controlled cognition trials in remitted patients with mood disorders published between 2015 and 2021: A systematic review by the international society for bipolar disorders targeting cognition task force. Bipolar. Disord. 2022, 24, 354–374. [Google Scholar] [CrossRef]
- Srisurapanont, M.; Suttajit, S.; Likhitsathian, S.; Maneeton, B.; Maneeton, N. A network meta-analysis of the dose-response effects of lurasidone on acute schizophrenia. Sci. Rep. 2021, 11, 5571. [Google Scholar] [CrossRef]
- Leucht, S.; Crippa, A.; Siafis, S.; Patel, M.X.; Orsini, N.; Davis, J.M. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am. J. Psychiatry 2020, 177, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, L.; Wang, H.L.; Wang, G.H. Efficacy and safety of lurasidone versus placebo as adjunctive to mood stabilizers in bipolar i depression: A meta-analysis. J. Affect. Disord. 2020, 264, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, D.E.; Szot, P.; Kohen, R.; Hamblin, M.W. Function and distribution of three rat 5-hydroxytryptamine7 (5-ht7) receptor isoforms produced by alternative splicing. Neuropharmacology 1998, 37, 1621–1632. [Google Scholar] [CrossRef]
- Heidmann, D.E.; Metcalf, M.A.; Kohen, R.; Hamblin, M.W. Four 5-hydroxytryptamine7 (5-ht7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron-exon organization. J. Neurochem. 1997, 68, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Krobert, K.A.; Bach, T.; Syversveen, T.; Kvingedal, A.M.; Levy, F.O. The cloned human 5-ht7 receptor splice variants: A comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Andressen, K.W.; Manfra, O.; Brevik, C.H.; Ulsund, A.H.; Vanhoenacker, P.; Levy, F.O.; Krobert, K.A. The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-ht7 receptors. Br. J. Pharmacol. 2015, 172, 3846–3860. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, C.R.; Murray, A.T.; Franklin, A.A.; Hamblin, M.W. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J. Pharmacol. Exp. Ther. 2005, 313, 1003–1010. [Google Scholar] [CrossRef]
- Kvachnina, E.; Liu, G.; Dityatev, A.; Renner, U.; Dumuis, A.; Richter, D.W.; Dityateva, G.; Schachner, M.; Voyno-Yasenetskaya, T.A.; Ponimaskin, E.G. 5-ht7 receptor is coupled to g alpha subunits of heterotrimeric g12-protein to regulate gene transcription and neuronal morphology. J. Neurosci. 2005, 25, 7821–7830. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.P.; Nielsen, M.D.; Impey, S.; Metcalf, M.A.; Poser, S.W.; Chan, G.; Obrietan, K.; Hamblin, M.W.; Storm, D.R. Stimulation of type 1 and type 8 ca2+/calmodulin-sensitive adenylyl cyclases by the gs-coupled 5-hydroxytryptamine subtype 5-ht7a receptor. J Biol Chem 1998, 273, 17469–17476. [Google Scholar] [CrossRef]
- Grimes, M.T.; Powell, M.; Gutierrez, S.M.; Darby-King, A.; Harley, C.W.; McLean, J.H. Epac activation initiates associative odor preference memories in the rat pup. Learn. Mem. 2015, 22, 74–82. [Google Scholar] [CrossRef]
- Lin, S.L.; Johnson-Farley, N.N.; Lubinsky, D.R.; Cowen, D.S. Coupling of neuronal 5-ht7 receptors to activation of extracellular-regulated kinase through a protein kinase a-independent pathway that can utilize epac. J. Neurochem. 2003, 87, 1076–1085. [Google Scholar] [CrossRef]
- Speranza, L.; Chambery, A.; Di Domenico, M.; Crispino, M.; Severino, V.; Volpicelli, F.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; Perrone-Capano, C. The serotonin receptor 7 promotes neurite outgrowth via erk and cdk5 signaling pathways. Neuropharmacology 2013, 67, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Sardone, L.M.; Bonaccorso, C.M.; D’Antoni, S.; Spatuzza, M.; Gulisano, W.; Tropea, M.R.; Puzzo, D.; Leopoldo, M.; Lacivita, E.; et al. Activation of serotonin 5-ht7 receptors modulates hippocampal synaptic plasticity by stimulation of adenylate cyclases and rescues learning and behavior in a mouse model of fragile x syndrome. Front. Mol. Neurosci. 2018, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-related changes in ampk activation: Role for ampk phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.P.; Springborn, S.; Mitchell, G.S. Spinal 5-ht7 receptors induce phrenic motor facilitation via epac-mtorc1 signaling. J. Neurophysiol. 2015, 114, 2015–2022. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Pravir, K. Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell. Mol. Life Sci. 2021, 78, 8001–8047. [Google Scholar] [CrossRef]
- Speranza, L.; Giuliano, T.; Volpicelli, F.; De Stefano, M.E.; Lombardi, L.; Chambery, A.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; et al. Activation of 5-ht7 receptor stimulates neurite elongation through mtor, cdc42 and actin filaments dynamics. Front. Behav. Neurosci. 2015, 9, 62. [Google Scholar] [CrossRef]
- Ponimaskin, E.; Voyno-Yasenetskaya, T.; Richter, D.W.; Schachner, M.; Dityatev, A. Morphogenic signaling in neurons via neurotransmitter receptors and small gtpases. Mol. Neurobiol. 2007, 35, 278–287. [Google Scholar] [CrossRef]
- Wirth, A.; Holst, K.; Ponimaskin, E. How serotonin receptors regulate morphogenic signalling in neurons. Prog. Neurobiol. 2017, 151, 35–56. [Google Scholar] [CrossRef]
- Crispino, M.; Volpicelli, F.; Perrone-Capano, C. Role of the serotonin receptor 7 in brain plasticity: From development to disease. Int. J. Mol. Sci. 2020, 21, 505. [Google Scholar] [CrossRef]
- Ibata, K.; Sun, Q.; Turrigiano, G.G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 2008, 57, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Hübener, M.; Bonhoeffer, T. Neuronal plasticity: Beyond the critical period. Cell 2014, 159, 727–737. [Google Scholar] [CrossRef]
- Kobe, F.; Guseva, D.; Jensen, T.P.; Wirth, A.; Renner, U.; Hess, D.; Muller, M.; Medrihan, L.; Zhang, W.; Zhang, M.; et al. 5-ht7r/g12 signaling regulates neuronal morphology and function in an age-dependent manner. J. Neurosci. 2012, 32, 2915–2930. [Google Scholar] [CrossRef]
- Beaudet, G.; Jozet-Alves, C.; Asselot, R.; Schumann-Bard, P.; Freret, T.; Boulouard, M.; Paizanis, E. Deletion of the serotonin receptor type 7 disrupts the acquisition of allocentric but not egocentric navigation strategies in mice. Behav. Brain Res. 2017, 320, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vasefi, M.S.; Yang, K.; Li, J.; Kruk, J.S.; Heikkila, J.J.; Jackson, M.F.; MacDonald, J.F.; Beazely, M.A. Acute 5-ht7 receptor activation increases nmda-evoked currents and differentially alters nmda receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol. Brain 2013, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Bacon, W.L.; Beck, S.G. 5-hydroxytryptamine(7) receptor activation decreases slow afterhyperpolarization amplitude in ca3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 2000, 294, 672–679. [Google Scholar] [PubMed]
- Tokarski, K.; Zahorodna, A.; Bobula, B.; Hess, G. 5-ht7 receptors increase the excitability of rat hippocampal ca1 pyramidal neurons. Brain Res 2003, 993, 230–234. [Google Scholar] [CrossRef]
- Costa, L.; Trovato, C.; Musumeci, S.A.; Catania, M.V.; Ciranna, L. 5-ht(1a) and 5-ht(7) receptors differently modulate ampa receptor-mediated hippocampal synaptic transmission. Hippocampus 2012, 22, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Andreetta, F.; Carboni, L.; Grafton, G.; Jeggo, R.; Whyment, A.D.; van den Top, M.; Hoyer, D.; Spanswick, D.; Barnes, N.M. Hippocampal 5-ht7 receptors signal phosphorylation of the glua1 subunit to facilitate ampa receptor mediated-neurotransmission in vitro and in vivo. Br. J. Pharmacol. 2016, 173, 1438–1451. [Google Scholar] [CrossRef]
- Leo, D.; Adriani, W.; Cavaliere, C.; Cirillo, G.; Marco, E.; Romano, E.; Di Porzio, U.; Papa, M.; Perrone-Capano, C.; Laviola, G. Methylphenidate to adolescent rats drives enduring changes of accumbal htr7 expression: Implications for impulsive behavior and neuronal morphology. Genes Brain Behav. 2009, 8, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, M.S.; Morelli, E.; Gingrich, J.A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 2008, 28, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, G.; Bouet, V.; Jozet-Alves, C.; Schumann-Bard, P.; Dauphin, F.; Paizanis, E.; Boulouard, M.; Freret, T. Spatial memory deficit across aging: Current insights of the role of 5-ht7 receptors. Front. Behav. Neurosci. 2014, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-O.; Ip, N.Y. Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2257–2263. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Parra-Bueno, P.; Laviv, T.; Szatmari, E.M.; Lee, S.-J.R.; Yasuda, R. Camkii autophosphorylation is necessary for optimal integration of Ca2+ signals during ltp induction, but not maintenance. Neuron 2017, 94, 800–808.e804. [Google Scholar] [CrossRef]
- Bosch, M.; Hayashi, Y. Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 2012, 22, 383–388. [Google Scholar] [CrossRef]
- Rusheen, A.E.; Gee, T.A.; Jang, D.P.; Blaha, C.D.; Bennet, K.E.; Lee, K.H.; Heien, M.L.; Oh, Y. Evaluation of electrochemical methods for tonic dopamine detection in vivo. Trends Analyt. Chem. 2020, 132, 116049. [Google Scholar] [CrossRef]
- Fukuyama, K.; Fukuzawa, M.; Shiroyama, T.; Okada, M. Pathomechanism of nocturnal paroxysmal dystonia in autosomal dominant sleep-related hypermotor epilepsy with s284l-mutant alpha4 subunit of nicotinic ach receptor. Biomed. Pharmacother. 2020, 126, 110070. [Google Scholar] [CrossRef]
- Fukuyama, K.; Nakano, T.; Shiroyama, T.; Okada, M. Chronic administrations of guanfacine on mesocortical catecholaminergic and thalamocortical glutamatergic transmissions. Int. J. Mol. Sci. 2021, 22, 4122. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Serotonergic mechanisms as targets for existing and novel antipsychotics. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2012; pp. 87–124. [Google Scholar]
- Yamamura, S.; Ohoyama, K.; Hamaguchi, T.; Kashimoto, K.; Nakagawa, M.; Kanehara, S.; Suzuki, D.; Matsumoto, T.; Motomura, E.; Shiroyama, T.; et al. Effects of quetiapine on monoamine, gaba, and glutamate release in rat prefrontal cortex. Psychopharmacology 2009, 206, 243–258. [Google Scholar] [CrossRef]
- Yamamura, S.; Ohoyama, K.; Hamaguchi, T.; Nakagawa, M.; Suzuki, D.; Matsumoto, T.; Motomura, E.; Tanii, H.; Shiroyama, T.; Okada, M. Effects of zotepine on extracellular levels of monoamine, gaba and glutamate in rat prefrontal cortex. Br. J. Pharmacol. 2009, 157, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ohoyama, K.; Yamamura, S.; Hamaguchi, T.; Nakagawa, M.; Motomura, E.; Shiroyama, T.; Tanii, H.; Okada, M. Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur. J. Pharmacol. 2011, 653, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, S.; Yamamura, S.; Nakagawa, M.; Motomura, E.; Okada, M. Dopamine d2 and serotonin 5-ht1a receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology 2012, 62, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Bourdelais, A.J.; Deutch, A.Y. The effects of haloperidol and clozapine on extracellular gaba levels in the prefrontal cortex of the rat: An in vivo microdialysis study. Cereb. Cortex 1994, 4, 69–77. [Google Scholar] [CrossRef]
- Fukuyama, K.; Hasegawa, T.; Okada, M. Cystine/glutamate antiporter and aripiprazole compensate nmda antagonist-induced dysfunction of thalamocortical l-glutamatergic transmission. Int. J. Mol. Sci. 2018, 19, 3645. [Google Scholar] [CrossRef]
- Fukuyama, K.; Kato, R.; Murata, M.; Shiroyama, T.; Okada, M. Clozapine normalizes a glutamatergic transmission abnormality induced by an impaired nmda receptor in the thalamocortical pathway via the activation of a group iii metabotropic glutamate receptor. Biomolecules 2019, 9, 234. [Google Scholar] [CrossRef]
- Nakano, T.; Hasegawa, T.; Suzuki, D.; Motomura, E.; Okada, M. Amantadine combines astroglial system xc(-) activation with glutamate/nmda receptor inhibition. Biomolecules 2019, 9, 191. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Ueda, Y. Memantine protects thalamocortical hyper-glutamatergic transmission induced by nmda receptor antagonism via activation of system xc(). Pharmacol. Res. Perspect. 2019, 7, e00457. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Nakano, T.; Ueda, Y. Pharmacological discrimination of effects of mk801 on thalamocortical, mesothalamic, and mesocortical transmissions. Biomolecules 2019, 9, 746. [Google Scholar] [CrossRef]
- Fukuyama, K.; Fukuzawa, M.; Okada, M. Upregulated and hyperactivated thalamic connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with s284l-mutant alpha4 subunit of nicotinic ach receptor. Pharmaceuticals 2020, 13, 99. [Google Scholar] [CrossRef]
- Fukuyama, K.; Fukuzawa, M.; Okubo, R.; Okada, M. Upregulated connexin 43 induced by loss-of-functional s284l-mutant alpha4 subunit of nicotinic ach receptor contributes to pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Pharmaceuticals 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Fukuzawa, M.; Shiroyama, T.; Okada, M. Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with s284l-mutant alpha4 subunit of nicotinic ach receptor. Br. J. Pharmacol. 2020, 177, 2143–2162. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Suzuki, D.; Ueda, Y. Effects of acute and sub-chronic administrations of guanfacine on catecholaminergic transmissions in the orbitofrontal cortex. Neuropharmacology 2019, 156, 107547. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K. Interaction between mesocortical and mesothalamic catecholaminergic transmissions associated with nmda receptor in the locus coeruleus. Biomolecules 2020, 10, 990. [Google Scholar] [CrossRef]
- Hwang, K.; Shine, J.M.; Bruss, J.; Tranel, D.; Boes, A. Neuropsychological evidence of multi-domain network hubs in the human thalamus. eLife 2021, 10, e69480. [Google Scholar] [CrossRef]
- Hwang, K.; Shine, J.M.; Cole, M.W.; Sorenson, E. Thalamocortical contributions to cognitive task activity. eLife 2022, 11, e81282. [Google Scholar] [CrossRef] [PubMed]
- Reber, J.; Hwang, K.; Bowren, M.; Bruss, J.; Mukherjee, P.; Tranel, D.; Boes, A.D. Cognitive impairment after focal brain lesions is better predicted by damage to structural than functional network hubs. Proc. Natl. Acad. Sci. USA 2021, 118, e2018784118. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. High frequency oscillations play important roles in development of epileptogenesis/ictogenesis via activation of astroglial signallings. Biomed. Pharmacother. 2022, 149, 112846. [Google Scholar] [CrossRef]
- Fernandez, L.M.J.; Luthi, A. Sleep spindles: Mechanisms and functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Brivaracetam and levetiracetam suppress astroglial l-glutamate release through hemichannel via inhibition of synaptic vesicle protein. Int. J. Mol. Sci. 2022, 23, 4473. [Google Scholar] [CrossRef]
- Yang, M.; Logothetis, N.K.; Eschenko, O. Occurrence of hippocampal ripples is associated with activity suppression in the mediodorsal thalamic nucleus. J. Neurosci. 2019, 39, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Can rodent models elucidate the pathomechanisms of genetic epilepsy? Br. J. Pharmacol. 2022, 179, 1620–1639. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kawano, Y.; Fukuyama, K.; Motomura, E.; Shiroyama, T. Candidate strategies for development of a rapid-acting antidepressant class without neuropsychiatric adverse effects: Prevention of ketamine-induced neuropsychiatric adverse reactions. Int. J. Mol. Sci. 2020, 21, 7951. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Murata, M. A working hypothesis regarding identical pathomechanisms between clinical efficacy and adverse reaction of clozapine via the activation of connexin43. Int. J. Mol. Sci. 2020, 21, 7019. [Google Scholar] [CrossRef]
- Maurin, T.; Zongaro, S.; Bardoni, B. Fragile x syndrome: From molecular pathology to therapy. Neurosci. Biobehav. Rev. 2014, 46 Pt 2, 242–255. [Google Scholar] [CrossRef]
- Osterweil, E.K.; Krueger, D.D.; Reinhold, K.; Bear, M.F. Hypersensitivity to mglur5 and erk1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile x syndrome. J. Neurosci. 2010, 30, 15616–15627. [Google Scholar] [CrossRef]
- Huber, K.M.; Gallagher, S.M.; Warren, S.T.; Bear, M.F. Altered synaptic plasticity in a mouse model of fragile x mental retardation. Proc. Natl. Acad. Sci. USA 2002, 99, 7746–7750. [Google Scholar] [CrossRef]
- Kelley, D.J.; Davidson, R.J.; Elliott, J.L.; Lahvis, G.P.; Yin, J.C.; Bhattacharyya, A. The cyclic amp cascade is altered in the fragile x nervous system. PLoS ONE 2007, 2, e931. [Google Scholar] [CrossRef]
- Fu, C.; Armstrong, D.; Marsh, E.; Lieberman, D.; Motil, K.; Witt, R.; Standridge, S.; Nues, P.; Lane, J.; Dinkel, T.; et al. Consensus guidelines on managing rett syndrome across the lifespan. BMJ Paediatr. Open 2020, 4, e000717. [Google Scholar] [CrossRef]
- Neul, J.L.; Fang, P.; Barrish, J.; Lane, J.; Caeg, E.B.; Smith, E.O.; Zoghbi, H.; Percy, A.; Glaze, D.G. Specific mutations in methyl-cpg-binding protein 2 confer different severity in rett syndrome. Neurology 2008, 70, 1313–1321. [Google Scholar] [CrossRef]
- Kulikovskaja, L.; Sarajlija, A.; Savic-Pavicevic, D.; Dobricic, V.; Klein, C.; Westenberger, A. Wdr45 mutations may cause a mecp2 mutation-negative rett syndrome phenotype. Neurol. Genet. 2018, 4, e227. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.L.; Levenson, J.M.; Vilaythong, A.P.; Richman, R.; Armstrong, D.L.; Noebels, J.L.; David Sweatt, J.; Zoghbi, H.Y. Mild overexpression of mecp2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 2004, 13, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Nativio, P.; Fabbri, A.; Ricceri, L.; Adriani, W.; Lacivita, E.; Leopoldo, M.; Passarelli, F.; Fuso, A.; Laviola, G. Pharmacological stimulation of the brain serotonin receptor 7 as a novel therapeutic approach for rett syndrome. Neuropsychopharmacology 2014, 39, 2506–2518. [Google Scholar] [CrossRef]
- De Filippis, B.; Chiodi, V.; Adriani, W.; Lacivita, E.; Mallozzi, C.; Leopoldo, M.; Domenici, M.R.; Fuso, A.; Laviola, G. Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling rett syndrome. Front. Behav. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Lassig, J.P.; Vachirasomtoon, K.; Hartzell, K.; Leventhal, M.; Courchesne, E.; Courchesne, R.; Lord, C.; Leventhal, B.L.; Cook, E.H., Jr. Physical mapping of the serotonin 5-ht(7) receptor gene (htr7) to chromosome 10 and pseudogene (htr7p) to chromosome 12, and testing of linkage disequilibrium between htr7 and autistic disorder. Am. J. Med. Genet. 1999, 88, 472–475. [Google Scholar] [CrossRef]
- Blankenship, K.; Erickson, C.A.; Stigler, K.A.; Posey, D.J.; McDougle, C.J. Aripiprazole for irritability associated with autistic disorder in children and adolescents aged 6-17 years. Ped Health 2010, 4, 375–381. [Google Scholar] [CrossRef]
- Knight, J.A.; Smith, C.; Toohey, N.; Klein, M.T.; Teitler, M. Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-hydroxytryptamine7 receptor by risperidone, 9-oh-risperidone, and other inactivating antagonists. Mol. Pharmacol. 2009, 75, 374–380. [Google Scholar] [CrossRef]
- Loebel, A.; Brams, M.; Goldman, R.S.; Silva, R.; Hernandez, D.; Deng, L.; Mankoski, R.; Findling, R.L. Lurasidone for the treatment of irritability associated with autistic disorder. J. Autism. Dev. Disord. 2016, 46, 1153–1163. [Google Scholar] [CrossRef]
- Hull, M.; Berger, M.; Heneka, M. Disease-modifying therapies in alzheimer’s disease: How far have we come? Drugs 2006, 66, 2075–2093. [Google Scholar]
- Lalut, J.; Karila, D.; Dallemagne, P.; Rochais, C. Modulating 5-ht(4) and 5-ht(6) receptors in alzheimer’s disease treatment. Future Med. Chem. 2017, 9, 781–795. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Komaki, A.; Soleimani Asl, S.; Shahidi, S. The effects of the 5-ht7 receptor on hippocampal long-term potentiation and apoptosis in a rat model of alzheimer’s disease. Brain Res. Bull. 2017, 135, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Villegas, A.Q.; Manzo, H.S.A.; Almada, M.O.V.; Modragon, C.B.; Guzman, R.G. Procognitive and neuroprotective effect of 5-ht7 agonist in an animal model by icv amyloid-b injection (p1.1-005). Neurology 2019, 92, P1.1-005. [Google Scholar]
- Miller-Thomas, M.M.; Sipe, A.L.; Benzinger, T.L.; McConathy, J.; Connolly, S.; Schwetye, K.E. Multimodality review of amyloid-related diseases of the central nervous system. Radiographics 2016, 36, 1147–1163. [Google Scholar] [CrossRef]
- Meneses, A. Neural activity, memory, and dementias: Serotonergic markers. Behav. Pharmacol. 2017, 28, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Werner, F.M.; Covenas, R. Serotonergic drugs: Agonists/antagonists at specific serotonergic subreceptors for the treatment of cognitive, depressant and psychotic symptoms in alzheimer’s disease. Curr. Pharm. Des. 2016, 22, 2064–2071. [Google Scholar] [CrossRef]
- Bourson, A.; Kapps, V.; Zwingelstein, C.; Rudler, A.; Boess, F.G.; Sleight, A.J. Correlation between 5-ht7 receptor affinity and protection against sound-induced seizures in dba/2j mice. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 356, 820–826. [Google Scholar] [CrossRef]
- Graf, M.; Jakus, R.; Kantor, S.; Levay, G.; Bagdy, G. Selective 5-ht1a and 5-ht7 antagonists decrease epileptic activity in the wag/rij rat model of absence epilepsy. Neurosci. Lett. 2004, 359, 45–48. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Yin, Y.; Sun, S.; Deng, X. Involvement of 5-ht(7) receptors in the pathogenesis of temporal lobe epilepsy. Eur. J. Pharmacol. 2012, 685, 52–58. [Google Scholar] [CrossRef]
- Witkin, J.M.; Baez, M.; Yu, J.; Barton, M.E.; Shannon, H.E. Constitutive deletion of the serotonin-7 (5-ht(7)) receptor decreases electrical and chemical seizure thresholds. Epilepsy Res. 2007, 75, 39–45. [Google Scholar] [CrossRef]
- Pai, V.P.; Marshall, A.M.; Hernandez, L.L.; Buckley, A.R.; Horseman, N.D. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009, 11, R81. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Hancock, K.J.; Kisely, S. The gap in life expectancy from preventable physical illness in psychiatric patients in western australia: Retrospective analysis of population based registers. BMJ 2013, 346, f2539. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.A.N.; Asai, I.; Detraux, J.; Gautam, S.; MÖLler, H.-J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.F.; Huang, A.S.; Snowman, A.M.; Teuscher, C.; Snyder, S.H. From the cover: Antipsychotic drug-induced weight gain mediated by histamine h1 receptor-linked activation of hypothalamic amp-kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 3456–3459. [Google Scholar] [CrossRef]
- Lopez, M. Hypothalamic ampk as a possible target for energy balance-related diseases. Trends Pharmacol. Sci. 2022, 43, 546–556. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Okada, M.; Yoshida, S.; Zhu, G.; Hirose, S.; Kaneko, S. Biphasic actions of topiramate on monoamine exocytosis associated with both soluble n-ethylmaleimide-sensitive factor attachment protein receptors and ca(2+)-induced ca(2+)-releasing systems. Neuroscience 2005, 134, 233–246. [Google Scholar] [CrossRef]
- Yoshida, S.; Okada, M.; Zhu, G.; Kaneko, S. Carbamazepine prevents breakdown of neurotransmitter release induced by hyperactivation of ryanodine receptor. Neuropharmacology 2007, 52, 1538–1546. [Google Scholar] [CrossRef]
- Fukuyama, K.; Tanahashi, S.; Nakagawa, M.; Yamamura, S.; Motomura, E.; Shiroyama, T.; Tanii, H.; Okada, M. Levetiracetam inhibits neurotransmitter release associated with cicr. Neurosci. Lett. 2012, 518, 69–74. [Google Scholar] [CrossRef]
- de Brito, O.M.; Scorrano, L. An intimate liaison: Spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010, 29, 2715–2723. [Google Scholar] [CrossRef]
- Lian, J.; Huang, X.F.; Pai, N.; Deng, C. Betahistine ameliorates olanzapine-induced weight gain through modulation of histaminergic, npy and ampk pathways. Psychoneuroendocrinology 2014, 48, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okada, M. Effects of an atypical antipsychotic, zotepine, on astroglial l-glutamate release through hemichannels: Exploring the mechanism of mood-stabilising antipsychotic actions and antipsychotic-induced convulsion. Pharmaceuticals 2021, 14, 1116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).