The Endocannabinoid System and Physical Exercise
Abstract
1. Introduction
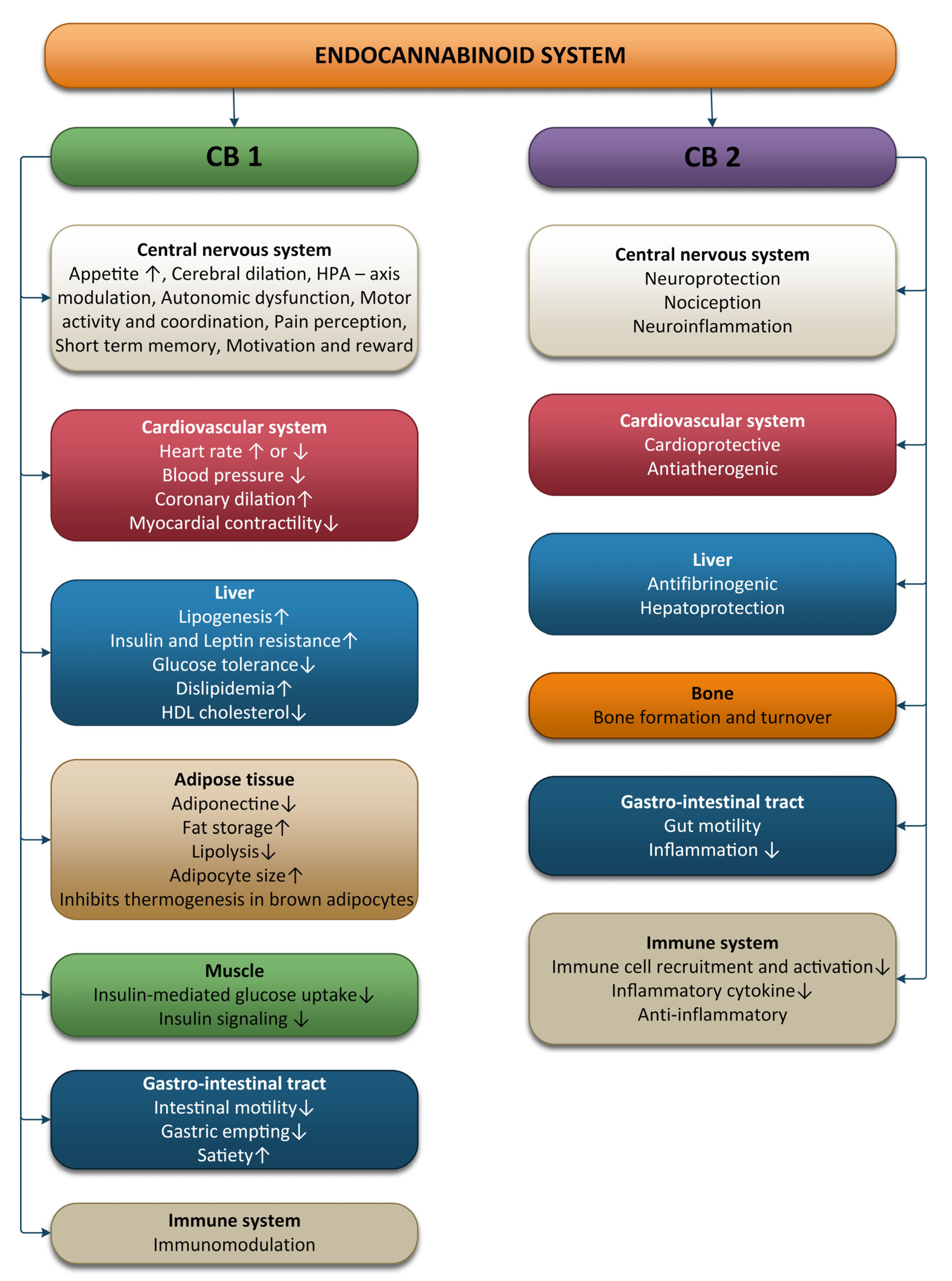
2. Physiology of the Endocannabinoid System
2.1. The ECS and the Nervous System
2.2. The ECS and the Cardiovascular System
2.3. The ECS and the Immune System
2.4. The ECS and the Digestive System
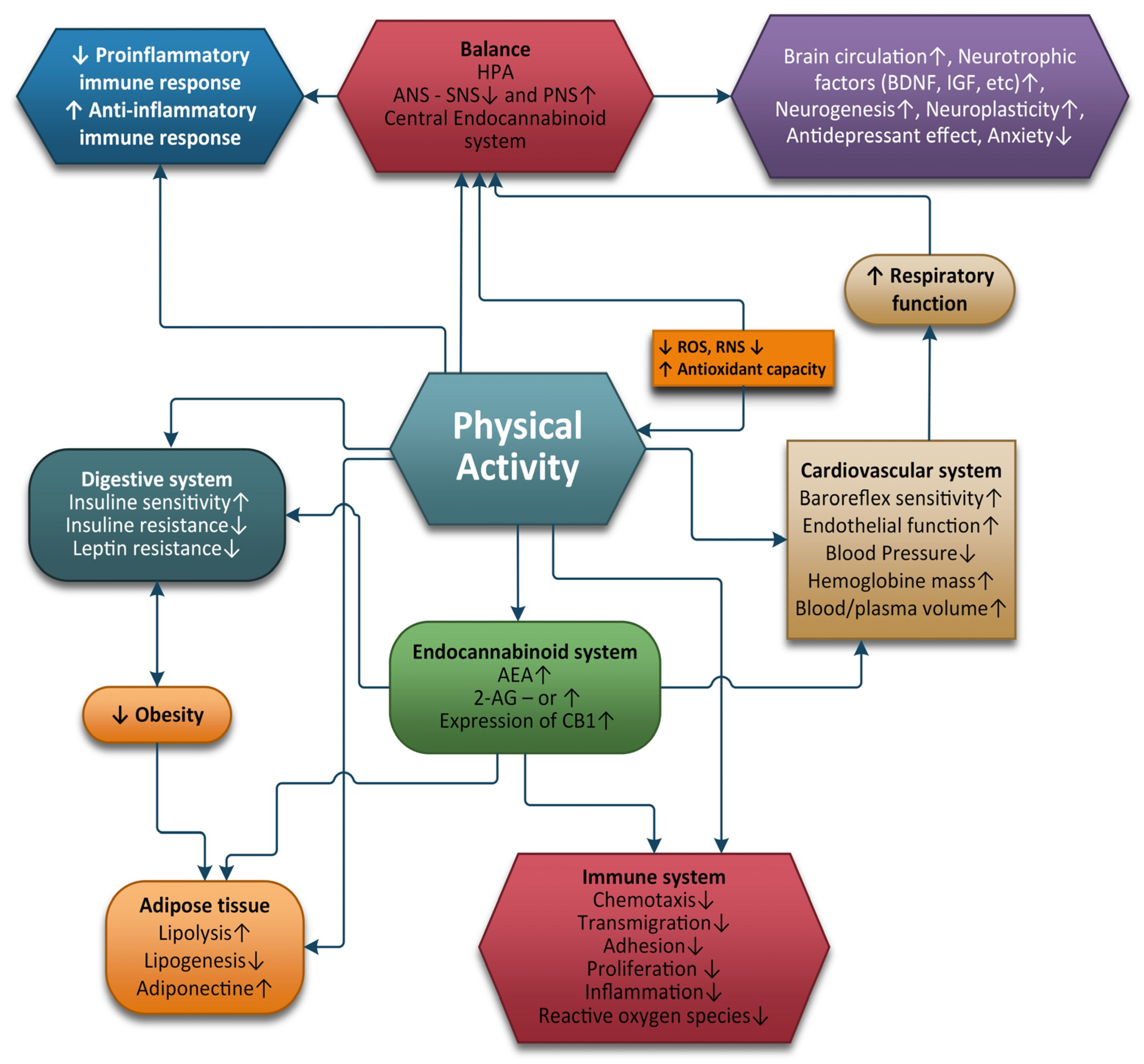
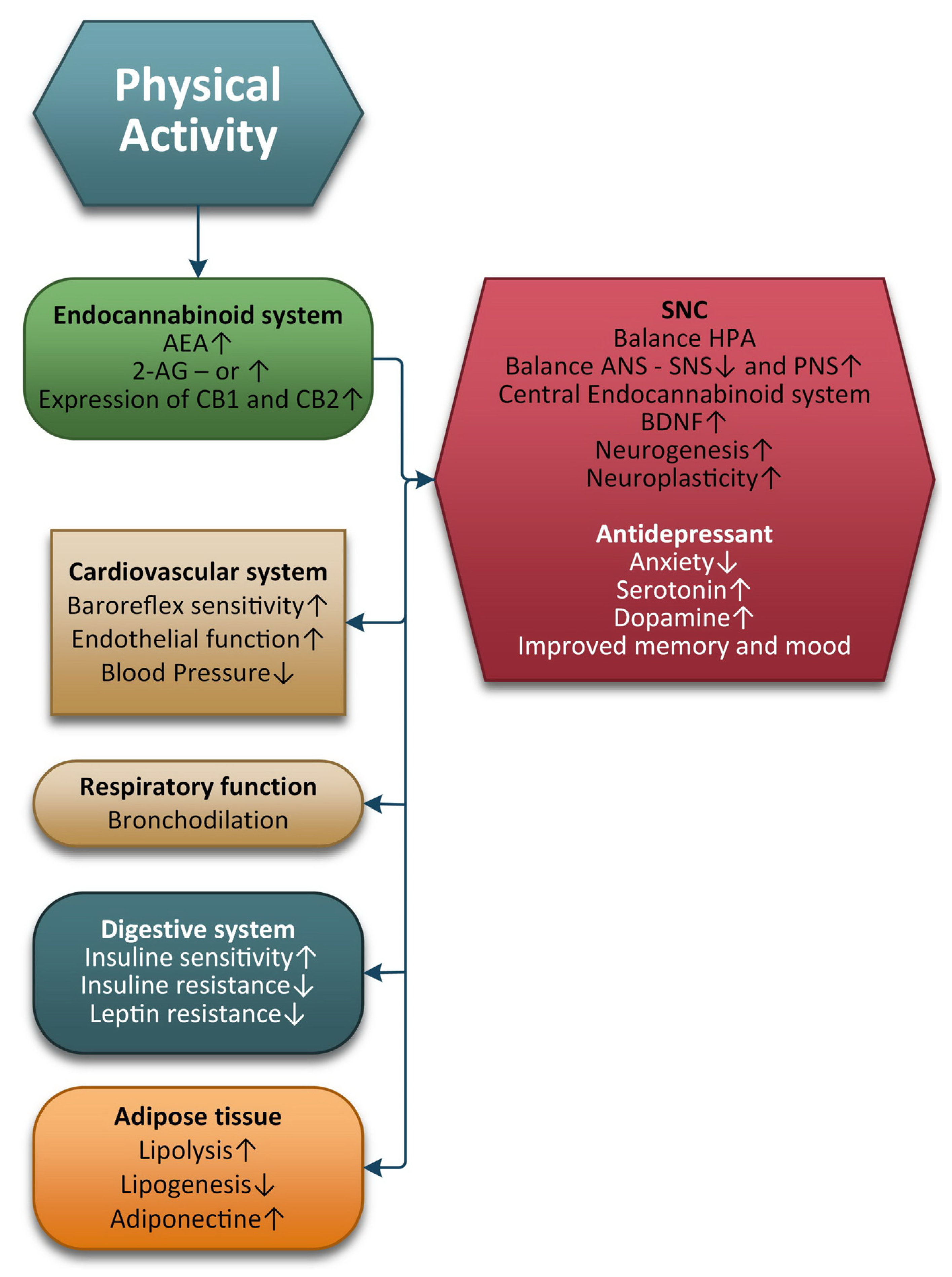
3. Physical Exercise and the ECS
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goncalves:, R.L.; Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Brand, M. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015, 290, 209. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Morrow, J.D.; Utter, A.C.; Dumke, C.L. Effect of resistance exercise and carbohydrate ingestion on oxidative stress. Free Radic. Res. 2005, 3, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H. Endorphins: The basis of pleasure? J. Neurol. Neurosurg. Psychiatry 1992, 55, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef]
- Reynolds, Gretchen, Homing in on the Source of Runner’s High, October 7, 2015, Retrieved from the New York Times, PHYS ED. Available online: https://well.blogs.nytimes.com/2015/10/07/homing-in-on-the-source-of-runners-high/ (accessed on 1 August 2022).
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Boecker, H.; Sprenger, T.; Spilker, M.E.; Henriksen, G.; Koppenhoefer, M.; Wagner, K.J.; Valet, M.; Berthele, A.; Tolle, T.R. The runner’s high: Opioidergic mechanisms in the human brain. Cereb Cortex. 2008, 18, 2523–2531. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.; Gass, P. Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp. Neurol. 2010, 224, 103–105. [Google Scholar] [CrossRef]
- Carr, D.B.; Bullen, B.A.; Skrinar, G.S.; Arnold, M.A.; Rosenblatt, M.; Beitins, I.Z.; McArthur, J.W. Physical conditioning facilitates the exercise-induced secretion of beta-endorphin and beta-lipotropin in women. N. Engl. J. Med. 1981, 305, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, S.; Hommel, B.; Kibele, A.; Colzato, L.S. Neuromodulation of Aerobic Exercise-A Review. Front. Psychol. 2016, 6, 1890. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; Biedermann, S.V.; Bindila, L.; Lutz, B.; Fussa, J. Exercise-Induced Euphoria and Anxiolysis Do Not Depend on Endogenous Opioids in Humans. Psychoneuroendocrinology 2021, 126, 105173. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- McPartland, J.; Guy, G.; Di Marzo, V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PloS ONE 2014, 9, e89566. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345. [Google Scholar] [CrossRef]
- Maldonado, R.; Baños, J.E.; Cabañero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, S23–S32. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Porreca, F.; Lai, J.; Albrecht, P.J.; Rice, F.L.; Khodorova, A.; Davar, G.; Makriyannis, A.; Vanderah, T.W.; Mata, H.P.; et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. USA 2005, 102, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Nadal, X.; La Porta, C.; Andreea Bura, S.; Maldonado, R. Involvement of the opioid and cannabinoid systems in pain control: New insights from knockout studies. Eur. J. Pharmacol. 2013, 716, 142–157. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.; Bura, S.A.; Aracil-Fernández, A.; Manzanares, J.; Maldonado, R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 2013, 154, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.W.; Connor, M.; Bagley, E.E.; Christie, M.J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol. Pharmacol. 2000, 57, 288–295. [Google Scholar] [PubMed]
- Berger, B.G.; Owen, D.R. Relation of low and moderate intensity exercise with acute mood change in college joggers. Percept. Mot. Skills 1998, 87, 611–621. [Google Scholar] [CrossRef]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Opioid System Interactions in Exercise-Induced Hypoalgesia. Pain Med. 2018, 19, 118–123. [Google Scholar] [CrossRef]
- Basavarajappa, B. Neuropharmacology of the Endocannabinoid Signaling System Molecular Mechanisms, Biological Actions and Synaptic Plasticity. Curr. Neuropharmacol. 2007, 5, 81–97. [Google Scholar] [CrossRef]
- Cabral, G.; Raborn, R.; Griffin, L.; Dennis, J.; Marciano-Cabral, F. CB2 receptors in the brain: Role in central immune. Br. J. Pharmacol. 2009, 153, 240–251. [Google Scholar] [CrossRef]
- Wright, K.; Duncan, M.; Sharkey, K. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Br. J. Pharmacol. 2008, 153, 263–270. [Google Scholar] [CrossRef]
- Howlett, A.; Breivogel, C.; Childers, S.; Deadwyler, S.; Hampson, R.; Porrino, L. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47, 345–358. [Google Scholar] [CrossRef]
- Gündel, D.; Deuther-Conrad, W.; Ueberham, L.; Kaur, S.; Otikova, E.; Teodoro, R.; Moldovan, R.P. Structure-Based Design, Optimization, and Development of [18F]LU13: A Novel Radioligand for Cannabinoid Receptor Type 2 Imaging in the Brain with PET. J. Med. Chem. 2022, 65, 9034–9049. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Bellocchio, L.; Soria-Gómez, E.; Quarta, C.; Metna-Laurent, M.; Cardinal, P.; Binder, E.; Marsicano, G. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc. Natl. Acad. Sci. USA 2013, 110, 4786–4791. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Pagotto, U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Hill, M.N.; Karatsoreos, I.N.; Hillard, C.J.; McEwen, B.S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 2010, 35, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Itoga, C.A.; Fisher, M.O.; Solomonow, J.; Roltsch, E.A.; Gilpin, N.W.; Tasker, J.G. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 2016, 36, 8461–8670. [Google Scholar] [CrossRef]
- Gray, J.M.; Vecchiarelli, H.A.; Morena, M.; Lee, T.T.; Hermanson, D.J.; Kim, A.B.; Hill, M.N. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015, 35, 3879–3892. [Google Scholar] [CrossRef]
- Hartmann, A.; Fassini, A.; Scopinho, A.; Correa, F.M.; Guimarães, F.S.; Lisboa, S.F.; Resstel, L.B. Role of the endocannabinoid system in the dorsal hippocampus in the cardiovascular changes and delayed anxiety-like effect induced by acute restraint stress in rats. J. Psychopharmacol. 2019, 33, 606–614. [Google Scholar] [CrossRef]
- Navarria, A.; Tamburella, A.; Iannotti, F.A.; Micale, V.; Camillieri, G.; Gozzo, L.; Di Marzo, V. The dual blocker of FAAH/TRPV1 N-arachidonoylserotonin reverses the behavioral despair induced by stress in rats and modulates the HPA-axis. Pharmacol. Res. 2014, 87, 151–159. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsycho. Pharmacol. 2016, 41, 80–102. [Google Scholar] [CrossRef]
- Straub, R.H.; Herfarth, H.; Falk, W.; Andus, T.; Scholmerich, J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J. Neuroimmunol. 2002, 126, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Gu, N.; Wang, Y.; Wang, F.; Zhu, J.; Fang, Y.; Zhang, X. Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. J. Psychiatry Neurosci. 2017, 42, 230–241. [Google Scholar] [CrossRef]
- Yasmin, F.; Colangeli, R.; Morena, M.; Filipski, S.; van der Stelt, M.; Pittman, Q.J.; Chattarji, S. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl. Acad. Sci. USA 2020, 117, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering Myocardial Infarction by Marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef]
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kossl, M.; Holsboer, F.; Wotjak, C.T. Reduced Anxiety, Conditioned Fear, and Hippocampal Long-Term Potentiation in Transient Receptor Potential Vanilloid Type 1 Receptor-Deficient Mice. J. Neurosci. 2007, 27, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Bölcskei, K.; Helyes, Z.; Szabó, Á.; Sándor, K.; Elekes, K.; Németh, J.; Almási, R.; Szolcsányi, J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005, 117, 368–376. [Google Scholar] [CrossRef]
- Ho, B.Y.; Uezono, Y.; Takada, S.; Takase, I.; Izumi, F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Recept. Channels 1999, 6, 363–374. [Google Scholar]
- Fioravanti, B.; De Felice, M.; Stucky, C.L.; Medler, K.A.; Luo, M.-C.; Gardell, L.R.; Vanderah, T.W. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: Antinociceptive actions of CB1 inverse agonists. J. Neurosci. 2008, 28, 11593–11602. [Google Scholar] [CrossRef]
- Zhang, Y.; Popović, Z.B.; Bibevski, S.; Fakhry, I.; Sica, D.A.; Van Wagoner, D.R.; Mazgalev, T.N. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ. Heart Fail. 2009, 2, 692–699. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008, 54, 244–249. [Google Scholar] [CrossRef]
- Hall, W.; Degenhardt, L. Adverse health effects of non-medical cannabis use. Lancet 2009, 374, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Sachdeva, R.; Mehta, J.L. Cannabinoids and atherosclerotic coronary heart disease. Clin. Cardiol. 2012, 35, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Penner, E.A.; Buettner, H.; Mittleman, M.A. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am. J. Med. 2013, 126, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Cook, B. TRP Channel Proteins and Signal Transduction. Physiol. Rev. 2002, 82, 429–472. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef]
- Thayer, J.; Yamamoto, S.; Cardiology, J.B.-I. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Elsevier 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.; Warth, M. Body mass index is related to autonomic nervous system activity as measured by heart rate variability—A replication using short term measurements. J. Nutr. Health Aging 2014, 18, 300–302. [Google Scholar] [CrossRef]
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012, 53, 272–282. [Google Scholar] [CrossRef]
- Sgoifo, A.; Carnevali, L.; Pico Alfonso M de los, A.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Luca, C.; Ilie, O.; Matei, P.; Iordan, D.-A.; Buculei, I. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar]
- Anghel, L.; Baroiu, L.; Popazu, C.R.; Pătraș, D.; Fotea, S.; Nechifor, A.; Ciubara, A.B. Benefits and adverse events of melatonin use in the elderly (Review). Exp. Ther. Med. 2022, 23, 219. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The Role of Cannabinoids in Adult Neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef]
- Mechoulam, R.; Spatz, M.; Shohami, E. Endocannabinoids and Neuroprotection. Sci. STKE 2002, 2002, re5. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734. [Google Scholar] [CrossRef]
- Ros, J.; Clària, J.; To-Figueras, J.; Planagumà, A.; Cejudo-Martín, P.; Fernández-Varo, G.; Martín-Ruiz, R.; Arroyo, V.; Rivera, F.; Rodüs, J.; et al. Endogenous Cannabinoids: A New System Involved in the Homeostasis of Arterial Pressure in Experimental Cirrhosis in the Rat. Gastroenterology 2002, 122, 85–93. [Google Scholar] [CrossRef]
- Kunos, G.; Járai, Z.; Bátkai, S.; Goparaju, S.K.; Ishac, E.J.N.; Liu, J.; Wang, L.; Wagner, J.A. Endocannabinoids as Cardiovascular Modulators. Chem. Phys. Lipids 2000, 108, 159–168. [Google Scholar] [CrossRef]
- Mourtakos, S.; Vassiliou, G.; Kontoangelos, K.; Philippou, A.; Tzavellas, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J.; Papageorgiou, C.; Sidossis, L.S.; Papageorgiou, C. Endocannabinoids and Heart Rate Variability Alterations after Exposure to Prolonged Intensive Physical Exercise of the Hellenic Navy Seals. Int. J. Environ. Res. Public Health 2022, 19, 28. [Google Scholar] [CrossRef]
- Jones, R.T. Cardiovascular System Effects of Marijuana. J. Clin. Pharmacol. 2002, 42, 48S–63S. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takáts, Z.; Bátkai, S.; et al. Endocannabinoid-Mediated Modulation of Gq/11 Protein-Coupled Receptor Signaling-Induced Vasoconstriction and Hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, O.; Ganguly, D. Endocannabinoids in Immune Regulation and Immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef]
- Miller, A.M.; Stella, N. CB2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lee, S.T.; Lin, W.W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: Involvement of eicosanoids. J. Cell. Biochem. 2001, 81, 715–723. [Google Scholar] [CrossRef]
- Sardinha, J.; Kelly, M.E.; Zhou, J.; Lehmann, C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair. Regeneration 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.K.; Zhang, M.; Li, J.Y.; Wang, L.L.; Guan, D.W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Reimer, R.A.; Sharkey, K.A. Cannabinoid signaling regulates inflammation and energy balance: The importance of the brain–gut axis. Brain Behav. Immun. 2012, 26, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Wiley, J.W. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Jansma, J.; Brinkman, F.; van Hemert, S.; El Aidy, S. Targeting the endocannabinoid system with microbial interventions to improve gut integrity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110169. [Google Scholar] [CrossRef]
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol. Ther. 2010, 126, 21–38. [Google Scholar] [CrossRef]
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Storr, M.A. Endocannabinoid and cannabinoid-like fatty acid amide levels correlate with pain-related symptoms in patients with IBS-D and IBS-C: A pilot study. PLoS ONE 2013, 8, e85073. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Nomura, D.K.; O’Neal, S.T.; Long, J.Z.; Mahadevan, A.; Cravatt, B.F.; Grider, J.R.; Lichtman, A.H. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J. Pharmacol. Exp. Ther. 2011, 338, 795–802. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef]
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2018, 51, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Ligresti, A.; Di Marzo, V. Peripheral effects of the endocannabinoid system in energy homeostasis: Adipose tissue, liver and skeletal muscle. Rev. Endocr. Metab. Disord. 2011, 12, 153–162. [Google Scholar] [CrossRef]
- Vettor, R.; Pagano, C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Gamelin, F.X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Rani Sagar, D.; Burston, J.J.; Woodhams, S.G.; Chapman, V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos. Trans. R Soc. Lond. B Biol. Sci. 2012, 367, 3300–3311. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef]
- Paszcuk, A.F.; Dutra, R.C.; da Silva, K.A.; Quintão, N.L.; Campos, M.M.; Calixto, J.B. Cannabinoid agonists inhibit neuropathic pain induced by brachial plexus avulsion in mice by affecting glial cells and MAP kinases. PLoS ONE 2011, 6, e24034. [Google Scholar] [CrossRef]
- Da Silva, S.R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar]
- Galdino, G.; Romero, T.; da Silva, J.P.; Aguiar, D.; de Paula, A.M.; Cruz, J.; Perez, A. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth. Analg. 2014, 119, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.; Matthys, D.; Hryhorczuk, C.; Sharma, S.; Mogra, S.; Alquier, T.; Fulton, S. Leptin Suppresses the Rewarding Effects of Running via STAT3 Signaling in Dopamine Neurons. Cell Metab. 2015, 22, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Butler, N. Sports participation and emotional wellbeing in adolescents. Lancet 1996, 347, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Dubreucq, S.; Koehl, M.; Abrous, D.N.; Marsicano, G.; Chaouloff, F. CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp. Neurol. 2010, 224, 106–113. [Google Scholar] [CrossRef]
- Aberg, E.; Hofstetter, C.P.; Olson, L.; Brené, S. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int. J. Neuropsychopharmacol. 2005, 8, 557–567. [Google Scholar] [CrossRef]
- Brené, S.; Bjørnebekk, A.; Aberg, E.; Mathé, A.A.; Olson, L.; Werme, M. Running is rewarding and antidepressive. Physiol. Behav. 2007, 92, 136–140. [Google Scholar] [CrossRef]
- Siebers, M.; Biedermann, S.V.; Fuss, J. Do Endocannabinoids Cause the Runner’s High? Evidence and Open Questions. Neuroscientist 2022, 10738584211069981. [Google Scholar] [CrossRef]
- Feuerecker, M.; Hauer, D.; Toth, R.; Demetz, F.; Hölzl, J.; Thiel, M.; Kaufmann, I.; Schelling, G.; Choukèr, A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur. J. Appl. Physiol. 2012, 112, 2777–2781. [Google Scholar] [CrossRef]
- Hoffmann, P.; Thorén, P.; Ely, D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR). Behav. Neural. Biol. 1987, 47, 346–355. [Google Scholar] [CrossRef]
- Revest, J.M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.V.; Abrous, D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Dranovsky, A.; Hen, R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychiatry 2006, 59, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sci. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.D.; Crombie, K.M.; Cook, D.B.; Hillard, C.J.; Koltyn, K.F. Serum Endocannabinoid and Mood Changes after Exercise in Major Depressive Disorder. Med. Sci. Sports Exerc. 2019, 51, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Jaromin, E.; Sadowska, E.T.; Koteja, P. Is Experimental Evolution of an Increased Aerobic Exercise Performance in Bank Voles Mediated by Endocannabinoid Signaling Pathway? Front. Physiol. 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef]
- Crombie, K.M.; Cisler, J.M.; Hillard, C.J.; Koltyn, K.F. Aerobic exercise reduces anxiety and fear ratings to threat and increases circulating endocannabinoids in women with and without PTSD. Ment. Health Phys. Act. 2021, 20, 100366. [Google Scholar] [CrossRef]
- Marin Bosch, B.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. Effect of acute physical exercise on motor sequence memory. Sci. Rep. 2020, 10, 15322. [Google Scholar] [CrossRef]
- Amatriain-Fernández, S.; Budde, H.; Gronwald, T.; Quiroga, C.; Carreón, C.; Viana-Torre, G.; Murillo-Rodríguez, E. The Endocannabinoid System as Modulator of Exercise Benefits in Mental Health. Curr. Neuropharmacol. 2021, 19, 1304–1322. [Google Scholar] [CrossRef]
- Forteza, F.; Giorgini, G.; Raymond, F. Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome. Cells 2021, 10, 938. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Berk, K.; Bzdega, W.; Kolakowski, A.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3083. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications. J. Diabetes Res. 2017, 2017, 1478294. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Vaanholt, L.M.; Davidova, A.; Mitchell, S.E.; Storey-Gordon, E.; Hambly, C.; Irving, A.J.; Speakman, J.R.; Hundal, H.S. CB1 receptor blockade counters age-induced insulin resistance and metabolic dysfunction. Aging Cell 2016, 15, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Côté, M.; Matias, I.; Lemieux, I.; Arsenault, B.J.; Cartier, A.; Piscitelli, F.; Petrosino, S.; Alméras, N.; Després, J.P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: Associations with changes in metabolic risk factors. Diabetologia 2009, 52, 213–217. [Google Scholar] [CrossRef]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aranda, F.; Sauchelli, S.; Pastor, A.; Gonzalez, M.L.; De La Torre, R.; Granero, R.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; Fernández-Real, J.M.; et al. Moderate-vigorous physical activity across body mass index in females: Moderating effect of endocannabinoids and temperament. PLoS ONE 2014, 9, e104534. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.C.; Liu, D.Y.; Zhang, L.L.; Shen, C.Y.; Ma, Q.L.; Cao, T.B.; Wang, L.J.; Nie, H.; Zidek, W.; Tepel, M.; et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-δ. Biochem. Biophys. Res. Commun. 2007, 354, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Zhu, X.; Zhao, G.; Yang, Q. Effect of diet and physical exercise on endocannabinoid system and energy homeostasis in obese mice. Int. J. Clin. Exp. Med. 2021, 14, 1445–1454. [Google Scholar]
- Gamelin, F.X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Leclair, E.; et al. Exercise training and high-fat diet elicit endocannabinoid system modifications in the rat hypothalamus and hippocampus. J. Physiol. Biochem. 2016, 73, 335–347. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Montel, V.; et al. Effects of chronic exercise on the endocannabinoid system in Wistar rats with high-fat diet-induced obesity. J. Physiol. Biochem. 2016, 72, 183–199. [Google Scholar] [CrossRef]
- Forteza, F.; Bourdeau-Julien, I.; Nguyen, G.Q.; Guevara Agudelo, F.A.; Rochefort, G.; Parent, L.; Raymond, F. Influence of diet on acute endocannabinoidome mediator levels post exercise in active women, a crossover randomized study. Sci. Rep. 2022, 12, 8568. [Google Scholar] [CrossRef]
- Schonke, M.; Martinez-Tellez, B.; Rensen, P.C.N. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Curr. Opin. Pharmacol. 2020, 52, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Fanelli, F.; Fazzini, A.; Pagotto, U.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Schiöth, H.B.; Benedict, C. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 2016, 74, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An Analysis of Endocannabinoid Concentrations and Mood Following Singing and Exercise in Healthy Volunteers. Front. Behav. Neurosci. 2018, 26, 269. [Google Scholar] [CrossRef]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S. Lancet Low Back Pain SeriesWorking Group. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Dorner, T.E. Pain and chronic pain epidemiology: Implications for clinical and public health fields. Wien. Klin. Wochenschr. 2018, 130, 1–3. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Rice, A.S.C.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef]
- Fisher, E.; Moore, R.A.; Fogarty, A.E.; Degenhardt, L.; Finn, D.P.; Finnerup, N.B.; Gilron, I. Cannabinoids, cannabis, and cannabis-based medicine for pain management: A systematic review of randomised controlled trials. Pain 2021, 162 (Suppl. 1), S45–S66. [Google Scholar] [CrossRef]
- Gedin, F.; Blome, S.; Ponten, M.; Lalouni, M.; Fust, J.; Raquette, A.; Vadenmark Lundquist, V.; Thompson, W.H.; Jensen, K. Placebo Response and Media Attention in Randomized Clinical Trials Assessing Cannabis-Based Therapies for Pain. A Systematic Review and Meta-analysis. JAMA Network Open 2022, 5, e2243848. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exerciseyour heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Szapora, A.; Pannekoek, J.N.; Hommel, B. The impact of physical exercise on convergent and divergent thinking. Front. Hum. Neurosci. 2013, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the life span on the brain, behavior and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Kool, W.; Hommel, B. Stress modulation of visuomotor binding. Neuropsychologia 2008, 46, 1542–1548. [Google Scholar] [CrossRef]
- Lecie, R.L.; Oberlin, L.E.; Voss, M.W.; Prakash, R.S.; Szabo-Reed, A.; Chaddock-Heyman, L.; Erickson, K.I. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front. Hum. Neurosci. 2014, 8, 985. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A.R. A role for the endocannabinoid system in exercise- induced spatial memory enhancement in mice. Hippocampus 2014, 24, 79–88. [Google Scholar] [CrossRef]
- Martinowich, K.; Lu, B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N.; Vitamin, D. The omega-3 fatty acids control serotonin synthesis and action, part2: Relevance for ADHD, bipolar, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
- Harmer, C.J. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology 2008, 55, 1023–1028. [Google Scholar] [CrossRef]
- Haider, S.; Khaliq, S.; Ahmed, S.P.; Haleem, D.J. Long-term tryptophan administration enhances cognitive performance and increases 5HT metabolism in the hippocampus of female rats. Amino Acids 2006, 31, 421–425. [Google Scholar] [CrossRef]
- Fattore, L.; Fadda, P.; Spano, M.S.; Pistis, M.; Fratta, W. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol. 2008, 286, S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.E.; Fleshner, M. Neuroplasticity of dopamine circuits after exercise: Implications for central fatigue. Neuromol. Med. 2008, 10, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; DeMeirleir, K. Exercise and brain neurotransmission. Sports Med. 1995, 20, 160–188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. https://doi.org/10.3390/ijms24031989
Matei D, Trofin D, Iordan DA, Onu I, Condurache I, Ionite C, Buculei I. The Endocannabinoid System and Physical Exercise. International Journal of Molecular Sciences. 2023; 24(3):1989. https://doi.org/10.3390/ijms24031989
Chicago/Turabian StyleMatei, Daniela, Dan Trofin, Daniel Andrei Iordan, Ilie Onu, Iustina Condurache, Catalin Ionite, and Ioana Buculei. 2023. "The Endocannabinoid System and Physical Exercise" International Journal of Molecular Sciences 24, no. 3: 1989. https://doi.org/10.3390/ijms24031989
APA StyleMatei, D., Trofin, D., Iordan, D. A., Onu, I., Condurache, I., Ionite, C., & Buculei, I. (2023). The Endocannabinoid System and Physical Exercise. International Journal of Molecular Sciences, 24(3), 1989. https://doi.org/10.3390/ijms24031989








