Oleracone F Alleviates Cognitive Impairment and Neuropathology in APPswe/PSEN1dE9 Mice by Reducing the Expression of Vascular Cell Adhesion Molecule and Leukocyte Adhesion to Brain Vascular Endothelial Cells
Abstract
1. Introduction
2. Results
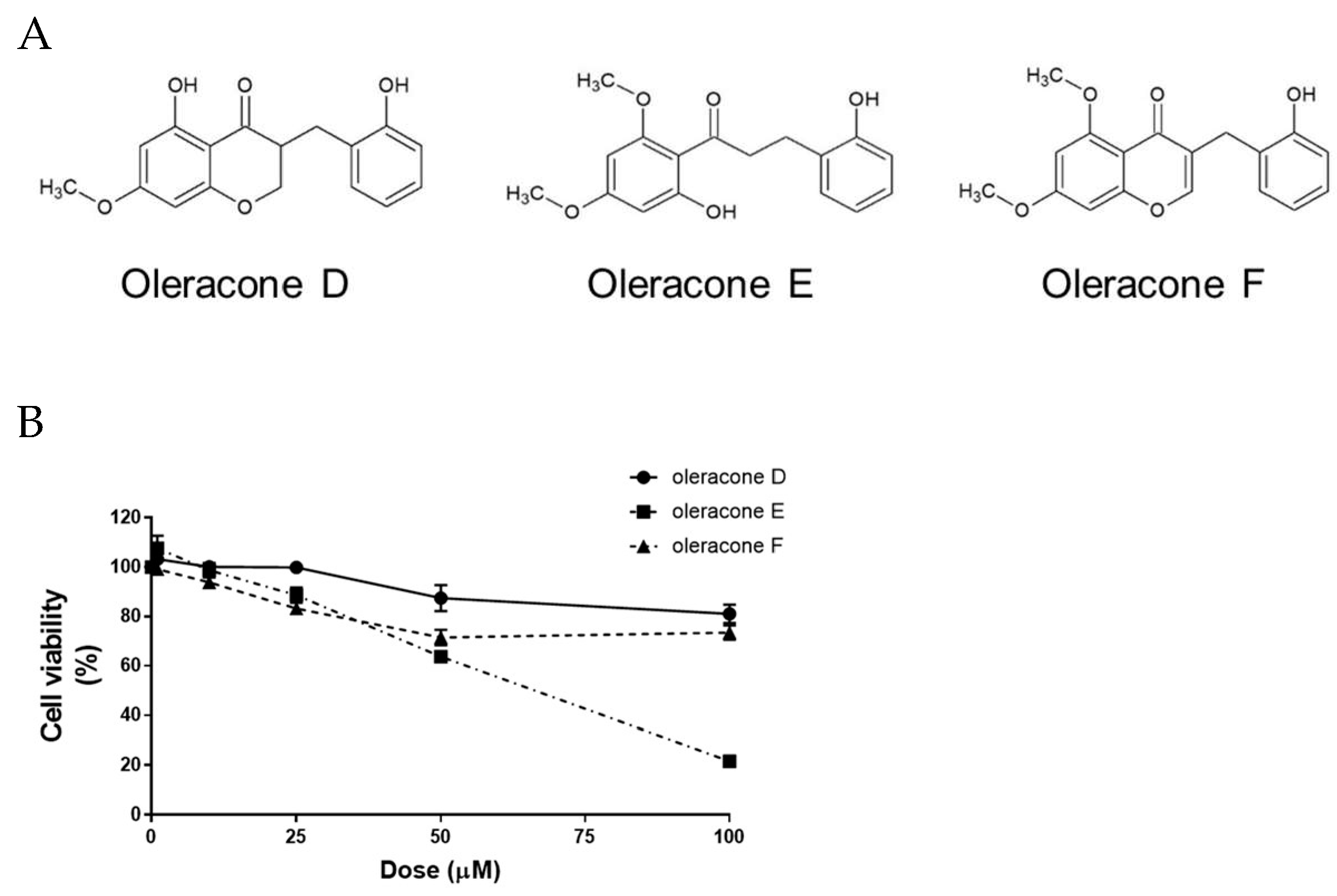
2.1. Effect of Oleracones on Cell Viability
2.2. Oleracones Blocked TNF-α-Induced Increases in Leukocyte Adhesion to Endothelial Cells and VCAM-1 Protein Expression
2.3. Oleracone F Attenuated Cognitive Impairment in AD Model Mice
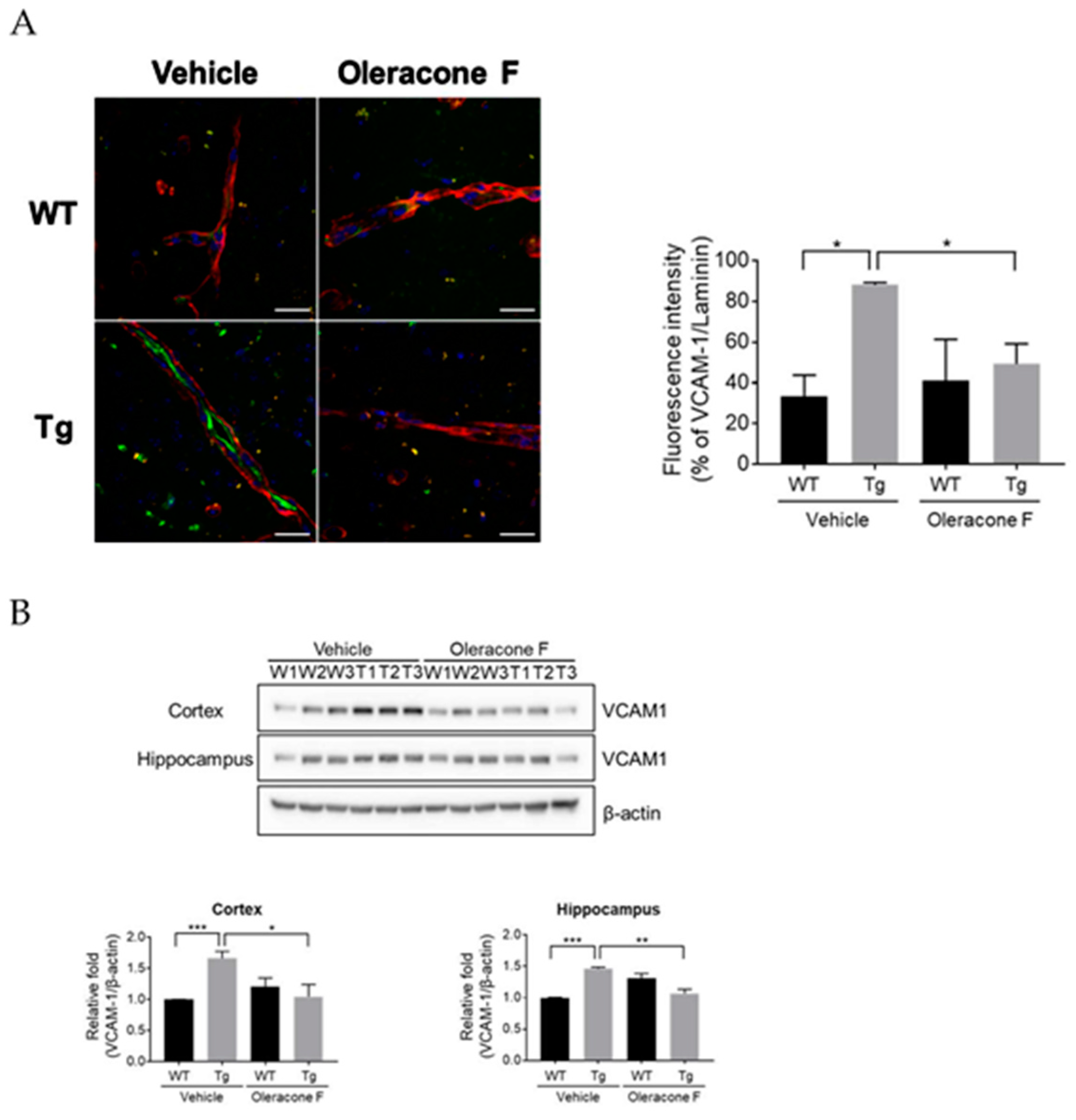
2.4. Oleracone F Blocked VCAM-1 Expression in the Vessels of Tg Mice
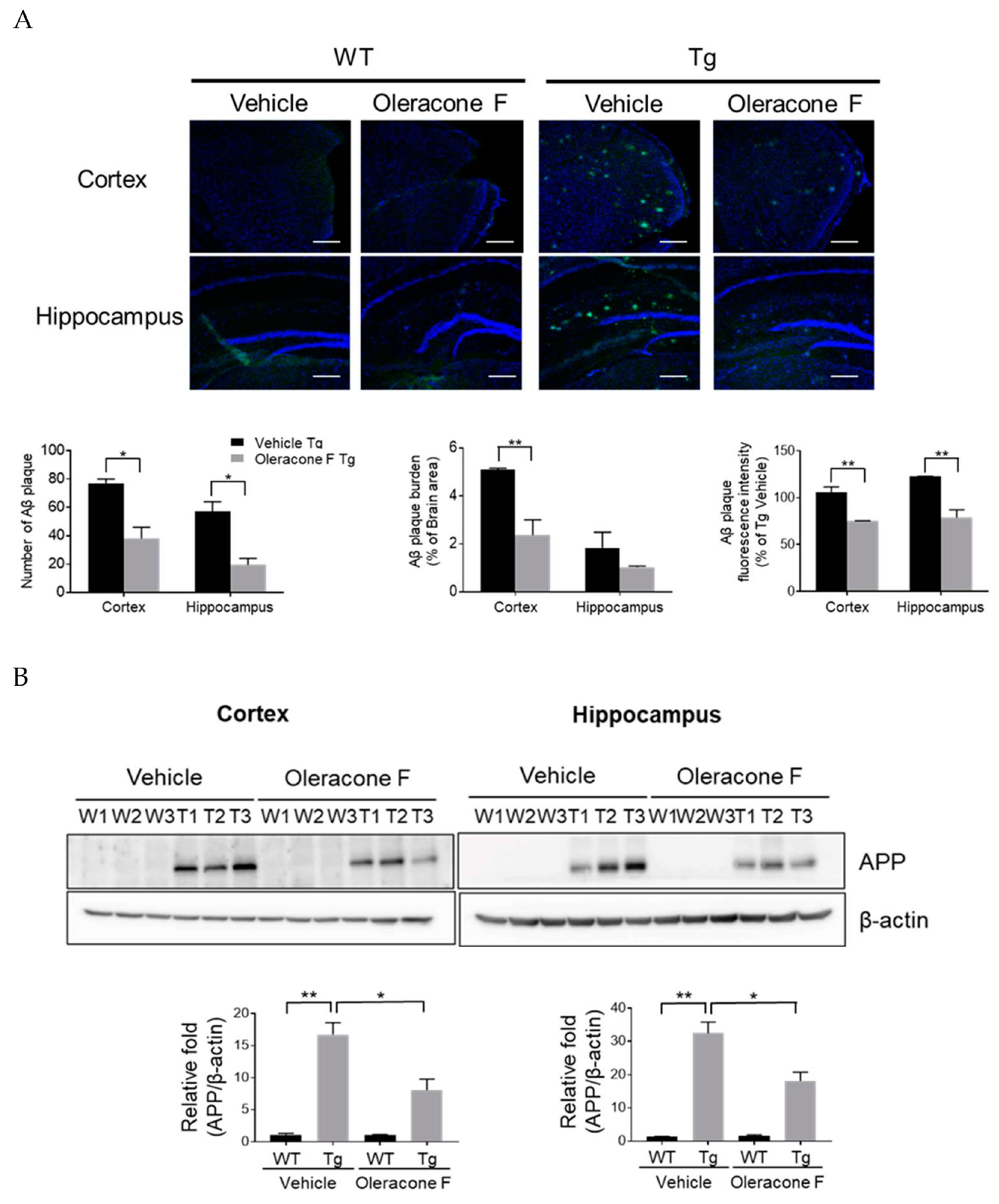
2.5. The Effect of Oleracone F on the Levels of Brain Amyloids and APP Protein in Tg Mice
2.6. Effect of Oleracone F on Brain Inflammation in Tg Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Adhesion Assay
4.4. Cell Toxicity Assay
4.5. Transgenic Mice and Genotyping
4.6. Drug Administration
4.7. Passive Avoidance Test
4.8. Brain Section Preparation
4.9. Immunofluorescence Staining
4.10. Western Blot Analysis
4.11. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, J.L. Alzheimer’s disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Kurz, C.; Walker, L.; Rauchmann, B.; Perneczky, R. Dysfunction of the blood-brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of immune cells in the central nervous system. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef]
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation. Colloq. Ser. Integr. Syst. Physiol. 2010, 2, 1–87. [Google Scholar] [CrossRef]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Woehrl, B.; Klein, M.; Rupprecht, T.A.; Schmetzer, H.; Angele, B.; Hacker, H.; Hacker, G.; Pfister, H.; Koedel, U. CXCL16 contributes to neutrophil recruitment to cerebrospinal fluid in pneumococcal meningitis. J. Infect. Dis. 2010, 202, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Puig, I.; Miro-Mur, F.; Ferrer-Ferrer, M.; Gelpi, E.; Pedragosa, J.; Justicia, C.; Urra, X.; Chamorro, A.; Planas, A.M. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015, 129, 239–257. [Google Scholar] [CrossRef]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Pietronigro, E.; Bianca, V.D.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, W.; Ying, X.; Stien, D. New flavonoids from Portulaca oleracea L. and their activities. Fitoterapia 2018, 127, 257–262. [Google Scholar] [CrossRef]
- Lee, A.S.; Kim, J.S.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Anti-TNF-alpha activity of Portulaca oleracea in vascular endothelial cells. Int. J. Mol. Sci. 2012, 13, 5628–5644. [Google Scholar] [CrossRef]
- Li, C.; Meng, Y.; Ying, Z.; Xu, N.; Hao, D.; Gao, M.; Zhang, W.; Xu, L.; Gao, Y.; Ying, X. Three Novel Alkaloids from Portulaca oleracea L. and Their Anti-inflammatory Effects. J. Agric. Food Chem. 2016, 64, 5837–5844. [Google Scholar] [CrossRef]
- Yang, X.; Ying, Z.; Liu, H.; Ying, X.; Yang, G. A new homoisoflavone from Portulaca oleracea L. and its antioxidant activity. Nat. Prod. Res. 2019, 33, 3500–3506. [Google Scholar] [CrossRef]
- Duan, Y.; Ying, Z.; Zhang, M.; Ying, X.; Yang, G. Two new homoisoflavones from Portulaca oleracea L. and their activities. Nat. Prod. Res. 2022, 36, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Youssef, A.M.; Magharbeh, M.K.; Al-Dalaen, S.M.; Al-Jawabri, N.A.; Al-Nawaiseh, T.N.; Al-Jwanieh, A.; Al-Ani, F.S. Protective Effect of Portulaca oleracea Extract Against Lipopolysaccharide-Induced Neuroinflammation, Memory Decline, and Oxidative Stress in Mice: Potential Role of miR-146a and miR-let 7. J. Med. Food 2022, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, W.; Li, Y.; Li, L.; Zhang, H.; Pang, Y.; Xiao, Z.; Xiao, H.; Xiao, Y. TNF-alpha enhances vascular cell adhesion molecule-1 expression in human bone marrow mesenchymal stem cells via the NF-kappaB, ERK and JNK signaling pathways. Mol. Med. Rep. 2016, 14, 643–648. [Google Scholar] [CrossRef]
- Otgongerel, D.; Lee, H.; Jo, S.A. Induction of ICAM1 in Brain Vessels is Implicated in an Early AD Pathogenesis by Modulating Neprilysin. NeuroMolecular Med. 2022. [Google Scholar] [CrossRef]
- Errede, M.; Annese, T.; Petrosino, V.; Longo, G.; Girolamo, F.; de Trizio, I.; d’Amati, A.; Uccelli, A.; Kerlero de Rosbo, N.; Virgintino, D. Microglia-derived CCL2 has a prime role in neocortex neuroinflammation. Fluids Barriers CNS 2022, 19, 68. [Google Scholar] [CrossRef]
- Van de Haar, H.J.; Jansen, J.F.A.; Jeukens, C.R.L.P.N.; Burgmans, S.; van Buchem, M.A.; Muller, M.; Hofman, P.A.M.; Verhey, F.R.J.; van Osch, M.J.P.; Backes, W.H. Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Med. Phys. 2017, 44, 4112–4125. [Google Scholar] [CrossRef]
- Park, J.; Ryu, S.Y.; Jung, I.; Lee, Y.; Kang, K.J.; Lee, M.; Lee, M.; Sonn, S.K.; Lee, J.H.; Lee, H.; et al. Evaluation of VCAM-1 antibodies as therapeutic agent for atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2013, 226, 356–363. [Google Scholar] [CrossRef]
- Grammas, P.; Ovase, R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol. Aging 2001, 22, 837–842. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, W.J.; Jung, Y. Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-kappaB Signaling. Int. J. Mol. Sci. 2017, 18, 1424. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Doody, R.; Stoffler, A.; Schmitt, F.; Ferris, S.; Mobius, H.J. Memantine Study Group Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Lim, C.; Cha, D.S.; Han, Y.T. Synthesis and Evaluation of the Lifespan-Extension Properties of Oleracones D–F, Antioxidative Flavonoids from Portulaca oleracea L. Appl. Sci. 2019, 9, 4014. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Yoon, S.-S.; Kim, S.-E.; Jo, S.A. KDM4B histone demethylase and G9a regulate expression of vascular adhesion proteins in cerebral microvessels. Sci. Rep. 2017, 7, 45005. [Google Scholar] [CrossRef]



| Antibodies | Host | Company | Catalog Number |
|---|---|---|---|
| Anti-ICAM-1 | Mouse monoclonal | Santa Cruz | sc8439 |
| Anti-VCAM-1 | Rabbit polyclonal | Abcam | ab134047 |
| Anti-Laminin | Rabbit polyclonal | Abcam | ab11575 |
| Anti-Iba1 | Rabbit polyclonal | Wako | 019-19741 |
| Anti-GFAP | Rabbit polyclonal | Abcam | ab53554 |
| Anti-GFAP | Rabbit polyclonal | Dako | Z0334 |
| Anti-β-amyloid (6E10) | Mouse monoclonal | Biolegend | SIG-39320 |
| Anti-β-amyloid (4G8) | Mouse monoclonal | Biolegend | SIG-39200 |
| Anti-BACE1 | Rabbit polyclonal | Abcam | Ab2077 |
| Anti-β-actin | Mouse monoclonal | Abcam | Ab6276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-S.; Ko, J.-S.; Oh, S.-Y.; Han, Y.T.; Jo, S.A. Oleracone F Alleviates Cognitive Impairment and Neuropathology in APPswe/PSEN1dE9 Mice by Reducing the Expression of Vascular Cell Adhesion Molecule and Leukocyte Adhesion to Brain Vascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 2056. https://doi.org/10.3390/ijms24032056
Kwon Y-S, Ko J-S, Oh S-Y, Han YT, Jo SA. Oleracone F Alleviates Cognitive Impairment and Neuropathology in APPswe/PSEN1dE9 Mice by Reducing the Expression of Vascular Cell Adhesion Molecule and Leukocyte Adhesion to Brain Vascular Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(3):2056. https://doi.org/10.3390/ijms24032056
Chicago/Turabian StyleKwon, Young-Sun, Jin-Sung Ko, Se-Young Oh, Young Taek Han, and Sangmee Ahn Jo. 2023. "Oleracone F Alleviates Cognitive Impairment and Neuropathology in APPswe/PSEN1dE9 Mice by Reducing the Expression of Vascular Cell Adhesion Molecule and Leukocyte Adhesion to Brain Vascular Endothelial Cells" International Journal of Molecular Sciences 24, no. 3: 2056. https://doi.org/10.3390/ijms24032056
APA StyleKwon, Y.-S., Ko, J.-S., Oh, S.-Y., Han, Y. T., & Jo, S. A. (2023). Oleracone F Alleviates Cognitive Impairment and Neuropathology in APPswe/PSEN1dE9 Mice by Reducing the Expression of Vascular Cell Adhesion Molecule and Leukocyte Adhesion to Brain Vascular Endothelial Cells. International Journal of Molecular Sciences, 24(3), 2056. https://doi.org/10.3390/ijms24032056






