Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Silibinin Inhibits the Phenotypical Maturation of DC Induced by LPS Stimulation
2.2. Silibinin Inhibits Proinflammatory Cytokine Production by LPS-Stimulated DC
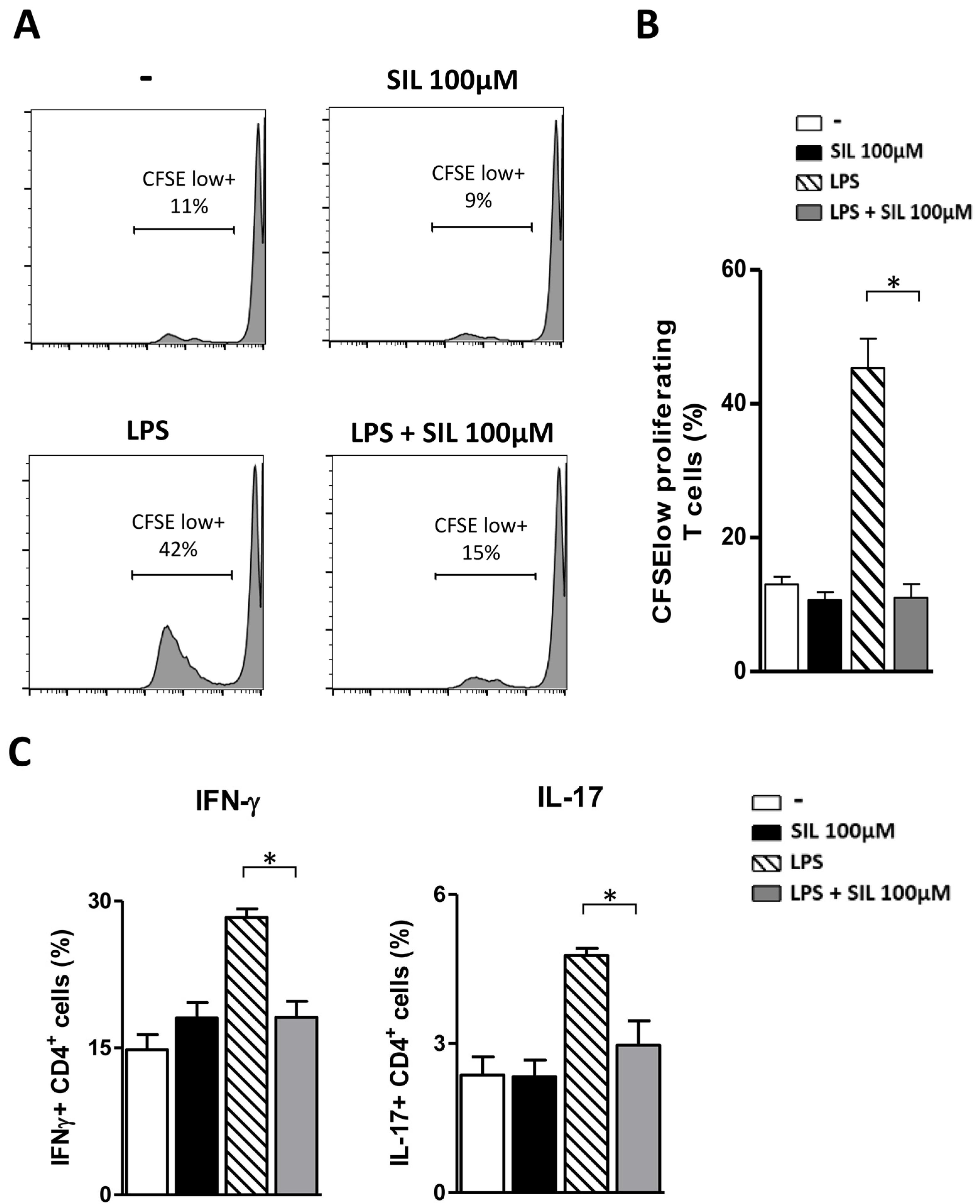
2.3. Mature DC Treated with Silibinin Do Not Present Alloantigens to Memory CD4+ T Lymphocytes
3. Materials and Methods
3.1. Monocyte Isolation and DC Generation
3.2. Abs and Other Reagents
3.3. Flow Cytometric Analysis
3.4. Cytokine Production Analysis
3.5. Mixed Leukocyte Reaction (MLR)
3.6. Analysis of Intracellular Cytokine Production
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zhang, Z.; Wu, S.-C. Health Benefits of Silybum Marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk Thistle in Liver Diseases: Past, Present, Future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Li, X.; Wang, S.; Dong, Y.; Yin, H.; Gu, Z.; Na, X.; Wei, X.; Yuan, J.; Cao, J.; et al. Silibinin Ameliorates Formaldehyde-Induced Cognitive Impairment by Inhibiting Oxidative Stress. Oxidative Med. Cell. Longev. 2022, 2022, 5981353. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to Improve Cancer Therapeutic, as an Apoptotic Inducer, Autophagy Modulator, Cell Cycle Inhibitor, and MicroRNAs Regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin Impacts on Immune System as an Immunomodulator: One Key for Many Locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Bannwart, C.F.; Nakaira-Takahagi, E.; Golim, M.A.; de Medeiros, L.T.L.; Romão, M.; Weel, I.C.; Peraçoli, M.T.S. Downregulation of Nuclear Factor-Kappa B (NF-KappaB) Pathway by Silibinin in Human Monocytes Challenged with Paracoccidioides Brasiliensis. Life Sci. 2010, 86, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyàs, E.; Llorach-Parés, L.; Pérez-Sánchez, A.; Micol, V.; Nonell-Canals, A.; Joven, J.; Valiente, M.; Sánchez-Martínez, M.; Bosch-Barrera, J.; et al. Silibinin Is a Direct Inhibitor of STAT3. Food Chem. Toxicol. 2018, 116, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Jafari, S.; Esmaeil, N.; Javid, E.N.; Bagherpour, B.; Rezaei, A. Immunosuppressive Effect of Silymarin on Mitogen-Activated Protein Kinase Signalling Pathway: The Impact on T Cell Proliferation and Cytokine Production. Basic Clin. Pharmacol. Toxicol. 2013, 113, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Essa, S.; Siddique, I.; Saad, M.; Raghupathy, R. Modulation of Production of Th1/Th2 Cytokines in Peripheral Blood Mononuclear Cells and Neutrophils by Hepatitis C Virus Infection in Chronically Infected Patients. Pathogens 2021, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.L.; Conti, F.; Maselli, A.; Pagano, M.T.; Ruggieri, A.; Anticoli, S.; Fragale, A.; Gabriele, L.; Gagliardi, M.C.; Sanchez, M.; et al. The Natural Agonist of Estrogen Receptor β Silibinin Plays an Immunosuppressive Role Representing a Potential Therapeutic Tool in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-L.; Shi, X.-W. Silybin Alleviates Experimental Autoimmune Encephalomyelitis by Suppressing Dendritic Cell Activation and Th17 Cell Differentiation. Front. Neurol. 2021, 12, 659678. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.-H.; Jeong, Y.-I.; Lee, C.-M.; Yoon, M.-S.; Na, Y.J.; Suh, D.-S.; Park, N.C.; et al. Silibinin Polarizes Th1/Th2 Immune Responses through the Inhibition of Immunostimulatory Function of Dendritic Cells. J. Cell. Physiol. 2007, 210, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, H.; Thomson, A.W. Dendritic Cells: Emerging Pharmacological Targets of Immunosuppressive Drugs. Nat. Rev. Immunol. 2004, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, M.T.; Fecchi, K.; Pierdominici, M.; Ortona, E.; Peruzzu, D. Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin. Int. J. Mol. Sci. 2022, 23, 10417. https://doi.org/10.3390/ijms231810417
Pagano MT, Fecchi K, Pierdominici M, Ortona E, Peruzzu D. Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin. International Journal of Molecular Sciences. 2022; 23(18):10417. https://doi.org/10.3390/ijms231810417
Chicago/Turabian StylePagano, Maria Teresa, Katia Fecchi, Marina Pierdominici, Elena Ortona, and Daniela Peruzzu. 2022. "Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin" International Journal of Molecular Sciences 23, no. 18: 10417. https://doi.org/10.3390/ijms231810417
APA StylePagano, M. T., Fecchi, K., Pierdominici, M., Ortona, E., & Peruzzu, D. (2022). Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin. International Journal of Molecular Sciences, 23(18), 10417. https://doi.org/10.3390/ijms231810417







