Distribution of DC Subtypes: CD83+, DC-LAMP+, CD1a+, CD1c+, CD123+, and DC-SIGN+ in the Tumor Microenvironment of Endometrial Cancers—Correlation with Clinicopathologic Features
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
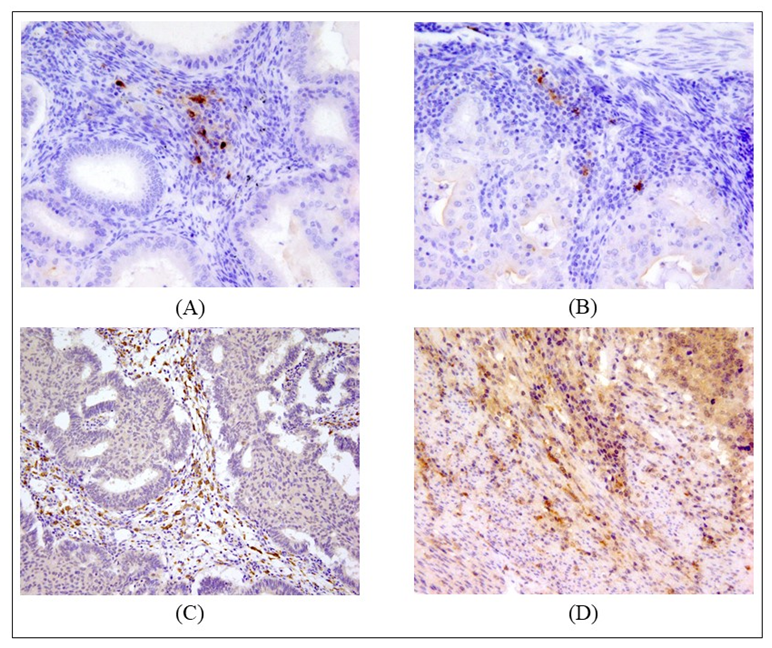
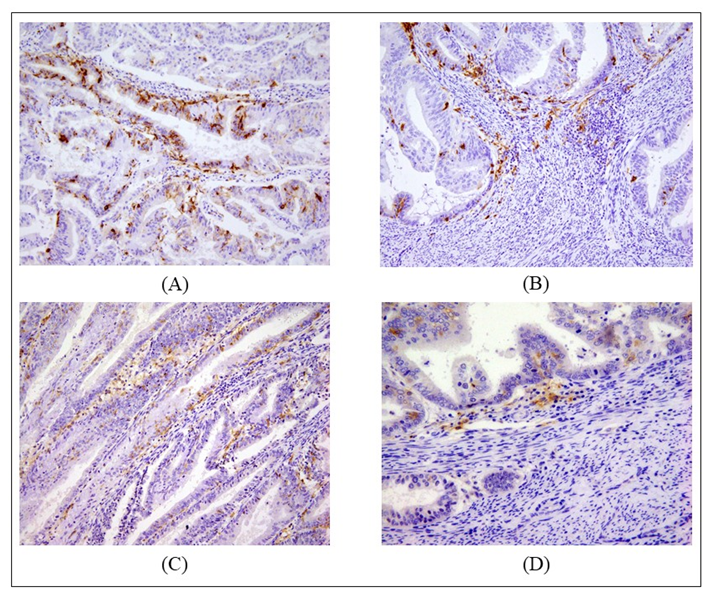
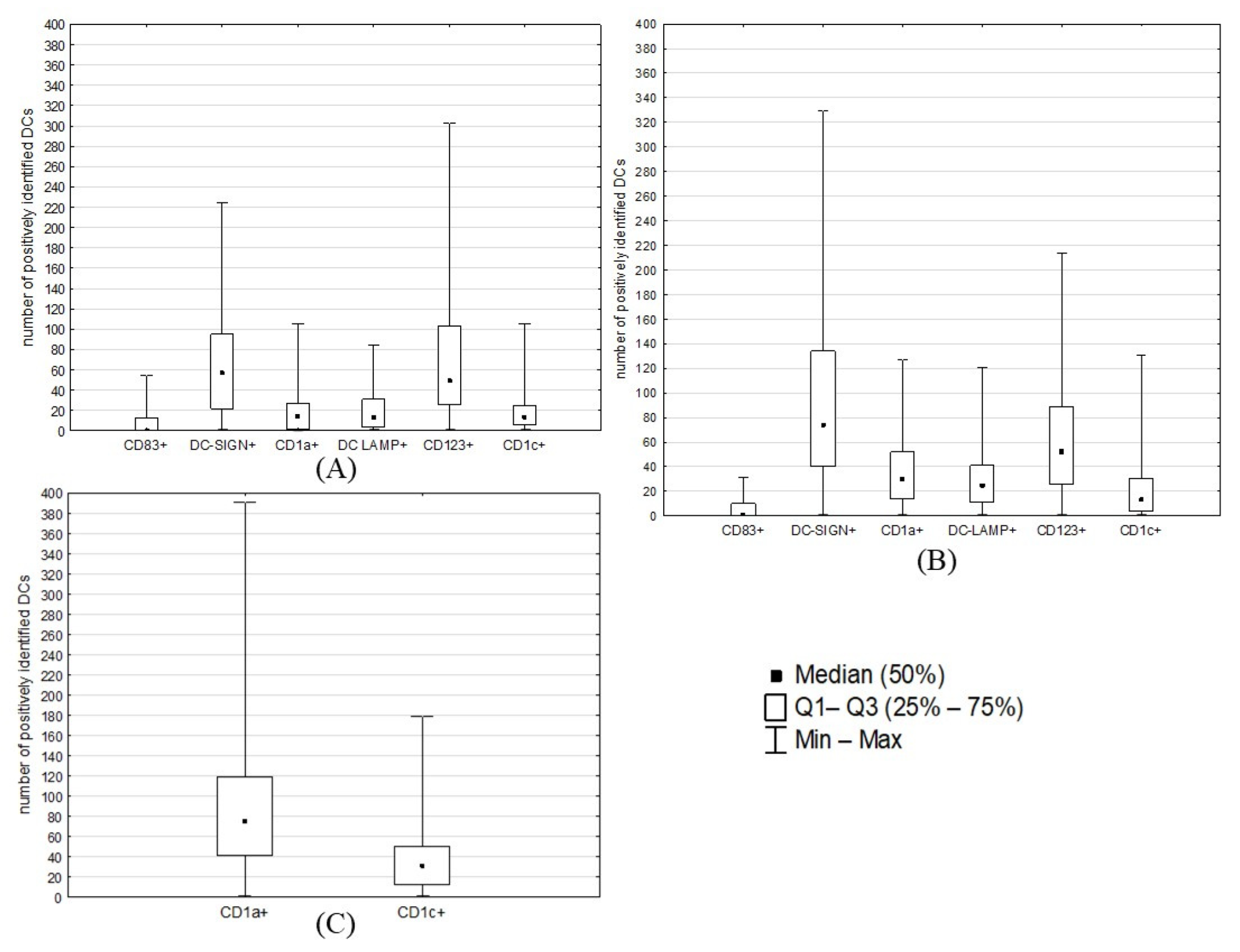
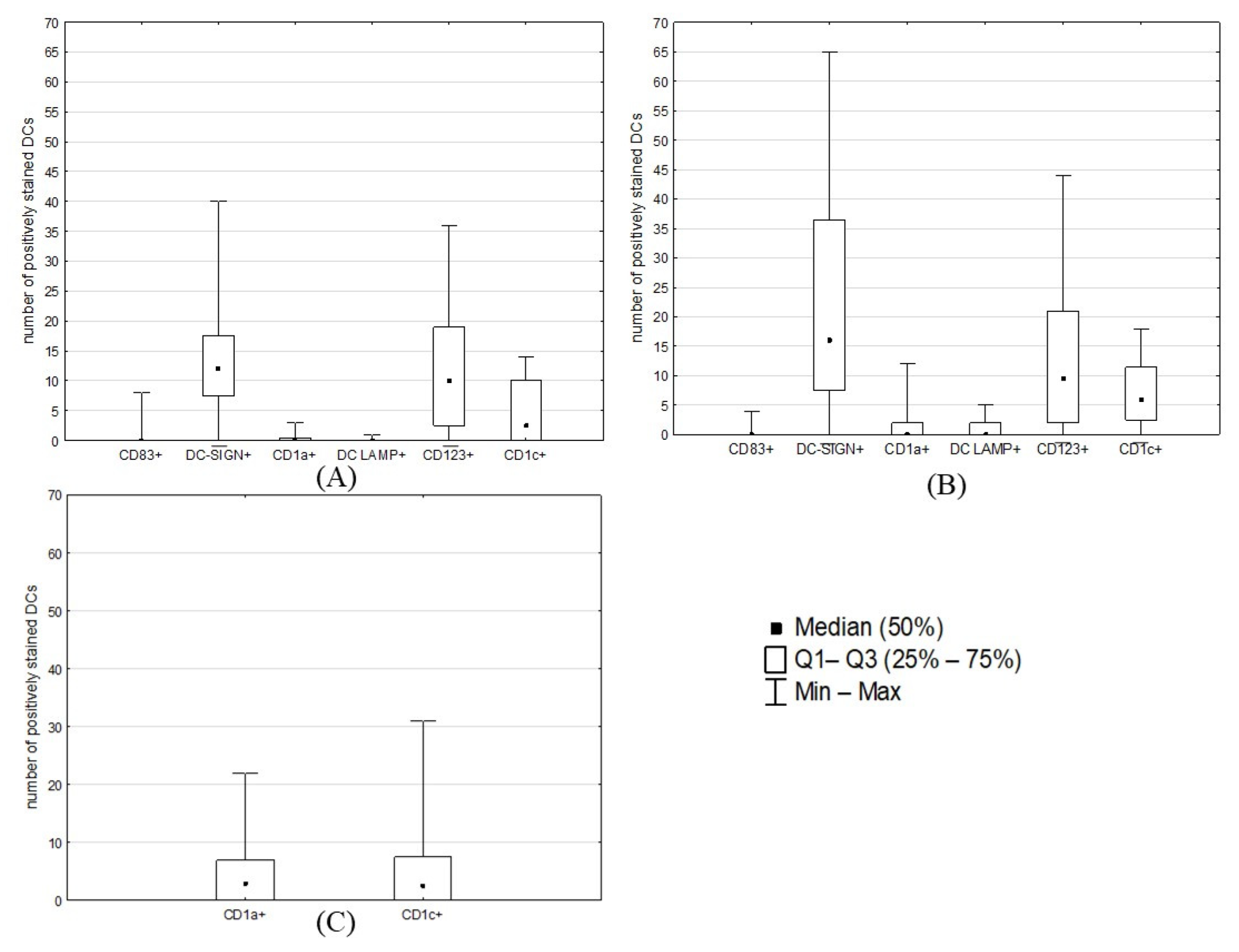
2.2. Counts of Different DC Subsets
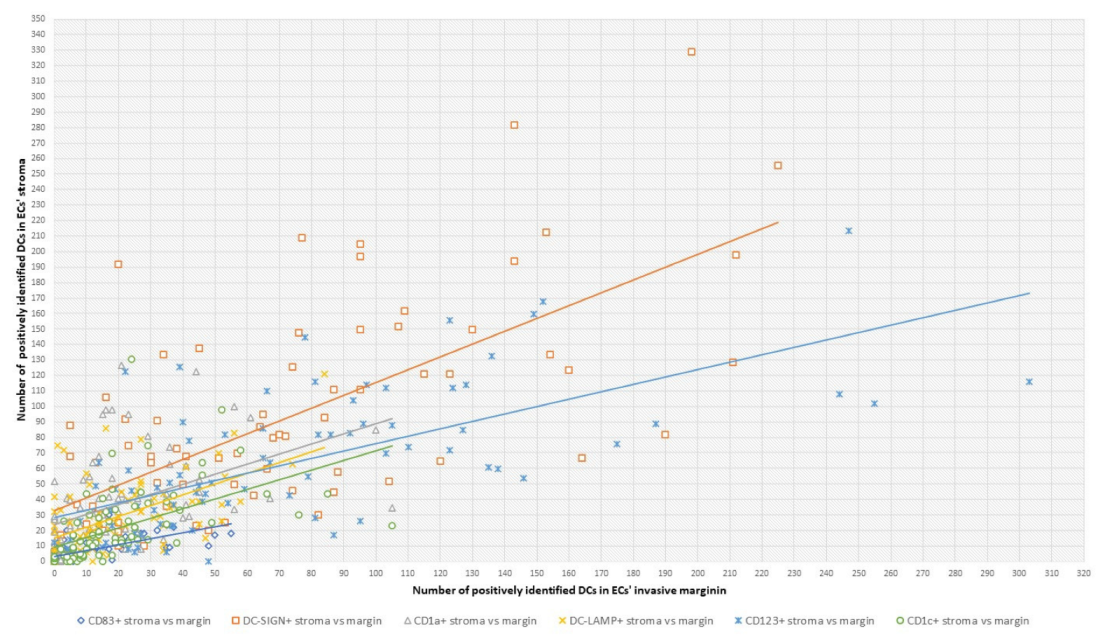
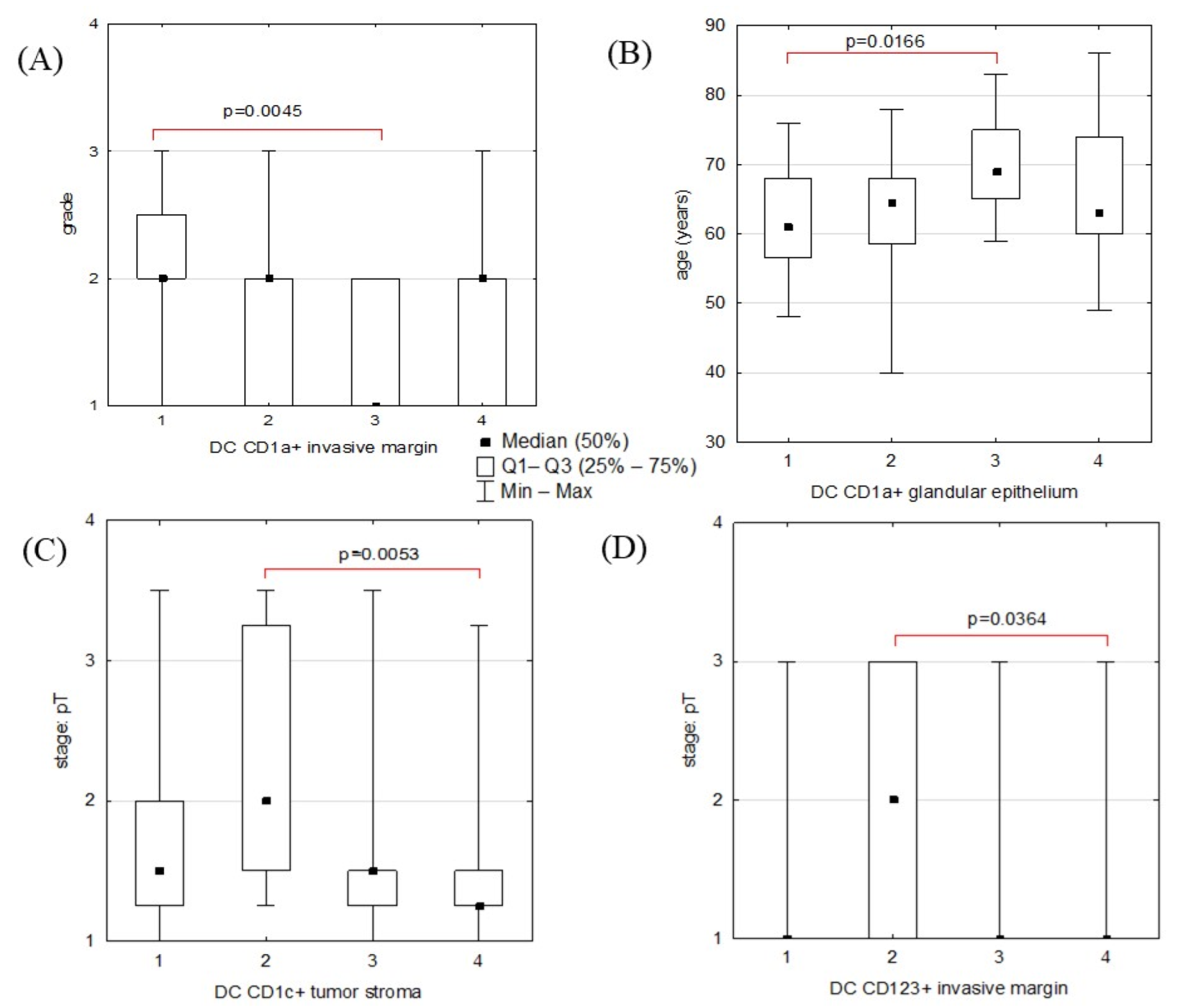
2.3. Statistical Analyses: Correlations between DC Counts and ECs’ Clinicopathological Features
3. Discussion
4. Materials and Methods
4.1. Tissue Specimens
4.2. Immunohistochemistry
4.3. Evaluation of Immunostaining
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Zhang, X.D.; Hondermarck, H.; Tanwar, P.S. The Emerging Role of the Microenvironment in Endometrial Cancer. Cancers 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wan, X. Prognostic significance of immune landscape in tumour microenvironment of endometrial cancer. J. Cell Mol. Med. 2020, 24, 7767–7777. [Google Scholar] [CrossRef]
- Diao, J.; Zhao, J.; Winter, E.; Cattral, M.S. Recruitment and Differentiation of Conventional Dendritic Cell Precursors in Tumors. J. Immunol. 2009, 184, 1261–1267. [Google Scholar] [CrossRef]
- Zhang, S.; Chopin, M.; Nutt, S.L. Type 1 conventional dendritic cells: Ontogeny, function, and emerging roles in cancer immunotherapy. Trends Immunol. 2021, 42, 1113–1127. [Google Scholar] [CrossRef]
- Böttcher, J.P.; e Sousa, C.R. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef]
- Plaxe, C.S.; Mundt, J.A.; Overview of Endometrial Carcinoma. UpToDate. 2022. Available online: https://www.uptodate.com/contents/overview-of-endometrial-carcinoma. (accessed on 13 January 2023).
- Guo, F.; Dong, Y.; Tan, Q.; Kong, J.; Yu, B. Tissue Infiltrating Immune Cells as Prognostic Biomarkers in Endometrial Cancer: A Meta-Analysis. Dis. Markers 2020, 2020, 1805764. [Google Scholar] [CrossRef] [PubMed]
- Kara, P.P.; Ayhan, A.; Caner, B.; Gultekin, M.; Ugur, O.; Bozkurt, M.F.; Usubutun, A.; Uner, A. Analysis of Dendritic Cells in Sentinel Lymph Nodes of Patients with Endometrial and Patients with Cervical Cancers. Int. J. Gynecol. Cancer 2009, 19, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Lijun, Z.; Xin, Z.; Danhua, S.; Xiaoping, L.; Jianliu, W.; Huilan, W.; Lihui, W. Tumor-Infiltrating Dendritic Cells May Be Used as Clinicopathologic Prognostic Factors in Endometrial Carcinoma. Int. J. Gynecol. Cancer 2012, 22, 836–841. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Li, X.; Wang, Z.; Wang, X. Morphological characteristics and co-stimulatory molecule (CD80, CD86, CD40) expression in tumor infiltrating dendritic cells in human endometrioid adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 160, 223–227. [Google Scholar] [CrossRef]
- Li, Z.; Ju, X.; Silveira, P.A.; Abadir, E.; Hsu, W.-H.; Hart, D.N.J.; Clark, G. CD83: Activation Marker for Antigen Presenting Cells and Its Therapeutic Potential. Front. Immunol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Krzyzak, L.; Seitz, C.; Urbat, A.; Hutzler, S.; Ostalecki, C.; Gläsner, J.; Hiergeist, A.; Gessner, A.; Winkler, T.H.; Steinkasserer, A.; et al. CD83 Modulates B Cell Activation and Germinal Center Responses. J. Immunol. 2016, 196, 3581–3594. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Hamada, T.; Asagoe, K.; Umemura, H.; Mizuno-Ikeda, K.; Aoyama, Y.; Otsuka, M.; Yamasaki, O.; Iwatsuki, K. Increase of DC-LAMP+ mature dendritic cell subsets in dermatopathic lymphadenitis of mycosis fungoides. Eur. J. Dermatol. 2014, 24, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Giorello, M.B.; Matas, A.; Marenco, P.; Davies, K.M.; Borzone, F.R.; Calcagno, M.D.L.; García-Rivello, H.; Wernicke, A.; Martinez, L.M.; Labovsky, V.; et al. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer 2021, 28, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Minesaki, A.; Kai, K.; Kuratomi, Y.; Aishima, S. Infiltration of CD1a-positive dendritic cells in advanced laryngeal cancer correlates with unfavorable outcomes post-laryngectomy. BMC Cancer 2021, 21, 973. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, W.; Gu, Y.; Chang, X.; Wei, G.; Rong, Z.; Qin, L.; Chen, X.; Zhou, F. Non-small Cell Lung Cancer Cells Modulate the Development of Human CD1c+ Conventional Dendritic Cell Subsets Mediated by CD103 and CD205. Front. Immunol. 2019, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Ma, B.Y.; Murai, R.; Nakamura, N.; Baba, M.; Kawasaki, N.; Hodohara, K.; Asano, S.; Kawasaki, T. Glycosylation-Dependent Interactions of C-Type Lectin DC-SIGN with Colorectal Tumor-Associated Lewis Glycans Impair the Function and Differentiation of Monocyte-Derived Dendritic Cells. J. Immunol. 2008, 180, 3347–3356. [Google Scholar] [CrossRef]
- Fraga, G.R.; Chow, P. Plasmacytoid dendritic cells in keratoacanthoma and squamous cell carcinoma: A blinded study of CD123 as a diagnostic marker. J. Cutan. Pathol. 2019, 47, 17–21. [Google Scholar] [CrossRef]
- Minkov, P.; Gulubova, M.; Ivanova, K.; Obretenov, E.; Ananiev, J. CD11c- and CD123-positive dendritic cells in development of antitumour immunity in non-small cell lung cancer patients. Pol. J. Pathol. 2019, 70, 109–114. [Google Scholar] [CrossRef]
- Cao, W.; Ma, X.; Fischer, J.V.; Sun, C.; Kong, B.; Zhang, Q. Immunotherapy in endometrial cancer: Rationale, practice and perspectives. Biomark. Res. 2021, 9, 49. [Google Scholar] [CrossRef]
- Van der Woude, H.; Hally, K.E.; Currie, M.J.; Gasser, O.; Henry, C.E. Importance of the endometrial immune environment in endometrial cancer and associated therapies. Front. Oncol. 2022, 12, 975201. [Google Scholar] [CrossRef]
- Azad, N.S.; Gray, R.J.; Overman, M.J.; Schoenfeld, J.D.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Streicher, H.; Li, S.; McShane, L.M.; et al. Nivolumab Is Effective in Mismatch Repair–Deficient Noncolorectal Cancers: Results From Arm Z1D—A Subprotocol of the NCI-MATCH (EAY131) Study. J. Clin. Oncol. 2020, 38, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Ascierto, P.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.; Bariani, G.; Acosta, A.D.J.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Sadeghzadeh, M.; Bornehdeli, S.; Mohahammadrezakhani, H.; Abolghasemi, M.; Poursaei, E.; Asadi, M.; Zafari, V.; Aghebati-Maleki, L.; Shanehbandi, D. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020, 254, 117580. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Kvedaraite, E.; Ginhoux, F. Human dendritic cells in cancer. Sci. Immunol. 2022, 7, eabm9409. [Google Scholar] [CrossRef]
- Maciejewski, R.; Radej, S.; Furmaga, J.; Chrościcki, A.; Rudzki, S.; Roliński, J.; Wallner, G. Evaluation of immature monocyte-derived dendritic cells generated from patients with colorectal cancer. Ann. Surg. 2013, 85, 714–720. [Google Scholar] [CrossRef]
- den Dunnen, J.; Gringhuis, S.I.; Geijtenbeek, T.B.H. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol. Immunother. 2009, 58, 1149–1157. [Google Scholar] [CrossRef]
- Schwingshackl, P.; Obermoser, G.; Nguyen, V.; Fritsch, P.; Sepp, N.; Romani, N. Distribution and Maturation of Skin Dendritic Cell Subsets in Two Forms of Cutaneous T-Cell Lymphoma: Mycosis Fungoides and Sézary Syndrome. Acta Derm. Venereol. 2012, 92, 269–275. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.K.; Mick, R.; Feldman, M.; Hino, S.; Wang, Y.; Brose, M.S.; Muschel, R.J. Distribution of dendritic cell subtypes in primary oral squamous cell carcinoma is inconsistent with a functional response. Cancer Lett. 2007, 255, 145–152. [Google Scholar] [CrossRef]
- van Gisbergen, K.P.; Aarnoudse, C.A.; Meijer, G.A.; Geijtenbeek, T.B.; van Kooyk, Y. Dendritic Cells Recognize Tumor-Specific Glycosylation of Carcinoembryonic Antigen on Colorectal Cancer Cells through Dendritic Cell–Specific Intercellular Adhesion Molecule-3–Grabbing Nonintegrin. Cancer Res 2005, 65, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, X.; Zhang, J.; Wang, K.; Zhang, Y.; Shang, W.; Zhang, Y.; Cui, J.; Shi, X.; Na, H.; et al. DC-SIGN–LEF1/TCF1–miR-185 feedback loop promotes colorectal cancer invasion and metastasis. Cell Death Differ. 2019, 27, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Aerts-Toegaert, C.; Heirman, C.; Tuyaerts, S.; Corthals, J.; Aerts, J.L.; Bonehill, A.; Thielemans, K.; Breckpot, K. CD83 expression on dendritic cells and T cells: Correlation with effective immune responses. Eur. J. Immunol. 2007, 37, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Grosche, L.; Knippertz, I.; König, C.; Royzman, D.; Wild, A.B.; Zinser, E.; Sticht, H.; Muller, Y.A.; Steinkasserer, A.; Lechmann, M. The CD83 Molecule—An Important Immune Checkpoint. Front. Immunol. 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.B.; Krzyzak, L.; Peckert, K.; Stich, L.; Kuhnt, C.; Butterhof, A.; Seitz, C.; Mattner, J.; Grüner, N.; Gänsbauer, M.; et al. CD83 orchestrates immunity toward self and non-self in dendritic cells. J. Clin. Investig. 2019, 4, e126246. [Google Scholar] [CrossRef]
- Kashimura, S.; Saze, Z.; Terashima, M.; Soeta, N.; Ohtani, S.; Osuka, F.; Kogure, M.; Gotoh, M. CD83+ dendritic cells and Foxp3+ regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 2011, 15, 144–153. [Google Scholar] [CrossRef]
- Yu, K.J.; Rader, J.S.; Borecki, I.; Zhang, Z.; Hildesheim, A. CD83 polymorphisms and cervical cancer risk. Gynecol. Oncol. 2009, 114, 319–322. [Google Scholar] [CrossRef]
- Martinet, L.; Filleron, T.; Le Guellec, S.; Rochaix, P.; Garrido, I.; Girard, J.-P. High Endothelial Venule Blood Vessels for Tumor-Infiltrating Lymphocytes Are Associated with Lymphotoxin β–Producing Dendritic Cells in Human Breast Cancer. J. Immunol. 2013, 191, 2001–2008. [Google Scholar] [CrossRef]
- Akhave, N.; Zhang, J.; Bayley, E.; Frank, M.; Chiou, S.-H.; Behrens, C.; Chen, R.; Hu, X.; Parra, E.R.; Lee, W.-C.; et al. Immunogenomic profiling of lung adenocarcinoma reveals poorly differentiated tumors are associated with an immunogenic tumor microenvironment. Lung Cancer 2022, 172, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Medler, T.R.; Blair, T.C.; Crittenden, M.R.; Gough, M.J. Defining Immunogenic and Radioimmunogenic Tumors. Front. Oncol. 2021, 11, 667075. [Google Scholar] [CrossRef]
- Villablanca, E.; Raccosta, L.; Zhou, D.; Fontana, R.; Maggioni, D.; Negro, A.; Sanvito, F.; Ponzoni, M.; Valentinis, B.; Bregni, M.; et al. Tumor-mediated liver X receptor-α activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 2009, 16, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hilly, O.; Rath-Wolfson, L.; Koren, R.; Mizrachi, A.; Hamzany, Y.; Bachar, G.; Shpitzer, T. CD1a-positive dendritic cell density predicts disease-free survival in papillary thyroid carcinoma. Pathol. Res. Pract. 2015, 211, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Amoueian, S.; Attaranzadeh, A.; Montazer, M. Intratumoral CD68-, CD117-, CD56-, and CD1a-positive immune cells and the survival of Iranian patients with non-metastatic intestinal-type gastric carcinoma. Pathol. Res. Pract. 2015, 211, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Jardim, J.F.; Gondak, R.; Galvis, M.M.; Pinto, C.A.L.; Kowalski, L.P. A decreased peritumoral CD1a+ cell number predicts a worse prognosis in oral squamous cell carcinoma. Histopathology 2017, 72, 905–913. [Google Scholar] [CrossRef]
- Iliadis, A.; Koletsa, T.; Patsatsi, A.; Georgiou, E.; Sotiriadis, D.; Kostopoulos, I. The cellular microenvironment and neoplastic population in mycosis fungoides skin lesions: A clinicopathological correlation. Eur. J. Dermatol. 2016, 26, 566–571. [Google Scholar] [CrossRef]
- Shurin, M.R.; Shurin, G.V.; Chatta, G.S. Aging and the dendritic cell system: Implications for cancer. Crit. Rev. Oncol. 2007, 64, 90–105. [Google Scholar] [CrossRef]
- Roda, Â.; Travassos, A.R.; Soares-De-Almeida, L.; Kutzner, H. Lupus erythematosus mimicking mycosis fungoides: CD123+ plasmacytoid dendritic cells as a useful diagnostic clue. J. Cutan. Pathol. 2018, 46, 167–170. [Google Scholar] [CrossRef]
- Mitchell, D.; Chintala, S.; Dey, M. Plasmacytoid dendritic cell in immunity and cancer. J. Neuroimmunol. 2018, 322, 63–73. [Google Scholar] [CrossRef]
- Conrad, C.; Gregorio, J.; Wang, Y.-H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.-J.; et al. Plasmacytoid Dendritic Cells Promote Immunosuppression in Ovarian Cancer via ICOS Costimulation of Foxp3+ T-Regulatory Cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef] [PubMed]
- Faget, J.; Sisirak, V.; Blay, J.-Y.; Caux, C.; Bendriss-Vermare, N.; Ménétrier-Caux, C. ICOS is associated with poor prognosis in breast cancer as it promotes the amplification of immunosuppressive CD4+T cells by plasmacytoid dendritic cells. Oncoimmunology 2013, 2, e23185. [Google Scholar] [CrossRef] [PubMed]
- Nierkens, S.; Brok, M.H.D.; Garcia, Z.; Togher, S.; Wagenaars, J.; Wassink, M.; Boon, L.; Ruers, T.J.; Figdor, C.G.; Schoenberger, S.P.; et al. Immune Adjuvant Efficacy of CpG Oligonucleotide in Cancer Treatment Is Founded Specifically upon TLR9 Function in Plasmacytoid Dendritic Cells. Cancer Res. 2011, 71, 6428–6437. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 339–367. [Google Scholar] [CrossRef] [PubMed]

| Patients’ Characteristics | |||
|---|---|---|---|
| Parameters | All patients (94) | ||
| Age, median in years (range) | 64 (40–86) | ||
| Histological type, % in column (n) | Endometroid cancer | 92.55 (87) | |
| Clear cell carcinoma | 1.06 (1) | ||
| Mucinous carcinoma | 1.06 (1) | ||
| Mixed carcinoma | 1.06 (1) | ||
| Carcinosarcoma | 2.13 (2) | ||
| Not specified | 2.13 (2) | ||
| Data for endometroid cancers only: | |||
| Parameters | All patients (87) | ||
| Age, median in years (range) | 64 (40–86) | ||
| Age groups (y.o.), % in column (n) | 1: <40; 50) | 4.60 (4) | |
| 2: <50; 60) | 20.69 (18) | ||
| 3: <60; 70) | 44.83 (39) | ||
| 4: <70; 80) | 24.14 (21) | ||
| 5: ≥80 | 5.75 (5) | ||
| Stage | pT, % in column (n) | 1 | 21.75 (4) |
| 1a | 35.63 (31) | ||
| 1b | 28.74 (25) | ||
| 2 | 12.64 (11) | ||
| 3a | 11.49 (10) | ||
| 3b | 6.90 (6) | ||
| not reported | 0 (0) | ||
| pN, % in column (n) | 0 | 81.61 (71) | |
| 1 | 3.45 (3) | ||
| 2 | 1.15 (1) | ||
| not reported | 13.80 (12) | ||
| Grade, % in column (n) | 1 | 39.08 (34) | |
| 2 | 50.57 (44) | ||
| 3 | 9.20 (8) | ||
| not reported | 1.15 (1) | ||
| Compared Areas | DCs Subtype | p-Value * |
|---|---|---|
| tumor stroma vs. tumor invasive margin | DC CD83+ | 0.6818 |
| DC DC-SIGN+ | 0.0278 | |
| DC CD1a+ | <0.00001 | |
| DC DC-LAMP+ | 0.0061 | |
| DC CD123+ | 0.6312 | |
| DC CD1c+ | 0.9522 |
| Compared Areas | DCs Subtype | p-Value * |
|---|---|---|
| tumor stroma vs. normal endometrial stroma | DC CD83+ | 0.0016 |
| DC DC-SIGN+ | <0.00001 | |
| DC CD1a+ | <0.00001 | |
| DC DC-LAMP+ | <0.00001 | |
| DC CD123+ | <0.00001 | |
| DC CD1c+ | 0.0099 | |
| tumor invasive margin vs. normal endometrial margin | DC CD83+ | 0. 0155 |
| DC DC-SIGN+ | <0.00001 | |
| DC CD1a+ | <0.00001 | |
| DC DC-LAMP+ | <0.00001 | |
| DC CD123+ | <0.00001 | |
| DC CD1c+ | 0.0001 | |
| tumor glands vs. normal endometrial glands | DC CD1a+ | <0.00001 |
| DC CD1c+ | <0.00001 |
| Dependent Variable | Independent (Grouping) Variable | p-Value * |
|---|---|---|
| Grade | DC CD83+ stroma | 0.7527 † |
| DC DC-SIGN+ stroma | 0.5165 | |
| DC CD1a+ stroma | 0.2713 | |
| DC DC-LAMP+ stroma | 0.1511 | |
| DC CD123+ stroma | 0.7394 | |
| DC CD1c+ stroma | 0.8745 | |
| DC CD83+ margin | 0.4618 † | |
| DC DC-SIGN+ margin | 0.2071 | |
| DC CD1a+ margin | 0.0379 (0.0045) ** | |
| DC DC-LAMP+ margin | 0.0942 | |
| DC CD123+ margin | 0.2027 | |
| DC CD1c+ margin | 0.4012 | |
| DC CD1a+ glands | 0.5371 | |
| DC CD1c+ glands | 0.2765 | |
| Stage: pT | DC CD83+ stroma | 0.5902 † |
| DC DC-SIGN+ stroma | 0.1281 | |
| DC CD1a+ stroma | 0.2415 | |
| DC DC-LAMP+ stroma | 0.9356 | |
| DC CD123+ stroma | 0.7739 | |
| DC CD1c+ stroma | 0.0054 (0.0053) ** | |
| DC CD83+ margin | 0.1669 † | |
| DC DC-SIGN+ margin | 0.0670 | |
| DC CD1a+ margin | 0.8728 | |
| DC DC-LAMP+ margin | 0.0705 | |
| DC CD123+ margin | 0.0037 (0.0364) ** | |
| DC CD1c+ margin | 0.4080 | |
| DC CD1a+ glands | 0.4124 | |
| DC CD1c+ glands | 0.6116 | |
| Stage: pN | DC CD83+ stroma | 0.3070 † |
| DC DC-SIGN+ stroma | 0.9977 | |
| DC CD1a+ stroma | 0.1801 | |
| DC DC-LAMP+ stroma | 0.6962 | |
| DC CD123+ stroma | 0.5843 | |
| DC CD1c+ stroma | 0.4771 | |
| DC CD83+ margin | 0.7191 † | |
| DC DC-SIGN+ margin | 0.5881 | |
| DC CD1a+ margin | 0.9788 | |
| DC DC-LAMP+ margin | 0.6839 | |
| DC CD123+ margin | 0.6076 | |
| DC CD1c+ margin | 0.5913 | |
| DC CD1a+ glands | 0.0725 | |
| DC CD1c+ glands | 0.4004 |
| DCs Subtype | Criteria for Group Allocation | No of Samples |
|---|---|---|
| DC CD83+ stroma * | 1st group: No DCs ≤ 1 | 41 |
| 2nd group: No DCs >1 | 43 | |
| missing data | 3 | |
| DC CD83+ margin * | 1st group: No DCs ≤ 0 | 48 |
| 2nd group: No DCs > 0 | 36 | |
| missing data | 3 | |
| DC DC-SIGN+ stroma | 1st group: No DCs < 40 | 20 |
| 2nd group: 40 ≤No DCs < 74 | 20 | |
| 3rd group: 74 ≤No DCs < 134 | 19 | |
| 4th group: No DCs ≥ 134 | 21 | |
| missing data | 7 | |
| DC DC-SIGN+ margin | 1st group: No DCs < 22 | 19 |
| 2nd group: 22 ≤No DCs < 57 | 19 | |
| 3rd group: 57 ≤No DCs < 95 | 17 | |
| 4th group: No DCs ≥ 95 | 22 | |
| missing data | 10 | |
| DC CD1a+ stroma | 1st group: No DCs <14 | 19 |
| 2nd group: 14 ≤No DCs < 30 | 21 | |
| 3rd group: 30 ≤No DCs < 52 | 20 | |
| 4th group: No DCs ≥ 52 | 21 | |
| missing data | 6 | |
| DC CD1a+ margin | 1st group: No DCs < 2 | 16 |
| 2nd group: 2 ≤No DCs < 14.50 | 24 | |
| 3rd group: 14.50 ≤No DCs < 27 | 19 | |
| 4th group: No DCs ≥ 27 | 21 | |
| missing data | 7 | |
| DC CD1a+ glands | 1st group: No DCs < 41 | 20 |
| 2nd group: 41 ≤No DCs < 75 | 20 | |
| 3rd group: 75 ≤No DCs < 119 | 18 | |
| 4th group: No DCs ≥ 119 | 23 | |
| missing data | 6 | |
| DC LAMP+ stroma | 1st group: No DCs < 11 | 20 |
| 2nd group: 11 ≤No DCs < 25 | 20 | |
| 3rd group: 25 ≤No DCs < 41 | 22 | |
| 4th group: No DCs ≥ 41 | 21 | |
| missing data | 4 | |
| DC LAMP+ margin | 1st group: No DCs < 4 | 18 |
| 2nd group: 4 ≤No DCs < 14 | 20 | |
| 3rd group: 14 ≤No DCs < 31 | 23 | |
| 4th group: No DCs ≥ 31 | 22 | |
| missing data | 4 | |
| CD123+ stroma | 1st group: No DCs < 25.50 | 21 |
| 2nd group: 25.50 ≤No DCs < 52.50 | 21 | |
| 3rd group: 52.50 ≤No DCs < 88.50 | 21 | |
| 4th group: No DCs ≥ 88.50 | 21 | |
| missing data | 3 | |
| CD123+ margin | 1st group: No DCs < 26 | 21 |
| 2nd group: 26 ≤No DCs < 49 | 21 | |
| 3rd group: 49 ≤No DCs < 103 | 21 | |
| 4th group: No DCs ≥ 103 | 22 | |
| missing data | 3 | |
| CD1c+ stroma | 1st group: No DCs < 4 | 20 |
| 2nd group: 4 ≤No DCs < 13 | 21 | |
| 3rd group: 13 ≤No DCs < 30 | 20 | |
| 4th group: No DCs ≥ 30 | 22 | |
| missing data | 4 | |
| CD1c+ margin | 1st group: No DCs < 6 | 19 |
| 2nd group: 6 ≤No DCs < 14 | 22 | |
| 3rd group: 14 ≤No DCs < 25 | 21 | |
| 4th group: No DCs ≥ 25 | 21 | |
| missing data | 4 | |
| CD1c+ glands | 1st group: No DCs < 12 | 20 |
| 2nd group: 12 ≤No DCs < 31 | 18 | |
| 3rd group: 31 ≤No DCs < 50.50 | 22 | |
| 4th group: No DCs ≥ 50.50 | 20 | |
| missing data | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyduch, G.; Miążek, A.; Laskowicz, Ł.; Szpor, J. Distribution of DC Subtypes: CD83+, DC-LAMP+, CD1a+, CD1c+, CD123+, and DC-SIGN+ in the Tumor Microenvironment of Endometrial Cancers—Correlation with Clinicopathologic Features. Int. J. Mol. Sci. 2023, 24, 1933. https://doi.org/10.3390/ijms24031933
Dyduch G, Miążek A, Laskowicz Ł, Szpor J. Distribution of DC Subtypes: CD83+, DC-LAMP+, CD1a+, CD1c+, CD123+, and DC-SIGN+ in the Tumor Microenvironment of Endometrial Cancers—Correlation with Clinicopathologic Features. International Journal of Molecular Sciences. 2023; 24(3):1933. https://doi.org/10.3390/ijms24031933
Chicago/Turabian StyleDyduch, Grzegorz, Apolonia Miążek, Łukasz Laskowicz, and Joanna Szpor. 2023. "Distribution of DC Subtypes: CD83+, DC-LAMP+, CD1a+, CD1c+, CD123+, and DC-SIGN+ in the Tumor Microenvironment of Endometrial Cancers—Correlation with Clinicopathologic Features" International Journal of Molecular Sciences 24, no. 3: 1933. https://doi.org/10.3390/ijms24031933
APA StyleDyduch, G., Miążek, A., Laskowicz, Ł., & Szpor, J. (2023). Distribution of DC Subtypes: CD83+, DC-LAMP+, CD1a+, CD1c+, CD123+, and DC-SIGN+ in the Tumor Microenvironment of Endometrial Cancers—Correlation with Clinicopathologic Features. International Journal of Molecular Sciences, 24(3), 1933. https://doi.org/10.3390/ijms24031933







