Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development
Abstract
1. Introduction
2. Results
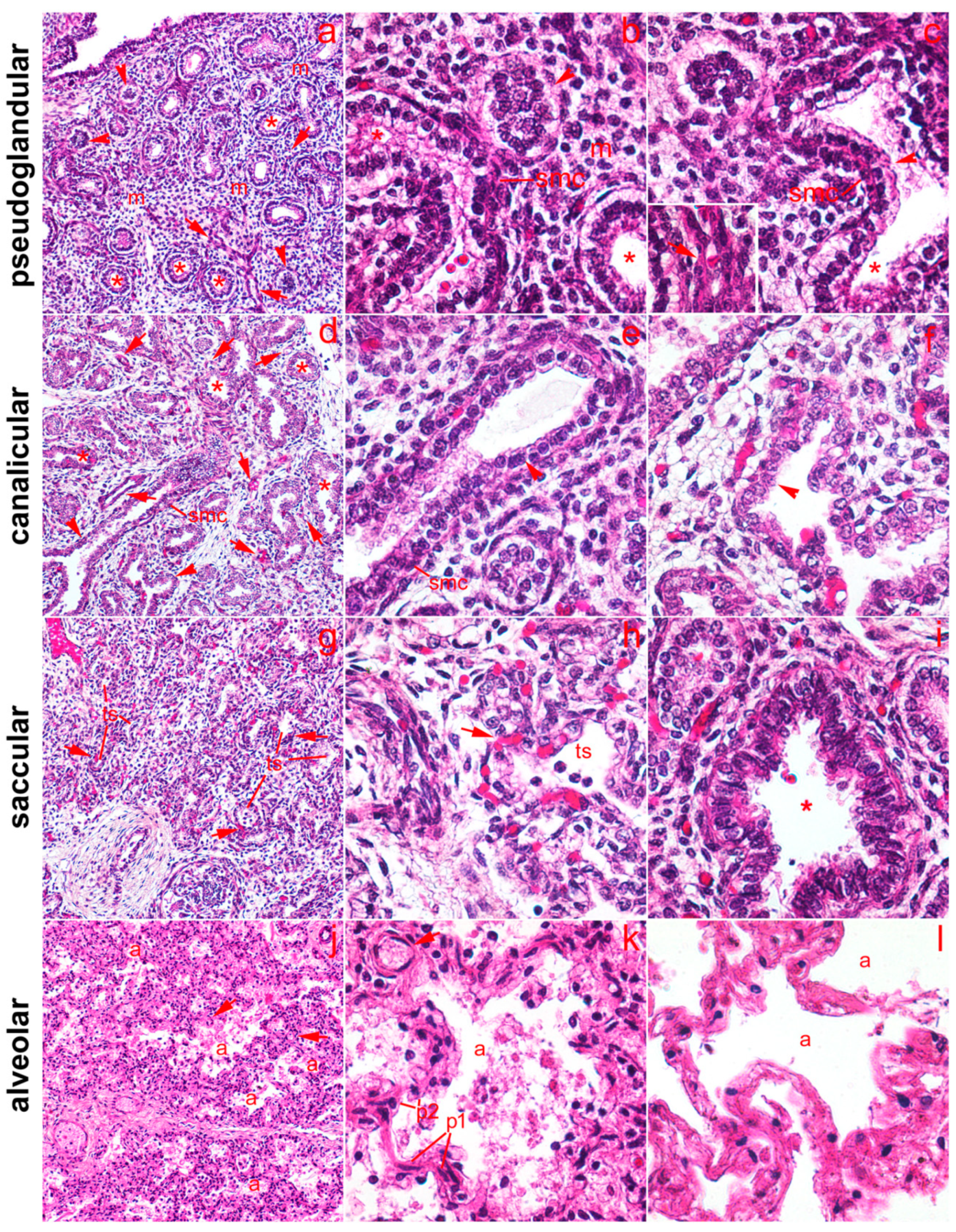
2.1. General Remarks
2.2. 5-HT1A Expression
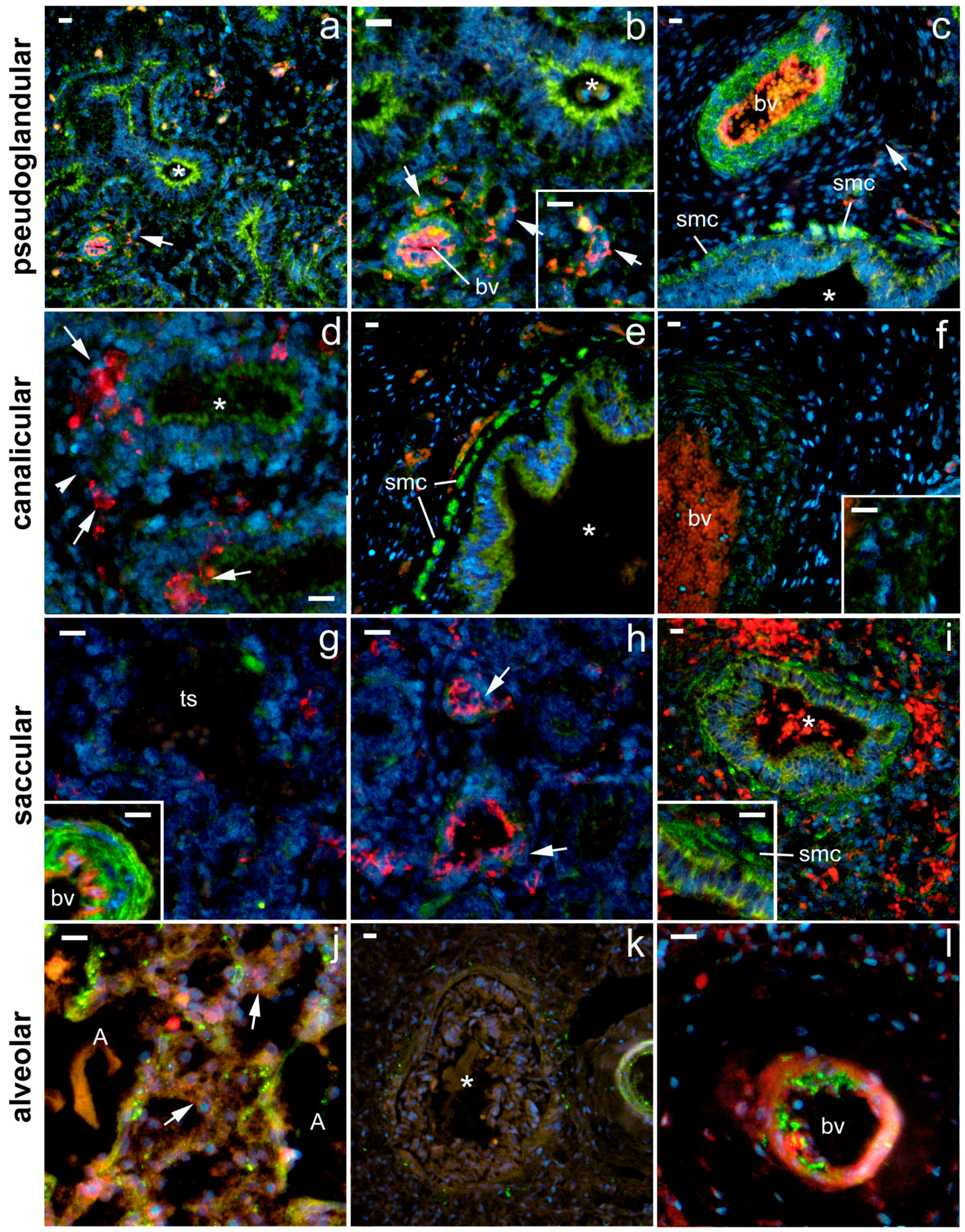
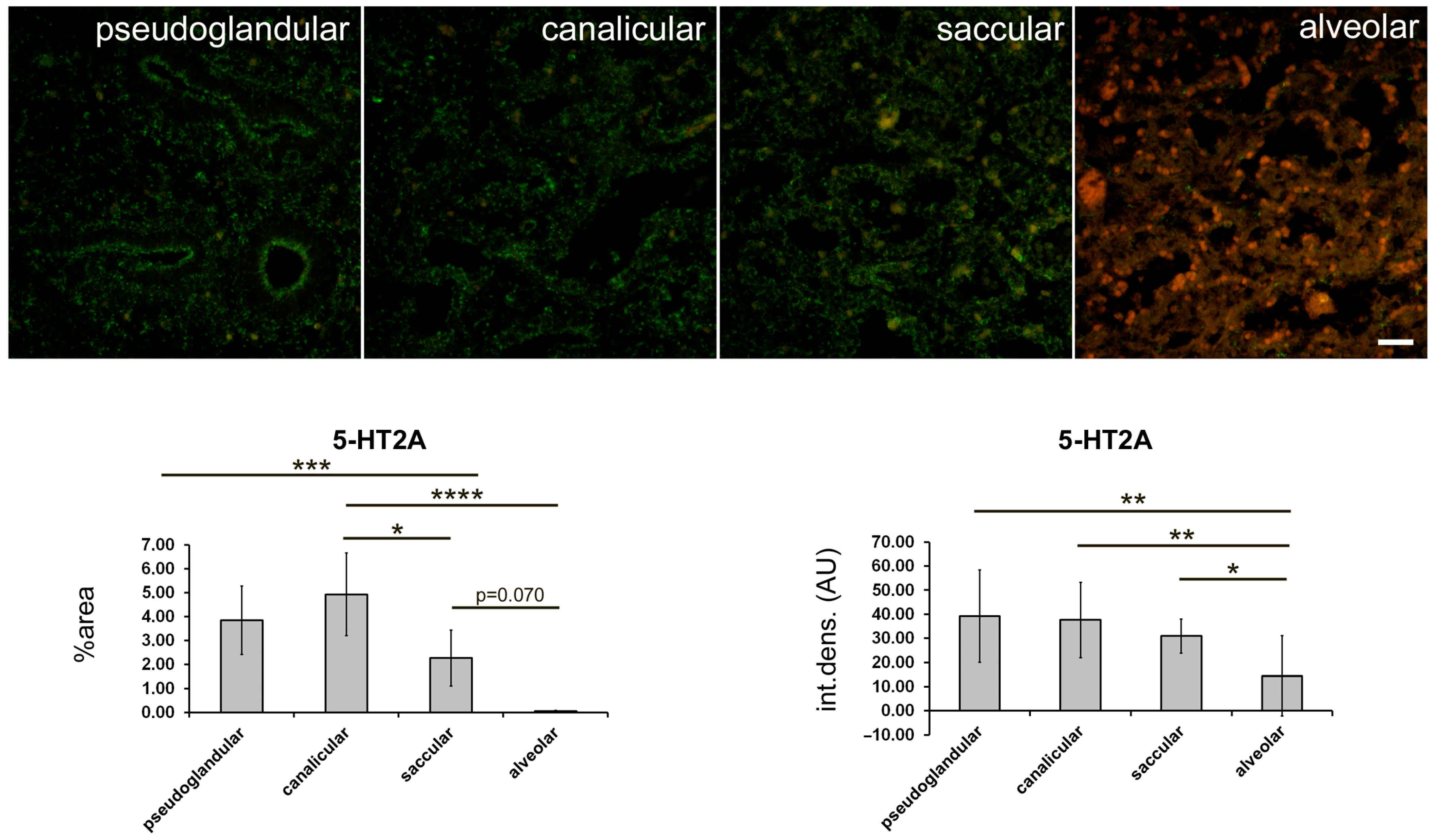
2.3. 5-HT2A Expression
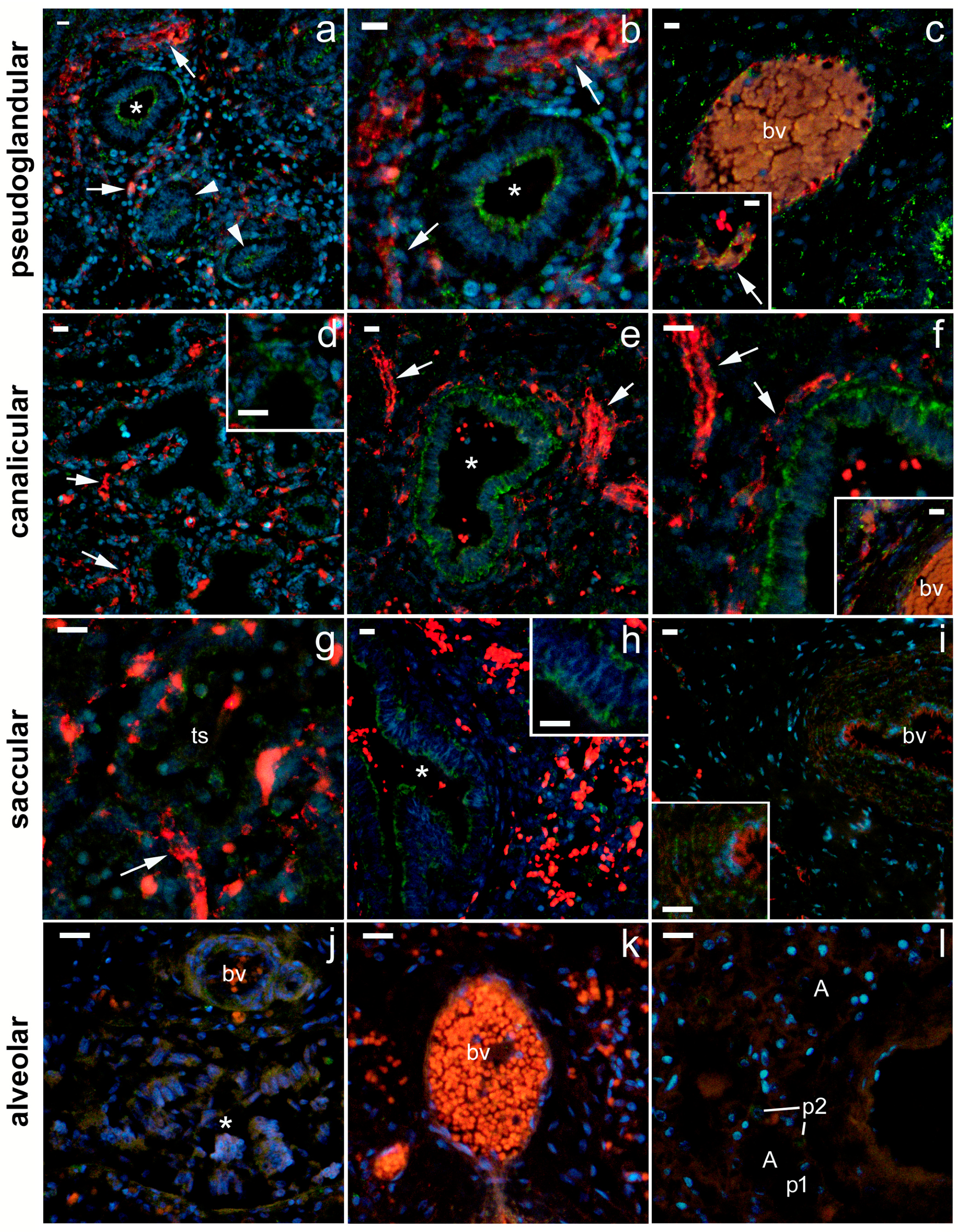
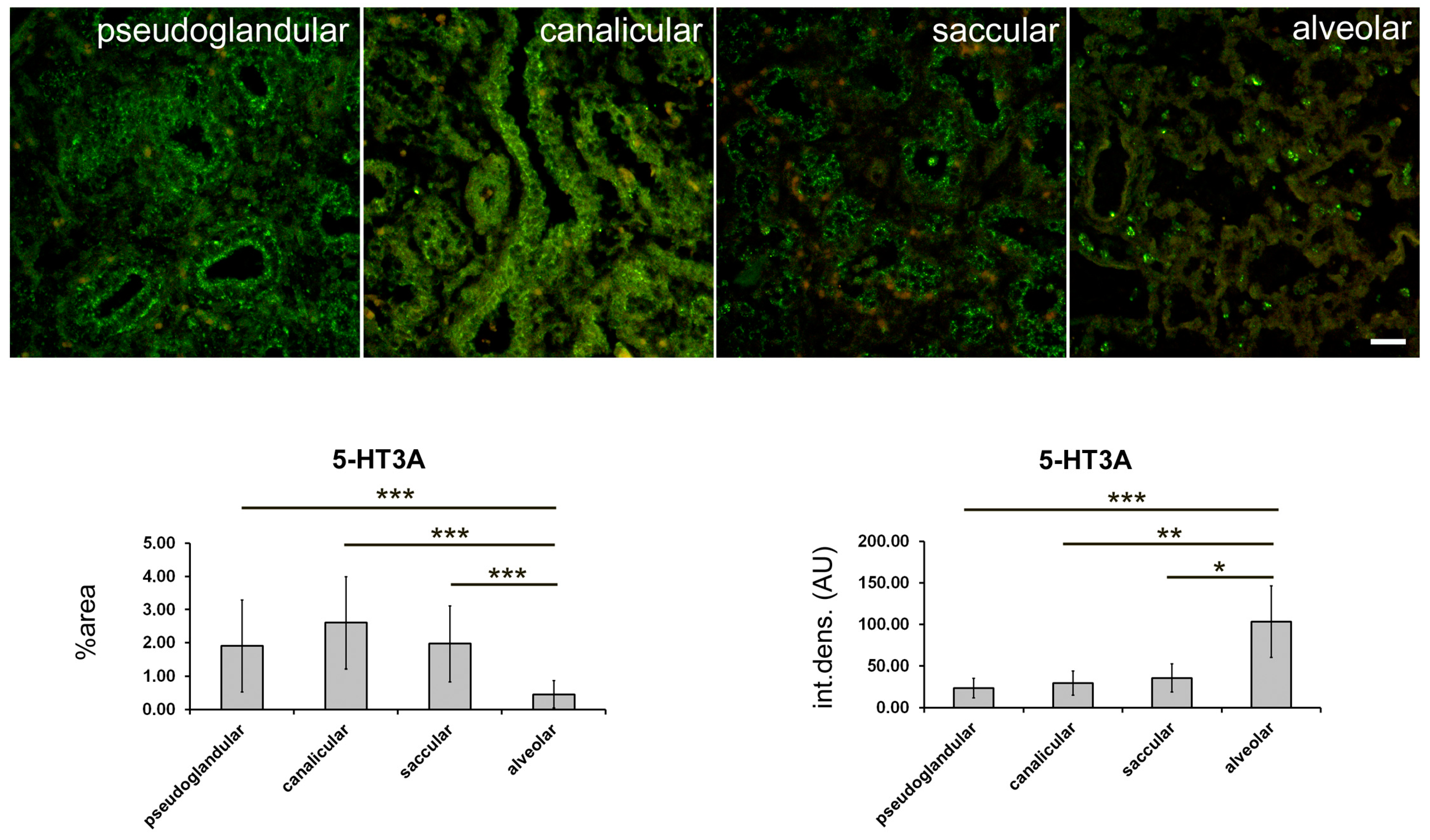
2.4. 5-HT3A Expression
2.5. Expression of Serotonin Receptors in Pneumocytes
3. Discussion
4. Materials and Methods
4.1. Collecting Tissue Samples and Defining Fetus Gestation
4.2. Sample Preparation
4.3. Immunohistochemistry and Immunofluorescence Staining
4.4. Data Acquisition and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M. Before We Are Born, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nikolic, M.Z.; Sun, D.; Rawlins, E.L. Human lung development: Recent progress and new challenges. Development 2018, 145, dev163485. [Google Scholar] [CrossRef] [PubMed]
- Herriges, M.; Morrisey, E.E. Lung development: Orchestrating the generation and regeneration of a complex organ. Development 2014, 141, 502–513. [Google Scholar] [CrossRef]
- Narayanan, M.; Owers-Bradley, J.; Beardsmore, C.S.; Mada, M.; Ball, I.; Garipov, R.; Panesar, K.S.; Kuehni, C.E.; Spycher, B.D.; Williams, S.E.; et al. Alveolarization continues during childhood and adolescence: New evidence from helium-3 magnetic resonance. Am. J. Respir. Crit. Care Med. 2012, 185, 186–191. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharm. 2017, 239, 319–342. [Google Scholar] [CrossRef]
- Punda, H.; Mardesic, S.; Filipovic, N.; Kosovic, I.; Benzon, B.; Ogorevc, M.; Bocina, I.; Kolic, K.; Vukojevic, K.; Saraga-Babic, M. Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida. Int. J. Mol. Sci. 2021, 22, 7320. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Gien, J.; Grover, T.R.; Roe, G.; Abman, S.H. Pulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late-gestation ovine fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L937–L944. [Google Scholar] [CrossRef]
- Adnot, S.; Houssaini, A.; Abid, S.; Marcos, E.; Amsellem, V. Serotonin transporter and serotonin receptors. Handb. Exp. Pharm. 2013, 218, 365–380. [Google Scholar]
- Lv, J.; Liu, F. The Role of Serotonin beyond the Central Nervous System during Embryogenesis. Front. Cell. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Eddahibi, S.; Adnot, S. Serotonin and pulmonary arterial hypertension. Rev. Mal. Respir. 2006, 23 (Suppl. 2), 4S45–4S51. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, W.K.; Kristiansen, K.; Roth, B.L. Molecular biology of serotonin receptors structure and function at the molecular level. Curr. Top. Med. Chem. 2002, 2, 507–528. [Google Scholar] [CrossRef]
- Hilaire, G.; Voituron, N.; Menuet, C.; Ichiyama, R.M.; Subramanian, H.H.; Dutschmann, M. The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 2010, 174, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Marchal-Somme, J.; Marchand-Adam, S.; Quesnel, C.; Borie, R.; Dehoux, M.; Ruffie, C.; Callebert, J.; Launay, J.M.; Henin, D.; et al. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 2008, 32, 426–436. [Google Scholar] [CrossRef]
- Frille, A.; Rullmann, M.; Becker, G.A.; Patt, M.; Luthardt, J.; Tiepolt, S.; Wirtz, H.; Sabri, O.; Hesse, S.; Seyfarth, H.J. Increased pulmonary serotonin transporter in patients with chronic obstructive pulmonary disease who developed pulmonary hypertension. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1081–1092. [Google Scholar] [CrossRef]
- Cummings, K.J.; Hodges, M.R. The serotonergic system and the control of breathing during development. Respir. Physiol. Neurobiol. 2019, 270, 103255. [Google Scholar] [CrossRef]
- Delaney, C.; Gien, J.; Roe, G.; Isenberg, N.; Kailey, J.; Abman, S.H. Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L894–L901. [Google Scholar] [CrossRef]
- Hodge, E.; Nelson, C.P.; Miller, S.; Billington, C.K.; Stewart, C.E.; Swan, C.; Malarstig, A.; Henry, A.P.; Gowland, C.; Melen, E.; et al. HTR4 gene structure and altered expression in the developing lung. Respir. Res. 2013, 14, 77. [Google Scholar] [CrossRef]
- Castro, E.C.; Sen, P.; Parks, W.T.; Langston, C.; Galambos, C. The Role of Serotonin Transporter in Human Lung Development and in Neonatal Lung Disorders. Can. Respir. J. 2017, 2017, 9064046. [Google Scholar] [CrossRef]
- Cazzola, I.; Matera, M.G. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol. Sci. 2000, 21, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Vicencio, A.G.; Yeager, M.E.; Kashgarian, M.; Haddad, G.G.; Eickelberg, O. Differential expression of matrix metalloproteinases and their inhibitors in human and mouse lung development. Thromb. Haemost. 2005, 94, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Adamson, I.Y.; King, G.M. Sex differences in development of fetal rat lung. I. Autoradiographic and biochemical studies. Lab. Investig. J. Tech. Methods Pathol. 1984, 50, 456–460. [Google Scholar]
- Maniscalco, W.M.; Watkins, R.H.; O’Reilly, M.A.; Shea, C.P. Increased epithelial cell proliferation in very premature baboons with chronic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L991–L1001. [Google Scholar] [CrossRef] [PubMed]
- Kraljevic, D.; Vukojevic, K.; Karan, D.; Rajic, B.; Todorovic, J.; Miskovic, J.; Tomic, V.; Kordic, M.; Soljic, V. Proliferation, apoptosis and expression of matrix metalloproteinase-9 in human fetal lung. Acta Histochem. 2015, 117, 444–450. [Google Scholar] [CrossRef]
- Liu, Y.; Suzuki, Y.J.; Day, R.M.; Fanburg, B.L. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ. Res. 2004, 95, 579–586. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef]
- Jones, M.R.; Lingampally, A.; Wu, J.; Sedighi, J.; Ahmadvand, N.; Wilhelm, J.; Vazquez-Armendariz, A.I.; Herold, S.; Chen, C.; Zhang, J.S.; et al. Evidence for Overlapping and Distinct Biological Activities and Transcriptional Targets Triggered by Fibroblast Growth Factor Receptor 2b Signaling between Mid- and Early Pseudoglandular Stages of Mouse Lung Development. Cells 2020, 9, 1274. [Google Scholar] [CrossRef]
- Joshi, S.; Kotecha, S. Lung growth and development. Early Hum. Dev. 2007, 83, 789–794. [Google Scholar] [CrossRef]
- Rubarth, L.B.; Quinn, J. Respiratory Development and Respiratory Distress Syndrome. Neonatal Netw. NN 2015, 34, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Surate Solaligue, D.E.; Rodriguez-Castillo, J.A.; Ahlbrecht, K.; Morty, R.E. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1101–L1153. [Google Scholar] [CrossRef]
- Bustani, P.; Hodge, R.; Tellabati, A.; Li, J.; Pandya, H.; Kotecha, S. Differential response of the epithelium and interstitium in developing human fetal lung explants to hyperoxia. Pediatr. Res. 2006, 59, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Schwarz, H.; Hartmann, P.; Wiegand, S.; Skill, M.; Althaus, M.; Kummer, W.; Krasteva-Christ, G. Caveolin-1: Functional Insights into Its Role in Muscarine- and Serotonin-Induced Smooth Muscle Constriction in Murine Airways. Front. Physiol. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Koos, B.J.; Rajaee, A. Fetal breathing movements and changes at birth. Adv. Exp. Med. Biol. 2014, 814, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.; Muller, T.; Myrtek, D.; Sorichter, S.; Ziegenhagen, M.; Norgauer, J.; Zissel, G.; Idzko, M. Serotoninergic receptors on human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 36, 85–93. [Google Scholar] [CrossRef]
- Brandt, J.P.; Mandiga, P. Histology, Alveolar Cells; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pechkovsky, D.V.; Zissel, G.; Ziegenhagen, M.W.; Einhaus, M.; Taube, C.; Rabe, K.F.; Magnussen, H.; Papadopoulos, T.; Schlaak, M.; Muller-Quernheim, J. Effect of proinflammatory cytokines on interleukin-8 mRNA expression and protein production by isolated human alveolar epithelial cells type II in primary culture. Eur. Cytokine Netw. 2000, 11, 618–625. [Google Scholar]
- Eghtesad, M.; Jackson, H.E.; Cunningham, A.C. Primary human alveolar epithelial cells can elicit the transendothelial migration of CD14+ monocytes and CD3+ lymphocytes. Immunology 2001, 102, 157–164. [Google Scholar] [CrossRef]
- Miskovic, J.; Brekalo, Z.; Vukojevic, K.; Miskovic, H.R.; Kraljevic, D.; Todorovic, J.; Soljic, V. Co-expression of TTF-1 and neuroendocrine markers in the human fetal lung and pulmonary neuroendocrine tumors. Acta Histochem. 2015, 117, 451–459. [Google Scholar] [CrossRef]
- Streeter, G.L. Weight, sitting-height, head size, foot-length, and menstrual age in the human embryo. Contrib. Embriol. 1920, 10, 274. [Google Scholar]



| Developmental Stage | Proximal Airways /Bronchioli * —Epithelium | Proximal Airways /Bronchioli * —SMC | Mesenchime | Capillary Endothelium | Artery —Endothelium | Artery SMC | Distal Airways ** | Alveoli *** | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | |||||||||
| 5-HT1A | Pseudoglandular | +++ | ++++ | + | ++ | +++ | ++++ | / | / | / |
| Canalicular | ++(+) | ++++ | − | + | − | + | ++ | / | / | |
| Saccular | +++ | +++(+) | −/+ | (+) | + | ++++ | + | / | / | |
| Alveolar | − | ++(+) | / | +(+) | ++++ | +++ | / | +(+) | +++ | |
| 5-HT2A | Pseudoglandular | ++ | ++ | + | ++ | ++(+) | ++(+) | / | / | / |
| Canalicular | +++ | + | + | + | +++ | +++ | + | / | / | |
| Saccular | ++ | −/+ | −/+ | −/+ | −/+ | +(+) | +(+) | / | / | |
| Alveolar | −/+ | − | / | − | − | − | / | − | + | |
| 5-HT3A | Pseudoglandular | +++(+) | − | + | + | ++ | ++ | / | / | / |
| Canalicular | +++(+) | + | + | + | + | + | ++ | / | / | |
| Saccular | +++(+) | + | −/+ | − | − | − | +++ | / | / | |
| Alveolar | ++ | ++ | / | −/+ | +(+) | ++ | / | −/+ | ++++ | |
| Antibody | Code No. | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-5HT1A receptor antibody | ab227165 | Rabbit | 1:150 | Abcam, Cambridge, UK |
| Anti-SR-2A (A-4) | sc-166775 | Mouse | 1:150 | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA | |
| Anti-5HT3A receptor antibody | GTX54151 | Rabbit | 1:150 | GeneTex, Irvine, CA, USA | |
| Anti-CD31/PECAM-1 (H3), Alexa fluor 546 conjugated | sc-376764 | Mouse | 1:50 | Santa Cruz Biotechnology Inc. | |
| Anti-CD31/PECAM-1 | NB100-2284 | Rabbit | 1:100 | Novus Biologicals Englewood, CO, USA | |
| Secondary | Anti-Mouse lgG, Alexa Fluor®488 | 715-545-150 | Donkey | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Anti-Rabbit lgG, Alexa Fluor®488 | 711-545-152 | Donkey | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | ||
| Anti-Rabbit IgG, Rhodamine Red™-X | 711-295-152 | Donkey | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, J.; Vukojević, K.; Šoljić, V.; Mišković, J.; Orlović Vlaho, M.; Saraga-Babić, M.; Filipović, N. Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development. Int. J. Mol. Sci. 2023, 24, 2965. https://doi.org/10.3390/ijms24032965
Nikolić J, Vukojević K, Šoljić V, Mišković J, Orlović Vlaho M, Saraga-Babić M, Filipović N. Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development. International Journal of Molecular Sciences. 2023; 24(3):2965. https://doi.org/10.3390/ijms24032965
Chicago/Turabian StyleNikolić, Jelena, Katarina Vukojević, Violeta Šoljić, Josip Mišković, Martina Orlović Vlaho, Mirna Saraga-Babić, and Natalija Filipović. 2023. "Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development" International Journal of Molecular Sciences 24, no. 3: 2965. https://doi.org/10.3390/ijms24032965
APA StyleNikolić, J., Vukojević, K., Šoljić, V., Mišković, J., Orlović Vlaho, M., Saraga-Babić, M., & Filipović, N. (2023). Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development. International Journal of Molecular Sciences, 24(3), 2965. https://doi.org/10.3390/ijms24032965







