Age-Related Properties of Aquaponics-Derived Tilapia Skin (Oreochromis niloticus): A Structural and Compositional Study
Abstract
1. Introduction
2. Results
2.1. General Composition
| Composition | ST | BT | [28] | [29] | [30] | [31] | [33] | [34] | [34] |
|---|---|---|---|---|---|---|---|---|---|
| Water % | 51.6 ± 3.2 | 64.4 ± 2.4 | 72.5 ± 2.1 | 64.1 ± 1.0 | 71.7 ± 0.3 | 57.7 ± 4.1 | 72.6 ± 2.5 | 76.5 ± 3.0 | 71.0 ± 0.6 |
| Proteins % | 38.9 ± 1.7 | 30.6 ± 2.1 | 22.8 ± 0.4 | 21.9 ± 0.1 | 21.3 ± 1.2 | 41.4 ± 1.2 | 21.3 ± 2.5 | 65.5 ± 3.3 | 60.8 ± 8.1 |
| Lipids % | 16.5 ± 3.8 | 4.4 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 1.1 | 1.6 ± 0.5 | 10.4 ± 2.9 | 3.9 ± 0.2 | 10.2 ± 1.8 | 11.3 ± 4.1 |
| Ashes % | 0.12 ± 0.03 | 0.70 ± 0.5 | 0.17 ± 0.03 | 11.7 ± 1.1 | 3.9 ± 0.4 | 0.20 ± 0.03 | 4.24 ± 0.4 | 7.3 ± 1.1 | 10.2 ± 0.2 |
2.2. Amino Acid Composition
2.3. Fatty Acid Composition
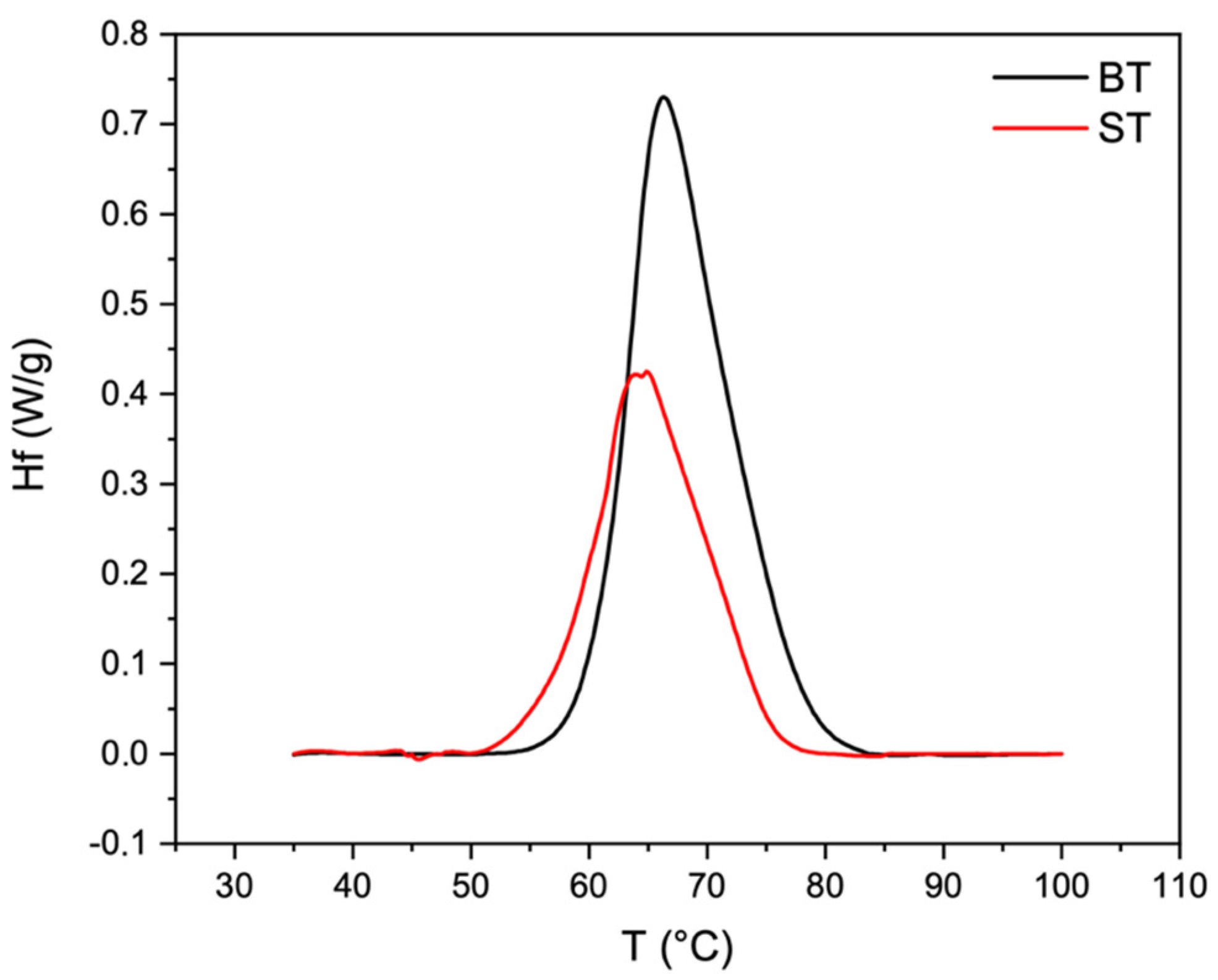
2.4. Thermal Properties
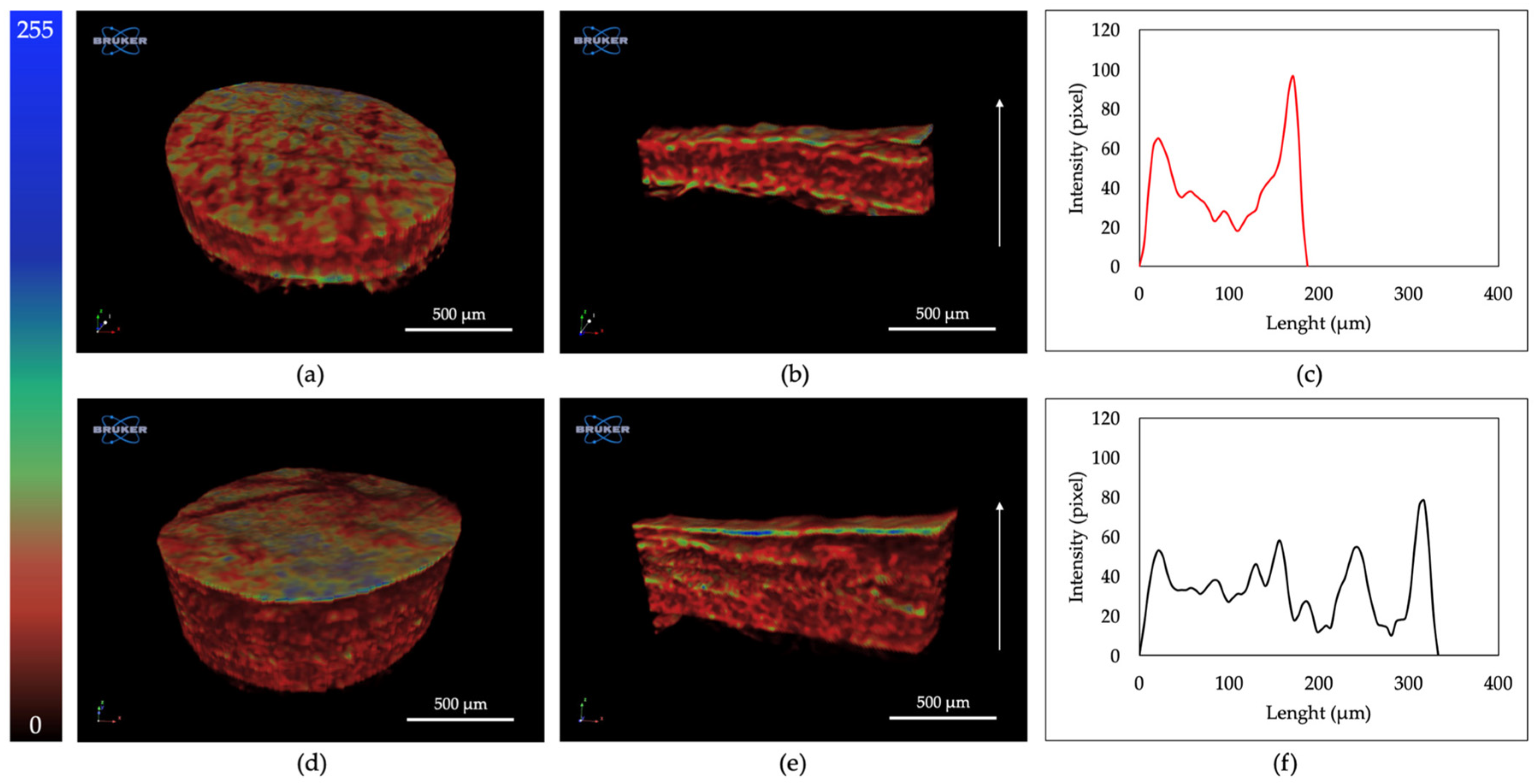
2.5. Three-Dimensional Structural Investigation
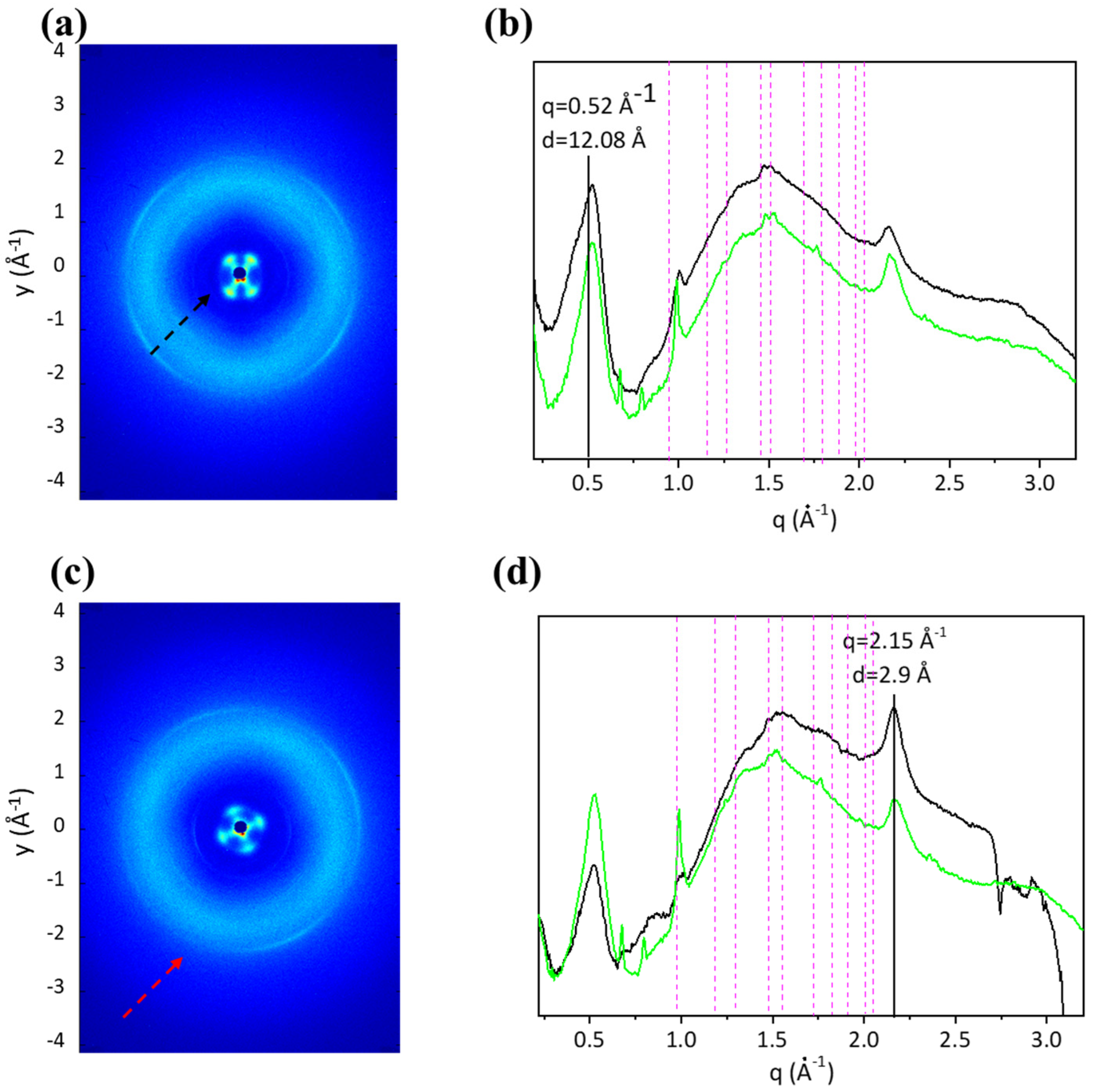
2.6. Two-Dimensional Structural Analysis
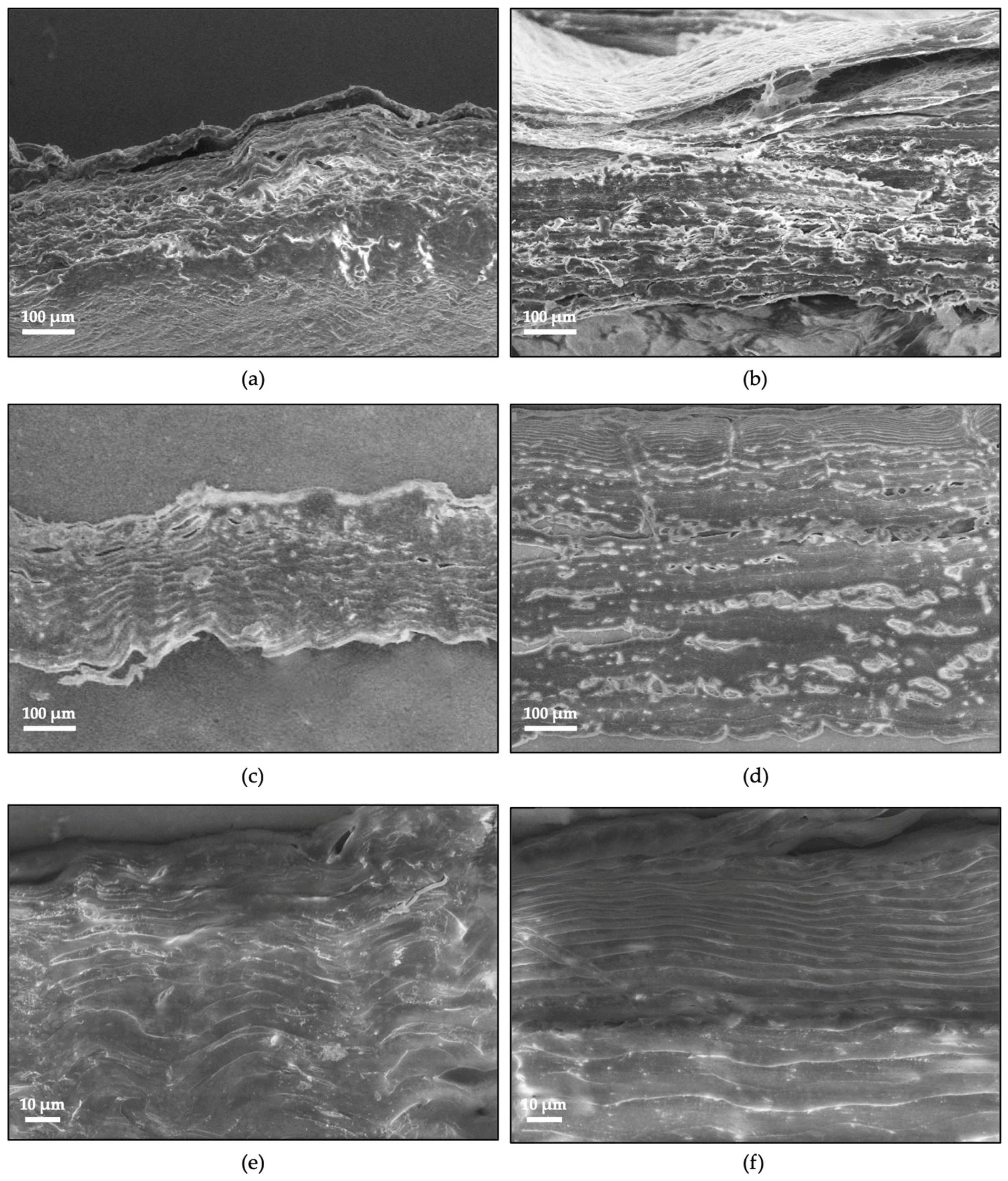
2.7. Ultrastructural Analysis
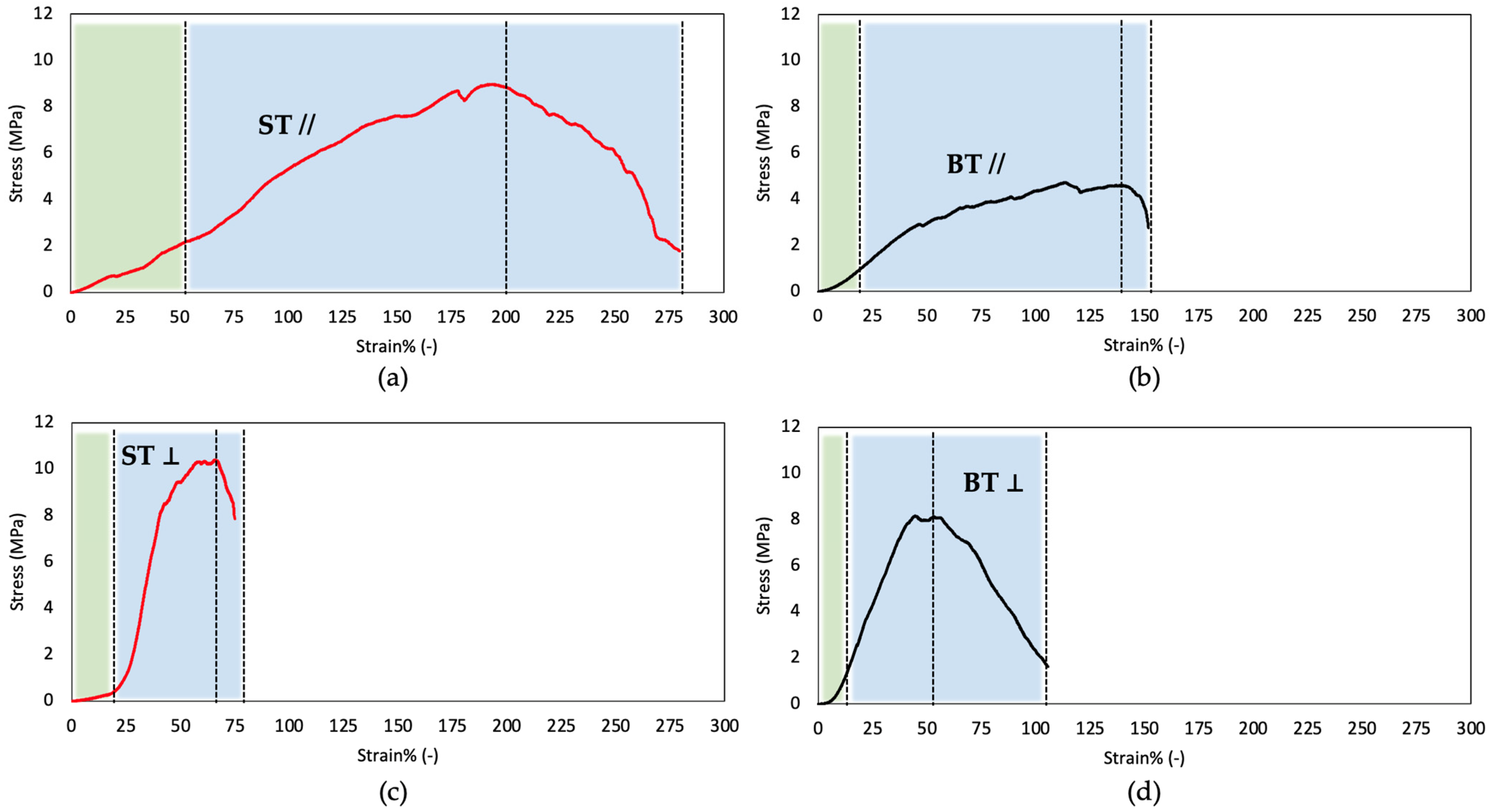
2.8. Mechanical Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Composition
4.3. Amino Acid Composition
4.4. Fatty Acid Composition
4.5. Thermal Properties
4.6. Three-Dimensional Structural Investigation
4.7. Two-Dimensional Structural Analysis
4.8. Ultrastructural Analysis
4.9. Mechanical Properties
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Birk, D.E.; Brückner, P. Collagens, Suprastructures, and Collagen Fibril Assembly. In The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–115. [Google Scholar]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2011, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.-Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of Collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Tampieri, A.; Salvatore, L.; Sannino, A.; Ghiron, J.H.L.; Condorelli, G. Collagen Based Scaffold for Biomedical Applications. J. Biotechnol. 2010, 150, 29. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine Collagen and Its Derivatives: Versatile and Sustainable Bio-Resources for Healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 197–209. [Google Scholar] [CrossRef]
- Rana, S.; Singh, A.; Surasani, V.K.R.; Kapoor, S.; Desai, A.; Kumar, S. Fish Processing Waste: A Novel Source of Non-conventional Functional Proteins. Int. J. Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Guan, Y.; He, J.; Chen, J.; Li, Y.; Zhang, X.; Zheng, Y.; Jia, L. Valorization of Fish Processing By-Products: Microstructural, Rheological, Functional, and Properties of Silver Carp Skin Type I Collagen. Foods 2022, 11, 2985. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Kikionis, S.; Karkatzoulis, L.; Bethanis, K.; Roussis, V.; Ioannou, E. Valorization of Fish Waste: Isolation and Characterization of Acid- and Pepsin-Soluble Collagen from the Scales of Mediterranean Fish and Fabrication of Collagen-Based Nanofibrous Scaffolds. Mar. Drugs 2022, 20, 664. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Front. Sustain. Food Syst. 2021, 5, 132. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical Applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Zata, H.F.; Chiquita, P.; Shafira, K. Collagen from Marine Source for Regenerative Therapy: A Literature Review. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2314, p. 050017. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable Valorisation of Seafood By-Products: Recovery of Collagen and Development of Collagen-Based Novel Functional Food Ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Martins, M.E.O.; Sousa, J.R.; Claudino, R.L.; Lino, S.C.O.; do Vale, D.A.; Silva, A.L.C.; Morais, J.P.S.; de Souza Filho, M.d.S.M.; de Souza, B.W.S. Thermal and Chemical Properties of Gelatin from Tilapia (Oreochromis niloticus) Scale. J. Aquat. Food Prod. Technol. 2018, 27, 1120–1133. [Google Scholar] [CrossRef]
- Nurilmala, M.; Suryamarevita, H.; Husein Hizbullah, H.; Jacoeb, A.M.; Ochiai, Y. Fish Skin as a Biomaterial for Halal Collagen and Gelatin. Saudi. J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Melon Barroso, R.; Pizarro Munoz, A.E.; Cai, J. Social and Economic Performance of Tilapia Farming in Brazil; FAO: Rome, Italy, 2019. [Google Scholar]
- Liu, D.; Zhang, X.; Li, T.; Yang, H.; Zhang, H.; Regenstein, J.M.; Zhou, P. Extraction and Characterization of Acid- and Pepsin-Soluble Collagens from the Scales, Skins and Swim-Bladders of Grass Carp (Ctenopharyngodon idella). Food Biosci. 2015, 9, 68–74. [Google Scholar] [CrossRef]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Zeng, S.K.; Zhang, C.H.; Lin, H.; Yang, P.; Hong, P.Z.; Jiang, Z. Isolation and Characterisation of Acid-Solubilised Collagen from the Skin of Nile Tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Potaros, T.; Raksakulthai, N.; Runglerdkreangkrai, J.; Worawattanamateekul, W. Characteristics of Collagen from Nile Tilapia (Oreochromis niloticus) Skin Isolated by Two Different Methods. Kasetsart J.—Nat. Sci. 2009, 43, 584–593. [Google Scholar]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of Acid- and Pepsin-Soluble Collagen Extracted from the Skin of Nile Tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-A.; Shin, E.S.; Park, Y.M.; Ma, A.; Yang, H.; Kim, S.; Shin, T.-S. Composition of Collagen Extracted from the Skin of Three Different Varieties of Fish. J. Korean Soc. Food Sci. Nutr. 2022, 51, 71–81. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and Characterization of Type I Collagen from Skin of Tilapia (Oreochromis niloticus) and Its Potential Application in Biomedical Scaffold Material for Tissue Engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Akter, S.; Sheikh, B.; Rahman, M.; Bhowmik, S.; Alam, N.; Rahman, M.A.; Alam, A.N. Assessment of Fishery Wastes and Suitability of Its Utilization in the Manufacture of Fish Glue. Am. J. Food Nutr. 2016, 6, 77–81. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, K.; de Melo, E.S.; da Paz Lima, I.C.; Bacelar, R.G.A.; dos Anjos, A.M.P.; Muratori, M.C.S.; Nobrega, M.M.G.P. Drying of Residual Tilapia Skin from Filleting Using a Thermophotovoltaic Solar Dehydrator. Acta Vet. Bras. 2021, 15, 166–172. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and Physico-Chemical Characterisation of Nile Perch (Lates niloticus) Skin and Bone Gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Alfaro, A.D.T.; Fonseca, G.G.; Balbinot, E.; Machado, A.; Prentice, C. Physical and Chemical Properties of Wami Tilapia Skin Gelatin. Food Sci. Technol. 2013, 33, 592–595. [Google Scholar] [CrossRef]
- Moses, S.; Agbaji, E.B.; Ajibola, V.O.; Gimba, C.E. Amino Acid Composition and Proximate Analysis in Tilapia (Oreochromis mossambicus) Fish from Dams and Rivers in Zamfara State, Nigeria. J. Appl. Sci. Environ. Manag. 2018, 22, 899. [Google Scholar] [CrossRef]
- Lima Verde, M.E.Q.; Ferreira-Júnior, A.E.C.; de Barros-Silva, P.G.; de Castro Miguel, E.; Mathor, M.B.; Lima-Júnior, E.M.; de Moraes-Filho, M.O.; Alves, A.P.N.N. Nile Tilapia Skin (Oreochromis niloticus) for Burn Treatment: Ultrastructural Analysis and Quantitative Assessment of Collagen. Acta Histochem. 2021, 123, 151762. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Quarta, A.; Gaballo, A.; Terzi, A.; Sibillano, T.; Giannini, C.; de Benedetto, G.E.; Lunetti, P.; Capobianco, L.; et al. Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development. Polymers 2022, 14, 1865. [Google Scholar] [CrossRef]
- Bella, J. Collagen Structure: New Tricks from a Very Old Dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E. Collagen: Biochemistry, Biomechanics, Biotechnology; CRC Press Inch.: Boca Raton, FL, USA, 1988. [Google Scholar]
- Yata, M.; Yoshida, C.; Fujisawa, S.; Mizuta, S.; Yoshinaka, R. Identification and Characterization of Molecular Species of Collagen in Fish Skin. J. Food Sci. 2001, 66, 247–251. [Google Scholar] [CrossRef]
- Alves, A.P.N.N.; Lima Júnior, E.M.; Piccolo, N.S.; de Miranda, M.J.B.; Lima Verde, M.E.Q.; Ferreira Júnior, A.E.C.; de Barros Silva, P.G.; Feitosa, V.P.; de Bandeira, T.J.P.G.; Mathor, M.B.; et al. Study of Tensiometric Properties, Microbiological and Collagen Content in Nile Tilapia Skin Submitted to Different Sterilization Methods. Cell Tissue Bank. 2018, 19, 373–382. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An Insight on Type I Collagen from Horse Tendon for the Manufacture of Implantable Devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef]
- Love, R.M.; Yamaguchi, K.; Créac’h, Y.; Lavéty, J. The Connective Tissues and Collagens of Cod during Starvation. Comp. Biochem. Physiol.—Part B Biochem. 1976, 55, 487–492. [Google Scholar] [CrossRef]
- Rosmawati; Abustam, E.; Tawali, A.B.; Said, M.I.; Sari, D.K. Effect of Body Weight on the Chemical Composition and Collagen Content of Snakehead Fish Channa Striata Skin. Fish. Sci. 2018, 84, 1081–1089. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H.; Toppe, J.; Albrektsen, S. The Effect of Dietary Hydroxyproline Supplementation on Salmon (Salmo salar L.) Fed High Plant Protein Diets. Aquaculture 2008, 275, 242–249. [Google Scholar] [CrossRef]
- Miller, A.T.; Karmas, E.; Lu, M.F. Age-Related Changes in the Collagen of Bovine Corium: Studies on Extractability, Solubility and Molecular Size Distribution. J. Food Sci. 1983, 48, 681–685. [Google Scholar] [CrossRef]
- Cohen-Solal, L.; le Lous, M.; Allain, J.-C.; François, M. Absence of Maturation of Collagen Crosslinks in Fish Skin? FEBS Lett. 1981, 123, 282–284. [Google Scholar] [CrossRef]
- Kinsman, D.M.; Kotula, A.W.; Breidenstein, B.C. Muscle Food Meat Poultry and Seafood; Springer Science + Business Media: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier Transform Infrared (FTIR) Spectroscopic Study of Acid Soluble Collagen and Gelatin from Skins and Bones of Young and Adult Nile Perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Lee, D.-S.; Yoon, H.-D.; Kim, Y.-K.; Yoon, N.-Y.; Moon, S.-K.; Kim, I.-S.; Jeong, B.-Y. Proximate and Fatty Acid Compositions of 14 Species of Coastal and Offshore Fishes in Korea. Korean J. Fish. Aquat. Sci. 2011, 44, 569–576. [Google Scholar] [CrossRef]
- Rault, I.; Frei, V.; Herbage, D. Evaluation of Different Chemical Methods for Cross-Linking Collagen Gel, Films and Sponges. J. Mater. Sci. Mater. Med. 1996, 7, 215–221. [Google Scholar] [CrossRef]
- Terzi, A.; Sibillano, T.; de Caro, L.; Altamura, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L.; Blasi, F.S.; Corallo, A.; et al. WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant. Crystals 2022, 12, 700. [Google Scholar] [CrossRef]
- Ibrahim, A.; Soliman, M.; Kotb, S.; Ali, M.M. Evaluation of Fish Skin as a Biological Dressing for Metacarpal Wounds in Donkeys. BMC Vet. Res. 2020, 16, 472. [Google Scholar] [CrossRef]
- Szewciw, L.; Barthelat, F. Mechanical Properties of Striped Bass Fish Skin: Evidence of an Exotendon Function of the Stratum Compactum. J. Mech. Behav. Biomed. Mater. 2017, 73, 28–37. [Google Scholar] [CrossRef]
- Mumford, S.; Heidel, J.; Smith, C.; Morrison, J.; MacConnell, B.; Blazer, V. Fish Histology and Histopathology; US Fish & Wildlife Service: Elkins, WV, USA, 2007. [Google Scholar]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of Processing on Structural, Mechanical and Biological Properties of Collagen-Based Substrates for Regenerative Medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; de Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Guo, C.; Li, L.; Ma, Y. Unique Morphology and Mechanical Property of Chinese Sturgeon (Acipenser sinensis) Fish Skin. IET Nanobiotechnol. 2020, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Valenzuela-Rojo, D.R.; López-Cervantes, J.; Sánchez-Machado, D.I. Tilapia (Oreochromis aureus) Collagen for Medical Biomaterials; IntechOpen: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Ghannam, H.E.; Soliman, H.A. The Protective Role of Vitamins (E + C) on Nile Tilapia (Oreochromis niloticus) Exposed to ZnO NPs and Zn Ions: Bioaccumulation and Proximate Chemical Composition. Ann. Anim. Sci. 2021, 22, 633–642. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lambert, P.; Dehnel, P.A. Seasonal Variations in Biochemical Composition during the Reproductive Cycle of the Intertidal Gastropod Thais Lamellose Gmelin (Gastropoda, Prosobranchia). Can. J. Zool. 1974, 52, 305–318. [Google Scholar] [CrossRef]
- Lunetti, P.; Damiano, F.; de Benedetto, G.E.; Siculella, L.; Pennetta, A.; Muto, L.; Paradies, E.; Marobbio, C.; Marya, T.; Dolce, V.; et al. Characterization of Human and Yeast Mitochondrial Glycine Carriers with Implications for Heme Biosynthesis and Anemia. J. Biol. Chem. 2016, 291, 19746–19759. [Google Scholar] [CrossRef]
- Fico, D.; Margapoti, E.; Pennetta, A.; de Benedetto, G.E. An Enhanced GC/MS Procedure for the Identification of Proteins in Paint Microsamples. J. Anal. Methods Chem. 2018, 2018, 6032084. [Google Scholar] [CrossRef]
- Salvatore, L.; Calò, E.; Bonfrate, V.; Pedone, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Madaghiele, M. Exploring the Effects of the Crosslink Density on the Physicochemical Properties of Collagen-Based Scaffolds. Polym. Test 2021, 93, 106966. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Curci, C.; Picerno, A.; Gallone, A.; Vulpi, M.; Vitarelli, A.; Ditonno, P.; Cascione, M.; Sallustio, F.; et al. Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration. Materials 2021, 14, 7648. [Google Scholar] [CrossRef] [PubMed]
- Altamura, D.; Lassandro, R.; Vittoria, F.A.; de Caro, L.; Siliqi, D.; Ladisa, M.; Giannini, C. X-Ray Microimaging Laboratory (XMI-LAB). J. Appl. Crystallogr. 2012, 45, 869–873. [Google Scholar] [CrossRef]
- Siliqi, D.; de Caro, L.; Ladisa, M.; Scattarella, F.; Mazzone, A.; Altamura, D.; Sibillano, T.; Giannini, C. SUNBIM: A Package for X-Ray Imaging of Nano- and Biomaterials Using SAXS, WAXS, GISAXS and GIWAXS Techniques. J. Appl. Crystallogr. 2016, 49, 1107–1114. [Google Scholar] [CrossRef]


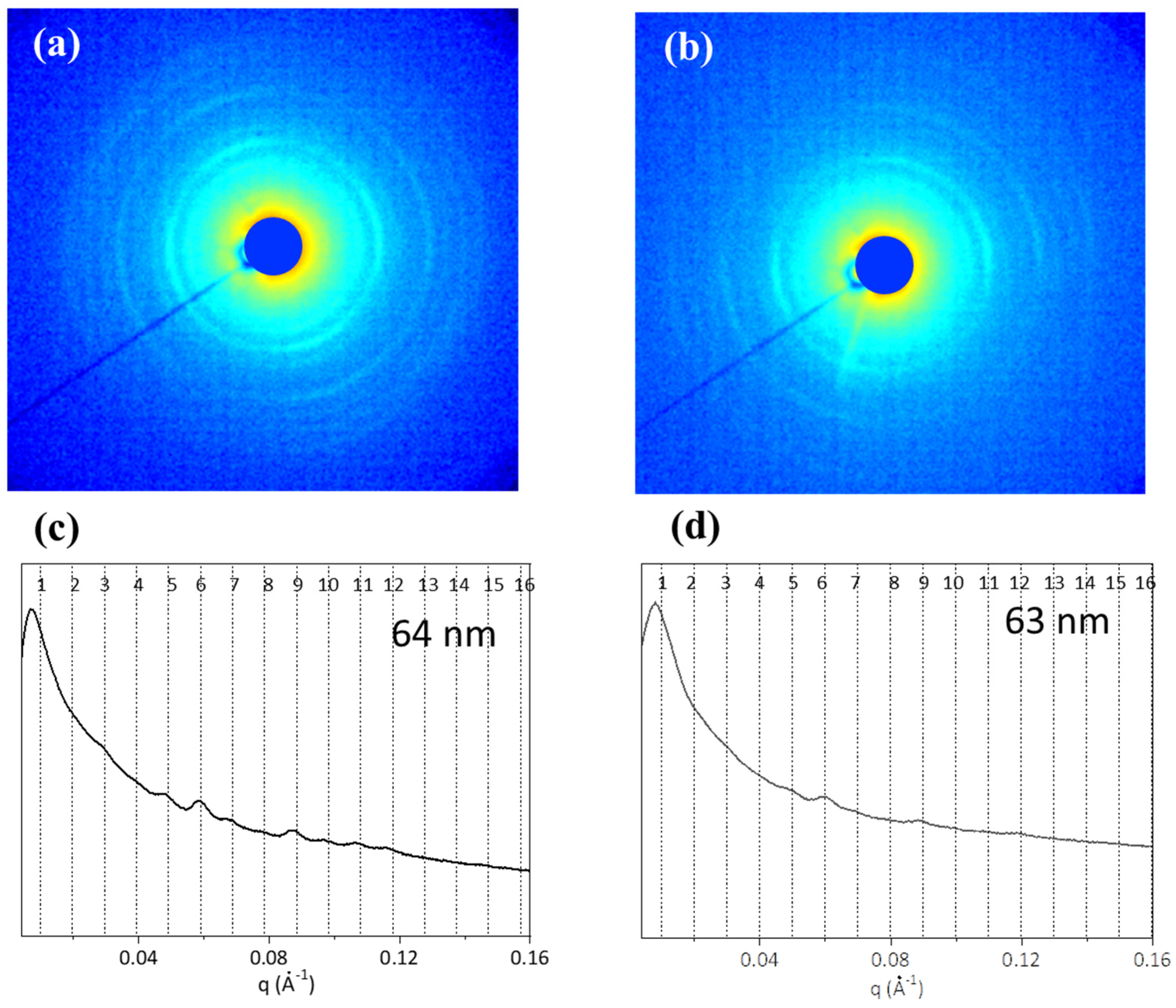
| Amino Acids | ST | BT | [28] |
|---|---|---|---|
| Alanine | 139 ± 20 | 144 ± 11 | 157.0 ± 1.5 |
| Glycine | 287 ± 52 | 289 ± 15 | 381.1 ± 5.2 |
| Valine | 23 ± 1 | 20 ± 1 | 17.5 ± 0.8 |
| Leucine | 36 ± 8 | 23 ± 6 | 24.7 ± 0.7 |
| Isoleucine | 15 ± 2 | 9 ± 1 | 9.0 ± 0.2 |
| Proline | 90 ± 13 | 119 ± 7 | 97.8 ± 9.9 |
| Methionine | 4 ± 3 | 4 ± 2 | 7.7 ± 0.5 |
| Serine | 42 ± 3 | 38 ± 5 | 30.7 ± 0.5 |
| Threonine | 61 ± 7 | 29 ± 6 | 26.8 ± 0.8 |
| Phenylalanine | 18 ± 6 | 15 ± 6 | 12.6 ± 1.1 |
| Aspartate | 59 ± 4 | 51 ± 7 | 32.0 ± 0.5 |
| Glutamate | 87 ± 4 | 83 ± 9 | 56.6 ± 0.9 |
| Lysine | 23 ± 9 | 18 ± 4 | 21.9 ± 0.4 |
| Histidine | 1 ± 1 | 1 ± 0 | 5.0 ± 0.4 |
| Tyrosine | 5 ± 3 | 3 ± 0 | 4.3 ± 0.3 |
| Hydroxyproline | 65 ± 14 | 95 ± 13 | 53.3 ± 1.0 |
| Arginine | 35 ± 5 | 38 ± 4 | 61.4 ± 1.8 |
| Imino acids | 145 ± 26 | 220 ± 15 | 151.1 ± 8.9 |
| Tot. | 1000 | 1000 | 1000 |
| Fatty Acids | ST | BT | [28] |
|---|---|---|---|
| C14:1 | 0.05 ± 0.02 | 0.06 ± 0.01 | 0.30 ± 0.04 |
| C14:0 | 3.05 ± 0.54 | 4.03 ± 0.18 | 3.90 ± 0.57 |
| C15:1 | 0.010 ± 0.004 | 0.05 ± 0.05 | ND |
| C15:0 | 0.52 ± 0.02 | 0.90 ± 0.06 | 0.28 ± 0.04 |
| C16:1 | 5.40 ± 0.56 | 7.23 ± 0.96 | 7.25 ± 0.94 |
| C16:0 | 19.81 ± 2.17 | 20.78 ± 0.69 | 19.39 ± 2.69 |
| C17:1 | ND | ND | 0.53 ± 0.09 |
| C17:0 | 0.22 ± 0.01 | 0.30 ± 0.05 | ND |
| C18:2t | 0.08 ± 0.04 | 0.15 ± 0.04 | ND |
| C18:1t | 3.30 ± 0.37 | 3.26 ± 0.23 | ND |
| C18:1n9c | 56.36 ± 2.07 | 43.59 ± 2.15 | 23.72 ± 11.37 |
| C18:2n6c | 2.78 ± 0.98 | 7.40 ± 0.97 | 10.83 ± 1.55 |
| C18:3n6 | 0.005 ± 0.001 | 0.03 ± 0.01 | 0.93 ± 0.13 |
| C18:3n3 | 0.13 ± 0.14 | 0.43 ± 0.16 | 8.89 ± 1.63 |
| C18:0 | 4.82 ± 0.82 | 6.36 ± 0.47 | 8.16 ± 1.48 |
| C19:0 | 0.07 ± 0.01 | 0.11 ± 0.04 | ND |
| C20:0 | 0.12 ± 0.02 | 0.11 ± 0.09 | 0.31 ± 0.07 |
| C20:1n9 | 1.68 ± 0.05 | 1.17 ± 0.33 | 0.88 ± 0.11 |
| C20:2n6 | 0.11 ± 0.8 | 0.27 ± 0.14 | 1.07 ± 0.19 |
| C20:3n6 | 0.06 ± 0.09 | 0.04 ± 0.02 | 1.41 ± 0.24 |
| C20:3n3 | 0.015 ± 0.016 | 0.08 ± 0.03 | 2.74 ± 0.50 |
| C20:4n6 | 0.007 ± 0.007 | 0.32 ± 0.27 | 0.31 ± 0.02 |
| C20:5n3 | 0.014 ± 0.006 | 0.12 ± 0.13 | 0.90 ± 0.11 |
| C21:1n9 | 0.02 ± 0.01 | 0.02 ± 0.06 | 0.44 ± 0.09 |
| C22:0 | 0.013 ± 0.014 | 0.12 ± 0.08 | 0.34 ± 0.09 |
| C22:1n9 | 0.05 ± 0.05 | 0.08 ± 0.03 | ND |
| C22:2n6 | 0.007 ± 0.006 | 0.02 ± 0.01 | 0.02 ± 0.04 |
| C22:4n6 | 0.14 ± 0.22 | 1.24 ± 0.96 | ND |
| C22:6n3 | 0.05 ± 0.08 | 0.47 ± 0.36 | ND |
| C23:0 | 0.003 ± 0.003 | 0.017 ± 0.012 | ND |
| C24:0 | 0.02 ± 0.02 | 0.06 ± 0.05 | ND |
| C24:1n9 | 0.02 ± 0.01 | 0.16 ± 0.27 | 0.23 ± 0.10 |
| C24:6n3 | ND | ND | 7.16 ± 1.21 |
| Saturates | 28.6 | 31.7 | 32.4 |
| Monoenes | 65.2 | 53.1 | 33.4 |
| Polyenes | 6.1 | 15.2 | 34.3 |
| ST | BT | |||
|---|---|---|---|---|
| // | ⊥ | // | ⊥ | |
| E (MPa) | 2.6 ± 0.9 (2–10%) 5.7 ± 2.0 (60–80%) | 1.4 ± 0.4 (2–10%) 10.3 ± 1.3 (20–25%) 27.5 ± 7.0 (30–40%) | 2.4 ± 1.2 (2–10%) 4.1 ± 1.4 (60–80%) | 3.7 ± 1.7 (2–5%) 15.5 ± 3.0 (5–10%) 18.6 ± 6.9 (30–40%) |
| σmax (MPa) | 8.5 ± 1.1 | 8.6 ± 1.9 | 5.3 ± 0.7 | 7.2 ± 1.0 |
| εr (%) | 207 ± 75 | 71 ± 17 | 187 ± 79 | 53 ± 18 |
| T (MPa) | 1687 ± 286 | 371 ± 147 | 565 ± 121 | 477 ± 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, N.; Terzi, A.; Sibillano, T.; Giannini, C.; Masi, A.; Sicuro, A.; Blasi, F.S.; Corallo, A.; Pennetta, A.; De Benedetto, G.E.; et al. Age-Related Properties of Aquaponics-Derived Tilapia Skin (Oreochromis niloticus): A Structural and Compositional Study. Int. J. Mol. Sci. 2023, 24, 1938. https://doi.org/10.3390/ijms24031938
Gallo N, Terzi A, Sibillano T, Giannini C, Masi A, Sicuro A, Blasi FS, Corallo A, Pennetta A, De Benedetto GE, et al. Age-Related Properties of Aquaponics-Derived Tilapia Skin (Oreochromis niloticus): A Structural and Compositional Study. International Journal of Molecular Sciences. 2023; 24(3):1938. https://doi.org/10.3390/ijms24031938
Chicago/Turabian StyleGallo, Nunzia, Alberta Terzi, Teresa Sibillano, Cinzia Giannini, Annalia Masi, Alessandro Sicuro, Federica Stella Blasi, Angelo Corallo, Antonio Pennetta, Giuseppe Egidio De Benedetto, and et al. 2023. "Age-Related Properties of Aquaponics-Derived Tilapia Skin (Oreochromis niloticus): A Structural and Compositional Study" International Journal of Molecular Sciences 24, no. 3: 1938. https://doi.org/10.3390/ijms24031938
APA StyleGallo, N., Terzi, A., Sibillano, T., Giannini, C., Masi, A., Sicuro, A., Blasi, F. S., Corallo, A., Pennetta, A., De Benedetto, G. E., Montagna, F., Maffezzoli, A., Sannino, A., & Salvatore, L. (2023). Age-Related Properties of Aquaponics-Derived Tilapia Skin (Oreochromis niloticus): A Structural and Compositional Study. International Journal of Molecular Sciences, 24(3), 1938. https://doi.org/10.3390/ijms24031938











