Abstract
Sodium nitroprusside (SNP), as a single minuscule signaling molecule, has been employed to alleviate plant stress in recent years. This approach has a beneficial effect on the biological and physiological processes of plants. As a result, an in vitro tissue culture experiment was carried out to investigate the effect of high and low levels of SNP on the amelioration of manganese (Mn) and chromium (Cr) toxicity in a one-year-old bamboo plant, namely Pleioblastus pygmaea L. Five different concentrations of SNP were utilized as a nitric oxide (NO) donor (0, 50, 80, 150, 250, and 400 µM) in four replications of 150 µM Mn and 150 µM Cr. The results revealed that while 150 µM Mn and 150 µM Cr induced an over-generation of reactive oxygen species (ROS) compounds, enhancing plant membrane injury, electrolyte leakage (EL), and oxidation in bamboo species, the varying levels of SNP significantly increased antioxidant and non-antioxidant activities, proline (Pro), glutathione (GSH), and glycine betaine (GB) content, photosynthesis, and plant growth parameters, while also reducing heavy metal accumulation and translocation in the shoot and stem. This resulted in an increase in the plant’s tolerance to Mn and Cr toxicity. Hence, it is inferred that NO-induced mechanisms boosted plant resistance to toxicity by increasing antioxidant capacity, inhibiting heavy metal accumulation in the aerial part of the plant, restricting heavy metal translocation from root to leaves, and enhancing the relative water content of leaves.
1. Introduction
In recent years, due to rising anthropogenic activity, both natural resources and the human environment have been contaminated by heavy metals, thus posing a hazard to human society’s health [1]. When plants uptake nutrients from the soil, some heavy metals and non-heavy metals are absorbed through their roots, which can induce fundamental alterations in cellular metabolism [2]. Manganese (Mn) is one of the essential micronutrients with a widespread abundance in China’s forestland and agricultural soils [3]. Although Mn can be used as a plant nutrient and a trace element to promote plant growth and development, excess levels of Mn induce toxicity in plants [4]. Extreme levels of Mn2+ can induce a destructive impact on the plant photosynthesis process with generation of ROS through the Fenton reaction, which inhibits the co-factoring role of the element in vital enzymatic reactions and disturbs the photosynthetic processes in plants [5]. Moreover, the preponderance of Mn2+ reduces uptake and translocation of some plant essential elements such as potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) [3], which can directly impact on plant growth and development. Chromium (Cr) can be released into the environment due to its wide use in industries, such as leather processing, mining, wood preservation, petroleum refining, and textile electropainting and manufacturing, etc., and this has resulted in an environmental dilemma [6,7]. Cr and Cr compounds can affect humans through skin contact, eating, breathing, as well as drinking [8]. In China, Cr (VI) ions are the most abundant toxic metal ion resulting from pollution into river waters [9]. In addition, China is the country with the largest production of Cr slag, with 329,000 tons produced and 450,000 tons discharged annually. It is possible that there is stored more than 400 million tons of untreated Cr slags from previous years’ production (State Ministry of Environmental Protection (MEP), 2007) [10]. The chromium in agricultural soil remarkably impacts on the grain quality of plants as well as crop yield, which is a threat to the human food chain [8]. In plants, Cr toxicity reduces plant growth with an impact on chloroplasts and cell membranes and by inducing root cell damage. Chromium, with accompanying chlorosis, influences plant morphology. In a physiological aspect, Chromium can affect mineral nutrition and water retention, enzymatic activities, and pigment content, and could disrupt nitrogen assimilation, transpiration as well as plant photosynthesis and growth [11,12,13]. Chromium leads to the over generation of ROS, which can disrupt the plant redox balance [13]. When a plant is exposed to abiotic stress, it responds typically by generating ROS [14]. Thus, the synthesis of ROS has altered the cellular redox homeostasis, which is the main factor of oxidative stress in plants under heavy metal stress [15]. Additionally, oxidative stress has a debilitating effect on cell damage and plant necrosis, leading to plant cell death [16]. Nitric oxide acts as a signaling molecule in this process by regulating ROS compounds [17].
Nitric oxide is a small signaling molecule and a crucial regulator of the plant life cycle [11], which acts positively during various stages of the plant life cycle, such as germination, flowering, development, and senescence, as well as in the amelioration of abiotic stress [2,18]. Thus, this neutral and tiny redox molecule has a great capacity to spread through the cell membrane and is frequently referred to as a dynamic molecule [11]. SNP, a nitric oxide donor, has been implicated in signaling responses to abiotic and biotic stress in plants [11]. This has revealed the role of SNP as an exogenous NO donor in alleviating abiotic stresses, particularly those caused by heavy metals [2,12,19,20,21,22]. Nitric oxide has been observed to both suppress and induce cell death, depending on a variety of factors such as the dose and flux of local nitric oxide, plant species, and various concentrations of heavy metals. For instance, in cell suspension cultures of ARABIDOPSIS, grown at two different concentrations of CdCl2, nitric oxide with accelerated senescence produced cell death [23]. On the other hand, NO can help to mitigate and detoxify the high concentrations of ROS that cause detrimental effects on plant cells [24]. The protective role of NO in reducing ROS in plants has been indicated in several studies [13,25]. However, the positive action of nitric oxide depends on the flux and concentrations of nitric oxide and heavy metals, plant species, and nitric oxide quantification methods [26,27]. Numerous studies have reported that SNP contributes to the preservation of plant cells during stressful conditions [28,29,30]. However, there is not enough knowledge regarding the effects of different SNP doses as a nitric oxide donor on heavy metals. In this attempt, we will explore the role of high and low SNP concentrations in the reduction of toxicity in Pleioblastus pygmaea L. in order to determine the optimal SNP detoxification levels. To the best of the authors’ knowledge, this is the first study in this field on bamboo species (Pleioblastus pygmaea L.). We hypothesize that increasing the antioxidant capacity of SNP alleviates oxidative stress and reduces ROS compounds in plants, and that high concentrations of SNP have a significant effect on the reduction of H2O2 content and ROS compounds. Bamboo plants belong to the Poaceae family, which is classified as Bambusoideas in a subfamily [31,32]. Every year, new bamboo shoots emerge from the bamboo rhizomes in bud sites and bamboo shoots expand into a new culm and emerge in spring, while bamboo rhizomes and their root systems expand during the year. Bamboo growth increases in summer and autumn [33]. Bamboo, as a perennial evergreen woody plant, is known to be a cost-effective forestry product [34,35]. It is widespread across the tropics and subtropics and covers a large area (>6 million hectares) of Chinese forestland. Bamboo plants have been categorized into 500 species and 70 genera [36,37]. Bamboo’s rapid growth and huge biomass make it an excellent candidate for pollutant bioaccumulation as well as plant phytoremediation purposes [33]. In addition, Bamboo leaves are known as an effective tool for the removal of pollution in wastewater [38]. On the other hand, this remarkable plant, as an Asia native, is one of the primary economic resources and livelihoods of indigenous people in the southern and southeast regions of Asia [33]. It contributes significantly to the local populace’s economy, with over US $19.7 billion granted by the State Forestry Administration of China Beijing, China, 2012 [39]. Some bamboo species as ornamental plants have been used as eco-friendly and sustainable solutions to remove contamination and clean up air pollution in urban areas, thereby enhancing the commercial aspect of gardening and beautification by serving as a pollution indicator for phytoremediation purposes [40]. Pleioblastus pygmaea L. has been used in numerous landscaping projects as an ornamental and evergreen bamboo species. This bamboo species (which grows to a height of 30–50 cm height) was introduced into China from Japan in the last few decades. It has been known as a resistant bamboo in a variety of soil types, including neutral, basic (alkaline), and acidic soils [41]. Additionally, in some Chinese provinces, such as Jiangsu, it has been employed for urban beautification, and additionally for landscape purposes. Pleioblastus pygmaea L. has been identified as a viable plant for soil, air, and environmental pollution clearance. As a result of growing anthropogenic activity and industrialization in recent decades, a huge portion of China’s agricultural and forest land has become contaminated with toxic metals such as Mn and Cr, posing a serious threat to human health [42]. Southeastern China is the largest bamboo shoot-producing region, in recent research on six bamboo species in this region, Mn and Cr are introduced as two main polluting metals which can threaten the human food chain with high accumulation in shoots [43]. Thus, it is essential to focus on identifying the most effective means for reducing pollution from soil and plants in this area, as well as investigating techniques to increase bamboo’s tolerance against metal toxicity. The primary objective of this research is to evaluate the possibility of increasing plant tolerance by stimulating involved mechanisms such as antioxidant activity and relative water content, as well as reducing heavy metal accumulation and limiting root to shoot translocation, using high and low doses of SNP as a nitric oxide donor to bamboo plants under Mn and Cr.
2. Results
2.1. Mn and Cr Accumulation in Root, Stem, and Leaves in Bamboo Species
Metal accumulation in various plant organs is one way for plants to respond to the increased stress caused by heavy metals. According to the findings of the present study, increasing the concentration of SNP significantly reduces the Mn and Cr accumulation in Pleioblastus pygmaea L. The results of this experiment revealed a significant difference between the various levels of SNP alone and in combination with Mn and Cr (p < 0.001). Thus, the greatest reduction in Mn and Cr was attributed to a combination of 400 µM SNP, 150 µM Mn, and 150 µM Cr, which resulted in 55 and 48% reductions in bamboo leaves, 61 and 48% reductions in bamboo stems, and 59 and 50% decrements in bamboo roots, respectively, in comparison to their control. On the other hand, as reported in Table 1, the content of heavy metals (i.e., Mn and Cr) in roots is greater than that in stems and leaves, demonstrating the role of SNP in reducing metal translocation from roots to stems and leaves. As a result, we hypothesized that SNP as a nitric oxide (NO) donor has the potential to significantly reduce metal accumulation in plant organs such as roots, stems, and leaves, which could be due to nitric oxide’s ability to absorb and bind Mn and Cr ions, or it could be due to nitric oxide’s role as a physical barrier to metal translocation to stems and leaves. In any case, SNP as a nitric oxide (NO) donor increases the uptake of nutrient elements while reducing the absorbance of toxic metals such as Mn and Cr in Pleioblastus pygmaea L. (Table 1).

Table 1.
The content of various levels of SNP and Mn and Cr accumulation in bamboo stems, leaves, and roots. The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between different concentrations of SNP used individually or in combination with 150 μM Mn and 150 μM Cr, as determined by Tukey′s test (p < 0.05).
2.2. Tocopherols, Flavonols, and Total Phenolics in Plants under Mn and Cr
The levels of flavonols, tocopherols, and total phenolics were measured to ascertain the effect of SNP on the stimulation of plants’ non-antioxidant activity in the presence of Mn and Cr toxicity. Our results indicate that there is a significant difference in the indices of flavonols, tocopherols, and total phenolics between the different concentrations of SNP in a combination of Mn and Cr (p < 0.001), which has demonstrated that SNP concentrations significantly increase flavonols, tocopherols, and total phenolics. Thus, the greatest enhancement was associated with 400 µM and 250 µM SNP concentrations, which increased flavonols by 22% and 19%, tocopherol by 22% and 18% and total phenolics by 24% and 21%, respectively, in comparison to their control treatments. On the other hand, the results indicated that 400 µM SNP in a combination of Mn and Cr enhanced non-antioxidant activity the most, with 51% and 47% increases in flavonols, 53% and 44% increases in tocopherols, and 53% and 39% increases in total phenolics, respectively, when compared to their control treatments. We hypothesized that varying the concentration of SNP enhances non-antioxidant activity as a second metabolism in Pleioblastus pygmaea L. (Figure 1).
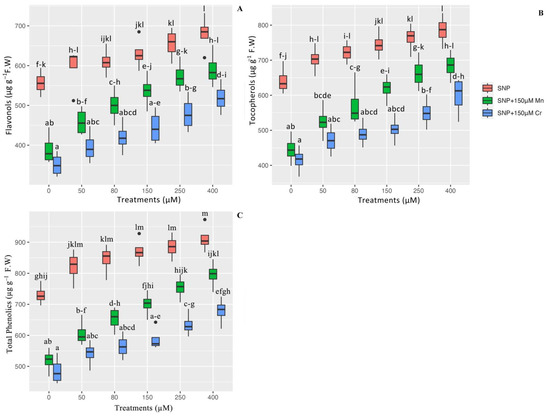
Figure 1.
The effect of various concentrations of SNP individually and in combination with Mn and Cr on non-enzyme antioxidant activity (flavonols (A), tocopherols (B), and total phenolics (C)). The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences across all treatments based on Tukey′s test (p < 0.05).
2.3. Proline Contents (Pro), Glycine Betaine (GB), Glutathione (GSH), and Relative Water Content (RWC) in Pleioblastus pygmaea L. under Mn and Cr
To determine the efficacy of SNP in reducing heavy metal toxicity, several important indices such as the content of GB, Pro, GSH, and RWC were measured. According to the data analysis, there is a significant difference in the effects of SNP in combination with Mn and Cr on GB, Pro, GSH, and RWC (p < 0.001). While the 150 µM Mn and 150 µM Cr reduced the content of GB, Pro, GSH, and RWC in Pleioblastus pygmaea L, the addition of various concentrations of SNP significantly increased the content of GB, Pro, GSH, and RWC in Pleioblastus pygmaea L. exposed to Mn and Cr. According to the results, the greatest increase in the content of these indices was attributed to high concentrations of SNP (400 µM and 250 µM), which increased GB by 44% and 36%, proline (Pro) by 38% and 35%, GSH by 48% and 42%, and RWC by 8% and 6%, respectively. On the other hand, the results indicated that 150 µM Mn and 150 µM Cr had the greatest reductions in the content of GB, Pro, GSH, and RWC, with 46%, 51%, 43%, and 26% reductions for 150 µM Mn and 58%, 69%, 54%, and 31% reductions for 150 µM Cr, respectively, in comparison to their control treatment. We can conclude that all concentrations of SNP significantly increased the content of GB, Pro, GSH, and RWC in plants exposed to Mn and Cr. However, the highest concentrations of SNP were more effective at increasing these indices (Figure 2).
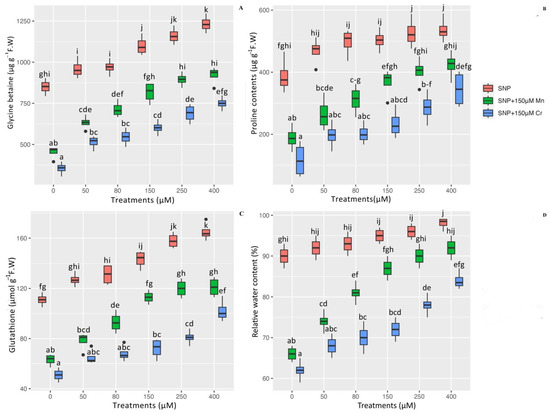
Figure 2.
The effect of various concentrations of SNP individually and in combination with Mn and Cr on glycine betaine (GB) (A), proline content (Pro) (B), glutathione (GSH) (C), and relative water content (RWC) (D) in Pleioblastus pygmaea L. exposed to Mn and Cr. The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between treatments as determined by Tukey′s test (p < 0.05).
2.4. ROS Component and Amelioration Lipid Peroxidation in Pleioblastus pygmaea L. under Mn and Cr Stress
The investigation of the detrimental effects of metal toxicity on the generation of ROS compounds and plant cell membranes is a critical issue in plants under stress. As a result, it is necessary to determine the ROS compounds, such as H2O2 and O2•− as well as lipid peroxidation indicators, such as MDA and electrolyte leakage, in plants under stress. The results of this study revealed a significant difference in the indices of MDA, H2O2, EL, and O2•− between different concentrations of SNP with 150 µM Mn and 150 µM Cr (p < 0.001). Thus, while 150 µM Mn and 150 µM Cr increased cell oxidation and lipid peroxidation, SNP significantly reduced MDA, H2O2, O2•−, and EL in Pleioblastus pygmaea L. exposed to Mn and Cr, respectively. This result showed the greatest reduction was the high concentration of SNP (400 µM SNP), which reduced MDA, H2O2, O2•−, and EL by 70%, 47%, 58%, and 66%, respectively, in comparison to their control treatment. On the other hand, the results revealed that the combination form of 400 µM SNP with heavy metals (150 µM Mn and 150 µM Cr) resulted in the greatest reduction in the indices of Malondialdehyde (MDA), hydrogen peroxide (H2O2), superoxide radical (O2•−), and electrolyte leakage (EL) with enhancements of 70%, 44%, 79%, and 93% by 150 µM Mn and 84%, 52%, 96%, and 99% by 150 µM Cr, respectively, in comparison to their control treatments. We hypothesized that different concentrations of SNP mitigated the detrimental effects of ROS components (H2O2 and O2•−) on plant cells and protected the cell membrane from oxidation, resulting in a decrease in MDA content and EL percentage (Figure 3).
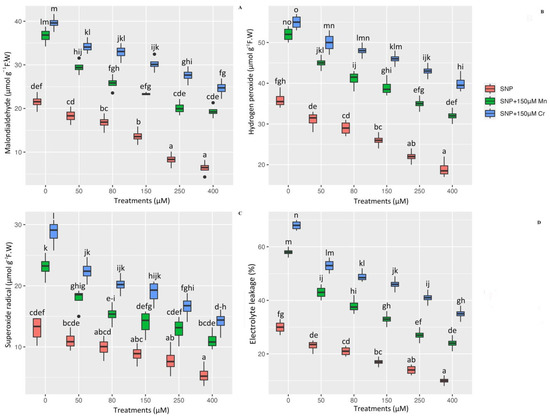
Figure 3.
The effect of various concentrations of SNP individually and in combination with Mn and Cr on malondialdehyde (MDA) content (A), hydrogen peroxide (H2O2) (B), superoxide radical (O2•−) (C), and electrolyte leakage (EL) (D) in Pleioblastus pygmaea L. exposed to Mn and Cr. The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between different concentrations of SNP used individually or in combination with 150 μM Mn and 150 μM Cr, as determined by Tukey′s test (p < 0.05).
2.5. Antioxidant Enzyme Activity in Plants Exposed to Mn and Cr Toxicity
Antioxidants are the first line of plant defense for plants exposed to heavy metal stress. They play a critical role in protecting plant cells from ROS compounds and free radicals, as well as preventing plant oxidation under metal stress. The present study’s data analysis revealed a significant difference in the activity of antioxidant enzymes such as SOD, POX, CAT, APX, GR, and PAL between the different concentrations of nitric oxide and 150 µM Mn and 150 µM Cr (p < 0.001), which showed that various concentrations of SNP significantly increased antioxidant enzyme activity. However, the results indicated that the highest concentration of nitric oxide (400 µM) is the most effective at enhancing plant antioxidant activity under stress, with increases in SOD, POD, CAT, APX, GR, and PAL activity of 78%, 34%, 62%, 30%, 28%, and 24%, respectively, when compared to the control treatments. Additionally, the results indicated that Mn and Cr had the lowest increase in antioxidant capacity, with 150 µM Mn and 150 µM Cr increasing SOD by 62% and 77%, POD by 44% and 59%, CAT by 43% and 61%, APX by 33% and 42%, GR by 49% and 64%, and PAL by 31% and 48%, respectively, when compared to control treatments. We can suggest that all concentrations of SNP increase the antioxidant activity of Mn and Cr in plants, thereby protecting cells from oxidation. However, the results indicated that as SNP levels increased, the antioxidant capacity increased as well (Figure 4).
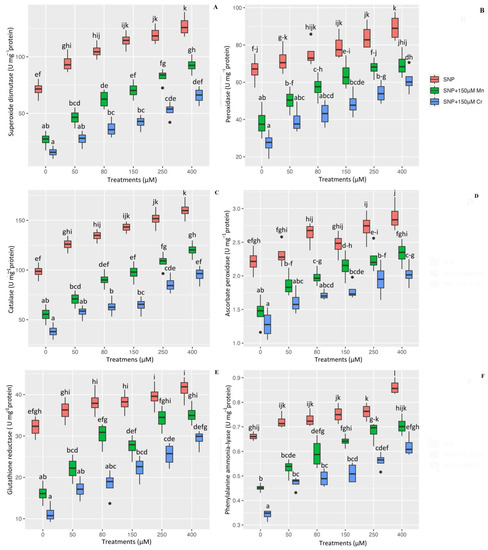
Figure 4.
The effect of various concentrations of SNP individually or in combination with Mn and Cr on antioxidant enzyme activity, such as superoxide dismutase (SOD) (A), peroxidase (POX) (B), catalase (CAT) (C), ascorbate peroxidase (APX) (D), glutathione reductase (GR) (E), and phenylalanine ammonia-lyase (PAL) (F) in Pleioblastus pygmaea L. exposed to Mn and Cr. The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between different concentrations of SNP used individually or in combination with 150 μM Mn and 150 μM Cr based on Tukey′s test (p < 0.05).
2.6. Plant Photosynthetic Pigments, including Chlorophyll a, Chlorophyll b, Total Chlorophyll and Carotenoids Exposed to Mn and Cr
Photosynthesis parameters are critical for evaluating plant metabolism under stress conditions that can directly affect plant growth and development. For this purpose, we measured photosynthetic pigments, including chlorophyll a and chlorophyll b as well as carotenoids, in Pleioblastus pygmaea L. under Mn and Cr with and without the addition of various levels of SNP. We hypothesized that the SNP could enhance photosynthesis properties. The results indicated that there is a significant difference between the various levels of SNP when combined with Mn and Cr (p < 0.001). As a result, we discovered that increasing SNP levels increased photosynthetic pigments in plants exposed to metal toxicity. However, the greatest increase in chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids was associated with 400 µM and 250 µM SNPs, with increases of 13% and 10% in chlorophyll a, 35% and 30% in chlorophyll b, 39%, and 33% in total chlorophyll, and 34% and 29% in carotenoid, respectively, in comparison to their control treatment. On the other hand, 400 µM SNP had the greatest effect on increasing photosynthesis parameters in plants grown in 150 µM Mn and 150 µM Cr, with 57% and 58% increases in chlorophyll-a, 90% and 74% increases in chlorophyll-b, 72% and 65% increases in total chlorophyll, and 70% and 95% increases in carotenoid content, respectively, in comparison to their control treatments. Samples treated with 150 µM Mn and 150 µM Cr reduce photosynthesis pigments by 35% and 41% in chlorophyll-a, 41% and 48% in chlorophyll-b, 30%, and 37% in total chlorophyll, and 30% and 50% in carotenoids, respectively, in comparison to control treatment (Table 2).

Table 2.
The effect of various SNP concentrations on chlorophyll pigments (Chl-a, Chl-b, Total Chl, and Carotenoid). The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between different concentrations of SNP used individually or in combination form with 150 μM Mn and 150 μM Cr, as determined by Tukey′s test (p < 0.05).
2.7. Plant Growth, and Plant Biomass on the Dry Weight of Shoot and Root as Well as Plant Shoot Length under Mn and Cr
Shoot and root dry weights, as well as shoot length, are used as indicators of plant biomass and growth in this study, which are necessary to demonstrate the morphologic impact of SNP on heavy metal toxicity. According to the results, there was a significant difference in shoot and root dry weight, as well as plant shoot length, between the different levels of SNP in combination with 150 µM Mn and 150 µM Cr (p < 0.001) (Figure 5). This indicated that various concentrations of SNP enhance plant growth and biomass indexes both individually and in combination with Mn and Cr. However, the greatest increase was observed with 400 µM and 250 µM SNP, respectively, with 1.08 g and 1.02 g shoot dry weight, 1.38 g and 1.27 g root dry weight, and 17.02 cm and 16.65 cm in shoot length, respectively. Additionally, the results revealed that 400 µM SNP in plants under Mn and Cr increases plant biomass by 61% and 54% in shoot dry weight, 61% and 44% in root dry weight, and 27% and 24% in shoot length, respectively, in comparison to their control treatment. This demonstrated that varying concentrations of SNP mitigated the destructive effects of heavy metals on plant growth. According to Table 3, 150 µM Mn and 150 µM Cr significantly reduce shoot dry weight by 33% and 42%, root dry weight by 32% and 36%, and plant shoot length by 18% and 22%, respectively when compared to the control treatment (Table 3). We hypothesized that varying levels of SNP increase plant biomass as well as plant growth exposed to Mn and Cr, which is associated with an increase in Pleioblastus pygmaea L. antioxidant capacity. This in turn increases chlorophyll pigments and, ultimately, plants’ growth.
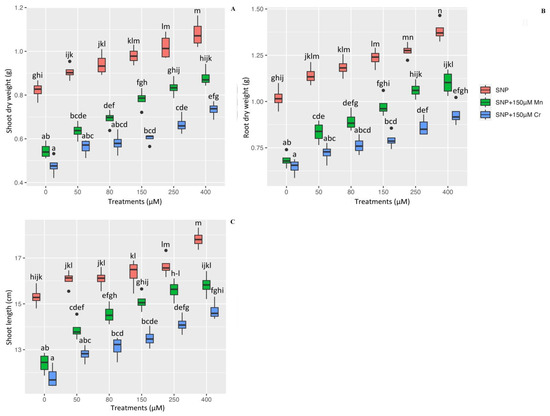
Figure 5.
The effect of various concentrations of SNP individually and in combination with Mn and Cr on shoot dry weight (A), root dry weight (B), and shoot length (C) in bamboo species exposed to Mn and Cr. The data indicated the mean ± standard error of four repetitions. The treatments include five different concentrations of SNP, either individually or in combination with 150 μM manganese and 150 μM chromium. Different lower-case letters indicate significant differences between different concentrations of SNP used individually or in combination with 150 μM Mn and 150 μM Cr based on Tukey′s test (p < 0.05).

Table 3.
The effects of different concentrations of SNP on Pleioblastus pygmaea L. biomass in terms of shoot and root dry weights and shoot length, both individually and in combination with manganese and chromium, in comparison to the control treatment. ↑ indicates increases and ↓ indicates decreases.
2.8. Effects of Nitric Oxide on Plant Tolerance Indices (TI) in Shoot and Root, as Well as Bio Accumulation Factor (BAF) and Plant Translocation Factor (TF) in Pleioblastus pygmaea L. Exposed to Mn and Cr Toxicity
The TI, BAF, and TF all play critical roles in determining a plant’s resistance to metal toxicity. In fact, these are some of the major mechanisms by which nitric oxide can reduce metal toxicity in plants. Our findings indicate that there is a statistically significant difference between various levels of SNP in combination with Mn and Cr in TI, BAF, and TF (p < 0.001) (Table 4). Thus, the results indicate that TF from root to leaf decreases with the addition of SNP, with the greatest reduction attributed to the combination of high concentrations of SNP with Mn and Cr, which resulted in a 9% and 8% decrease in the translocation factor of Mn and Cr, respectively. Additionally, the results indicated that BAF of Mn and Cr decreased by 56% and 49% in the leaves, respectively, when compared to the control treatment. However, the BAF in the leaves and stem was less than that in the roots, indicating that nitric oxide helps to accumulate Mn and Cr in roots, thereby limiting metal toxicity transfer to the stem and leaves, potentially improving the tolerance factor in Pleioblastus pygmaea L. Our results demonstrate that the 400 µM and 250 µM SNP concentrations have the highest TI in the bamboo species, increasing the TI of the shoot by 31% and 24%, and the TI of the root by 35% and 25%, respectively, when compared to the control treatment. On the other hand, evaluation of the various levels of SNP indicated that nitric oxide has the potential to increase TI in Pleioblastus pygmaea L. exposed to Mn and Cr; thus, the greatest increase in TI in shoot and root was attributed to the combination of 400 µM SNP with 150 µM Mn and 150 µM Cr, which resulted in a 61% and 54% increase in shoot tolerance index and a 61% and 43% increase in root tolerance index, respectively, when compared to control treatments. According to Table 1, roots accumulate more Mn and Cr than stems and leaves, indicating that bamboo roots are capable of accumulating a significant amount of Mn and Cr. This is consistent with previous research indicating that bamboo accumulates in tissues in the rhizome and culm, primarily in the radius of the vacuole, cell wall, and cytoplasm of a plant cell. As a result, we concluded that SNP enhances the phytoremediation potential of Pleioblastus pygmaea L. in polluted areas by increasing the accumulation of Mn and Cr on the root surface.

Table 4.
Changes in the translocation factor and tolerance index of shoots and roots, as well as the bioaccumulation factor, in response to SNPs individually or in combination with 150 μM Mn or 150 μM Cr, when compared to the control treatment. The data indicated the mean ± standard error of four repetitions. Different lower-case letters The lowercase letters (a, b, c, d, etc.) indicate significant differences between different concentrations of SNP used individually or in combination form with 150 µM Mn and 150 µM Cr, as determined by Tukey’s test (p < 0.05).
3. Discussion
Nitric oxide is a gaseous molecule with multifunctions (lipophilic, paramagnetic gas, free radical, and charge-free with a short half-life) that has the ability to mitigate the toxicity of heavy metals at both exogenous and endogenous levels [44,45,46]. It has been implicated as a NO-mediated mediator in a variety of physiological processes in plants exposed to different types of stresses, including heavy metals [47]. Nitric oxide has two significant mechanisms by which it can alleviate oxidative stress in plants. Firstly, nitric oxide acts directly as a scavenger of ROS compounds, forming peroxynitrite when it reacts with superoxide radicals. Therefore, nitric oxide reduces cell toxicity, resulting in less cell damage in plants [48]. The second action is related to the signaling role of nitric oxide in the reduction of stress, as nitric oxide is a signaling molecule involved in antioxidative gene expression alterations [49]. On the other hand, in plants under stress, ROS regulates electron transport pathways in plant organs and tissues such as mitochondria. Where ROS modulation occurs, it stimulates the plant’s defense mechanism, resulting in an increase in the activity of antioxidant enzymes [50]. Exogenous nitric oxide with activating antioxidant capacity scavenges free radicals such as organic radicals, superoxide anion, and lipid O2 as well as H2O2 [51,52]. Numerous studies have demonstrated that nitric oxide increases antioxidant activity in plants exposed to heavy metals [53,54,55,56,57], which was also observed in the present study. Thus, in order to determine whether the decrease in Mn and Cr toxicity in bamboo plants is related to the antioxidant capacity of nitric oxide, the activity of antioxidant enzymes such as SOD, POD, CAT, APX, GR, and PAL was examined. Our results demonstrated that increasing the concentration of SNP as one nitric oxide donor increased antioxidant enzyme activity, which is consistent with the activation of the antioxidant enzyme’s gene expression induced by high and low SNP doses. Flavonols, tocopherols, and total phenolics are all important non-enzyme antioxidants that protect against heavy metal toxicity. In fact, increasing flavonols and total phenolics is a critical strategy for plants exposed to heavy metals [58,59]. Due to the electron-donating agents, phenolic compounds act as antioxidants, scavenging ROS compounds and free radicals [60,61]. Additionally, flavonols have antioxidant properties in abiotic stores [62]. When a plant is stressed, it accumulates more flavonols to protect itself [63]. It appears that SNP as a nitric oxide donor stimulates gene expression involved in flavonoid biosynthesis, thereby increasing flavonoid accumulation in plants exposed to metal toxicity [64]. Our data analysis revealed that varying SNP concentrations can enhance non-antioxidant activity in Pleioblastus pygmaea L. exposed to Mn and Cr. As a result, we hypothesized that SNP, through its effects on gene expression involved in the biosynthesis of non-antioxidant enzyme activity (flavonols, tocopherols, and total phenolics), may enhance plant second metabolism under stress conditions.
MDA is one indicator of increased lipid peroxidation in plants exposed to excessive amounts of heavy metals [65]. H2O2 is generated in plant cells in response to metal toxicity [66]. Exogenous nitric oxide has been shown to reduce MDA levels as well as ROS compounds in a variety of plant species, including rice and mung bean [44,45]. On the other hand, nitric oxide has the potential to directly eliminate the toxic effects of O2•− through the conversion of O2•− to ONOO− [67]. As we hypothesized in our current study, the addition of SNP as a nitric oxide donor to Mn and Cr reduces the superoxide radical (O2•−) content. Our findings indicated that 150 µM Mn and 150 µM Cr increased H2O2, resulting in increased lipid peroxidation and electronic leakage. Our findings, however, indicate that the addition of SNP remarkably reduces Mn and Cr toxicity, thereby limiting lipid peroxidation and electrolyte leakage across the plant cell membrane. Thus, we hypothesized that increased antioxidant activity of SNP scavenges ROS compounds such as H2O2 and O2•−, which contribute to increased lipid peroxidation and electrolyte leakage across the cell membrane. Methylglyoxal (MG) is a component of a reactive cytotoxic α-oxo aldehyde that is abundantly produced in stressed plants [68,69]. Stimulating GSH synthesis via nitric oxide levels is one of the primary defense mechanisms against plant cell oxidation [70], which is associated with the GSH-mediated regulation of MG levels [71,72,73]. Thus, Gly I, the first enzyme in the glyoxalase pathway, was co-factored by GSH during MG reduction, and nitric oxide can impact the glyoxalase pathway enzymes [74]. Our findings indicated that increasing the concentration of SNP significantly increased the GSH concentration in plants exposed to Mn and Cr, demonstrating the protective role of nitric oxide in a plant cell.
By accumulating osmotic constituents, nitric oxide preserves cell water content and thus alleviates plant stress [75]. Among osmolytes, glycine betaine and proline have beneficial effects on membrane stability, plant osmoregulation, and plant stress reduction. Glycine betaine and proline have been shown to act as co-factors in the activity of hydrated enzymes [76]. Glycine betaine is a major compatible substance that has been shown to regulate plant osmotic balance under stress conditions [77]. Additionally, it has been reported that glycine betaine plays a beneficial role in increasing photosynthetic pigments. Thus, accumulation of glycine betaine can improve photosynthesis efficiency in plants that are exposed to heavy metals [78]. Proline can either fix protein complexes in plant cells or directly scavenge oxygen free radicals. Additionally, proline acts as a signal for downstream events to occur [79]. SNPs have been shown to increase proline levels in stressed plants. The correlation between proline and nitric oxide is due to the fact that they both utilize L-arginine as a common precursor in their biosynthesis [56]. On the other hand, numerous studies have reported an increase in the glycine betaine and proline levels in plants exposed to heavy metals [80,81,82]. Our results establish a correlation between proline and glycine betaine levels, demonstrating that when nitric oxide is added, the content of proline and glycine betaine accumulates in Pleioblastus pygmaea L. exposed to Mn and Cr toxicity. The RWC of the leaves is a critical factor in the reduction of heavy metal stress. Nitric oxide increases water content by increasing wall extensibility, cell division, and plant morphological characteristics such as leaf area, weight, and length [82]. While 150 µM Mn and 150 µM Cr decreased the RWC of Pleioblastus pygmaea L., the addition of SNP increased the index of RWC in Pleioblastus pygmaea L. exposed to toxicity. This has already been demonstrated in numerous publications on bean seeds [45] and wheat (Triticum aestivum L.) [56,83].
The accumulation of heavy metals in plants reduces the synthesis of chlorophyll pigments, which is related to changes in the chlorophyll biosynthetic intermediates, and thus has a detrimental effect on the pigment–protein complex [84]. Nitric oxide preserves the chlorophyll pigment and chloroplast membrane through the viscosity of the cytoplasm, which is maintained through the preservation of osmotic pressure [82]. Numerous studies have documented the role of nitric oxide in alleviating the negative effects of heavy metals on photosynthesis as well as promoting photosynthesis properties [46,55,85,86,87]. This is consistent with our findings in Pleioblastus pygmaea L, indicating that different concentrations of SNP significantly increase photosynthesis pigments such as chl-a, chl-b, total chl, and carotenoid in plants grown in 150 µM Mn and 150 µM Cr. Nitric oxide has been shown to regulate plant abiotic stress tolerance through alteration of exogenous nitric oxide levels, resulting in increased crop and plant production under stressful conditions [82,88]. Typha angustifolia under cadmium [80], vicia faba under arsenic [89], and oryza sativa (rice) under cadmium [90] demonstrated the effect of nitric oxide on promoting plant biomass and growth. This can be explained by nitric oxide’s inducing effect on cell wall relaxation and expansion, as well as its protective effect on the phospholipid bilayer, which results in increased plant growth and development under stress [91,92]. On the other hand, it has elucidated the role of nitric oxide in the protective alteration of plant roots against oxidative damage in several plant species, including Citrus grandis [93], Oryza sativa [44,94], Brassica juncea [46], and Lupinus luteus [95], which can be an important issue in improving plant growth. Additionally, there is another reason that demonstrates the efficiency of nitric oxide in plant growth. That is attributed to the role of nitric oxide in enhancing the osmotic pressure within the cell as well as ameliorating the viscosity of the cytoplasm [96,97]. As a result, it can be concluded that SNP as a nitric oxide donor has the ability to increase plant growth and biomass in the presence of heavy metals, as demonstrated in our study. Thus, our study revealed that various concentrations of SNP increase plant biomass and growth (dry weight of shoot and root as well as shoot length) in the presence of Mn and Cr. This has been demonstrated by the beneficial effects of Mn on Oryza sativa (rice) growth [98] and Cr on triticum aestivum (wheat) growth [80]. Additionally, our results indicate that 400 µM SNP has the greatest effect on plant growth and biomass, implying that a high concentration of SNP could be significantly more effective at promoting plant growth in the presence of Mn and Cr toxicity.
It has been reported that the initial mechanisms of the protective role of nitric oxide in various doses and forms are likely to begin with the reduction of metal accumulation [99] and progress to the amelioration of metal-induced oxidative stress [100,101]. The findings of this study indicate that SNP as a nitric oxide donor limits the accumulation and uptake of heavy metals in plant organs, which can be a protective mechanism against metal stress, and this has been confirmed by other studies as well [82,102]. On the other hand, in one study on rice, nitric oxide resulted in the enhancement of hemicellulose and pectin, which could improve Cd detoxification by increasing the accumulation of Cd in the root cell walls while decreasing the Cd content in the leaves’ cell walls [103]. This is consistent with our findings in the current study. As a result, the findings revealed that metal accumulation in plant roots is greater than that in the shoot and stem, demonstrating the beneficial effect of SNP on the reduction of heavy metal translocation from root to shoot. Hence, SNP can absorb and bind with metal ions on the root surface, preventing the transfer of Mn and Cr from the root to the aerial parts, thereby increasing the tolerance factor of the plant under stress. Additionally, it has been suggested that the combination of SNP and heavy metal accumulation on the root surface and root cell walls increases plant phytoremediation and plant recovery.
4. Materials and Methods
4.1. Experiment Materials and Vitro Plant Tissue Culture
A single clone of P. pygmaeus, a one-year-old sapling, was provided by the bamboo research base garden at Nanjing Forestry University. For the pre-experimental study, approximately 10 mm-long nodal explants of P. pygmaeus treatments were used to initiate this experiment. The MS medium [104] was employed in the tissue culture of nodal explants in a vitro environment. It comprised of 4 μM 6-benzyl amino purine (6-BAP), 0.5 µM kinetin (KT), 25 g/L sucrose, and 7–10 g/L agar and the explant was left for two weeks. To promote root proliferation, shoots were transferred and cultured in an MS medium containing 1.2 μM thiamine –HCl, 4 μM nicotinic acid, 0.6 mM myo-inositol, and 3 μM pyridoxine, as well as 150 μM Mn and 150 μM Cr alone or in combination with various concentrations of SNP added to 30 g/L sucrose, 8–10 g/L agar, and 0.1 mg/L indole-3-acetic acid (IAA) (growth hormone regulator) at pH 5.8 ± 0.1. Thus, one liter of the prepared MS medium was placed in an oven (HiClave HVE-50) and sterilized for 30 min at 120–130 °C. After sterilizing, the medium was allowed to cool to room temperature before each treatment of bamboo species was cultured in a glass petri dish measuring 60 and 90 mm in diameter and height, respectively, and containing 100 mL of plant culture medium in an air tech ultraviolet-sterilized incubation hood. This lasted for five hours under white fluorescent light with a wavelength range of 20–410 nm at a temperature of 20 °C. The treatment of bamboo was then transferred to a single plant tissue culture room chamber for three weeks under controlled conditions for a 15-h photoperiod with light and dark phases of 28–24 °C and 18–23 °C, respectively (Table 5) (Figure 6).

Table 5.
The experimental design.
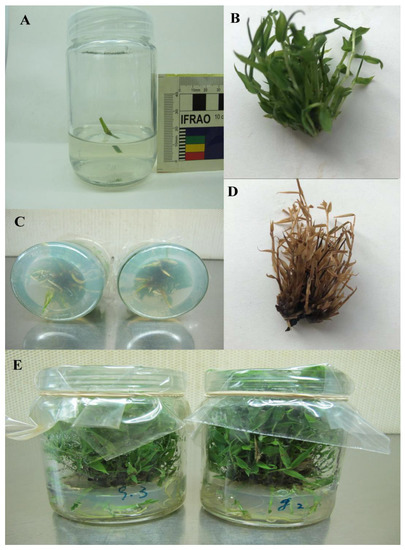
Figure 6.
Phenotypes of bamboo plants in vitro condition (A), 10 mm long nodal explants of P. pygmaeus treatments (B), control treatment of bamboo species (C), roots proliferation from bamboo shoots (D), bamboo treatment exposed to 150 µM Cr (E), bamboo treatments in plant tissue culture room chamber.
4.2. Estimation of Metal Content and Nitric Oxide Accumulation in Roots, Stems, and Leaves
To determine the Mn and Cr content of root stems and leaves, 0.5 g of plant samples was added to a solution containing 2 mL of HNO3 (67% w/v) and 2 mL of H2O2 (30% v/v). The resulting solution was then added to a mixture containing 10% (v/v) HCl, 10% KI (w/v), and 5% ascorbic acid (w/v). In the final step, a Shimadzu AA-6200 atomic absorption spectrometer (HG-AAS), was utilized to determine the accumulation of Mn and Cr using an external standard [105]. The accumulation of nitric oxide was detected by converting the oxygen–hemoglobin content to methemoglobin. Thus, in this approach, 0.5 g of root, stem, and leaf samples were added to a mixture containing sodium acetate (0.1 M), 3 mL of buffer (pH 6.0), 1 M NaCl, and 1% (w/v) ascorbic acid. It was then centrifuged for 25 minutes at 7000× g at 5 °C [106,107].
4.3. Determination of Tocopherols, Flavonols, and Total Phenolics as Non-Antioxidant Activity
To prepare the methanolic extract, 0.5 g of samples (dry leaf) were added to 4 mL of methanol (80%) and centrifuged at 6000× g for 20 min. The Conde method was used to determine the total phenolics [108]. A folin–ciocalteu reagent (10% v/v) (2.5 mL) was added to 0.1 M of methanolic extract. Subsequently, 7% (w/v) sodium bicarbonate was added to the mixture to neutralize the soluble phase. Finally, to estimate the total phenolics in the combination, its absorbance at 765 nm was assessed using a spectrometer (Beijing Purkinje TU-1810UV-vis spectrometer, Beijing, China). The flavonol content was measured using the Akkol technique [109]. To this end, 0.4 mL of aluminum chloride (2% w/v) and 1.5 mL of sodium acetate (5% w/v) were added to 0.5 mL of methanolic extract. The obtained supernatant was then kept at the room temperature for three hours. The content of flavonols was determined using a single spectrometer by measuring the absorbance of the supernatant at 445 nm. Tocopherol was determined in this investigation using a protocol developed by Kayden [110]. In this method, 0.1 g of samples (leaves) were added to 3 mL of ethanol and centrifuged at 6000× g for 20 min before being added to a mixture containing 0.001 M of 0.2 mL of ferric chloride, 0.1 mL of ethanol extract, 0.2 mL of bathophenanthroline (0.2% w/v), and 1 mM of phosphoric acid (0.2 mL). The tocopherol content was determined by measuring the absorbance of the resulting supernatant at 534 nm.
4.4. Relative Water Content (RWC), Proline Content (Pro), Glutathione (GSH), and Glycine Betaine (GB)
Relative water content (RWC) analysis was conducted according to the protocol proposed by Barrs and Weatherly [111]. For this means, to determine the fresh weight (FW), the leaf lamina was weighed, and then the fresh leaves were floated on water in a petri dish for 10 h under dark conditions. Then, a paper towel was used to dry the surface water from the leaves and then the turgid weights (TW) were measured. To determine the dry weights (DW), leaves were dried for two days at 75 °C. The final RWC was calculated using the formula below:
RWC (%) = (FW − DW)/(TW − DW) × 100
Glycine betaine (GB) was measured using the protocol proposed by Grieve and Grattan [112]. It was obtained by measuring the absorbance at 365 nm using a single standard curve. The proline content was determined by the protocol proposed by Bates [113]. To this end, sulfosalicylic acid was used to digest 330 mg of leaf samples, and supernatant absorbance at 520 nm was determined using a standard curve. Ellman’s methods [114] were used to determine the glutathione (GSH) content by recording an absorbance at 412 nm.
4.5. Lipid Peroxidation (MDA), Hydrogen Peroxide (H2O2), Electrolyte Leakage (EL), and Superoxide Radical (O2•−)
Malondialdehyde (MDA) was used as a lipid peroxidation biomarker in accordance with the Madhava Rao and Sresty’s methodology [115]. The MDA content was determined from spectrometer absorbance measurements at 600 and 532 nm. Hydrogen peroxide (H2O2) is a ROS compound that was obtained using the protocol proposed by Velikova et al. (2000) [116], with the final supernatant absorbance being measured at 390 nm using a spectrometer. The superoxide radical (O2•−) was determined using the protocol proposed by Li et al. (2010) [117]. It was obtained through the use of a nitrogen dioxide radical (NO2•) as a reference curve. Electrolyte leakage (EL) was obtained using the methods proposed by Valentovic et al. (2006) [118]. According to this method, 0.3 g of leaf samples were combined with 15 mL of deionized water. Next, the mixture was kept at a temperature of 20 °C for 3 h. The resulting solution was then used to determine the primary electrical conductivity (EC1). The samples were then placed in an autoclave set to 115 °C for 20 min. The resulting samples were then used to determine the secondary electrical conductivity (EC2). The final EC was calculated using the following formula:
EL (%) = (EC1/EC2) × 100
4.6. Antioxidant Activity
The 0.5 g of leaf samples were washed and cleaned before being cut into small species with scissors. The samples were then immersed in liquid nitrogen (LN) and crunched in a single mortar and pestle. The resulting powder was added to 4 mL of saline phosphate buffer (pH 7.2–7.4) at room temperature. Finally, the mixture was centrifuged at 3000–4000× g for 10 min to get the supernatant, which was used to estimate the antioxidant enzyme activity.
Superoxide dismutase (SOD) was measured using the protocol proposed by Zhang (1992) [119], which was based on the photo reduction of nitro blue tetrazolium (NBT). Peroxidase (POD) was estimated by measurement of the alteration in absorbance at 470 nm using the Zhang (1992) [119] method. Catalase (CAT) was measured using the Aebi et al. (1984) [120] method, which involved analyzing two H2O2 reactions at an absorbance of 240 nm. Glutathione reductase (GR) was determined using the protocol proposed by Foster and Hess (1980) [121], which used an absorbance of 340 nm. The ascorbate peroxidase (APX) was determined using the protocol proposed by Nakano and Asada (1981) [122] at 290 nm. The phenylalanine ammonia-lyase (PAL) activity was determined using the protocol proposed by Berner et al. (2006) [123].
4.7. Photosynthetic Pigments including Chlorophyll a, Chlorophyll b, Total Chlorophyll, and Carotenoids Content
Photosynthetic pigments contents, such as chlorophyll-a, chlorophyll-b, total chlorophyll, and content of carotenoids were estimated using the protocol proposed by Lichtenthaler and Buschmann (2001) [124]. According to this protocol, bamboo leaf samples weighing 0.5 g were crunched in an adequate amount of liquid nitrogen (LN). The resulting powder was homogenized in a single mortar and pestle in 20 mL of 80% (v/v) acetone at a temperature of 0 to 5 °C. The mixture treatments were then centrifuged at 7000× g for 15 min to determine the photosynthetic pigments. The resulting supernatant was transferred to a single spectrometer machine to determine chlorophyll a, and b, as well as carotenoid at absorbances of 663, 645, and 470 nm, respectively. The following formulae were used to determine the final content of photosynthetic pigments in mg/g F.W. (Fresh Weight) unit:
Total Chlorophyll = Chlorophyll a + Chlorophyll b
Chlorophyll a = 12.25A663 − 2.79A647
Chlorophyll b = 21.50A647 − 5.10A663
Carotenoid = (1000A470 − 1.82Chl a − 95.15 Chl b)/225
Chlorophyll a = 12.25A663 − 2.79A647
Chlorophyll b = 21.50A647 − 5.10A663
Carotenoid = (1000A470 − 1.82Chl a − 95.15 Chl b)/225
4.8. The Calculation of Tolerance Index (TI) in shoot and Root, The Translocation Factor (TF) in Leaves and Stem, as Well as Bioaccumulation Factor (BAF) in Root, Stem, and Leaves
TF, TI, and BAF were calculated in this study using the method proposed by Souri and Karimi (2017) [125] according to phytoextraction efficiency, which is the primary indicator of plant phytoremediation.
The bioaccumulation factor (BAF) was calculated using the formula below:
BAF = (Concentrations of Mn and Cr in stem and shoot)/(Concentrations of Mn and Cr in the medium)
The following formula was used to calculate the translocation factor (TF):
TF = (Concentrations of Mn and Cr in the shoot)/(Concentrations of Mn and Cr in the root)
The tolerance index (TI) was determined using the formulae below:
TISH = (Shoot dry weight of Mn and Cr treatments)/(Shoot dry weight of control treatment)
TIR = (Root dry weight of Mn and Cr treatment)/(Root dry weight of control treatment)
4.9. Root Dry Weight, Shoot Dry Weight, and Shoot Length
After washing and cleaning the plant shoots and roots, they were placed in a vacuum drying oven (DZF-6090) at 115 °C for 20 min. Following that, treatments were dried in a fixed-dry weight mode at an oven temperature of 80 °C. Finally, dried samples were used as root dry weight DW and shoot dry weight DW, with each treatment repeated four times. To determine the shoot length, bamboo shoots were measured in two steps—one at the inception and the other at the end of the experiment.
4.10. Statistical Analysis
The research was conducted using a two-way factorial design with four repetitions using a completely randomized design (CRD). The data were analyzed using a statistical package for the analysis of variance (ANOVA) provided by R software. Tukey’s test was used to determine the mean difference between treatments at the probability level of p < 0.05.
5. Conclusions
This study implies that SNP as a signaling molecule could play a pivotal role in the amelioration of metal toxicity in bamboo species (Pleioblastus pygmaea L.), which is consistent with previous findings. While the addition of 150 um Mn and 150 um Cr increased oxidative stress in Pleioblastus pygmaea L., our data suggest that the addition of various levels of SNP as a nitric oxide donor improved plant tolerance and growth, as well as other toxicity. These improvements were achieved through enhancing antioxidant activity, regulating relative water content, reducing heavy metal accumulation, and restricting heavy metal translocation from root to shoot. According to the findings of the current study, increasing the concentrations of heavy metals results in an increase in: (1) Antioxidant activity such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione reductase (GR), ascorbate peroxidase (APX), and phenylalanine ammonia-lyase (PAL); (2) non-antioxidant activity, such as tocopherols, flavonols, and total phenolics; (3) relative water content (RWC), proline contents (Pro), glycine betaine (GB), and glutathione (GSH); (4) photosynthesis pigments, such as total chlorophyll, chlorophyll-a, chlorophyll-b, and carotenoids content; and (5) plant biomass and plant growth, such as shoot dry weight, root dry weight, and length of shoot.
Alternatively, the results indicated that the addition of SNP reduced heavy metal accumulation in plant organs (shoot, stem, and root) as well as heavy metal translocation from root to shoot. However, when combined with heavy metal absorption and accumulation on the root surface, SNP enhances the phytoremediation potential of Pleioblastus pygmaea L. as well as soil heavy metal remediation, demonstrating that it could be used as an effective application in the phytoremediation induced process.
Author Contributions
Conceptualization, A.E., Y.D., J.B. and G.L.; statistical analysis, A.E. and Y.L.; investigation, A.E. and G.L.; supervision, A.E., Y.D. and G.L.; project administration, A.E.; funding acquisition, A.E. and Y.D.; writing—original draft and revised preparation, A.E., Y.D., F.M. and G.L.; writing—review and editing, F.M., Y.L., J.B. and A.E.; visualization, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from Jiangsu Agriculture Science and Technology Innovation Fund (CX(22)3049).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Nanjing Forestry University.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We would like to extend our sincere gratitude and appreciation to Peijian Shi, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, Jiangsu, China, for helping in the statistical analysis of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ekmekci, Y.; Bohms, A.; Thomson, J.A.; Mundree, S.G. Photochemical and Antioxidant Responses in the Leaves of Xerophyta viscosa Baker and Digitaria sanguinalis L. under Water Deficit. Z. Naturforsch. C J. Biosci. 2005, 60, 435–443. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Huang, Y.L.; Yang, S.; Long, G.X.; Zhao, Z.K.; Li, X.F.; Gu, M.H. Manganese Toxicity in Sugarcane Plantlets Grown on Acidic Soils of Southern China. PLoS ONE 2016, 11, e0148956. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Kumar, R.; Alamelu, D.; Acharya, R.; Rai, A.K. Determination of concentrations of chromium and other elements in soil and plant samples from leather tanning area by Instrumental Neutron Activation Analysis. J. Radioanal. Nucl. Chem. 2014, 300, 213–218. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J. Chromium Contamination Accident in China: Viewing Environment Policy of China. Environ. Sci. Technol. 2011, 45, 8605–8606. [Google Scholar] [CrossRef]
- State Ministry of Environmental Protection (MEP). Chromium Slays Pollution Control and Environmental Protection Technical Specifications. Rep. State Environ. China 2007. [Google Scholar]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric Oxide: A Multitasked Signaling Gas in Plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Liu, L.; Guo, Y.; Ren, H. Alleviating effect of exogenous nitric oxide in cucumber seedling against chilling stress. Afr. J. Biotechnol. 2011, 10, 4380–4386. [Google Scholar]
- Hao, G.-P.; Zhang, J.-H. The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In Nitric oxide in Plant Physiology; Hayat, S., Mori, M., Pichtel, J., Ahmad, A., Eds.; Wiley: Weinheim, Germany, 2010; pp. 115–138. [Google Scholar] [CrossRef]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Vranová, E.; Atichartpongkul, S.; Villarroel, R.; Van Montagu, M.; Inzé, D.; Van Camp, W. Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc. Natl. Acad. Sci. USA 2002, 99, 10870–10875. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wei, S.; Hu, D.; Liu, Y.; Feng, L.; Li, C.; Qi, N.; Wang, C.; Liao, W. Nitric Oxide Enhanced Salt Stress Tolerance in Tomato Seedlings, Involving Phytohormone Equilibrium and Photosynthesis. Int. J. Mol. Sci. 2022, 23, 4539. [Google Scholar] [CrossRef]
- Hasanuzzam, M.; Hoss, M.A.; Fujita, M. Physiological and Biochemical Mechanisms of Nitric Oxide Induced Abiotic Stress Tolerance in Plants. Am. J. Plant Physiol. 2010, 5, 295–324. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Gill, S.S.; Fujita, M. Physiological role of nitric oxide in plants grown under adverse environmental conditions. In Plant Acclimation to Environmental Stress; Tuteja, N., Gill, S.S., Eds.; Springer: New York, NY, USA, 2013; pp. 169–322. [Google Scholar] [CrossRef]
- Gupta, K.J.; Igamberdiev, A.U.; Manjunatha, G.; Segu, S.; Moran, J.F.; Neelawarne, B.; Bauwe, H.; Kaiser, W.M. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci. 2011, 181, 520–526. [Google Scholar] [CrossRef]
- De Michele, R.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; Di Valentin, M.; Careri, M.; Zottini, M.; Sanita di Toppi, L.; Lo Schiavo, F. Nitric Oxide Is Involved in Cadmium-Induced Programmed Cell Death in Arabidopsis Suspension Cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef]
- Lipton, S.A.; Choi, Y.-B.; Pan, Z.-H.; Lei, S.Z.; Chen, H.-S.V.; Sucher, N.J.; Loscalzo, J.; Singel, D.J.; Stamler, J.S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364, 626–632. [Google Scholar] [CrossRef]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef]
- Planchet, E.; Kaiser, W.M. Nitric Oxide Production in Plants. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef]
- Planchet, E. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006, 57, 3043–3055. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N.; Farooq, M.A.; Sandalio, L.M. Nitric oxide improves tolerance to arsenic stress in Isatis cappadocica desv. Shoots by enhancing antioxidant defenses. Chemosphere 2019, 239, 124523. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N.; Farooq, M.A.; Lobato, A.K.S. Improved physiological defense responses by application of sodium nitroprusside in Isatis cappadocica Desv. under cadmium stress. Physiol. Plant. 2021, 173, 100–115. [Google Scholar] [CrossRef]
- Jabeen, Z.; Fayyaz, H.A.; Irshad, F.; Hussain, N.; Hassan, M.N.; Li, J.; Rehman, S.; Haider, W.; Yasmin, H.; Mumtaz, S.; et al. Sodium nitroprusside application improves morphological and physiological attributes of soybean (Glycine max L.) under salinity stress. PLoS ONE 2021, 16, e0248207. [Google Scholar] [CrossRef]
- Emamverdian, Y.; Ding, Y.; Xie, Y. Phytoremediation potential of bamboo plant in China. Ecol. Environ. Conserv. 2018, 24, 530–539. [Google Scholar]
- Ahmad, Z.; Upadhyay, A.; Ding, Y.; Emamverdian, A.; Shahzad, A. Bamboo: Origin, habitat, distributions and global prospective. In Biotechnological Advances in Bamboo; Ahmad, Z., Ding, Y., Shahzad, A., Eds.; Springer: Singapore, 2021; pp. 1–31. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Huang, Z.; Jin, S.-H.; Guo, H.-D.; Zhong, X.-J.; He, J.; Li, X.; Jiang, M.-Y.; Yu, X.-F.; Long, H.; Ma, M.-D.; et al. Genome-wide identification and characterization of TIFY family genes in Moso Bamboo (Phyllostachys edulis) and expression profiling analysis under dehydration and cold stresses. Peerj 2016, 4, e2620. [Google Scholar] [CrossRef]
- Jin, Q.-Y.; Peng, H.-Z.; Lin, E.-P.; Li, N.; Huang, D.-N.; Xu, Y.-L.; Hua, X.-Q.; Wang, K.-H.; Zhu, T.-J. Identification and characterization of differentially expressed miRNAs between bamboo shoot and rhizome shoot. J. Plant Biol. 2016, 59, 322–335. [Google Scholar] [CrossRef]
- Hogarth, N.; Belcher, B. The contribution of bamboo to household income and rural livelihoods in a poor and mountainous county in Guangxi, China. Int. For. Rev. 2013, 15, 71–81. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhang, Y.; Booth, T.; He, X. Changes of carbon stocks in bamboo stands in China during 100 years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; He, T.; You, L.; Shen, X. Assessment of Potential Capability of Water Bamboo Leaves on the Adsorption Removal Efficiency of Cationic Dye from Aqueous Solutions. J. Polym. Environ. 2016, 24, 148–158. [Google Scholar] [CrossRef]
- State Forestry Administration of China. Statistical Yearbook of Forestry; State Forestry Administration of China: Beijing, China, 2012.
- Liu, J.-N.; Zhou, Q.-X.; Sun, T.; Ma, L.Q.; Wang, S. Growth responses of three ornamental plants to Cd and Cd–Pb stress and their metal accumulation characteristics. J. Hazard. Mater. 2008, 151, 261–267. [Google Scholar] [CrossRef]
- Huang, W.; Olson, E.; Wang, S.; Shi, P. The growth and mortality of Pleioblastus pygmaeus under different light availability. Glob. Ecol. Conserv. 2020, 24, e01262. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ramakrishnan, M.; Ahmad, Z.; Xie, Y. Different Physiological and Biochemical Responses of Bamboo to the Addition of TiO2 NPs under Heavy Metal Toxicity. Forests 2021, 12, 759. [Google Scholar] [CrossRef]
- RunHong, M.; Cheng, J.; Tang, F.; Yue, J.; Li, Z.; Ni, Z. Heavy metals in bamboo shoots from Southeastern China and risk assessment. Food Addit. Contam. Part B 2021, 14, 264–270. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Sharma, V.P.; Sharma, N.; Kohli, R.K. Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 2009, 20, 289–297. [Google Scholar] [CrossRef]
- Ismail, G.S.M. Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol. Plant. 2012, 34, 1303–1311. [Google Scholar] [CrossRef]
- Verma, K.; Mehta, S.K.; Shekhawat, G.S. Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: Cross-talk between ROS, NO and antioxidant responses. Biometals 2013, 26, 255–269. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul. 2004, 42, 227–238. [Google Scholar] [CrossRef]
- Wink, D.A.; Hanbauer, I.; Krishna, M.C.; DeGraff, W.; Gamson, J.; Mitchell, J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. USA 1993, 90, 9813–9817. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zottini, M.; Formentin, E.; Scattolin, M.; Carimi, F.; Schiavo, F.L.; Terzi, M. Nitric oxide affects plant mitochondrial functionality in vivo. FEBS Lett. 2002, 515, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Ding, F.; Wang, X.; Wei, M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol. Biochem. 2007, 45, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, S.; Xuan, Y.; Sun, M.; Zhao, L. Protective Effect of Nitric Oxide against Oxidative Damage in Arabidopsis Leaves under Ultraviolet-B Irradiation. J. Plant Biol. 2009, 52, 135–140. [Google Scholar] [CrossRef]
- Sun, F.; Guo, G.; Du, J.; Guo, W.; Peng, H.; Ni, Z.; Sun, Q.; Yao, Y. Whole-genome discovery of miRNAs and their targets in wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Y.; Wang, M.; Li, T.; Zhou, Y.; Wang, X.; Wei, S.; He, G.; Yang, G. Identification and comprehensive analyses of the CBL and CIPK gene families in wheat (Triticum aestivum L.). BMC Plant Biol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Farnese, F.S.; de Oliveira, J.A.; Gusman, G.S.; Leão, G.A.; Ribeiro, C.; Siman, L.I.; Cambraia, J. Plant Responses to Arsenic: The Role of Nitric Oxide. Water Air Soil Pollut. 2013, 224, 1660. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Talukdar, D. Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol. Mol. Biol. Plants 2012, 19, 69–79. [Google Scholar] [CrossRef]
- Márquez-García, B.; Fernández-Recamales, M.; Córdoba, F. Effects of Cadmium on Phenolic Composition and Antioxidant Activities of Erica andevalensis. J. Bot. 2012, 2012, 936950. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Jung, C.; Maeder, V.; Funk, F.; Frey, B.; Sticher, H.; Frossard, E. Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 2003, 252, 301–312. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Retracted: Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant. Cell Environ. 2011, 34, 909–921. [Google Scholar] [CrossRef]
- Dube, B.K.; Sinha, P.; Shukla, K.; Chatterjee, C.; Pandey, V.K.; Rai, A.D. Involvement of Excess Cadmium on Oxidative Stress and Other Physiological Parameters of Eggplant. J. Plant Nutr. 2009, 32, 996–1004. [Google Scholar] [CrossRef]
- Laloi, C.; Apel, K.; Danon, A. Reactive oxygen signalling: The latest news. Curr. Opin. Plant Biol. 2004, 7, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Singla-Pareek, S.L.; Sopory, S.K. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metab. Drug Interactions 2008, 23, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant. 2008, 31, 261–269. [Google Scholar] [CrossRef]
- Innocenti, G.; Pucciariello, C.; Le Gleuher, M.; Hopkins, J.; de Stefano, M.; Delledonne, M.; Puppo, A.; Baudouin, E.; Frendo, P. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 2006, 225, 1597–1602. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop. Sci. 2012, 6, 1314–1323. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Selenium Pretreatment Upregulates the Antioxidant Defense and Methylglyoxal Detoxification System and Confers Enhanced Tolerance to Drought Stress in Rapeseed Seedlings. Biol. Trace Element Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.; Sopory, S. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants—A review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Emamverdian, A.; Hasanuzzaman, M.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Liu, G. Zinc Oxide Nanoparticles Improve Pleioblastus pygmaeus Plant Tolerance to Arsenic and Mercury by Stimulating Antioxidant Defense and Reducing the Metal Accumulation and Translocation. Front. Plant Sci. 2022, 13, 841501. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric Oxide Stimulates Antioxidant System and Osmotic Adjustment in Soybean Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Altaf, M.M.; Diao, X.-P.; Rehman, A.U.; Imtiaz, M.; Shakoor, A.; Younis, H.; Fu, P.; Ghani, M.U. Effect of Vanadium on Growth, Photosynthesis, Reactive Oxygen Species, Antioxidant Enzymes, and Cell Death of Rice. J. Soil Sci. Plant Nutr. 2020, 20, 2643–2656. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Zhao, H.; Jin, Q.; Wang, Y.; Chu, L.; Li, X.; Xu, Y. Effects of nitric oxide on alleviating cadmium stress in Typha angustifolia. Plant Growth Regul. 2015, 78, 243–251. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Zhang, D.; Jiang, L.; Shao, Y. Exogenous nitric oxide effect on fructan accumulation and FBEs expression in chilling-sensitive and chilling-resistant wheat. Environ. Exp. Bot. 2013, 86, 2–8. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Moad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 649–668. [Google Scholar]
- Dong, Y.; Chen, W.; Xu, L.; Kong, J.; Liu, S.; He, Z. Nitric oxide can induce tolerance to oxidative stress of peanut seedlings under cadmium toxicity. Plant Growth Regul. 2015, 79, 19–28. [Google Scholar] [CrossRef]
- Khairy, A.I.H.; Oh, M.J.; Lee, S.M.; Kim, D.S.; Roh, K.S. Nitric oxide overcomes Cd and Cu toxicity in in vitro-grown tobacco plants through increasing contents and activities of rubisco and rubisco activase. Biochim. Open 2016, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, O.; Aghaei, P. Improving the growth of cowpea (Vigna unguiculata L. Walp.) by magnetized water. J. Biodivers. Environ. Sci. 2013, 3, 37–43. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2010, 248, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Latif, H.H.; Hanafy, R.S. Influence of Nitric Oxide Application on Some Biochemical Aspects, Endogenous Hormones, Minerals and Phenolic Compounds of Vicia faba Plant Grown under Arsenic Stress. Gesunde Pflanz. 2016, 68, 99–107. [Google Scholar] [CrossRef]
- Yang, L.; Ji, J.; Harris-Shultz, K.; Wang, H.; Wang, H.; Abd_Allah, E.; Luo, Y.; Hu, X. The Dynamic Changes of the Plasma Membrane Proteins and the Protective Roles of Nitric Oxide in Rice Subjected to Heavy Metal Cadmium Stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef]
- Seabra, A.B.; Oliveira, H.C. How nitric oxide donors can protect plants in a changing environment: What we know so far and perspectives. AIMS Mol. Sci. 2016, 3, 692–718. [Google Scholar] [CrossRef]
- Leshem, Y.Y.; Haramaty, E. The Characterization and Contrasting Effects of the Nitric Oxide Free Radical in Vegetative Stress and Senescence of Pisum sativum Linn. Foliage. J. Plant Physiol. 1996, 148, 258–263. [Google Scholar] [CrossRef]
- Yang, L.-T.; Qi, Y.-P.; Chen, L.-S.; Sang, W.; Lin, X.-J.; Wu, Y.-L.; Yang, C.-J. Nitric oxide protects sour pummelo (Citrus grandis) seedlings against aluminum-induced inhibition of growth and photosynthesis. Environ. Exp. Bot. 2012, 82, 1–13. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, L.; Wang, Q.; Fan, Z.; Kong, J.; Bai, X. Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J. Plant Interact. 2013, 9, 402–411. [Google Scholar] [CrossRef]
- Dong, Y.; Jinc, S.; Liu, S.; Xu, L.; Kong, J. Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J. Soil Sci. Plant Nutr. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.; Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Fu, G.; Tao, L.; Zhu, C. Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch. Biochem. Biophys. 2010, 497, 13–20. [Google Scholar] [CrossRef]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Gupta, D.K.; Archilla, A.; Romero-Puertas, M.C.; del Río, L.A. Reactive oxygen species and nitric oxide in plants under cadmium stress from toxicity to signaling. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 199–215. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A.A. An arsenic-accumulating, hypertolerant brassica, Isatis capadocica. New Phytol. 2009, 184, 41–47. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, A.K. Nitric oxide mitigates arsenic-induced oxidative stress and genotoxicity in Vicia faba L. Environ. Sci. Pollut. Res. 2015, 22, 13881–13891. [Google Scholar] [CrossRef]
- Murphy, M.E.; Noack, E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994, 233, 240–250. [Google Scholar] [CrossRef]
- Conde, E.; Cadahía, E.; Garcia-Vallejo, M.C. HPLC analysis of flavonoids and phenolic acids and aldehydes in Eucalyptus spp. Chromatographia 1995, 41, 657–660. [Google Scholar] [CrossRef]
- Akkol, E.K.; Göger, F.; Koşar, M.; Başer, K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008, 108, 942–949. [Google Scholar] [CrossRef]
- Kayden, H.J.; Chow, C.-K.; Bjornson, L.K. Spectrophotometric method for determination of tocopherol in red blood cells. J. Lipid Res. 1973, 14, 533–540. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Li, C.; Bai, T.; Ma, F.; Han, M. Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci. Hortic. 2010, 124, 274–279. [Google Scholar] [CrossRef]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 186–191. [Google Scholar] [CrossRef]
- Zhang, X. The Measurement and Mechanism of Lipid Peroxidation and SOD, POD and CAT Activities in Biological System. In Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Berner, M.; Krug, D.; Bihlmaier, C.; Vente, A.; Müller, R.; Bechthold, A. Genes and Enzymes Involved in Caffeic Acid Biosynthesis in the Actinomycete Saccharothrix espanaensis. J. Bacteriol. 2006, 188, 2666–2673. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–VIS specroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; p. 4. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N. Enhanced Phytoextraction by As Hyperaccumulator Isatis cappadocica Spiked with Sodium Nitroprusside. Soil Sediment Contam. Int. J. 2017, 26, 457–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).